Significance
In animal conflict, the more information individuals have about their social world the better decisions they can make about whom to fight. Determining what animals “know,” however, has proved difficult. We reverse-engineer how information about an individual’s rank structures their aggression. Applying this method to species from ants to primates, we found that while most groups use simple rules to choose fights some groups used more information-rich patterns. A key result is that these information-rich patterns were not restricted to species thought to be more cognitively sophisticated and that their use varied within species. Our work connects sociality with information, provides possibilities for comparative analyses, and opens avenues to study the relationship between individual decision-making and social outcomes.
Keywords: animal sociality, animal conflict, dominance hierarchy, self-organizing system
Abstract
Members of a social species need to make appropriate decisions about who, how, and when to interact with others in their group. However, it has been difficult for researchers to detect the inputs to these decisions and, in particular, how much information individuals actually have about their social context. We present a method that can serve as a social assay to quantify how patterns of aggression depend upon information about the ranks of individuals within social dominance hierarchies. Applied to existing data on aggression in 172 social groups across 85 species in 23 orders, it reveals three main patterns of rank-dependent social dominance: the downward heuristic (aggress uniformly against lower-ranked opponents), close competitors (aggress against opponents ranked slightly below self), and bullying (aggress against opponents ranked much lower than self). The majority of the groups (133 groups, 77%) follow a downward heuristic, but a significant minority (38 groups, 22%) show more complex social dominance patterns (close competitors or bullying) consistent with higher levels of social information use. These patterns are not phylogenetically constrained and different groups within the same species can use different patterns, suggesting that heuristic use may depend on context and the structuring of aggression by social information should not be considered a fixed characteristic of a species. Our approach provides opportunities to study the use of social information within and across species and the evolution of social complexity and cognition.
Social individuals can gain from social interactions, but close associations with potential competitors introduce the risk of costly aggression. Decisions about who and how to interact with others are often based on an individual’s assessment of its own abilities (e.g., refs. 1–3) or on the strength of relationships between pairs of individuals. These decisions can also be made on the basis of a larger social context—most notably, on the basis of rank in a dominance hierarchy. Evidence across multiple social situations shows that decisions about interactions can be affected by rank across both humans and animals: friendships among school children (4), messaging patterns in online social dating applications (5), and grooming in female primates (e.g., refs. 6 and 7) can all be affected by rank.
Almost 100 years of research on animal conflict has shown that rank matters. Dominance hierarchies structure group interactions in a vast array of animals, from primates and hyenas to fish and wasps (e.g., refs. 8–13), including humans (e.g., refs. 14 and 15). Previous research has shown that aggression networks underlying dominance hierarchies in species across the phylogenetic tree are built from remarkably similar basic structures (16). Other studies have documented the large effects rank can have on an individual’s stress, health, and fitness (e.g., refs. 17–19). In the last 30 to 70 years, studies in both empirical and theoretical contexts have provided insight into the major factors affecting the formation of hierarchies (e.g., refs. 20–22).
Although we now understand that dominance hierarchies are widespread, that rank is often important, and the basics of how hierarchies form in many species, a critical open question is what animals within these hierarchies “know” about their own rank and the ranks of others. Social information is increasingly recognized as a critical component for understanding the structure of animal societies (23, 24). Individuals can gather social information by attending to the signals and behaviors of their group members (1, 25, 26). If individuals can perceive something about their own rank or the ranks of others in their group, they could use that information to better maximize their potential gains from aggression and minimize potential losses or injury. In the context of conflict in hierarchically ordered groups, various kinds of social information can be gleaned from the outcomes of aggressive interactions such as social information about an individual’s own ability to win fights against opponents, the relationships it has with others, relationships among others in the group, an individual’s own rank and the rank of others, or the group’s overall dominance structure (1). The more information that individuals can access, process, and use in their decision-making the more patterns of micro-level aggressive actions become fundamentally entwined with macro-level structural information about rank in social groups (23).
Most previous studies to detect social information about rank in animals have required extensive experimental manipulation, involving reversing the apparent outcome of observed fights and testing whether uninvolved individuals are more attentive to fights which violate the order of rank in the hierarchy than fights with more expected outcomes. These experiments have been instrumental in demonstrating the extent of rank information contained in some animal groups (e.g., refs. 27 and 28). However, it has been difficult to assess rank information across many species because these experiments are time-intensive and require social systems in which fight outcomes can be easily artificially manipulated, which limits our abilities to conduct comparative analyses of information use across animals.
We take a different approach to address the question of what animals may “know” about rank by quantifying the presence, amount, and type of social information contained in animal dominance hierarchies. We define social information as any information about an individual’s interactions, relationships, or status held by that individual about itself or others in its group. To quantify social information, we developed computational methods to detect signatures of the kinds of social dominance patterns that characterize conflict in animal hierarchies. Our approach has three major benefits: 1) it provides computational rather than experimental methods that allow for detection of the presence and use of information, 2) the methods can be used with existing data, providing new opportunities for comparisons across a wide range of species, and 3) our focus on the structural properties and how information is contained and used in animal systems is agnostic to whether emergent patterns are based on complex cognition and strategic decision-making or are the result of much simpler mechanistic rules.
Our approach focuses on inferring the kinds of information about rank that are contained within patterns of social interactions. Our methods allow us to connect each individual’s micro-level decisions about aggression with macrolevel social properties like the structure of group dominance hierarchies. If the same decision-making process is used across individuals in a group, a group’s aggression patterns can be characterized. We refer to rank-dependent aggression as conflict in animal groups that is contingent on the relative rank differences between the individuals. Rank-dependent aggression forms the basis for the emergence of simple rules or heuristics about aggression, which we refer to as social dominance patterns. Different social dominance patterns may emerge depending on the detail of rank information individuals have.
We apply these methods to a large empirical dataset on aggression and dominance in 172 independent social groups across 85 species in 23 orders (16, 29).
To detect rank-dependent social dominance patterns, we developed a four-step process. First, we developed focus and position as summary measures to quantify the extent to which group conflict is affected by rank. Each individual in the group is assessed to determine how it aggresses against opponents based on relative rank difference (how many steps in rank above or below the aggressor its opponents are in rank). Focus quantifies the extent to which aggression is concentrated on a subset of opponents and measures the fraction of aggression that was directed between individuals separated a certain number of steps in relative rank compared to aggression that could have been directed the same number of steps away. If rank information is present and is used to concentrate aggression, then knowing where in relative-rank difference terms the peak of aggression is focused can tell us about the kind of social dominance pattern the group is using. To differentiate between different ways that rank may inform decision-making, we measure a second quantity, position, which reflects where in relative rank difference aggressors concentrate their aggression.
