Significance
N6-methyladenosine (m6A) is the most prevalent modification in eukaryotic messenger RNA (mRNA) and affects RNA metabolism including splicing, stability, and translation. The m6A methyltransferase complex (MTC) is responsible for generating the m6A modifications in mRNA; however, the regulation of m6A modification is still unclear. We have identified Mechanistic Target of Rapamycin Complex 1 (mTORC1) as a key regulator of MTC and demonstrate that mTORC1 can stabilize MTC via activation of the chaperonin CCT complex and upregulate m6A modification to promote the degradation of ATG transcripts. Thus, our study unveils an mTORC1-signaling cascade that regulates m6A RNA methylation and autophagy.
Keywords: mTORC1, m6A methyltransferase complex (MTC), chaperonin containing Tailless complex polypeptide 1 (CCT), m6A RNA methylation, autophagy
Abstract
Mechanistic Target of Rapamycin Complex 1 (mTORC1) is a central regulator of cell growth and metabolism that senses and integrates nutritional and environmental cues with cellular responses. Recent studies have revealed critical roles of mTORC1 in RNA biogenesis and processing. Here, we find that the m6A methyltransferase complex (MTC) is a downstream effector of mTORC1 during autophagy in Drosophila and human cells. Furthermore, we show that the Chaperonin Containing Tailless complex polypeptide 1 (CCT) complex, which facilitates protein folding, acts as a link between mTORC1 and MTC. The mTORC1 activates the chaperonin CCT complex to stabilize MTC, thereby increasing m6A levels on the messenger RNAs encoding autophagy-related genes, leading to their degradation and suppression of autophagy. Altogether, our study reveals an evolutionarily conserved mechanism linking mTORC1 signaling with m6A RNA methylation and demonstrates their roles in suppressing autophagy.
Mechanistic Target of Rapamycin Complex 1 (mTORC1), an evolutionarily conserved serine/threonine kinase, is a master regulator of cell growth, metabolism, and proliferation coupling different nutritional and environmental cues, including growth factors, energy levels, cellular stress, and amino acids, with metabolic programs (1). For example, insulin activates PI3K/AKT and inhibits the Tuberous Sclerosis Complex (TSC) 1/2, a negative regulator of mTORC1, thus promoting mTORC1 activation (2). Activated mTORC1 then phosphorylates multiple downstream effectors that control a wide range of anabolic and catabolic processes. Phosphorylation of the ribosomal S6 kinase 1 (S6K1) and eIF4E-binding protein 1 (4E-BP1) by mTORC1 promotes protein translation and enhances cell growth and proliferation (3). Moreover, autophagy, an intracellular degradation system that delivers cytoplasmic components to lysosomes, is inhibited by mTORC1 through phosphorylation of Atg13 that, in turn, inhibits ULK1 kinase activity (4).
Recent studies have highlighted a role for mTORC1 in regulating RNA metabolism. Through the phosphorylation of RNA metabolic proteins, mTORC1 modulates various RNA biogenesis and processing events. Phosphorylation of the SR protein kinase SRPK2 by S6K1 promotes its transport into the nucleus where it activates SR proteins and induces splicing of lipogenic pre-messenger RNAs (pre-mRNAs) for de novo synthesis of fatty acids and cholesterol, suggesting that SRPK2 is a critical mediator of mTORC1-dependent lipogenesis (5). In addition, mTORC1 regulates alternative splicing and polyadenylation of autophagic and metabolic genes to control autophagy, lipid, protein, and energy metabolism through the cleavage and polyadenylation complex (6). Furthermore, mTORC1 mediates phosphorylation of the decapping enzyme Dcp2. Phosphorylated Dcp2 associates with RNA helicase RCK family members and binds to transcripts of Autophagy-related genes (Atg) to degrade them, thereby suppressing autophagy (7). Altogether, these studies suggest an essential role for mTORC1 in controlling RNA biogenesis and processing, revealing a major function for mTORC1 in the regulation of protein diversity and in reshaping cellular metabolism and autophagy.
N6-methyl-adenosine (m6A) is one of the most abundant chemical modifications in eukaryotic mRNA, which is preferentially enriched in 3′ UTRs and around stop codons (8, 9). m6A modification affects almost all aspects of mRNA metabolism, such as splicing, translation, and stability, and plays essential roles in a wide range of cellular processes, including Drosophila sex determination and metabolism (10). The m6A methyltransferase complex (MTC) catalyzes m6A formation and is composed of the methyltransferase-like protein 3 (METTL3), the methyltransferase-like protein 14 (METTL14), WTAP (the ortholog of Drosophila Fl(2)d), and RBM15/RBM15B (the ortholog of Drosophila Nito). Although METTL3 is the only catalytic component of the MTC, its interaction with METTL14 is necessary for RNA substrate recognition and efficient m6A deposition. WTAP stabilizes the interaction between the two METTL proteins, and RBM15/RBM15B have been proposed to recruit the MTC to its target transcripts (10, 11).
