Significance
Resolution of inflammation, infection, and injury are essential for host defense and homeostasis. Cysteinyl-specialized proresolving mediators (cys-SPMs) are potent chemical signals that accelerate resolution of inflammation, control infection, and display proregenerative properties. We sought evidence for cys-SPM–activated primordial pathways that might link resolution of inflammation and regeneration using planaria because of their robust regenerative ability. RNA sequencing was carried out with surgically resected planaria exposed to cys-SPMs, and we identified genes and pathways regulated by cys-SPMs during the regeneration, including TRAF3. In mammalian systems, TRAF3 contributes to cys-SPM–stimulated phagocyte functions and resolution of infection.
Keywords: leukocytes, resolvins, planaria, chemical mediators, signaling
Abstract
The recently elucidated proresolving conjugates in tissue regeneration (CTR) maresin-CTR (MCTR), protectin-CTR (PCTR), and resolvin-CTR (RCTR), termed cysteinyl-specialized proresolving mediators (cys-SPMs) each promotes regeneration, controls infection, and accelerates resolution of inflammation. Here, we sought evidence for cys-SPM activation of primordial pathways in planaria (Dugesia japonica) regeneration that might link resolution of inflammation and regeneration. On surgical resection, planaria regeneration was enhanced with MCTR3, PCTR3, or RCTR3 (10 nM), each used for RNA sequencing. The three cys-SPMs shared up-regulation of 175 known transcripts with fold-change > 1.25 and combined false discovery rate (FDR) < 0.002, and 199 canonical pathways (FDR < 0.25), including NF-κB pathways and an ortholog of human TRAF3 (TNFR-associated factor 3). Three separate pathway analyses converged on TRAF3 up-regulation by cys-SPMs. With human macrophages, three cys-SPMs each dose-dependently increased TRAF3 expression in a cAMP-PKA–dependent manner. TRAF3 overexpression in macrophages enhanced Interleukin-10 (IL-10) and phagocytosis of Escherichia coli. IL-10 also increased phagocytosis in a dose-dependent manner. Silencing of mouse TRAF3 in vivo significantly reduced IL-10 and macrophage phagocytosis. TRAF3 silencing in vivo also relieved cys-SPMs’ actions in limiting polymorphonuclear neutrophil in E. coli exudates. These results identify cys-SPM–regulated pathways in planaria regeneration, uncovering a role for TRAF3/IL-10 in regulating mammalian phagocyte functions in resolution. Cys-SPM activation of TRAF3 signaling is a molecular component of both regeneration and resolution of infectious inflammation.
Resolution of inflammation is an active process, temporally and spatially regulated by cellular and molecular events, including the biosynthesis of endogenous lipid mediators (LM) that act on phagocytes (1–3). In addition to host defense, phagocytes are known to play essential roles in tissue repair and regeneration (4–8). Therefore, molecules that could link resolution of inflammation to tissue regeneration are of interest (9). Within the self-resolving phase of inflammation, LM class-switching occurs in contained exudates from initial production of prostaglandins and leukotrienes to activating biosynthesis of a superfamily of specialized proresolving mediators (SPMs), namely resolvins, protectins, and maresins. Each SPM member is antiinflammatory and proresolving (9). As such, SPMs play critical roles in widely occurring diseases, including sepsis (10), cardiovascular (11–13), autoimmune (14), metabolic (15), and neurological diseases [e.g., Alzheimer’s disease (16)], as well as age-related tissue degeneration (17).
Specific SPMs—such as resolvin (Rv) E1, RvD1, and maresin (MaR) 1—protect organs (18), stimulate tissue repair (19), and enhance regeneration (9, 20). Recently, we elucidated three new series of conserved bioactive chemical signals that are peptide–lipid molecules. These are produced during resolution of peritonitis in mice and screened for enhancing planaria tissue regeneration, which we’ve coined maresin conjugates in tissue regeneration (MCTR), protectin-CTR (PCTR), and resolvin-CTR (RCTR) based on their docosahexaenoic acid (DHA) backbone and family structures. Each series contains three bioactive members (i.e., MCTR1–3, PCTR1–3, and RCTR1–3) and the complete stereochemical assignments of these nine potent molecules are established and total organic synthesis achieved (for review, see ref. 9; cf. ref. 21 and references therein). Among these, MCTRs are produced by regenerating planaria (species Dugesia japonica), murine resolving exudates and human tissues (9, 21) that protect acute lung injury (21–23). Human M2-macrophages (MΦ) exposed to pathogenic bacteria biosynthesize PCTRs (24), and RCTRs are in human brain and spleen (21). Both MCTR1 and PCTR1 also prevent LPS-induced acute respiratory distress syndrome and multiple organ damage (25, 26). Collectively, these three series of bioactive lipid–peptide-containing molecules are grouped as cysteinyl-SPMs (cys-SPMs), given their potent tissue regenerative and classic proresolving functions, such as limiting polymorphonuclear neutrophil (PMN) infiltration and enhancing MΦ phagocytosis and efferocytosis (9).
Since planaria undergo robust regeneration via primordial pathways (27), and cys-SPMs enhance planaria tissue regeneration (9, 21), we therefore employed RNA sequencing (RNA-seq) with surgically resected planaria and cys-SPMs to identify genes and pathways activated by cys-SPMs. We selected the third member in each cys-SPM biosynthetic pathway for these studies, namely MCTR3 (13R-cysteinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid), PCTR3 (16R-cysteinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid), and RCTR3 (8R-cysteinyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid), because each potently enhances regeneration (21). We assessed the roles of these gene and pathway candidates from planaria in mammalian systems, and report on the proresolving and proregenerative functions of these cys-SPM–stimulated pathways with human MΦ and in vivo with mice.
Results
During D. japonica Head Regeneration, cys-SPMs Regulate Transcript Expression.
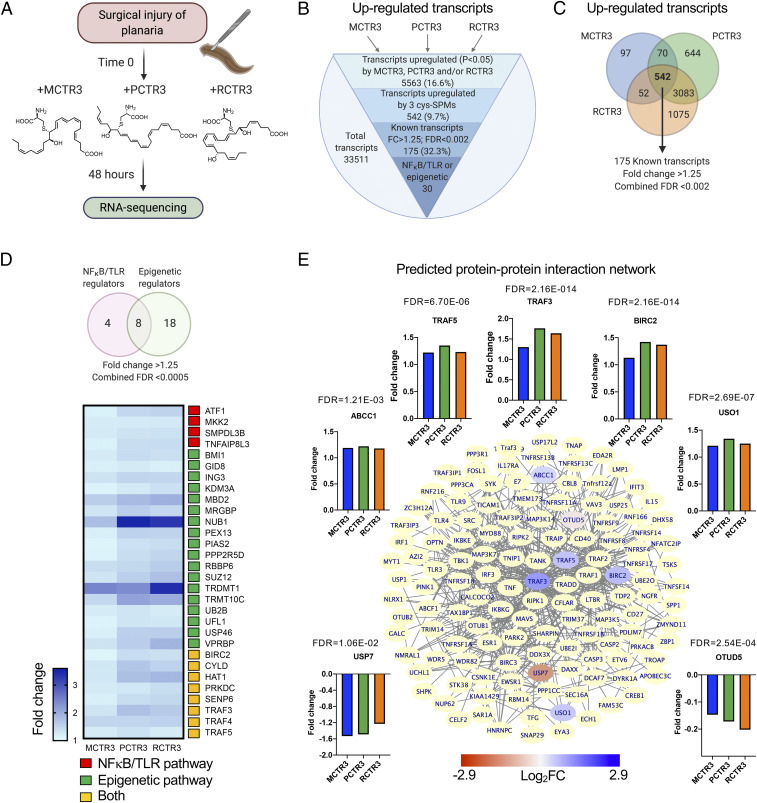
On head resection, freshwater planaria (species D. japonica; 10 planaria each condition) were incubated separately with MCTR3, PCTR3, RCTR3 (10 nM) or vehicle in water, and regeneration was assessed (Fig. 1A and SI Appendix, Fig. S1 A and B). Each of the three cys-SPM gave the highest fold increase in regeneration on day 2, compared with later time points [days 3–5; data from de la Rosa et al. (21) and replotted in SI Appendix, Fig. S1C], which permitted us to evaluate pathways used by each cys-SPM and compare these for the three different cys-SPMs to determine which are shared by the three cys-SPMs. To this end, planaria were collected at day 2 for RNA-seq and bioinformatics were carried out (Fig. 1A; see Materials and Methods for details).
Fig. 1.