We then defined three main social dominance patterns: 1) the downward heuristic, where individuals aggress against lower-ranked individuals regardless of their particular rank value relative to the aggressor; 2) close competitors, where individuals aggress preferentially toward those just below themselves in rank; and 3) bullying, where individuals aggress preferentially toward those ranked far below themselves in rank. Next, we categorized which social dominance pattern animals in each group followed. We assigned social dominance type by comparing focus and position values from the observed groups to those produced by an ensemble of permutation-based reference models (30, 31) simulating conflict via specified rules. These reference models allow us to simulate what aggression should look like if individuals in the group only follow the specified interaction rules rather than incorporating any additional information about the ranks of their opponents.
Finally, we compared the reference datasets to the empirical datasets to evaluate whether observed aggression patterns could plausibly have been generated by animals following the simplest social dominance pattern (the downward heuristic) or if more detailed information is needed to describe observed aggression patterns. Importantly, our methods are agnostic to the ways in which social information is encoded in these social systems. Information could be stored cognitively but may also be encoded in other less cognitively demanding ways, such as through observable signals.
Combined, our quantitative methods, our reference model comparisons, and our detection of social rules governing social dominance patterns within hierarchies provide insight into how animals structure their social relationships and how they make biologically relevant social decisions.
Results and Discussion
Rank-Dependent Social Dominance.
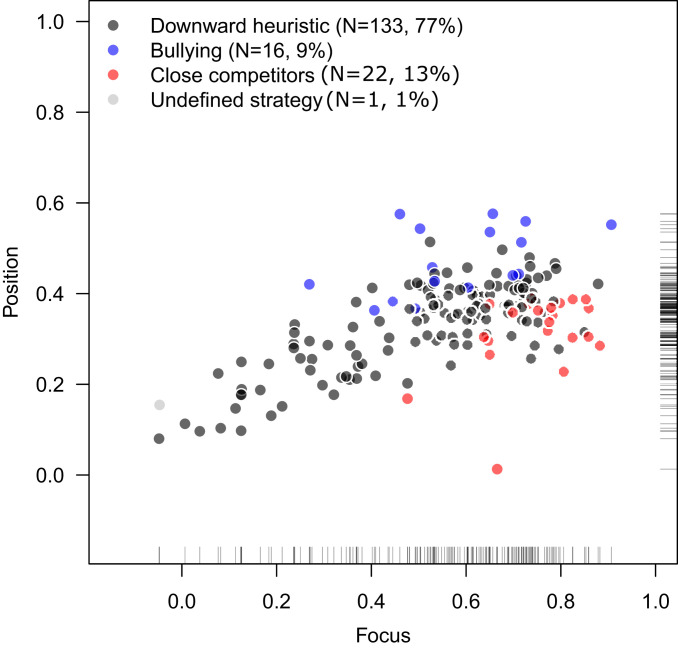
Our measures of focus show that the majority of animal social groups in our dataset had evidence of structured aggression (SI Appendix, sections SI 2 and SI 3). While focus values show how strong the hierarchical organization is, our measures of position allow us to diagnose the type of social dominance individuals used within the hierarchical structure. Based on our summary measures of focus and position for each group (Fig. 1), aggression patterns in nearly all groups ( of groups, ) could be categorized without ambiguity to one of three main aggression patterns: the downward heuristic, close competitors, or bullying (Fig. 2).
Fig. 1.
Focus and position values for observed social groups, colored by social dominance pattern type (see Fig. 2 for categorization). Focus is a measure of how concentrated aggression is, given the relative rank differences from all individuals to their potential opponents (as more aggression is restricted to a subset of opponents focus values become higher). Position values measure where in relative rank difference this aggression is concentrated: When individuals focus their aggression on opponents ranked just below themselves in the hierarchy, position values are near 0; when aggression is focused on opponents ranked far below, position is near 1.
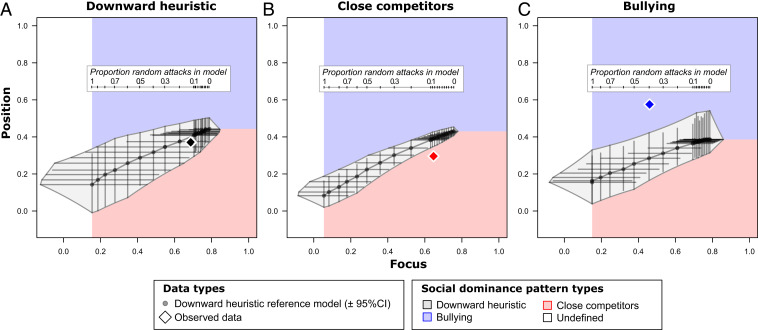
Fig. 2.
Each social group was categorized by which rank-dependent social dominance pattern they followed. Shown here are three examples of pattern assignment, to (A) downward heuristic [mule deer (32)], (B) close competitors [monk parakeet (29)], and (C) bullying [vervet monkey (33)]. Diamond points show observed focus and position values for each group. Gray circular points indicate focus and position values ( 95% CI) for reference model datasets generated using a downward heuristic with different proportions of randomly directed aggression (Inset). This ensemble of reference models shows how expected focus and position values change as the proportion of randomly directed aggression events increases, from no randomly directed aggression (100% adherence to the downward heuristic with all aggression directed toward lower-ranked opponents, right side) to fully randomly directed aggression (left side, where aggression is purely driven by individual aggressiveness, with no rank information). Social dominance patterns for each group were assigned by comparing focus and position values in each empirical group to the reference model ensemble for that group. If the observed value fell within the downward heuristic polygon, empirically observed focus and position values could have been produced by a downward heuristic; when values fell outside this polygon, another pattern is needed to explain the observed empirical patterns. When position values were lower than expected, and aggression toward opponents ranked close below in the hierarchy was more common, we categorized the aggression pattern as close competitors and when position values were higher than expected, and aggression toward opponents ranked far below in the hierarchy was more common, we categorized the pattern as bullying.