Using autophagy as a readout of mTORC1 signaling in Drosophila, we identified the MTC as a downstream effector of mTORC1 signaling. From the analysis of high-confidence Drosophila and human MTC proteomic data, we further identified the Chaperonin Containing Tailless complex polypeptide 1 (CCT) complex as an MTC interactor that mediates the effects of mTORC1 on m6A modification and autophagy. In mammalian cells, we also found that the CCT complex plays critical roles in the regulation of MTC protein stability and m6A RNA modification, suggesting that the mTORC1-CCT-MTC axis is conserved from Drosophila to mammals. Our studies thus unveil a mechanism linking mTORC1 signaling and the chaperonin CCT complex to RNA methylation and also uncover a layer of mTORC1 regulation of autophagy.
Results
Components of the MTC Modify TSC1RNAi-Inhibited Autophagy.
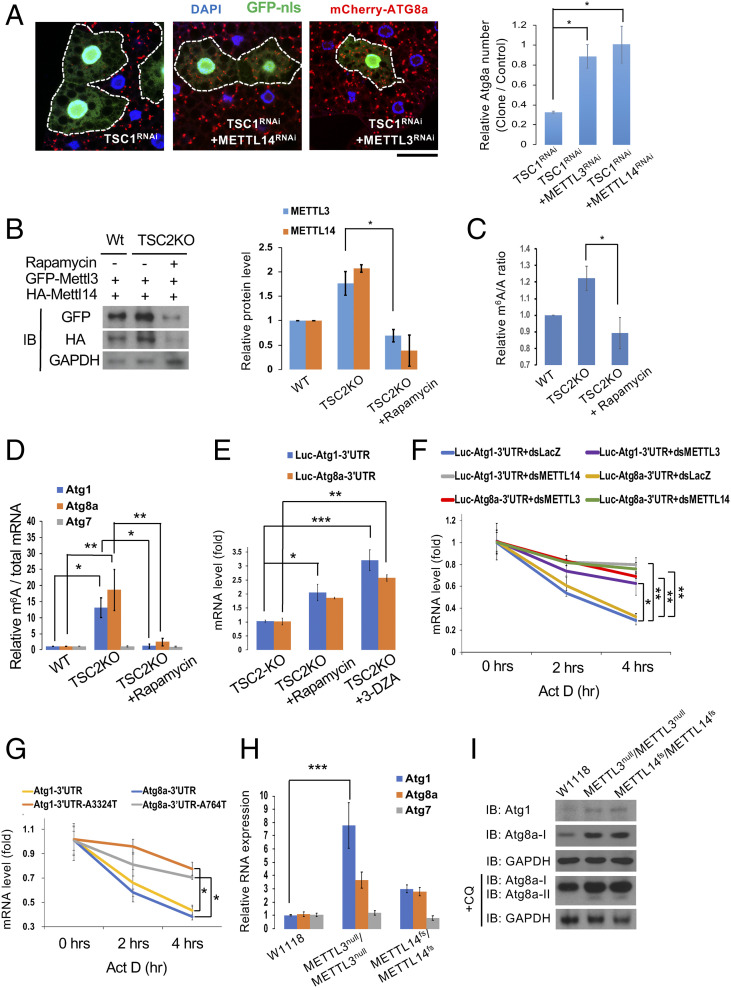
Inhibition of either TSC1 or TSC2 leads to mTORC1 overactivation which impairs autophagy (12). To identify downstream effectors of mTORC1 signaling, we performed an RNA interference (RNAi) screen for autophagy modifiers by generating flip-out clones expressing TSC1-RNAi and the autophagosomal marker mCherryAtg8a in larval fat bodies. While starvation induced autophagosome formation, clonal expression of TSC1-RNAi abolished starvation-induced mCherry-ATG8a punctae formation (Fig. 1A). Interestingly, RNAi lines against METTL3 or METTL14, components of the MTC, suppressed the TSC1-RNAi–induced effects, indicating that METTL3 and METTL14 are required for the effect of TSC1-inhibited autophagy (Fig. 1A). Furthermore, mCherryATG8a punctae were induced by depletion of either METTL3 or METTL14 (SI Appendix, Fig. S1A). Consistently, METTL3 null mutants also exhibited higher autophagy levels with or without chloroquine (CQ) treatment which blocks autophagosome degradation (13) (SI Appendix, Fig. S1B). Together, these results suggest that the MTC acts downstream of mTORC1 signaling to regulate autophagy.