Cys-SPMs regulate planaria transcripts during regeneration: RNA-seq analysis. (A) Following head resection, planaria (D. japonica; 10 planarians) were incubated with MCTR3, PCTR3, RCTR3 separately (10 nM each) or vehicle (0.1% ethanol [vol/vol]) in water. Planaria were collected at 48 h and mRNA isolated for RNA-seq. (B) The total of 33,511 transcripts were obtained, of which 5,563 were up-regulated (P < 0.05) by MCTR3, PCTR3, and/or RCTR3. Among these, 542 were up-regulated by the 3 cys-SPMs; 175 are known transcripts with predicted functions with FC > 1.25 by at least 1 of the 3 cys-SPMs, and 30 of them are involved in NF-κB and/or epigenetic regulation (SI Appendix, Table S1A). (C) Venn diagram illustrates the numbers of transcripts up-regulated (P < 0.05, FC > 1.25) by MCTR3, PCTR3, and/or PCTR3. Combined FDR < 0.002 for each of the 175 transcripts. (D, Upper) Venn diagram of cys-SPM up-regulated transcripts that have predicted functions in NF-κB/TLR and/or epigenetic-related pathways. Combined FDR < 0.0005 for each of the 30 transcripts. (Lower) The heatmap depicts FCs of these transcripts. (E) Predicted protein–protein interaction network with TRAF3 using Cytoscape. Within this network, TRAF3 was predicted to interact with proteins of other cys-SPM up-regulated transcripts, including TRAF5, ABCC1, BIRC2, and USO1 (blue), and down-regulated transcripts, including USP7 and OTUD5 (red). See Insets for FC of these transcripts, and the combined FDR values are shown above each graph. Additional proteins in the BioGrid database that are predicted to interact with TRAF3 are depicted in yellow.
In parallel, we profiled the presence of cysteinyl-conjugated LM in regenerating planaria using mass spectrometry-based LM metabololipidomics, and determined whether exogenous cys-SPMs increase endogenous cysteinyl-containing LM. Following head resection, planaria (40 planaria each condition) were incubated with internal standards consisting of three isotope-labeled cys-SPMs [MCTR3(13C)3(15N), PCTR3(13C)3(15N) and RCTR3(13C)3(15N); 10 nM each] or vehicle in water. Planaria were collected 48 h later for LM profiling. With injured planaria, we found unlabeled MCTR3 (∼3 to 6 fmol) and PCTR3 (∼15 to 33 fmol) together with cysteinyl-containing leukotriene C4 (SI Appendix, Fig. S1D). Thus, the addition of exogenous cys-SPMs did not alter endogenous cys-SPM production in a statistically significant manner.
Cys-SPMs Regulate Transcripts and Pathways.
Transcript analysis.
We next identified potential candidates that mediate cys-SPMs’ actions in accelerating planaria regeneration. A total of 33,511 transcripts were obtained from these regenerating planaria 48 h after head resection, and each cys-SPM was compared with control (injured planaria with vehicle alone). We also combined P values over the three comparisons to obtain combined P values and their corresponding false-discovery rates (FDRs). There were 5,563 (16.6%) transcripts up-regulated (P < 0.05) by MCTR3, PCTR3, and/or RCTR3 (Fig. 1B). Among these, 542 were nominally up-regulated by the three cys-SPMs; 175 are known transcripts with predicted functions and fold-change (FC) > 1.25 by at least one of the cys-SPMs. Each of these transcripts gave statistical significance with a combined FDR < 0.002 (Fig. 1 B and C). Of these, 30 transcripts were related to NF-κB and/or epigenetic regulation (combined FDR < 0.0005) (Fig. 1D and SI Appendix, Table S1A). These cys-SPM up-regulated transcripts include negative regulators of NF-κB activation, such as traf3 (TNFR-associated factor 3; a component of E3 ubiquitin-protein ligase complexes), cyld (a lysine 63 deubiquitinase), and hat1 (a histone acetyltransferase) (28–30). In addition to these transcripts shared by the three cys-SPMs, each regulated CTR family-specific transcripts: That is, MCTR3 up-regulated 97 transcripts, PCTR3 up-regulated 644, and RCTR3 up-regulated 1,075 transcripts (Fig. 1C). MCTR3, PCTR3, and RCTR3 also down-regulated 6,280 (18.7%) transcripts in total. Among these, 635 were down-regulated (P < 0.05) by the three cys-SPMs, and 394 of them have predicted names (SI Appendix, Fig. S2).
Among the transcripts up-regulated by the cys-SPMs, transcript and pathway analyses (Figs. 1 and 2 and SI Appendix, Tables S1–S3) converged on TRAF3 (vide infra "Gene ontology"). TRAF3 is considered a “gatekeeper” controlling Toll-like receptor (TLR) and tumor necrosis factor receptor (TNFR) signaling pathways (31, 32). Therefore, we next focused on TRAF3 network analysis using the Cytoscape and BioGrid interaction database (33). Fig. 1E illustrates the predicted protein–protein interaction network with TRAF3. Within this network, the protein products of cys-SPM up-regulated traf5, abcc1 (ATP binding cassette subfamily C member 1), birc2 (baculoviral IAP repeat-containing 2), and uso1 (USO1 vesicle transporter factor) and down-regulated usp7 (ubiquitin-specific peptidase 7) and otud5 (ovarian tumor deubiquitinase 5) could potentially interact with TRAF3.
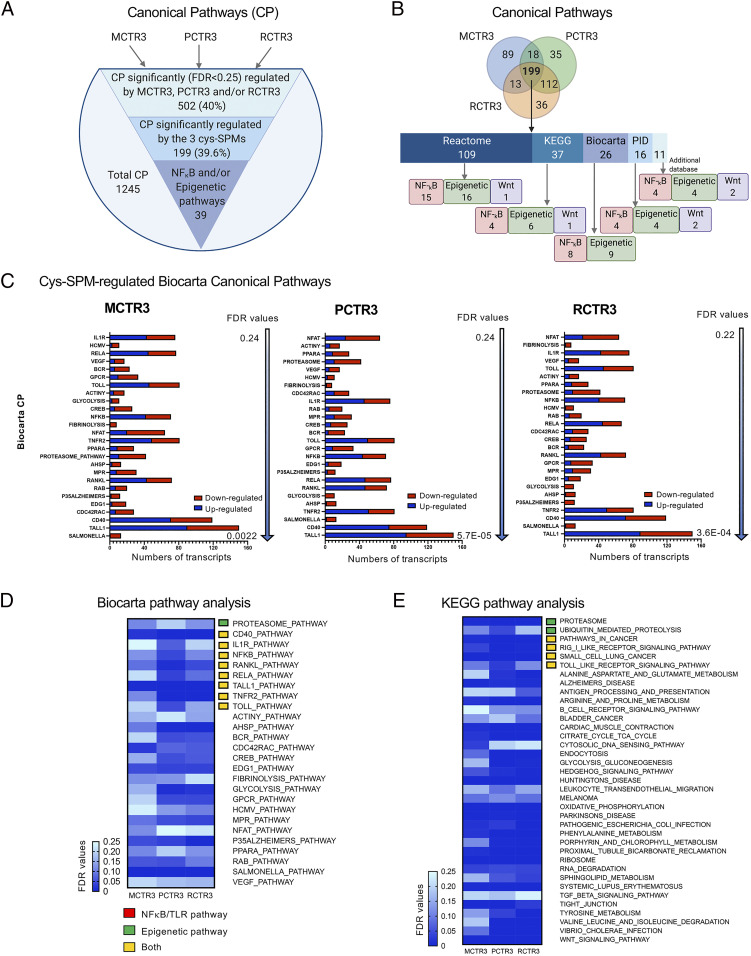
Fig. 2.
Cys-SPMs regulate planaria canonical pathways during regeneration. (A) Pathway analysis was carried out using CP, including Reactome, KEGG, Biocarta, and PID databases. The total of 1,245 CP were identified during regeneration, of which 502 were significantly (FDR < 0.25) regulated by MCTR3, PCTR3, and/or RCTR3. Among them, 199 CP were significantly regulated by three cys-SPMs, and within these CP, 39 are related to NF-κB and/or epigenetic pathways. (B) Venn diagram illustrates the CP that were significantly regulated (FDR < 0.25) by MCTR3, PCTR3, and/or RCTR3 during planaria regeneration. Among these, numbers of CP related to NF-κB, epigenetic modification and Wnt signaling are indicated for each database: Reactome, KEGG, Biocarta, PID, and additional databases. (C) Biocarta pathways that were significantly regulated by MCTR3, PCTR3, and RCTR3. Numbers of transcripts are expressed in blue (up-regulated) or red (down-regulated) in stacked bar graphs. (D and E) Heatmaps of significantly regulated CP by the three cys-SPMs in (D) Biocarta and (E) KEGG pathway database. For heatmaps of two pathway analysis using Reactome and PID databases, see SI Appendix, Fig. S4.