Each social dominance pattern emerges as individuals preferentially engage with a certain subset of opponents and may be based on the level of detail individuals have about the relative rank difference between themselves and potential opponents. The downward heuristic is the simplest of the three main social dominance patterns and emerges when individuals aggress indiscriminately toward lower-ranked opponents. The focus and position values in of empirical datasets () could have been produced by animals following a simple downward heuristic. However, (38 groups) used social dominance patterns where additional rank information is needed in order to produce the observed patterns. We classify both close competitors and bullying as more complex social dominance patterns because they are based on more detailed rank information than the downward heuristic, as aggressors need to differentiate between lower-ranked potential opponents by whether they are ranked just below or far below themselves in the hierarchy. A close competitors aggression pattern (preferentially aggress against opponents ranked slightly below themselves) was used by of groups and a bullying pattern (preferentially aggress against opponents ranked far below themselves) was used by of groups. Only one group had an undefined social dominance pattern.
Evaluating Other Potential Generative Processes.
Within our empirical datasets on aggression we found no evidence that a group’s social dominance pattern use could be consistently explained by the number of individuals in the social system or whether the group was observed in natural conditions or captivity (SI Appendix, section SI 4 and Figs. S4.1 and S4.2).
We use the presence of either a close competitor or bullying social dominance pattern as an indication of higher levels of social information. These patterns may emerge if individuals have access to that social information and use it to structure their fights with particular opponents beyond simply reacting to their own experiences and treating opponents as interchangeable or anonymous. However, individuals (especially across very different species, with different cognitive systems) may not have access to this more detailed social information (1).
To investigate the role of information in the emergence of more complex social dominance patterns, we constructed another model of aggression to determine how often more complex social dominance patterns might emerge when information is more limited (SI Appendix, section SI 5). We used a generative-process reference model (31) to simulate social groups with 10 individuals to examine how individual-level information about wins and losses results in the emergence of group-level social dominance patterns in the absence of the ability to collect information about the ranks of others in the group. Each individual in our model only has access to its own win/loss record and can only adjust its behavior based on outcomes of events (individuals do not have any information about which other individuals they interacted with or which individuals they have won or lost against). We modeled nine variants: a winner-effect-only model, a loser-effect-only model, and a mixed winner- and loser-effect model; each of these models was further investigated using a transient effect and a permanent effect (SI Appendix, Table S5.1), using winner- and loser-effect strengths from the literature (34) along with both a more extreme and a more moderate value for comparison.
Across all model variants, the majority of simulated groups showed aggression consistent with the downward heuristic social dominance pattern, demonstrating that basic hierarchical group structures can be produced when social information is limited. However, simulated group aggression rarely resulted in a bullying or close competitors pattern when information was limited (SI Appendix, Table S5.3). This pattern is even more apparent when we focused on “realistic” winner- and loser-effect values (34) and excluded simulated groups that differed strongly in structure from our empirical datasets (i.e., focus and/or position were less than 0; SI Appendix, Fig. S5.2): Bullying patterns were then only observed in 0% (transient effects) and 2.3% (persistent effects) of groups and close competitors were only observed in 1.8% (transient effects) and 1.14% (persistent effects) of groups (SI Appendix, Table S5.4). These results show that although it is possible to produce a close competitor or bullying social dominance pattern with individual-level information only, it is rare for these more complex patterns to emerge in the absence of additional social information. This is additional evidence for treating close competitor and bullying patterns as more information-rich patterns than the downward heuristic, and likely information beyond individual experience is required to reliably produce close competitor or bullying patterns.
Phylogenetic Signal and the Evolution of Rank-Dependent Aggression.
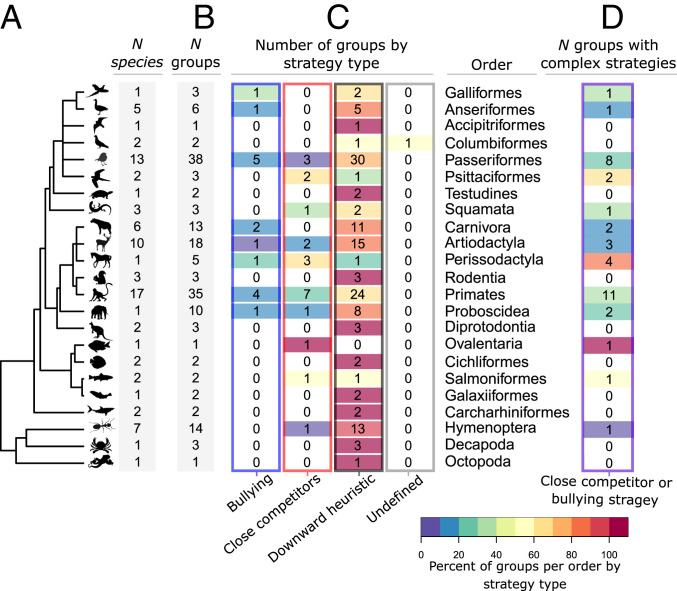
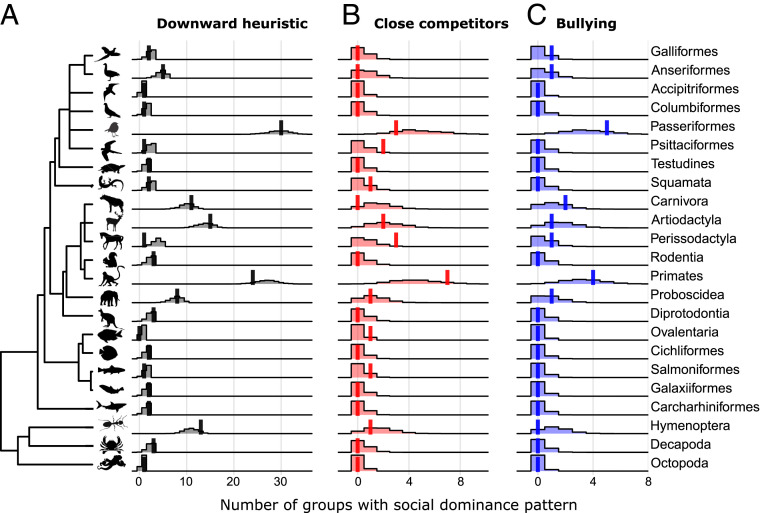
All three well-defined aggression patterns occurred in orders across the range of animal groups in our dataset of 172 social groups across 85 species and 23 orders (Fig. 3). We found no consistent evidence for phylogenetic signal in the evolution of any of the three social dominance patterns: The frequency at which each pattern occurred within each of the orders was consistent with the distribution expected from random allocation of patterns in almost every case (Fig. 4). With for two-tailed tests, Perissodactyla was the only order where the observed number of groups differed significantly from the randomized frequencies: The occurrence of downward heuristic social dominance patterns was lower than expected if patterns are randomly distributed (). Both Perissodactyla and Psittaciformes showed some evidence of unusually higher frequencies of observed close competitors patterns ( and , respectively). Due to the many comparisons shown here, these results should be interpreted with caution but are indications that future studies of species in these orders is warranted.