Fig. 1.
mTORC1 signaling regulates the MTC to suppress autophagy. (A) MTC acts downstream of mTORC1 signaling. Clonal depletion of TSC1 in GFP-labeled cells suppressed the formation of mCherry-ATG8a puncta under starvation conditions (compared to control cells outside the circled dashed lines). Coexpression of METTL3RNAi or METTL14RNAi reversed the TSC1RNAi-induced effect. Fat body cells were stained with DAPI. (Scale bar, 20 µm.) Quantification of the relative number of mCherry-ATG8a dots per cell. (B) mTORC1 activity regulates METTL3 and METTL14 protein levels. Wild-type S2R+ or TSC2 KO cells transfected with GFP-METTL3 or HA-METTL14 were treated with or without rapamycin. The protein levels of METTL3, METTL14, and GAPDH were analyzed by immunoblotting (IB) with antibodies as indicated and quantified. (C and D) mTORC1 signaling modulates global m6A RNA methylation levels as well as m6A levels in Atg transcripts. m6A levels were quantified using LC-MS in S2R+ cells treated as indicated. Compared with wild-type S2R+ cells, TSC2 KO cells showed enhanced m6A levels in their mRNAs while rapamycin treatment on TSC2 KO cells reduced it (C). Abundance of Atg1, Atg8a, and Atg7 transcripts among mRNA immunoprecipitated with anti-m6A antibody from S2R+ cells treated as indicated. m6A-modified Atg1 and Atg8a, but not Atg7 mRNAs, were increased in TSC2 KO cells, and rapamycin treatment in TSC2 KO cells can reduce it (D). (E) mTORC1 and MTC activities decrease Atg1 and Atg8a mRNA levels through their 3′ UTR regions. Firefly luciferase reporters with Atg1 or Atg8a 3′ UTRs were transfected into S2R+ cells. After 48 h, cells were treated with rapamycin (20 nM) or 3-DZA (100 μM) for 48 h and mRNA levels of Firefly luciferase were measured by qPCR. (F and G) m6A methylation is required for Atg1 and Atg8a mRNA degradation. Firefly luciferase reporters with the indicated 3′ UTRs were transfected into S2R+ cells treated with or without dsRNA against LacZ, METTL3, or METTL14. After 48 h, cells were treated with actinomycin D (10 μg/mL) for the indicated times to measure mRNA levels of Firefly luciferase by qPCR. (H and I) METTL3 and METTL14 mutants exhibit higher Atg1 and Atg8a transcripts as well as ATG1 and ATG8a protein levels in the larval fat body. RNA (H) or protein (I) extracts from larval fat bodies of wild-type (w1118), METTL3 null mutant (METTL3null/METTL3null), or METTL14 mutant (METTL14fs/METTL14fs), fed with or without CQ, were subjected to qPCR assay (H) or Western blot analysis using antibodies as indicated (I). One-way ANOVA test was performed followed by Tukey’s test to identify significant differences. Measurements shown are mean ± SEM of triplicates; *P < 0.05; **P < 0.01; ***P < 0.001.
mTORC1 Positively Regulates METTL3 and METTL14 Protein Levels to Control m6A RNA Methylation and Autophagy.
To investigate how mTORC1 regulates the MTC, we first tested whether METTL3 and/or METTL14 protein levels are affected by mTORC1 activity. As shown in Fig. 1B, both METTL3 and METTL14 levels were increased in TSC2 knockout (KO) cells, an effect that was inhibited by rapamycin (Fig. 1B). The time-course treatment of rapamycin further showed that METTL3 and METTL14 protein levels were significantly decreased after 48 h of rapamycin treatment (SI Appendix, Fig. S1 C and D). Consistent with this result, as METTL3 and METTL14 are essential for m6A RNA methylation, liquid chromatography-tandem mass spectrometry (LC-MS) analysis showed that global m6A RNA levels were enhanced in TSC2 KO cells and that the TSC2 KO-induced effect was suppressed by rapamycin (Fig. 1C). Together, these data show that mTORC1 activity induces METTL3 and METTL14 protein expression to increase m6A RNA methylation levels. In contrast, the protein levels of Nito, another MTC component, were not affected by rapamycin treatment (SI Appendix, Fig. S1D), indicating that METTL3 and METTL14 are specifically controlled by mTORC1 signaling.