To verify RNA-seq results for traf3, we carried out traf3 transcript expression by quantitative PCR during planaria regeneration. The traf3 transcript was statistically significantly up-regulated at day 2 following head resection (SI Appendix, Fig. S3). Incubations of injured planaria with MCTR3, PCTR3, or RCTR3 further significantly increased traf3 transcripts on day 3, suggesting that TRAF3 and its downstream effectors may play a role in regeneration and potentially in resolution of inflammation and infection.
Since MCTRs and PCTRs are in regenerating planaria (SI Appendix, Fig. S1D) (34), we searched for cys-SPM biosynthesis enzymes from planaria transcript database. A predicted ortholog of lipoxygenase loxhd1 was present, which did not appear to be significantly regulated by the three cys-SPMs. Planaria also possessed predicted orthologs of glutathione S-transferase and γ-glutamyltransferase that are essential for cys-SPM biosynthesis (9) (SI Appendix, Table S1B). These planaria orthologs of human enzymes could contribute to endogenous production of MCTR3 and PCTR3 in injured planaria (SI Appendix, Fig. S1D).
Canonical pathways.
The mean-rank gene set test was used for pathway analysis (Materials and Methods). For canonical pathway (CP) analyses, Reactome, Kyoto Encyclopedia of Genes and Genomes (KEGG), Biocarta, and Pathway Interaction Database (PID) databases were used. A total of 1,245 CPs were identified during regeneration, of which 502 (∼40%) were statistically significantly regulated by MCTR3, PCTR3, and RCTR3 (FDR < 0.25) (Fig. 2A). Among them, 199 CP were statistically significantly regulated by the three cys-SPMs, and 39 are related to NF-κB and epigenetic pathways (Fig. 2B). We further interrogated each database separately to identify overlapped and distinct pathways in each database. Within the Biocarta pathway database, 26 pathways were statistically significantly regulated (FDR < 0.25) by MCTR3, PCTR3, and RCTR3, including CD40, IL-1R, NF-κB, RANKL, RELA, TNFR2, and TOLL pathways (Fig. 2 C and D). The statistically significantly regulated pathways using KEGG, Reactome, PID, and additional databases are shown in Fig. 2E and SI Appendix, Fig. S4. Of interest, TRAF3 was up-regulated by MCTR3, PCTR3, and RCTR3 in transcript analysis, and also belonged to several CP that were regulated by the three cys-SPMs in the Biocarta and KEGG databases (SI Appendix, Table S2). In addition to these pathways shared by the three cys-SPMs, each cys-SPM regulated specific pathways that were not shared by the others (i.e., MCTR3 up-regulated 89 pathways, PCTR3 up-regulated 35, and RCTR3 up-regulated 36 pathways) (Fig. 2B).
Gene ontology.
A total of 5,535 gene ontology (GO) pathways were identified during regeneration, of which 2,112 (∼38.2%) were statistically significantly regulated by MCTR3, PCTR3, and RCTR3 (FDR < 0.25) (SI Appendix, Fig. S5). Among them, 790 GO were statistically significantly regulated by the three cys-SPMs, and 48 were related to NF-κB and innate immune pathways. In parallel, we carried out Ingenuity Pathway Analysis (IPA; https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/). Among the top-ranking canonical pathways regulated by cys-SPMs, several of them contain TRAF3, including NF-κB and TNF-α receptor superfamily (i.e., CD40 and lymphotoxin-β receptor) signaling (SI Appendix, Fig. S6A and Table S3). Within the CD40 signaling pathway, three components were significantly up-regulated by cys-SPMs, i.e., traf3, traf5, and atf1, a cyclic AMP-dependent transcription factor (SI Appendix, Fig. S6B). Taken together, these pathway analyses identified several CD40 and NF-κB–related pathways regulated by cys-SPMs during planaria regeneration.
To select candidate genes for further investigation in mammalian systems, we sought evidence for up-regulated molecules in transcript analysis that were also present in cys-SPM–regulated pathways. Among the 175 significantly up-regulated transcripts shared by the 3 cys-SPMs (Fig. 1C), 21 transcripts were also present in SPM-regulated Biocarta and KEGG pathways (Fig. 2 D and E and SI Appendix, Table S2). Of these, TRAF3-containing pathways gave the highest numbers of total hits within the 63 cys-SPM–regulated pathways (7 of 63) (SI Appendix, Table S2C). By comparison, CYLD, another negative regulator of NF-κB activation, appeared only in one pathway. Because both transcript and pathway analyses of cys-SPM–regulated molecules during planaria regeneration converged on TRAF3, we focused on TRAF3 and related pathways in mammalian systems.
Cys-SPMs Enhance TRAF3, Its Effectors, and Phagocytosis with Human Macrophages.
Given that macrophages (MΦ) are essential for tissue repair and regeneration (4, 7), we examined whether these cys-SPMs regulate TRAF3 and downstream effectors with human MΦ (Fig. 3A); TRAF3 is essential for counter regulation of TRAF6 and NF-κB activation as well as for induction of interleukin-10 (IL-10) (30–32), which is proregenerative and tissue reparatory (35). Along these lines, most SPMs reduce NF-κB expression, nuclear translocation, and activity, as well as up-regulate IL-10 (9). Here, we first determined if cys-SPMs can regulate traf3 on the transcriptional level with human MΦ stimulated with serum-treated zymosan (STZ; yeast wall particles). MCTR3, PCTR3, and RCTR3 at 10 nM each statistically significantly increase traf3 and il10 transcript levels at 6 h (Fig. 3B) (P < 0.05). In addition, PCTR3 statistically significantly up-regulated ifnγ and down-regulated traf6 (P < 0.05).
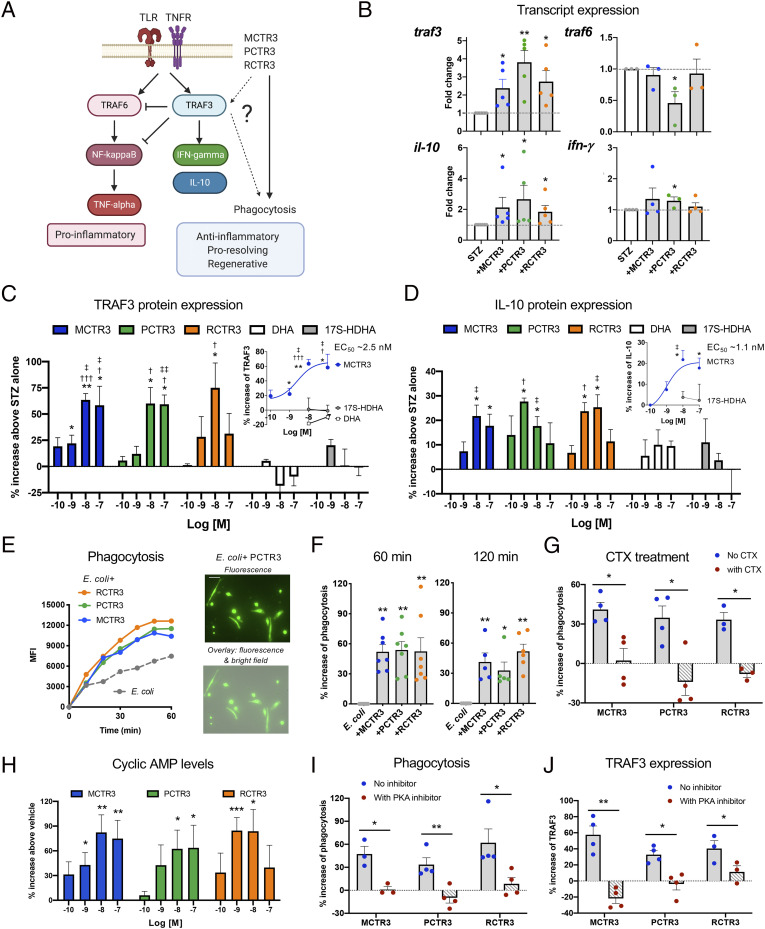
Fig. 3.