Fig. 3.
Social dominance pattern types are not phylogenetically restricted to particular orders. Studies of aggression in animals included in our dataset are unevenly distributed across orders (A) as well as whether multiple groups of a particular species have been sampled (B) (see also Fig. 5). When these totals are broken down by aggression pattern type (C), we see that many orders have groups with more than one aggression type (number of groups listed in the table, percent of groups by aggression pattern for each order indicated by color code, where red indicates 100% of sampled groups showed a particular type). In most cases, groups within many orders did not have consistently simple (downward heuristic) or consistently complex (bullying or close competitors) aggression patterns (D). Note: Ovalentaria is a group of fish families categorized as incertae sedis (“of uncertain placement”).
Fig. 4.
Little to no evidence of evolutionary relatedness on social dominance patterns is present at the order level. In almost every order the observed number of groups with each pattern (solid vertical lines) overlaps with the number of groups with each pattern when patterns are randomly allocated (shaded areas, density estimates) for each of the three main social dominance patterns: (A) downward heuristic, (B) close competitors, and (C) bullying. Note: Ovalentaria is a group of fish families categorized as incertae sedis (“of uncertain placement”).
Although certain kinds of conflict can be associated with phylogenetic relatedness, such as the occurrence of lethal violence in mammals (35) or the steepness of dominance hierarchies within a clade of primates (36), other studies have found more consistency in aggression and dominance across species. For example, studies of the structure and frequency of network motifs within aggression networks have found striking similarities across species at the micro social scale (16). Our work builds on these previous findings, although we take a complementary approach by addressing hierarchical structures from a macro-structural perspective. Rather than focusing on the building blocks of hierarchies, we looked at the social dominance patterns that may underlie aggression decisions. However, even coming at this question from the opposite scale we find similar patterns, where macro-level structures cannot be explained by phylogenetic relatedness. It is important to note that these historical datasets are taxonomically biased toward overrepresentation of certain clades (e.g., birds and primates) and an underrepresentation of studies in many others (Fig. 3A). Future work on a broader range of species will provide more balanced insight into evolutionary patterns.
Our methods allow us to detect social information within groups that could form the basis for simple heuristics to guide aggression but cannot differentiate between the availability or presence of information and the intentional use of that information. Although animals may vary widely in their underlying perception, memory, inference, and recognition skills, our results show that the social information contained in groups can be used to structure aggression. This raises the possibility the same social dominance patterns may emerge from very different cognitive mechanisms, decision-making heuristics, or social information processing abilities. Manipulative experiments are needed in order to differentiate the types of processes that generate and store information in high-information social groups. Of particular interest is better integrating research on signal evolution with the cognitive processes that allow individuals to react to or gain information from others. Status signals that affect conflict behavior are taxonomically widespread but there is variation in both the existence and use of status signals between species within taxa. Future experiments will provide valuable insight into whether the emergence of a particular social dominance pattern is indicative of a more complex social strategy, based on cognitive processing, strategic decision-making, and flexible social competence, or whether these patterns can be explained by simpler rules. Simple rules, or heuristics (37, 38), are a major factor that structures human social behavior and decision-making and characterizing these heuristics and the advantages and disadvantages of their use has allowed economists and psychologists to explain previously mystifying features of human behavior (e.g., refs. 39 and 40). A better understanding of the kinds of heuristics animals may use to make decisions and the ways animals may respond to changing social conditions by altering their heuristics has the potential to provide new and valuable insight into animal social complexity.
Intraspecific Variation in Aggression Pattern Use.
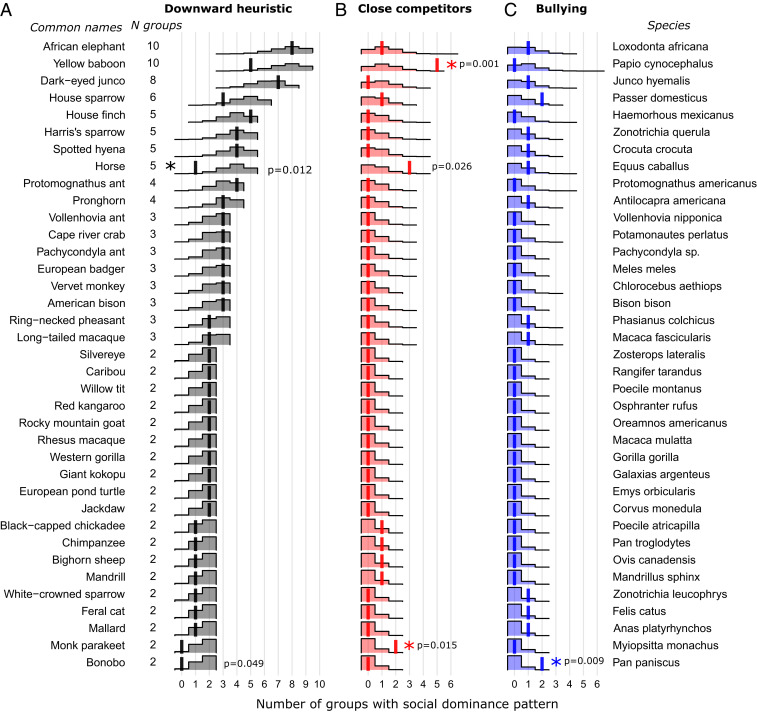
While the social dominance patterns used by different groups were sometimes consistent within a species, we found multiple cases where different groups followed different rank-dependent patterns. For the 37 species for which two or more groups were consistent with one of the three aggression patterns (downward heuristic, close competitors, or bullying), of species had groups that followed more than one aggression pattern (Fig. 5). For example, yellow baboons were evenly split between five groups which used a basic downward heuristic and five groups that used the more complex close competitors pattern. Three species, African elephants, house sparrows, and horses, had groups that followed each of the three social dominance patterns. Of these species with multiple observed groups, horses and bonobos showed some evidence of a lower-than-expected frequency of downward heuristic patterns ( and , respectively; Fig. 5). Yellow baboons, horses, and monk parakeets showed some evidence for higher-than-expected frequencies of close competitor dominance patterns (, , and respectively; Fig. 5) and bonobos showed evidence of higher-than-expected frequencies of bullying social dominance (; Fig. 5). Care must be taken in interpreting these results due to multiple comparisons, but they provide further indications of species which may be particularly interesting for future more detailed work.