Several Autophagy-related gene (Atg) transcripts have been shown to possess m6A methylation, which promotes mRNA degradation of these transcripts and suppresses autophagy (14, 15). We therefore examined whether mTORC1 regulation of METTL3 and METTL14 controls autophagy through this mechanism. m6A RNA immunoprecipitation (MeRIP) assays using an anti-m6A antibody revealed that Atg1 and Atg8a mRNAs from TSC2 KO cells show higher levels of m6A methylation than those from wild-type cells (8) (Fig. 1D). Consistently, rapamycin significantly reduced m6A levels in Atg1 and Atg8a transcripts from TSC2 KO cells (Fig. 1D), demonstrating that mTORC1 activity controls m6A methylation of Atg transcripts.
Next, to analyze the effects of the mTORC1-dependent m6A methylation of Atg transcripts, we tested whether m6A affects the Atg1 and Atg8a mRNA turnover. We generated luciferase reporter constructs with the short 3′ UTR region of either Atg1 or Atg8a and expressed them in S2R+ cells. Cells were then treated with either rapamycin or 3-DZA (the m6A inhibitor). qPCR analysis revealed that the amounts of luciferase mRNAs with either the Atg1 or Atg8a 3′ UTRs in 3-DZA- or rapamycin-treated cells were higher than those in wild-type cells, indicating that mTORC1-dependent m6A methylation reduces Atg1 and Atg8a mRNA levels through their 3′ UTRs (Fig. 1E). Moreover, double-stranded RNA (dsRNA)-mediated depletion of either METTL3 or METTL14 increased the half-life of luciferase mRNA with either Atg1 or Atg8a 3′ UTRs (Fig. 1F). Further, mutation of the m6A sites in the Atg1 and Atg8a 3′ UTR regions identified by miCLIP enhanced mRNA stabilities (16) (Fig. 1G). These results demonstrate that m6A RNA modification is required for Atg1 and Atg8a mRNA degradation. Consistent with these results, both mRNA and protein levels of Atg1 and Atg8a were increased in larval fat bodies of METTL3 and METTL14 null mutants, compared to wild type (Fig. 1 H and I). However, no significant changes of m6A and mRNA levels of Atg7 were detected in TSC2 KO cells or MTC mutants (Fig. 1 D and H). Taken together, these results indicate that mTORC1-regulated m6A methylation enhances degradation of specific Atg transcripts, which in turn inhibits autophagy.
Proteomic Identification of m6A RNA Methyltransferase Complex Protein–Protein Interaction Networks.
As mTORC1 increases protein expression through transcriptional, posttranscriptional, or translational regulation (5, 6, 17), we further investigated how mTORC1 up-regulates METTL3 and METTL14 protein levels. qPCR analysis of METTL3 and METTL14 revealed no significant changes in their transcript levels in TSC2 KO cells with or without rapamycin treatment (SI Appendix, Fig. S1E), indicating that the increase in METTL3 and METTL14 protein levels by mTORC1 signaling is not at the mRNA level. In addition, even though inhibition of protein translation by cycloheximide (CHX) reduced MTC protein levels, cotreatment of CHX with rapamycin further induced a dramatic decline of METTL3 and METTL14 levels (SI Appendix, Fig. S1F). In contrast, Nito protein levels were the same in these two conditions (SI Appendix, Fig. S1F). Together, these results suggest that mTORC1 enhances METTL3 and METTL14 protein levels in a transcription- and translation-independent manner.