Cys-SPMs increase TRAF3 and phagocytosis with macrophages (A) Schematic representation of cys-SPMs and TRAF3 regulation of downstream effectors and phagocytosis. (B) Transcript expression. Human MΦ were incubated with STZ with each cys-SPM (10 nM) or vehicle for 6 h. Transcript levels were determined by qPCR and expressed as FCs of STZ alone; mean ± SEM, n = 5 (traf3 and il-10), 3 or 4 (traf6 and ifnγ); *P < 0.05, **P < 0.01 vs. STZ alone; two-tailed Student’s t test. (C and D) Dose–responses. Human MΦ were incubated with STZ with each cys-SPM (0.1 to 100 nM), DHA, 17S-HDHA (1 to 100 nM), or vehicle for 24 h. (C) Cells were lysed for TRAF3 ELISA and (D) supernatants collected for IL-10 ELISA. Results are percent increase compared with STZ; mean± SEM, n = 3 to 5; *P < 0.05, **P < 0.01, vs. STZ alone; one-way ANOVA; †P < 0.05, †††P < 0.001 vs. DHA; ‡P < 0.05, ‡‡P < 0.01 vs. 17S-HDHA at the same concentrations; two-tailed Student’s t test. (C and D, Insets) Dose–response curves of MCTR3 with DHA and/or 17S-HDHA. EC50s were estimated using nonlinear regression with log (agonist) vs. response (three parameters). (E–G and I) Phagocytosis. Human MΦ were plated onto slide chambers (1 × 105 cells per well) and incubated with each cys-SPM (10 nM) or vehicle control for 24 h, followed by addition of BacLight green-labeled E. coli (5 × 106 CFU) to initiate phagocytosis. Fluorescent images were then recorded every 10 min for 120 min. In each experiment, four fields (20×) per condition (per well) were recorded. (E, Left) mean fluorescence intensity (MFI)/cell, average of 124 cells per field and 4 fields per condition from one representative experiment. (Right) Representative images. (Scale bar, 50 μm.) (F) Percent increase of phagocytosis compared with E. coli alone; mean± SEM, n = 5 to 7; *P < 0.05, **P < 0.01. (G and I) Cholera toxin (CTX; 1 μg/mL), PKA inhibitor (H89, 10 μM), or vehicle were incubated with MΦ together with cys-SPMs. Results are percent increase of phagocytosis compared with E. coli alone at 60 min; mean ± SEM, n = 3 or 4; *P < 0.05, **P < 0.01; (F) one-way ANOVA; (G and I) two-tailed Student’s t test. (H) cAMP. Human MΦ were incubated with each cys-SPM (0.1 to 100 nM) for 15 min. Cells were lysed and cAMP levels determined. Results are percent increase above vehicle; mean ± SEM, n = 5; *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle; one-way ANOVA. (J) TRAF3 expression was determined as in C in the absence or presence of a PKA inhibitor. Results are percent increase of STZ alone; mean ± SEM, n = 3 or 4; *P < 0.05, **P < 0.01; two-tailed Student’s t test.
Next, we investigated whether MCTR3, PCTR3, and RCTR3 also regulate protein expression of TRAF3 and IL-10. With MΦ stimulated with STZ, each cys-SPM dose-dependently (0.1–100 nM) increased TRAF3 protein levels, giving the highest increases at 10 nM (60–70% increases) (Fig. 3C). MCTR3 gave an estimated EC50 ∼2.5 nM (Fig. 3 C, Inset). For direct comparison, MΦ were incubated separately with precursor DHA or biosynthetic pathway markers for PCTR3 and RCTR3, namely 17S-HDHA (17S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid). Here, neither DHA nor 17S-HDHA significantly increased TRAF3 at 1–100 nM. Similar results were obtained with IL-10; each cys-SPM dose-dependently increased IL-10, giving the highest increases at 1 or 10 nM (Fig. 3D), with an estimated EC50 for MCTR3 ∼1.1 nM (Fig. 3 D, Inset). These increases by cys-SPMs were significantly higher than those by DHA or 17S-HDHA at equal molar concentrations. Thus, MCTR3, PCTR3, and RCTR3 each selectively enhanced transcript and protein levels of TRAF3 and its downstream effector IL-10 during phagocytosis of microbial particles.
Since MΦ clearance of microbes and cellular debris is a key cellular function in tissue resolution (9), we examined MCTR3, PCTR3, and RCTR3 side-by-side for their ability to stimulate phagocytosis with human MΦ. Using fluorescent-labeled live Escherichia coli with a real-time imaging microscope, we found that each cys-SPM (10 nM), when incubated with MΦ for 24 h followed by exposure to live E. coli, significantly increased phagocytosis compared with E. coli alone, with essentially equal potencies (∼50% increases at 60–120 min) (Fig. 3 E and F). By comparison, 15-min incubation of each cys-SPM (10 nM) with MΦ also significantly increased phagocytosis (SI Appendix, Fig. S7A). To address the mechanisms by which cys-SPMs increase TRAF3 and phagocytosis, we first investigated whether cys-SPM–stimulated phagocytosis with human MΦ was receptor-dependent. Treatment of human MΦ with cholera toxin (1 μg/mL) significantly diminished MCTR3-, PCTR3-, and RCTR3-stimulated phagocytosis (10 nM) (Fig. 3G), suggesting that these cys-SPMs activated Gαs protein-coupled G protein-coupled receptors (GPCRs). Cyclic AMP (cAMP) is a second messenger of Gαs protein, and a key signal for phagocyte functions via activating PKA (36); therefore, we assessed intracellular cAMP in MΦ and found that each of the three cys-SPMs increased cAMP in a dose-dependent manner (0.1–100 nM). At 10 nM, cys-SPMs gave 60–80% increases in cAMP (Fig. 3H). In addition, MΦ were incubated with a PKA inhibitor H89 that diminished cys-SPM-stimulated phagocytosis (Fig. 3I). H89 is known to block the catalytic ATP binding sites of PKA and substrate phosphorylation (37). These findings indicate that cys-SPMs activated the cAMP-PKA pathway, which contributed to phagocytosis of E. coli with human MΦ.
Next, we investigated cys-SPM-regulated TRAF3 expression. PKA is known to phosphorylate and activate ATF (activating transcription factor)/CREB (cAMP response element binding protein) family of transcription factors (38). Among them, atf1 was up-regulated in planaria by the three cys-SPMs (SI Appendix, Fig. S6B and Table S1A). Therefore, we searched the predicted transcriptional binding sites on human TRAF3 promoter with cistromic analysis (39) using chromatin immunoprecipitation sequencing (https://www.signalingpathways.org/ominer/query.jsf?geneSearchType=gene&findMax=y&gene=TRAF3&species=all&reportsBy=pathways&omicsCategory=cistromics&countMax=3000). Several predicted binding sites for ATF/CREB family members were present. When human MΦ were incubated with each cys-SPM (10 nM) in the presence of a PKA phosphorylation inhibitor H89, it significantly diminished cys-SPM–induced TRAF3 expression (Fig. 3J). Therefore, these results demonstrated that MCTR3, PCTR3, and RCTR3 each activated the G protein-dependent cAMP-PKA pathway in human MΦ, which up-regulated TRAF3 expression during microbial challenge and stimulated phagocytosis of E. coli.
Human Macrophage Overexpression of TRAF3 Stimulates IL-10 and Phagocytosis.
Since cys-SPMs increased TRAF3 expression and stimulated MΦ proresolving functions (Fig. 3), we questioned whether TRAF3 contributes to these functions. To address this, we overexpressed TRAF3 protein in human MΦ (46.5 ± 14.8% increase, P < 0.05) (Fig. 4A), which statistically significantly increased IL-10 release upon STZ stimulation, compared with mock-transfected MΦ at 24 h (Fig. 4B). In comparison, TRAF3 overexpression did not significantly alter TNF-α release following STZ stimulation. In TRAF3-overexpressing MΦ, MCTR3 and RCTR3 each significantly further increased IL-10 levels with TRAF3-overexpressing MΦ, compared with mock-transfected MΦ (Fig. 4B). These results indicate that cys-SPMs increased IL-10 release in a TRAF3-dependent manner.