Fig. 5.
Occurrence of social dominance patterns by species with multiple empirically sampled groups. In almost every species with multiple groups, the observed number of groups with each pattern (solid vertical lines) overlaps with the number of groups with each pattern when patterns are randomly allocated (shaded areas, density estimates) for each of the three main social dominance patterns: (A) downward heuristic, (B) close competitors, and (C) bullying. Asterisks indicate species with unusually low or high numbers of observed social dominance patterns compared to the randomized patterns ( for two-tailed test; species with indicated with annotated P values). Data are sorted by number of groups then by pattern type.
This variability we find in which social dominance pattern occurs within species shows that these patterns should be thought of as facts about particular groups rather than rigid species-level characteristics. Factors such as resource availability and distribution, environmentally mediated constraints, and direct environmental influences on physiology can all result in changes to individual aggression and group dominance structure (reviewed in ref. 41). These changes may shift which aggression pattern is optimal under new social, environmental, or ecological conditions. Temporal shifts in the behaviors underlying dominance interactions have been documented in human groups where dominance patterns and the behaviors used to mediate dominance interactions change with age (42). Dominance patterns can even change over time within the same social group, as we previously documented in aggression in parakeets (29). Our results support these earlier conclusions that sociality can vary within a single species.
Combined, these results suggest that experimental work on the emergence and dynamics of dominance hierarchies, social information, and social dominance patterns is needed to fully understand the conditions under which an information-based aggression pattern, like rank-dependent aggression, would emerge and be used in social groups. In particular, more studies are needed to determine the range of social dominance patterns that a particular species is able to use, whether there are similarities in the social or environmental conditions under which a more information-rich pattern generally emerges, and how flexible and on what time scale pattern use may vary within a particular social group.
Conclusions
A fundamental question in animal behavior is how much animals know about their social worlds and the extent to which they use this information in their decision-making processes (1, 23, 24, 43). Many approaches to social complexity seek to understand how much animals know about their social worlds, and recent work has advocated explicitly quantifying social information when attempting to assess social complexity (23, 44). There is growing evidence that social information is actively sought by individuals across a wide range of species (29, 45–47). However, while we can quantify many aspects of social structure, without additional experimental manipulation (e.g., refs. 27 and 28) it has not previously been possible to determine the extent of information that individuals in groups may have of their social worlds. In broader comparisons, it has also been difficult to find a way to quantify social information in a manner that is both feasible and general enough to be used in a wide range of species, as social interactions may differ in their salience and biological meaningfulness across species.
The computational methods presented here provide a way to assay interactions like aggression to determine the kinds of information encoded in social systems. We can now use these approaches to infer how much information animals have about their social worlds, based on their decisions about how to interact with each other. Using these tools, researchers can now categorize groups into a taxonomy of social dominance patterns, where the structuring of aggression is based on different types of social information.
The broad applicability of our quantitative tools provides opportunities to quantify the evolution of social structure across divergent taxa and groups with many different types of social organization. The tractability and wide applicability of our approach enables comparative analyses that can provide a better understanding of the evolutionary patterns underlying the distribution of social processing skills and complex sociality across taxa. Combined with recent results from empirical work and an understanding of the cognitive abilities of species, our approach provides opportunities to investigate the extent of rank-based information encoded in societies across species, compare the evolution of the use of social information, and better understand the effect of social information on individual behavior in within-group conflict.
The evidence we found for the role of social information in establishing social dominance patterns suggests that the question of what animals know about their social worlds should be thought of in two parts: First, how much do they know?, and second, how do they know it? Our results here deal with the extent of rank information animal groups have but cannot determine the mechanisms through which rank becomes “known” by individuals. A better understanding of the cognitive abilities of the species, including memory, recognition, and perceptive abilities, is needed to fully understand how information is encoded and the kinds of cognition that underlie the entire process. For species that have more detailed information about rank and use a close competitors or bullying aggression pattern, priorities for future research will be to differentiate between cases where individuals can follow a more information-rich social dominance pattern via a simple underlying rule that allows easy detection of relative rank differences compared to cases where the ability to use rank information is based instead on more cognitively demanding methods that require the recognition of particular individuals and memories of past outcomes. Manipulative experiments are needed in order to differentiate the types of processes that generate and store information in high-information social groups. These kinds of experiments are critical in distinguishing between social groups where information is contained in more or less cognitively demanding ways and will allow us to begin to identify those species that could have more- or less-complex social assessment and memory abilities than commonly assumed.
Methods
Empirical Data Sources.
We used a large openly accessible empirical dataset of aggression and dominance hierarchies (ref. 16, https://datadryad.org/stash/dataset/doi:10.5061/dryad.f76f2). We excluded two of these datasets due to apparent errors in the presentation of data in the original papers (table 4, nest 39 in ref. 48) and table 3 in ref. 49). We supplemented this dataset with data from aggression and rank in two groups of monk parakeets (ref. 29, https://datadryad.org/stash/dataset/doi:10.5061/dryad.p56q7, data from study quarters 2 through 4 for groups 1 and 2).
These datasets contain the number of times each individual “won” against each other individual. Depending on how the original study reported data, “wins” could be the outcome of aggressive contests, show the directionality of aggressive events, or indicate a submission display toward a dominant individual (they do not have information on which individual started a fight, only the outcome of the interaction). We use the general term “aggress” to describe the actions individuals take in these datasets and focus here on the perspective of the winners as initiators of aggression, although all of our analyses apply equally well to cases where the initiator of the fight chose to start a fight that it ultimately lost.
Rank and Distribution of Aggression.
For each group, we find individual ranks using a modified version of eigenvector centrality. In particular, we compute the probability that each individual aggresses with each other individual and then add a small regularization term, (see SI Appendix, section SI 1 for a Bayesian calculation of the optimal value of this term); the eigenvector centrality of the resulting matrix allows us to extract the relative ranks of individuals that are implicit in the patterns of aggression (50).
Plotting the overall distribution of observed aggression in each group by relative rank differences enables us to determine whether the distribution of aggression is structured by rank differences among individuals, whether individuals in the group focus their aggression on a subset of individuals based on relative rank differences, and where in relative rank distance space aggression is focused. We quantify these characteristics by measuring focus and position (defined below). In the measurement of both quantities, we correct for bias in our estimator using the statistical bootstrap method (for a pedagogical introduction see ref. 51), which also allows us to estimate standard errors about our estimated means.
Calculating Focus.
A group’s focus is high when individuals strongly concentrate their aggression toward opponents with a particular range of relative rank differences; it is low when aggression is spread across a wider range of individuals. Aggressive events in a group are summarized by the aggression matrix , whose elements count the number of times individual aggressed against individual .
To define focus we first construct the relative-aggression distribution, , which measures the level of aggression between individuals separated by steps in relative rank. If we define as the set of all pairs where is ranks above , then is defined as
| [1] |
where is the total amount of aggression by the attackers in the set’s pairs. is the average amount of aggression directed rank-steps away. When is positive, measures the average aggression directed “down” the hierarchy, from a higher-ranked individual to a lower-ranked individual.
In other words, is a measure of the fraction of events that are directed between individuals separated by steps in relative rank, given the total aggression in the system that could have been directed steps away. A plot of as a function of tells us a great deal about the flows of aggression through the system.
Focus, , is defined as how “sharp” this distribution is:
| [2] |
where is the -weighted variance of ,
| [3] |
and is the -weighted mean of ,
| [4] |
The normalization term is chosen so that a uniform (flat) distribution of aggression, that is, “rank ignorant,” gives a focus of zero. If focusing is very strong—for example, if all individuals direct their aggression toward the individual two ranks down from them in the hierarchy— is 1. As aggression is more evenly distributed decreases. In the case that aggression is completely uniform across all ranks, then the normalization is chosen such that will be precisely 0. (In rare cases, where the aggression is “overdispersed,” it is possible to have negative focus.)
Position of Focused Aggression.
If rank information is present and is used, and we can detect this via focus, then knowing the position of the peak of aggression gives us information about the specific relative rank-based aggression pattern that individuals are using. For example, individuals with focused aggression could direct most of their aggression toward those that are ranked directly beneath themselves in the hierarchy. Alternatively, individuals could focus their aggression on the very lowest-ranked individuals in the group. These two cases could result in similar levels of focus in aggression but could be differentiated from each other by differences in their position values. In the first case, position would be closer to each individual’s own rank (and closer to 0) while in the second case, position would move toward 1 as aggression is directed at individuals many ranks distant from an individual’s own rank.
We define the position of focused aggression as the average of the distribution of normalized aggression for each social group; that is, for each individual we compute the probability that the individual’s aggression is directed at an individual rank away, , and then average these probabilities over all individuals, formally,
| [5] |
where is the list of relative ranks available to individual ; higher-ranked individuals have more relative ranks available downward (positive ), while lower-ranked individuals have more available upward.
The measure accounts for the effects of both individual aggression levels and the number of potential aggressive targets as a function of rank and allows us to capture the extent to which decision-making on the individual level is sensitive to relative rank position.
Modeling the Structural Rules of Dominance Hierarchies.
We compare the focus and position values quantified from observed empirical data with those generated from 1) a set of permutation-based reference models (31), where we use a complex edge rewiring procedure that reproduces the basic hierarchical structure found in each empirical group, and 2) a set of generative models that use an agent-based framework to simulate social systems with winner and/or loser effects. This approach allows us to determine minimal models for group-level aggression patterns used in a particular group.
The simplest rank-based rule we considered is the “downward heuristic,” where individuals aggress only against those ranked below themselves. We used this rule to recreate aggression networks for each group, and compared it with the observed aggression, using an ensemble of agent-based model simulations to create a set of reference aggression networks. These models preserve some of the basic structure we observe in data, such as the overall aggressive dispositions of each individual, while potentially permuting other aspects.
We used these reference aggression networks to determine which values of focus and position we should expect to be generated if animals in the group were only using the downward heuristic. We use each individual’s rank, calculated from the data, and then allow individuals to aggress as much as they do in the empirical data, but, in the simplest case, with a uniform random preference for aggression against only the individuals ranked below themselves in the hierarchy. This process is consistent with best-practice recommendations for animal social network permutation, which supports event-level permutations of social interactions rather than relationship strengths (52).
Formally, given the aggression matrix , and the ranks , the first-ranked individual, , has equal to 1, and indicates that is lower ranked than . Then, for each individual , the row is then mapped to where
| [6] |
and is equal to 1 when the subscript is true (i.e., when then is lower ranked than ) and 0 otherwise. This mapping takes the total aggression by individual and distributes it equally toward all lower-ranked individuals.
Empirical systems may be somewhat noisy and may not follow a pure downward heuristic (e.g., due to mistakes in directing aggression, occasional opportunism in attacking a higher-ranked individual, or some level of stochasticity in directing aggression). At the extreme, individuals may direct aggression based only on their own levels of aggression, in complete disregard for rank differences.
To account for this, we introduced the possibility of randomness in aggression direction; mathematically, we allow for an probability that the individual simply directs aggression at a random individual,
| [7] |
We conducted a parameter sweep of the downward aggression heuristic in , gradually increasing the amount of randomly directed aggression from equal to 0 (perfect downward aggression) to unity (completely randomly directed aggression based only on individual aggressiveness) then examined how increasing randomness affected focus and position values. This process allowed us to simulate aggression along a continuum, from perfect use of basic rank information to completely random behavior dictated solely by each individual’s own levels of observed aggressiveness.
Social Dominance Pattern Assignment.
Our reference aggression networks generated by the downward heuristic serve as randomized reference models (30, 31) to which we can compare the observed datasets and as a form of null model for the downward heuristic: We fail to reject the downward heuristic as a plausible generating rule of focus and position in the observed datasets if observed focus and position values fall within the range that can be produced by our reference model datasets. For observed groups that fall outside of the region that could be generated by the downward heuristic we categorize these groups into social dominance patterns other than the basic downward heuristic.