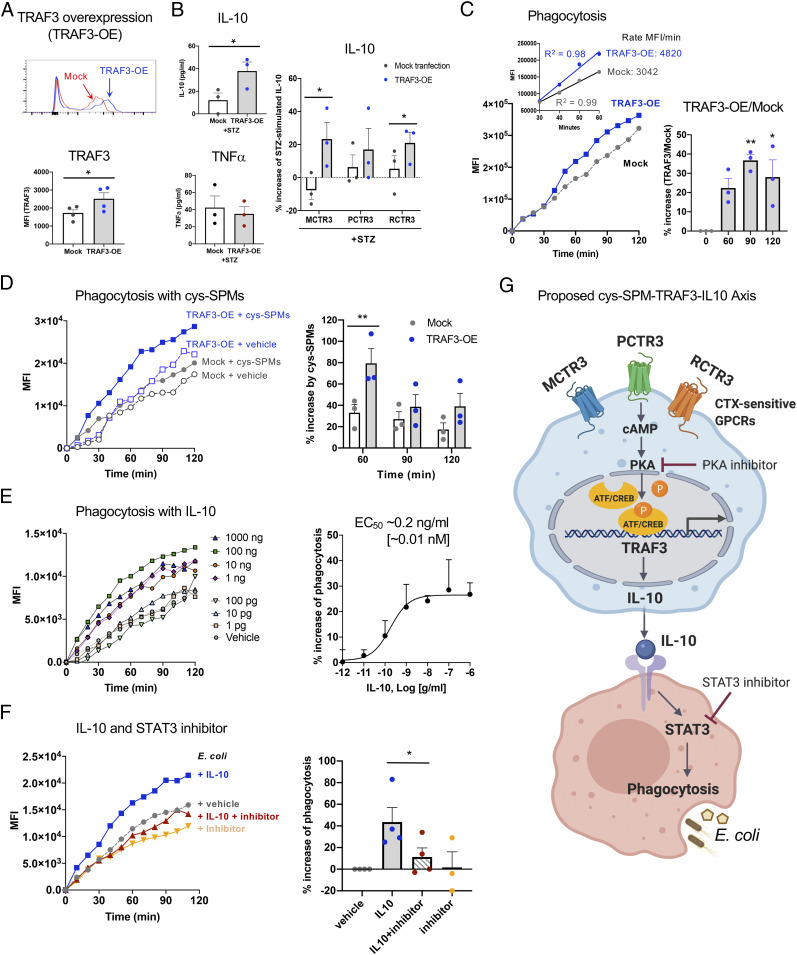
Fig. 4.
Overexpression of TRAF3 enhances IL-10 and phagocytosis with human macrophages. Human MΦ were transfected with Mock or TRAF3 plasmids. (A) TRAF3 protein expression was determined using flow cytometry with a specific anti-TRAF3 Ab. (Upper) Representative flow cytometry histograms; (Lower) mean ± SEM, n = 4; *P < 0.05. (B) Cytokine levels. MΦ were incubated with STZ with each cys-SPM (10 nM) or vehicle control for 24 h. Results are picograms per milliliter or percent increase of STZ alone; mean ± SEM, n = 3; *P < 0.05. (C and D) Phagocytosis was carried out as in Fig. 3E in the presence of a cys-SPM panel (MCTR3, PCTR3, and RCTR3 1 nM each) or vehicle control. (Left) MFI per cell from one representative experiment; (C, Inset) Kinetics 30 to 60 min (MFI/min). (C, Right) Percent increase of phagocytosis; MFI (TRAF3-OE)/MFI (mock); *P < 0.05, **P < 0.01 vs. time 0. (D, Right) Percent increase of phagocytosis by the cys-SPM panel; mean ± SEM, n = 3; **P < 0.01. For kinetics from three separate donors, see SI Appendix, Fig. S7B. (E and F) Phagocytosis was carried out as in Fig. 3E in the presence of (E) IL-10 (1 pg/ml to 1,000 ng/ml), (F) IL-10 (10 ng/ml) and/or a STAT3 inhibitor (NSC, 100 μM) for 24 h. (E and F, Left) MFI per cell from one representative experiment. (E, Right) Dose–response curve; mean ± SEM, n = 4; P < 0.05. EC50 was estimated using nonlinear regression with log (agonist) vs. response (three parameters). (F, Right) Percent increase of phagocytosis vs. E. coli alone; mean ± SEM, n = 3 or 4; *P < 0.05. Statistical analyses were carried out using (A, B, F) two-tailed Student’s t test, (C and E) one-way ANOVA, or (D) two-way ANOVA. (G) Proposed cys-SPMs/TRAF3/IL-10 axis.
We next investigated whether TRAF3 plays a role in MΦ phagocytosis of fluorescent-labeled E. coli using a real-time imaging microscope. TRAF3-overexpressing MΦ gave significantly increased phagocytosis at 90–120 min (30–40%), with higher rates from 30 to 60 min than those of mock-transfected MΦ (Fig. 4C). Combination of MCTR3, PCTR3, and RCTR3 (1 nM each) accelerated initial phagocytosis (0–30 min), and gave ∼80% increases at 60 min in TRAF3-overexpressing MΦ (Fig. 4D and SI Appendix, Fig. S7B). These increases were statistically significantly higher than those with mock-transfected cells (P < 0.01) (Fig. 4D). Taken together, these results demonstrated that overexpression of TRAF3 increased IL-10, accelerated phagocytosis, and contributed to cys-SPM–stimulated MΦ functions.
Since TRAF3 overexpression increased IL-10 levels and phagocytosis with human MΦ, we investigated whether IL-10 directly stimulates phagocytosis. IL-10 dose-dependently (1 pg to 1,000 ng/mL; P < 0.05, one-way ANOVA) enhanced MΦ phagocytosis of live E. coli (Fig. 4E), giving an estimated EC50∼0.2 ng/mL (∼0.01 nM). Because IL-10 protection of mouse infection is regulated by signal transducer and activator of transcription 3 (STAT3) (40, 41), we next tested whether IL-10-enhanced phagocytosis was mediated via STAT3. Incubation of MΦ with a STAT3 inhibitor (NSC 74859; 100 μM) together with IL-10 (10 ng/mL; ∼0.5 nM) for 24 h significantly diminished IL-10–stimulated phagocytosis by ∼75% (Fig. 4F). Thus, these results provide evidence that the IL-10/STAT3 pathway contributes to TRAF3-stimulated phagocytosis (Fig. 4G).
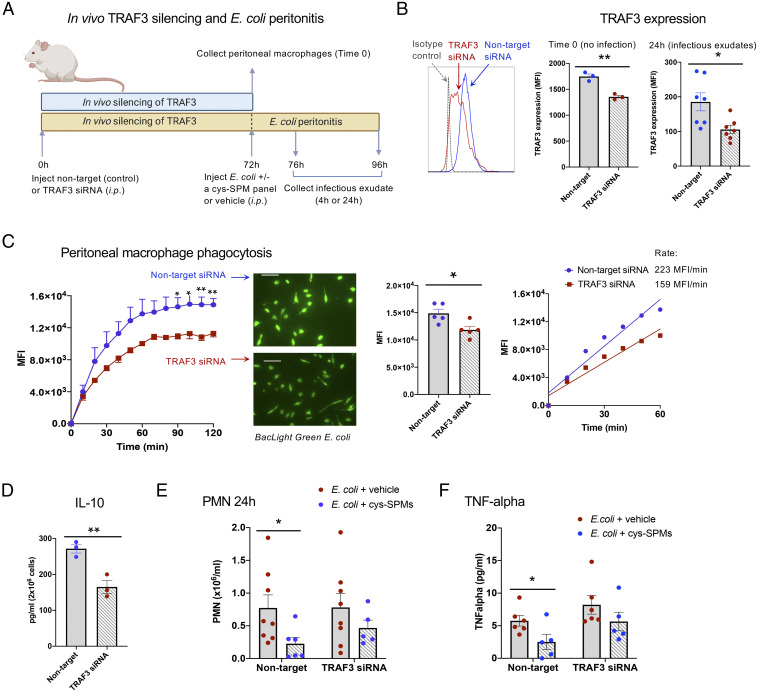
In Vivo Silencing of TRAF3 Hampers Phagocyte Functions and Resolution of E. coli Infection.
We questioned whether TRAF3 plays a role in mouse MΦ and contributes to cys-SPMs’ actions in regulating resolution of infection. To address this, we carried out in vivo silencing of TRAF3. TRAF3-specific small-interfering RNA (siRNA) or nontarget siRNA (10 μg per mouse, intraperitoneally) was injected and peritoneal MΦ collected at 72 h (see timeline in Fig. 5A). TRAF3 protein expression in MΦ was significantly reduced by siRNA specific for TRAF3, as demonstrated by flow cytometry (Fig. 5B) (P < 0.01). These MΦ from TRAF3 siRNA mice showed significant reduction (∼30%) in phagocytic capacity with E. coli, and also gave slower initial rates of phagocytosis (0–60 min) (Fig. 5C; see additional representative fluorescent images in SI Appendix, Fig. S8), as well as lowered IL-10 (∼45% decrease) (Fig. 5D), when directly compared with MΦ from nontarget siRNA control mice. These results (Fig. 5 C and D) are consistent with those obtained with human MΦ, where overexpression of TRAF3 increased IL-10 and phagocytosis (Fig. 4 B and C).
Fig. 5.