We categorized groups into three main social dominance pattern types: downward heuristic, close competitors, and bullying. We ran a suite of reference models of aggression under the downward heuristic pattern, scanning across values of from 0 (perfect use of categorical rank information) to 1 (completely random behavior based only on individual aggressiveness). This enabled us to delineate the focus and position parameter space in which these summary measures are consistent with those produced by the downward heuristic. We drew a polygon around the space traced out by different values of , using the extremes of error bars to set the edges of the polygon ( CI; Fig. 2). Observed data that intersected this downward heuristic polygon were scored as consistent with that model if any of the error bars for the observed data overlapped with the polygon (Fig. 2A). Fig. 2 provides an example of these assignments. This procedure, fit to aspects of each of the observed social groups, allowed us to discriminate between social dominance patterns on a case-by-case basis rather than using a generalized rule for all focus and position values (as a result, the same observed value of focus and position may be categorized as “close competitors” in some groups and “downward heuristic” in others; see Fig. 1).
We defined the close competitors social dominance pattern as having a lower position value than that produced by the downward heuristic model (i.e., aggression concentrated on opponents ranked just below themselves; Fig. 2B) and bullying as having a higher position value than the modeled data (i.e., aggression concentrated on opponents ranked far below themselves; Fig. 2C). Some groups had undefined social dominance patterns with focus values lower than those expected in fully random systems.
Phylogenetic Analyses.
We used the R package taxize (53, 54) to check all species names, assign them to order, and plot the phylogenetic relationships among the 23 orders (analyses run in summer 2020). To test for an effect of relatedness across taxa, we took the social dominance patterns for each group in our dataset and randomly reallocated all strategies (without replacement) so that each group had a new randomly assigned pattern. For all species, we then summarized the occurrence of social dominance patterns for each of the 23 orders for each of 1,000 randomization runs. We compared the frequency with which each of the three main social dominance patterns was observed in each order to the frequencies expected if social dominance is randomly assigned. If the observed frequency falls within this expected distribution, we concluded that we have no evidence that relatedness among taxa (at the order level) affects which groups use each social dominance pattern.
General Analyses and Code Availability.
All final analyses were run in R (55). We used R packages gplots (56) and ggtree (57, 58) to plot Fig. 3 and R package ggridges (59) to plot Fig. 4 and SI Appendix, Figs. S4.1 and S4.2. Code to enable running the social dominance analyses is contained in the R package domstruc (https://github.com/danm0nster/domstruc) and the model output from the winner/loser analyses is contained in a GitHub repository (https://github.com/danm0nster/social-dominance-patterns-winner-loser-effects; ref 60). All study data are included in the article and/or SI Appendix; raw social data are available on Dryad (https://datadryad.org/stash/dataset/doi:10.5061/dryad.f76f2; https://datadryad.org/stash/dataset/doi:10.5061/dryad.p56q7).
Supplementary Material
Acknowledgments
E.A.H. was supported by a postdoctoral fellowship from the ASU-SFI Center for Biosocial Complex Systems, with additional funding from the Santa Fe Institute. D.M. was funded in part by Independent Research Fund Denmark (grant 7089-00017B), Aarhus University Research Foundation, and The Interacting Minds Center and gratefully acknowledges the hospitality of the Santa Fe Institute during a sabbatical visit. This research was additionally supported by Army Research Office Grant W911NF1710502. E.A.H. thanks Joshua Garland, Brendan Tracey, Vanessa Ferdinand, and Andy Rominger for many helpful discussions that have improved the drafts. We thank several anonymous reviewers for improving the quality of the final paper.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022912118/-/DCSupplemental.
References
- 1.Hobson E. A., Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr. Opin. Psychol. 33, 209–215 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Chase I. D., Bartolomeo C., Dugatkin L. A., Aggressive interactions and inter-contest interval: How long do winners keep winning? Anim. Behav. 48, 393–400 (1994). [Google Scholar]
- 3.Landau H., On dominance relations and the structure of animal societies: II. Some effects of possible social factors. Bull. Math. Biophys. 13, 245–262 (1951). [Google Scholar]
- 4.Ball B., Newman M., Friendship networks and social status. Netw. Sci. 1, 16–30 (2013). [Google Scholar]
- 5.Bruch E. E., Newman M. E., Aspirational pursuit of mates in online dating markets. Sci. Adv. 4, eaap9815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henzi S. P., Barrett L., The value of grooming to female primates. Primates 40, 47–59 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Seyfarth R. M., A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Schjelderup-Ebbe T., Beiträge zur Sozialpsychologie des Haushuhns. Z. Psychol. 88, 225–252 (1922). [Google Scholar]
- 9.Vehrencamp S. L., A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667–682 (1983). [Google Scholar]
- 10.Shizuka D., McDonald D. B., A social network perspective on measurements of dominance hierarchies. Anim. Behav. 83, 925–934 (2012). [Google Scholar]
- 11.Strauss E. D., Holekamp K. E., Social alliances improve rank and fitness in convention-based societies. Proc. Natl. Acad. Sci. U.S.A. 116, 8919–8924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosenick L., Clement T. S., Fernald R. D., Fish can infer social rank by observation alone. Nature 445, 429–432 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Tibbetts E. A., Dale J., A socially enforced signal of quality in a paper wasp. Nature 432, 218–222 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Mascaro O., Csibra G., Human infants? Learning of social structures the case of dominance hierarchy. Psychol. Sci. 25, 250–255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascaro O., Csibra G., Representation of stable social dominance relations by human infants. Proc. Natl. Acad. Sci. U.S.A. 109, 6862–6867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shizuka D., McDonald D. B., The network motif architecture of dominance hierarchies. J. R. Soc. Interface 12, 20150080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sapolsky R. M., Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418 (2004). [Google Scholar]
- 18.Sapolsky R. M., The influence of social hierarchy on primate health. Science 308, 648–652 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Creel S., Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497 (2001). [Google Scholar]
- 20.Chase I. D., Dynamics of hierarchy formation: The sequential development of dominance relationships. Behaviour 3, 218–239 (1982). [Google Scholar]
- 21.Landau H. G., On dominance relations and the structure of animal societies: I. Effect of inherent characteristics. Bull. Math. Biophys. 13, 1–19 (1951). [Google Scholar]
- 22.Dugatkin L. A., Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583–587 (1997). [Google Scholar]
- 23.Hobson E. A., Ferdinand V., Kolchinsky A., Garland J., Rethinking animal social complexity measures with the help of complex systems concepts. Anim. Behav. 155, 287–296 (2019). [Google Scholar]
- 24.Seyfarth R. M., et al. , The central importance of information in studies of animal communication. Anim. Behav. 80, 3–8 (2010). [Google Scholar]
- 25.Page R. A., Bernal X. E., The challenge of detecting prey: Private and social information use in predatory bats. Funct. Ecol. 34, 344–363 (2019). [Google Scholar]
- 26.Danchin E., Giraldeau L. A., Valone T. J., Wagner R. H., Public information: From nosy neighbors to cultural evolution. Science 305, 487–491 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Bergman T. J., Beehner J. C., Cheney D. L., Seyfarth R. M., Hierarchical classification by rank and kinship in baboons. Science 302, 1234–1236 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Cheney D. L., Seyfarth R. M., Silk J. B., The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: Evidence for causal reasoning? J. Comp. Psychol. 109, 134 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Hobson E. A., DeDeo S., Social feedback and the emergence of rank in animal society. PLoS Comput. Biol. 11, 1–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gauvin L., et al. , Randomized reference models for temporal networks. arXiv[Preprint] (2020). https://arxiv.org/abs/1806.04032 (Accessed 14 December 2020).