Impact of TRAF3 on resolution of infection: In vivo silencing. (A) Timeline. Mice were injected with nontarget- or TRAF3-siRNA (10 μg per mouse, intraperitoneally). Peritoneal cells were collected at 72 h (blue bar). In separate experiments, mice were inoculated with E. coli (1 × 105 CFU per mouse, intraperitoneally) with a cys-SPM panel (MCTR3, PCTR3, and RCTR3, 50 ng each) or vehicle control (0.1% ethanol [vol/vol]) in sterile saline. Exudates were collected 4 h or 24 h later (yellow bar). (B) TRAF3 protein expression at Time 0 and 24 h using flow cytometry with a specific anti-TRAF3 Ab. (Left) Representative histograms. (Right) TRAF3 expression (MFI); mean ± SEM, n = 3 (0 h) or 7 (24 h); *P < 0.05, **P < 0.01. (C) Peritoneal MΦ phagocytosis of E. coli was carried out as in Fig. 3E. (Left) Fluorescence intensities (MFI per cell); mean ± SEM, n = 5 mice; average of 195 cells per field and 4 fields per mouse; *P < 0.05, **P < 0.01, nontarget vs. TRAF3 siRNA. (Inset) Representative fluorescent images. (Scale bar, 50 μm.) See additional images in SI Appendix, Fig. S8. (Center) MFI at 2 h; mean ± SEM, n = 5; *P < 0.05. (Right) Kinetics from 0 to 60 min; mean of 5 mice per group. (D) Peritoneal MΦ were plated onto 24-well plates and incubated with STZ (100 ng/mL) for 24 h. Supernatants were collected and IL-10 levels determined; mean ± SEM, n = 3; **P < 0.01. (E and F) Infectious exudates from 24 h E. coli infection were collected; (E) PMN numbers; mean ± SEM, n = 5 to 8. (F) Exudate TNF-α levels; mean ± SEM, n = 5 or 6; *P < 0.05. (B–F) Two-tailed unpaired Student’s t test.
Next, to address the functions of TRAF3 in E. coli infection, TRAF3-specific siRNA or nontarget siRNA (10 μg per mouse, intraperitoneally) was injected. Peritonitis was initiated at 72 h with E. coli (105 CFU), together with a panel of cys-SPMs (MCTR3, PCTR3, and RCTR3, 50 ng each, intraperotoneally), and exudates collected 4 h or 24 h later (see timeline in Fig. 5A). TRAF3 protein expression in peritoneal leukocytes at 24 h was significantly reduced by TRAF3-specific siRNA (∼40% reduction) (Fig. 5B). In mice that received nontarget siRNA, cys-SPMs significantly reduced PMN numbers at 4 h and 24 h, giving ∼42% (P < 0.01) and ∼70% (P < 0.05) reductions, respectively (Fig. 5E and SI Appendix, Fig. S9). This action of cys-SPMs was diminished in mice that received TRAF3-specific siRNA. Of interest, TRAF3 siRNA statistically significantly increased monocytes at 24 h, but did not alter MΦ numbers (SI Appendix, Fig. S9). Combination of three cys-SPMs significantly reduced exudate TNF-α at 24 h in nontarget siRNA-injected mice; this action was lost in TRAF3 siRNA-injected mice (Fig. 5F). Thus, in vivo silencing of TRAF3 hampered MΦ phagocytosis and reduced IL-10 (Fig. 5 C and D). IL-10 is known to limit PMN infiltration (40), and here it enhanced MΦ phagocytosis (Fig. 4E). Taken together, results with mice and human MΦ demonstrated that TRAF3 mediated cys-SPM–stimulated resolution of infectious inflammation and phagocyte functions via their shared effector IL-10 (Fig. 4G and SI Appendix, Fig. S10).
Discussion
In the present report, using unbiased RNA-seq of planaria incubated with cys-SPMs (MCTR3, PCTR3, and RCTR3) during head regeneration, we identified cys-SPM–regulated pathways and transcripts. Using GO and CP analyses, as well as IPA, we identified several pathways related to innate immunity, including CD40, NF-κB, TNF-α, TLR, and RIG-1–like receptor signaling pathways, that were significantly regulated by the three cys-SPMs (Fig. 2 and SI Appendix, Figs. S4–S6). Planaria are known to exhibit robust regeneration involving the innate immune systems (e.g., MAPK/ERK signaling that is highly active at the wound sites) (27, 42). Blocking the immune signaling pathways disrupted planaria regeneration (42). In addition, LPS stimulates the RIG-1–like receptor signaling pathway (e.g., rig-1, traf3, traf6, and p38, all components of the planaria innate immune system) (43, 44). Here, we found that traf3 and mkk2 (a p38 MAPK kinase-activated protein kinase) were each selectively up-regulated by the three different cys-SPMs (Fig. 1). TRAF3 was selected via unbiased transcript and pathway analyses (Figs. 1 and 2 and SI Appendix, Tables S1–S3) for further examination in mammalian systems.
The three cys-SPMs up-regulated planaria transcripts with a predicted protein–protein interaction network for TRAF3 (i.e., traf5, abcc1, birc2, and uso1) (Fig. 1E). Recently, human TRAF5 was shown to control IL-17A signaling, reducing IL-6 production in human retinal epithelial cells (45).
A homolog of BIRC2, BIRC3, plays a role in neural cell survival, and neuroprotection D1/Protectin D1 (NPD1/PD1), another SPM (9), up-regulates BIRC3 in retinal and neural cell integrity (43). ABCC1, an ATP-binding cassette transporter, regulates stem cell proliferation and neuroregeneration (46). USO1 belongs to the Golgin family, which plays important roles in membrane trafficking and integrity of the Golgi apparatus. USO1-deficient mice display early embryonic lethality (47). These molecules, also up-regulated by cys-SPMs, may contribute to the enhanced planaria head regeneration in the presence of cys-SPMs.
Within this predicted protein–protein interaction network for TRAF3, OTUD5 was down-regulated by the three cys-SPMs, and USP7 down-regulated by PCTR3 and RCTR3 (Fig. 1E). Both OTUD5 and USP7 are deubiquinating proteins, and USP7 exhibits opposing functions of TRAF3. TRAF3 is a component of E3 ubiquitin–protein ligase complexes, which triggers proteasomal degradation of target genes, such as NF-κB (32). In contrast, USP7 stabilizes NF-ĸB, promoting NF-ĸB–mediated transcription (48). Down-regulation of USP7 by cys-SPMs may contribute to cys-SPMs’ actions in reducing NF-ĸB responses (e.g., TNF-α during murine infection) (Fig. 5F). Along these lines, most SPMs are known to reduce NF-ĸB and TNF-α in mice and human cells (9). Both USP7 and OTUD5 deubiquinate the tumor suppressor P53, providing potential targets for oncotherapy (49, 50). Their precise roles in cys-SPM signaling remain to be established.
The three different cys-SPMs—namely MCTR3, PCTR3, and RCTR3—each up-regulated TRAF3 in both planaria and human MΦ (Figs. 1 and 3 and SI Appendix, Fig. S3). We focused on TRAF3 and found that it contributed to cys-SPM–stimulated human MΦ phagocytosis of E. coli and resolution of E. coli infection (Figs. 4 and 5). As part of the underlying mechanisms, cys-SPMs likely activated GPCR(s) and Gαs protein-initiated cAMP-PKA pathway to regulate TRAF3 and phagocytosis (Fig. 3 G–J). Of interest, MCTR3, PCTR3, and RCTR3 at 10 nM shared the actions in up-regulating TRAF3 and the downstream effector IL-10 (Fig. 3 B–D), while only PCTR3 at 10 nM significantly increased ifn-γ and down-regulated traf6 (Fig. 3B). These results indicate that TRAF3 and IL-10 regulation were shared among three cys-SPMs, and in addition, distinct pathways appeared to be regulated individually by MCTR3, PCTR3, or RCTR3. IL-10 is a key regulator of phagocyte functions (40, 51). Here, TRAF3 overexpression increased IL-10 and phagocytosis with human MΦ (Fig. 4 B and C), and IL-10 directly enhanced E. coli phagocytosis (Fig. 4E). In vivo TRAF3 silencing reduced IL-10 and phagocytosis with mouse peritoneal MΦ (Fig. 5 C and D), as well as limited PMN accumulation and TNF-α in murine E. coli exudates (Fig. 5 E and F). These are consistent with earlier results where IL-10 limits PMN infiltration in mouse models by reducing PMN chemoattractants (e.g., TNF-α and IL8/KC) (40). Thus, IL-10 contributed to TRAF3 actions in MΦ phagocytosis and may also play a role in limiting PMN accumulation in vivo. These results demonstrated that cys-SPM/TRAF3/IL10 constitutes a proresolving axis (Fig. 4G). They do not, however, preclude involvement of additional pathways.
TRAFs are well-known adaptor proteins connecting TNF receptors with downstream intracellular signaling pathways (32). The crystal structure of TRAF3 in complex with a cytoplasmic fragment of CD40, a member of the TNFR superfamily, provides direct evidence for TRAF3 interaction with CD40 (52) (https://www.ncbi.nlm.nih.gov/Structure/pdb/1FLL). TRAF3 reduces inflammation, infections and tumor development (30), and is suggested to be a “gatekeeper” of TLR and TNFR signaling pathways (32). Along these lines, in myeloma patients low TRAF3 expression is associated with reduced survival (53). Thus, it is likely that cys-SPMs increase TRAF3 and downstream signaling components that constitute a resolution signaling axis to help control inflammation, infection, and related collateral tissue damage. While mammalian tissue repair is significantly different from planaria tissue regeneration, we have used these systems to identify shared molecular links between primordial regeneration and resolution of infection in mice and human MΦ (Figs. 1–5) that could be explored for future therapeutics.
It is noteworthy to point out that TRAF3 is a negative regulator of platelet activation and thrombosis (54) and promotes antiviral signaling and type I interferon production upon RNA virus infections (55, 56). Along these lines, resolvins reduce thrombosis (57) and control influenza virus infections (9). These protective actions of SPMs and TRAF3 in viral infections and thromboinflammation are of interest and may be useful in light of the cytokine storms and increased coagulopathies associated with the SARS-CoV-2 pandemic (58–60). Of interest, both resolvin D1 and D2 reduce SARS-CoV-2–activated cytokine storm with human MΦ (61) and SPMs are identified in severe and moderate COVID-19 patients (62). Dexamethasone, which is found useful in treating COVID-19 patients (63), stimulates SPM production in mouse lungs (64).
Some of these cys-SPM–regulated planaria immune pathways (Fig. 2) are also significantly regulated by resolvins in mammalian systems. For example, RvD1 regulates specific miRNAs that target NF-κB pathways (TRAF6, NOS2), pathogen recognition (TLRs, CD1d), leukocyte activation (CD40, PAFR), as well as IL-1 family and receptors (IL-1R, IL-1RA) (65). In addition, RvD1 and RvD5 each counterregulate proinflammatory molecules in human MΦ (e.g., NF-κB, CD40, TNF-α, and phosphodiesterase 4B) when these cells engage phagocytosis of E. coli (66). Specific SPMs (e.g., RvE1, RvD1, RvD2, RvD3, PD1, and MaR1) each up-regulates IL-10, TGF-β, 15-LOX-1, HO-1, CD163, CD206, and/or arginase-1 (reviewed in ref. 9), as well as A20 and SIGIRR (67). These earlier identified SPM-stimulated resolvers. together with cys-SPM–regulated molecules identified herein (Fig. 1) (e.g., TRAF3, BIRC2, ABCC1 and USO1, ATF1, CYLD, HAT1) can thus serve as a potential resolution fingerprint, along with the direct SPM and eicosanoid signatures (9, 68).
In summation, using the model organism planaria with cys-SPMs (MCTR3, PCTR3, and RCTR3) -enhanced regeneration and RNA-seq, we identified 175 known transcripts (Fig. 1) and 199 canonical pathways (Fig. 2) with signaling molecules that converge for the cys-SPMs on TRAF3. In human MΦ, cys-SPMs increased TRAF3, activated by cAMP-PKA pathways. TRAF3 regulated phagocyte functions via IL-10 and promoted resolution of infection in mice (Fig. 4G; see the overall scheme in SI Appendix, Fig. S10). Thus, TRAF3 contributed to cys-SPM transduction pathways in regeneration in planaria, linked to innate immune resolution responses in mice and human phagocytes. Targeting cys-SPM networks could provide approaches for controlling resolution of infections and wound healing, as well as organ protection and inflammatory diseases where tissue regeneration and control of unresolved inflammation are critically needed.
Materials and Methods
Experimental details on planaria injury, cell culture, lipid mediator metabololipidomics, real-time imaging of phagocytosis, real-time quantitative PCR, ELISA, mouse protocols, in vivo siRNA knockdown, RNA-seq pathway analysis, and statistical analysis are described in detail in SI Appendix, Supplementary Materials and Methods. Animal experimental procedures were approved by the Standing Committee on Animals of Brigham and Women’s Hospital (protocol no. 2016N000145).
Planaria Head Resection and RNA-Seq.
Planaria (D. japonica) head resection was performed (postocular surgical injury), posterior portions were placed in water together with MCTR3, PCTR3, RCTR3 (10 nM), or vehicle (0.1% ethanol [vol/vol] in water). Forty-eight hours later, D. japonica were collected for RNA-seq.
Bioinformatics.
Transcript abundance was assessed with the R package Kallisto (69) as log2 transcripts per million using a D. japonica transcriptome from Chan et al. (70), which contains 44,857 sequences. All coding sequences were translated into peptide sequences using genome-wide functional annotation obtained via orthology assignment by eggNOG-mapper (71). Differential expression was assessed with the R package Sleuth (72) using a Wald test that leverages the bootstrap estimates of Kallisto. All groups were compared with vehicle to obtain FC, log2FC, P values, and FDR (i.e., adjusted P value using the Benjamini–Hochberg method). P values were combined over the comparisons by transforming each P value to a z-score of the same sign as the corresponding log2FC, adding up the three z-scores per transcript, transforming this sum to a z-score, and calculating its two-sided P value. This combination is valid because the three comparisons were statistically independent. The combined FDRs were then calculated from these P values. FDR < 0.25 was considered as statistically significant. Pathway enrichment was assessed with the mean-rank gene set test (geneSetTest function in the R package limma) (73) against the CP and GO pathway databases.
Phagocytosis.
MΦ were treated with MCTR3, PCTR3, or RCTR3 (10 nM, 15 min or 24 h), IL-10 (1 pg/ml–1,000 ng/mL, 24 h), PKA inhibitor (H89, 10 μM, 24 h), or STAT3 inhibitor (NSC, 100 μM, 24 h) prior to initiation of phagocytosis with BacLight Green-labeled E. coli (E. coli:MΦ 50:1). In select experiments, a combination of cys-SPMs (1 nM each) was added to MΦ for 15 min, followed by addition of E. coli. Images were then acquired using a Keyence BZ-9000 (BIOREVO) inverted fluorescence phase-contrast microscope.
Murine E. coli Peritonitis: In Vivo Knockdown of TRAF3.
Mice were injected intraperitoneally with mouse TRAF3-specific or nontarget siRNA. Seventy-two hours later, E. coli was inoculated together with a panel of cys-SPMs or vehicle to initiate infectious peritonitis (see Fig. 5A for timelines). Leukocyte phenotyping and TRAF3 expression were assessed by flow cytometry.
Supplementary Material
Acknowledgments
We thank Mary H. Small for expert assistance in manuscript preparations; Prof. Michael Levin (Tufts University) for providing D. japonica seed colonies; and Dr. Zach Herbert (Molecular Biology Core Facilities, Dana-Farber Cancer Institute) for RNA-sequencing. We thank Prof. Nicos Petasis (University of Southern California) for synthesis of labeled cys-SPMs. Figs. 1A, 3A, 4G, and 5A were prepared using https://biorender.com/. This work was supported in part by NIH Grants R01GM038765 and P01GM095467 (to C.N.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2013374118/-/DCSupplemental.
Data Availability
The planaria RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE160278).