- 31.Hobson E. A., et al. , A guide to choosing and implementing reference models for social network analysis. arXiv[Preprint] (2020). https://arxiv.org/abs/2012.04720 (Accessed 14 December 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutnik D. L., Sex-related differences in the seasonality of agonistic behavior in mule deer. J. Mammal. 62, 1–11 (1981). [Google Scholar]
- 33.Isbell L. A., Pruetz J. D., Differences between vervets (Cercopithecus aethiops) and patas monkeys (Erythrocebus patas) in agonistic interactions between adult females. Int. J. Primatol. 19, 837–855 (1998). [Google Scholar]
- 34.Rutte C., Taborsky M., Brinkhof M. W., What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Gómez J. M., Verdú M., González-Megías A., Méndez M., The phylogenetic roots of human lethal violence. Nature 538, 233 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Balasubramaniam K., et al. , Hierarchical steepness and phylogenetic models: Phylogenetic signals in macaca. Anim. Behav. 83, 1207–1218 (2012). [Google Scholar]
- 37.Tversky A., Kahneman D., Judgment under uncertainty: Heuristics and biases. Science 185, 1124–1131 (1974). [DOI] [PubMed] [Google Scholar]
- 38.Slovic P., Fischhoff B., Lichtenstein S., Behavioral decision theory. Annu. Rev. Psychol. 28, 1–39 (1977). [Google Scholar]
- 39.Hertwig R., Simple Heuristics in a Social World (Oxford University Press, New York), 2013). [Google Scholar]
- 40.Nagatsu M., et al. , Making good cider out of bad apples — Signaling expectations boosts cooperation among would-be free riders. Judgm. Decis.Mak. 13, 137–149 (2018). [Google Scholar]
- 41.Wong M. Y., Abiotic stressors and the conservation of social species. Biol. Conserv. 155, 77–84 (2012). [Google Scholar]
- 42.Hawley P. H., The ontogenesis of social dominance: A strategy-based evolutionary perspective. Dev. Rev. 19, 97–132 (1999). [Google Scholar]
- 43.Seyfarth R. M., Cheney D. L., Social cognition. Anim. Behav. 103, 191–202 (2015). [Google Scholar]
- 44.Bergman T. J., Beehner J. C., Measuring social complexity. Anim. Behav. 103, 203–209 (2015). [Google Scholar]
- 45.Barve S., Lahey A. S., Brunner R. M., Koenig W. D., Walters E. L., Tracking the warriors and spectators of acorn woodpecker wars. Curr. Biol. 30, R982–R983 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Hotta T., et al. , The use of multiple sources of social information in contest behavior: Testing the social cognitive abilities of a cichlid fish. Front. Ecol. Evol. 3, 85 (2015). [Google Scholar]
- 47.Tibbetts E. A., Wong E., Bonello S., Wasps use social eavesdropping to learn about individual rivals. Curr. Biol. 30, 3007–3010 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Blatrix R., Herbers J. M., Intracolonial conflict in the slave-making ant protomognathus americanus: Dominance hierarchies and individual reproductive success. Insectes Sociaux 51, 131–138 (2004). [Google Scholar]
- 49.Fairbanks W. S., Dominance, age and aggression among female pronghorn, antilocapra americana (family: Antilocapridae). Ethology 97, 278–293 (1994). [Google Scholar]
- 50.Brush E. R., Krakauer D. C., Flack J. C., A family of algorithms for computing consensus about node state from network data. PLoS Comput. Biol. 9, e1003109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeDeo S., Hawkins R., Klingenstein S., Hitchcock T., Bootstrap methods for the empirical study of decision-making and information flows in social systems. Entropy 15, 2246–2276 (2013). [Google Scholar]
- 52.Farine D. R., A guide to null models for animal social network analysis. Method. Ecol. Evol. 8, 1309–1320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlain S., Szocs E., Taxize - taxonomic search and retrieval in R. F1000Research 2, 191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chamberlain S., et al. , Taxize: Taxonomic information from around the web (R package version 0.9.95, 2020).
- 55.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna,2013). [Google Scholar]
- 56.Warnes G. R., et al. , Gplots: Various R programming tools for plotting data (R package version 3.0.3, 2020).
- 57.Yu G., Smith D., Zhu H., Guan Y., Lam T. T. Y., Ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Method. Ecol. Evol. 8, 28–36 (2017). [Google Scholar]
- 58.Yu G., Lam T. T. Y., Zhu H., Guan Y., Two methods for mapping and visualizing associated data on phylogeny using ggtree. Mol. Biol. Evol. 35, 3041–3043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilke C. O., ggridges: Ridgeline Plots in ’ggplot2’ (R package version 0.5.2, 2020).
- 60.Mønster D., Hobson E. A., DeDeo S., Simulation data for “Aggression heuristics underlie animal dominance hierarchies and provide evidence of group-level social information.” GitHub. https://github.com/danm0nster/social-dominance-patterns-winner-loser-effects. Deposited 18 February 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.