References
- 1.Serhan C. N., Savill J., Resolution of inflammation: The beginning programs the end. Nat. Immunol. 6, 1191–1197 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Rossi A. G., Sawatzky D. A., Eds., The Resolution of Inflammation (Birkhäuser Verlag AG, Basel, 2008). [Google Scholar]
- 3.Gordon S., Ed., Myeloid Cells in Health and Disease: A Synthesis (ASM Press, Washington, DC, 2017). [Google Scholar]
- 4.Forbes S. J., Rosenthal N., Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 20, 857–869 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Cartwright J. A., Lucas C. D., Rossi A. G., Inflammation resolution and the induction of granulocyte apoptosis by cyclin-dependent kinase inhibitor drugs. Front. Pharmacol. 10, 55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauseef W. M., Borregaard N., Neutrophils at work. Nat. Immunol. 15, 602–611 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Okabe Y., Medzhitov R., Tissue biology perspective on macrophages. Nat. Immunol. 17, 9–17 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Soehnlein O., Steffens S., Hidalgo A., Weber C., Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 17, 248–261 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Serhan C. N., Chiang N., Dalli J., New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects Med. 64, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padovan M. G., Norling L. V., Pro-resolving lipid mediators in sepsis and critical illness. Curr. Opin. Clin. Nutr. Metab. Care 23, 76–81 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Conte M. S., Desai T. A., Wu B., Schaller M., Werlin E., Pro-resolving lipid mediators in vascular disease. J. Clin. Invest. 128, 3727–3735 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredman G., Tabas I., Boosting inflammation resolution in atherosclerosis: The next frontier for therapy. Am. J. Pathol. 187, 1211–1221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirault J., Bäck M., Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 9, 1273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T., et al., HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 116, 23254–23263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Vicario C., et al., Association of a variant in the gene encoding for ERV1/ChemR23 with reduced inflammation in visceral adipose tissue from morbidly obese individuals. Sci. Rep. 7, 15724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu M., Wang X., Sun L., Schultzberg M., Hjorth E., Can inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 11, 1756286418791107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle R., Sadlier D. M., Godson C., Pro-resolving lipid mediators: Agents of anti-ageing? Semin. Immunol. 40, 36–48 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Sekheri M., El Kebir D., Edner N., Filep J. G., 15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc. Natl. Acad. Sci. U.S.A. 117, 7971–7980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colgan S. P., Resolvins resolve to heal mucosal wounds. Proc. Natl. Acad. Sci. U.S.A. 117, 10621–10622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannakis N., et al., Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat. Immunol. 20, 626–636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Rosa X., et al., Identification and complete stereochemical assignments of the new resolvin conjugates in tissue regeneration (RCTR) in human tissues that stimulate proresolving phagocyte functions and tissue regeneration. Am. J. Pathol. 188, 950–966 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godson C., Balancing the effect of leukotrienes in asthma. N. Engl. J. Med. 382, 1472–1475 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Levy B. D., et al., Cysteinyl maresins regulate the prophlogistic lung actions of cysteinyl leukotrienes. J. Allergy Clin. Immunol. 145, 335–344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liening S., Romp E., Werz O., Scriba G. K. E., Garscha U., Liquid chromatography-coupled mass spectrometry analysis of glutathione conjugates of oxygenated polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 144, 106350 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Wang Q., et al., MCTR1 enhances the resolution of lipopolysaccharide-induced lung injury through STAT6-mediated resident M2 alveolar macrophage polarization in mice. J. Cell. Mol. Med. 24, 9646–9657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P. H., et al., PCTR1 improves pulmonary edema fluid clearance through activating the sodium channel and lymphatic drainage in lipopolysaccharide-induced ARDS. J. Cell. Physiol. 235, 9510–9523 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Sánchez Alvarado A., Planarian regeneration: Its end is its beginning. Cell 124, 241–245 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Li Y. Y., et al., Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat. Commun. 8, 14121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadler A. J., et al., The acetyltransferase HAT1 moderates the NF-κB response by regulating the transcription factor PLZF. Nat. Commun. 6, 6795 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Lalani A. I., et al., Myeloid cell TRAF3 regulates immune responses and inhibits inflammation and tumor development in mice. J. Immunol. 194, 334–348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Häcker H., et al., Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Häcker H., Tseng P. H., Karin M., Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 11, 457–468 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Oughtred R., et al., The BioGRID interaction database: 2019 update. Nucleic Acids Res. 47, D529–D541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalli J., Chiang N., Serhan C. N., Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. U.S.A. 111, E4753–E4761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sok, et al., Dual delivery of IL-10 and AT-RvD1 from PEG hydrogels polarize immune cells towards pro-regenerative phenotype. Biomaterials 268, 120475 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bystrom J., et al., Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 112, 4117–4127 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engh R. A., Girod A., Kinzel V., Huber R., Bossemeyer D., Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J. Biol. Chem. 271, 26157–26164 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Sands W. A., Palmer T. M., Regulating gene transcription in response to cyclic AMP elevation. Cell. Signal. 20, 460–466 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Liu T., et al., Cistrome: An integrative platform for transcriptional regulation studies. Genome Biol. 12, R83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazzoni F., Tamassia N., Rossato M., Cassatella M. A., Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: Lessons from neutrophils. Eur. J. Immunol. 40, 2360–2368 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Kang M. J., et al., IL-10 protects mice from the lung infection of Acinetobacter baumannii and contributes to bacterial clearance by regulating STAT3-mediated MARCO expression in macrophages. Front. Immunol. 11, 270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umesono Y., et al., The molecular logic for planarian regeneration along the anterior-posterior axis. Nature 500, 73–76 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Calandria J. M., et al., NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Differ. 22, 1363–1377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang Q., et al., De novo transcriptome analysis provides insights into immune related genes and the RIG-I-like receptor signaling pathway in the freshwater planarian (Dugesia japonica). PLoS One 11, e0151597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGowan J., et al., 14-3-3ζ-TRAF5 axis governs interleukin-17A signaling. Proc. Natl. Acad. Sci. U.S.A. 117, 25008–25017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacher T., et al., ABC transporters B1, C1 and G2 differentially regulate neuroregeneration in mice. PLoS One 7, e35613 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S., Hill A., Warman M. L., Smits P., Golgi disruption and early embryonic lethality in mice lacking USO1. PLoS One 7, e50530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitxitorena I., et al., The deubiquitinase USP7 uses a distinct ubiquitin-like domain to deubiquitinate NF-ĸB subunits. J. Biol. Chem. 295, 11754–11763 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohol Y. M., et al., Novel, selective inhibitors of USP7 uncover multiple mechanisms of antitumor activity in vitro and in vivo. Mol. Cancer Ther. 19, 1970–1980 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Luo J., et al., OTUD5 regulates p53 stability by deubiquitinating p53. PLoS One 8, e77682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michlewska S., Dransfield I., Megson I. L., Rossi A. G., Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: Key role for TNF-alpha. FASEB J. 23, 844–854 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Ni C. Z., et al., Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. U.S.A. 97, 10395–10399 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dash A. B., et al., Clinical benefit of ixazomib plus lenalidomide-dexamethasone in myeloma patients with non-canonical NF-κB pathway activation. Eur. J. Haematol. 105, 274–285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R., et al., TRAF3 negatively regulates platelet activation and thrombosis. Sci. Rep. 7, 17112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beachboard D. C., et al., The small GTPase RAB1B promotes antiviral innate immunity by interacting with TNF receptor-associated factor 3 (TRAF3). J. Biol. Chem. 294, 14231–14240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu W., et al., TRAF3IP3 mediates the recruitment of TRAF3 to MAVS for antiviral innate immunity. EMBO J. 38, e102075 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cherpokova D., et al., Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 134, 1458–1468 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamond B., The renin-angiotensin system: An integrated view of lung disease and coagulopathy in COVID-19 and therapeutic implications. J. Exp. Med. 217, e20201000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giannis D., Ziogas I. A., Gianni P., Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 127, 104362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sousa L. P., Pinho V., Teixeira M. M., Harnessing resolving-based therapeutics to treat pulmonary viral infections: What can the future offer to COVID-19? Br. J. Pharmacol. 177, 3898–3904 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Recchiuti A., et al., Resolvin D1 and D2 reduce SARS-Cov-2-induced inflammation in cystic fibrosis macrophages. bioRxiv:10.1101/2020.08.28.255463 (28 August 2020).
- 62.Schwarz B., et al., Cutting edge: Severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J. Immunol. 206, 329–334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horby P.et al.; RECOVERY Collaborative Group , Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N. Engl. J. Med., 10.1056/NEJMoa2021436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pyrillou K., Chairakaki A. D., Tamvakopoulos C., Andreakos E., Dexamethasone induces ω3-derived immunoresolvents driving resolution of allergic airway inflammation. J. Allergy Clin. Immunol. 142, 691–695.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C. N., MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiang N., et al., Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sham H. P., et al., 15-epi-Lipoxin A4, resolvin D2, and resolvin D3 induce NF-κB regulators in bacterial pneumonia. J. Immunol. 200, 2757–2766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samuelsson B., Role of basic science in the development of new medicines: Examples from the eicosanoid field. J. Biol. Chem. 287, 10070–10080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bray N. L., Pimentel H., Melsted P., Pachter L., Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Chan J. D., et al., Dataset for a Dugesia japonica de novo transcriptome assembly, utilized for defining the voltage-gated like ion channel superfamily. Data Brief 9, 1044–1047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huerta-Cepas J., et al., eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 44, D286–D293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pimentel H., Bray N. L., Puente S., Melsted P., Pachter L., Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14, 687–690 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Goeman J. J., Bühlmann P., Analyzing gene expression data in terms of gene sets: Methodological issues. Bioinformatics 23, 980–987 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The planaria RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE160278).