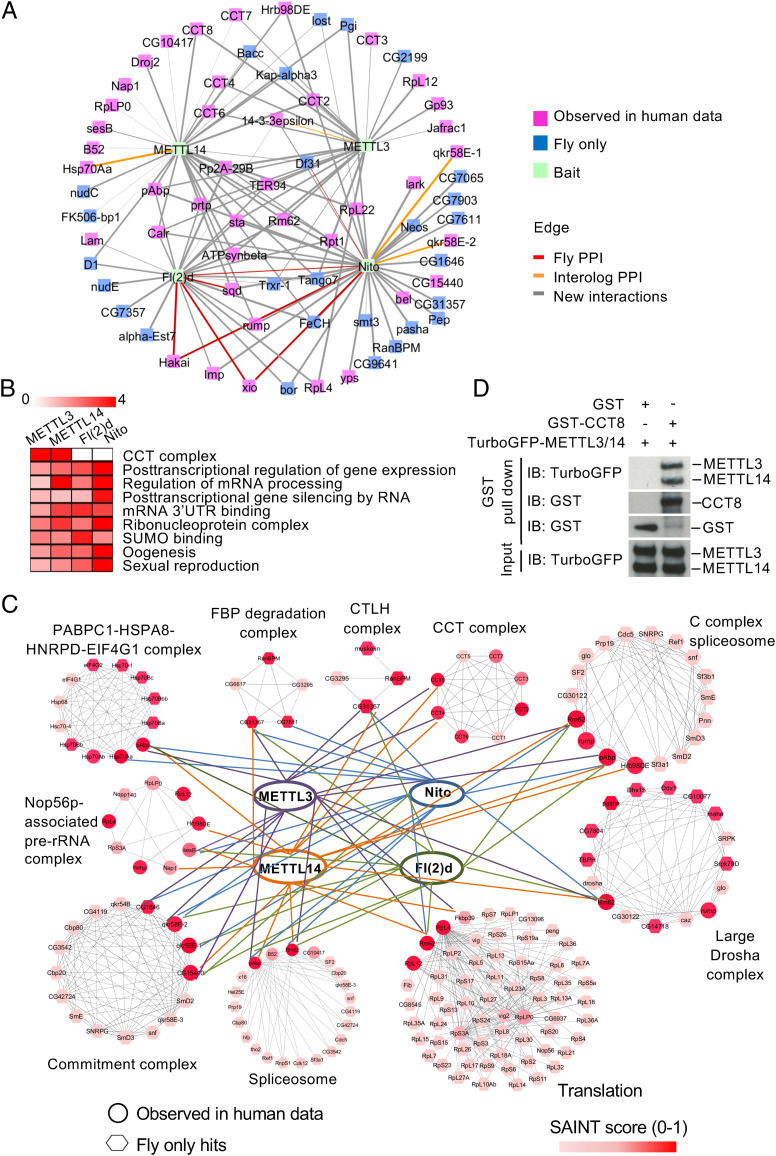
In order to characterize how mTORC1 regulates MTC, we generated Protein-Protein Interaction Networks (PPINs) centered on the Drosophila and human MTC by affinity purification and mass spectrometry (AP/MS) from Drosophila S2R+ and human HEK293T cells (Fig. 2A). We identified unfiltered networks of 1,462 and 1,504 interactions in Drosophila and human Methyltransferase Complex Protein–Protein Interaction Networks (MTC-PPINs), respectively, with more than 95% of the preys being evolutionarily conserved, and used the Significance Analysis of Interactome (SAINT) algorithm to evaluate the networks obtained with AP/MS (18). By comparing MTC-PPIN with published interactions (SI Appendix, Fig. S2 A and B), we further generated high-confidence MTC-PPIs with a SAINT score (SS) ≥ 0.2 (130 interactions for Drosophila as and 230 interactions for humans) (Fig. 2A). (See Dataset S1 for the full list of interactions and Dataset S2 for the list of interaction pairs with SS ≥ 0.2). Eleven known biochemical interactions were recovered in the Drosophila high-confidence MTC-PPIN (Fig. 2A, red and yellow edges). Moreover, the Drosophila MTC-PPIN was significantly enriched for hits from the published m6A RNA pull-down analysis (19) (SI Appendix, Fig. S2C), revealing that it is of high quality.
Fig. 2.
Identification of CCT complex as an interactor of MTC. (A) Network representation of the Drosophila MTC-PPIN. We collected the published PPIs deposited in public repositories from MIST (35) as well as the full LC-MS/MS datasets from two relevant studies (32, 36) and selected the interactions that are supported by at least two of three resources as the positive control set (SI Appendix, Fig. S2 A and B). Based on the analysis, comparing fly MTC-PPIN with interactions in the positive control set, we defined the SAINT cutoff and selected 130 and 230 high-confident interactions from fly and human MTC-PPINs, respectively, for follow up. PPIs with high confidence are shown. The pink node represents hits that can be found in both Drosophila and human MTC-PPINs, while the blue node indicates that the hits were found only in Drosophila MTC-PPIN. Green node indicates the bait. Red or yellow edges indicate the known interactions based on Drosophila data or data mapped from orthologous genes (interologs) annotated at MIST. Gray edges indicate interactions from the PPINs. The thickness of edges corresponds to the SAINT score. (B) Heatmap, based on COMPLEAT analysis, displaying interaction between baits (Top) and the –log10 (P value) for selected cellular processes (Right). Color represents the strength of significance. (C) Validated Drosophila MTC-PPIN with complexes involved in protein folding, RNA processing, and translation. Co-IP data are shown in SI Appendix, Fig. S3. (D) METTL3 and METTL14 directly interact with CCT8. In-vitro–translated Drosophila TurboGFP-METTL3, TurboGFP-METTL14, and GST-CCT8 proteins were subjected to GST pull-down assay. Pull-down fractions and input were analyzed by immunoblotting with antibodies as indicated.
The CCT Complex Directly Interacts with and Stabilizes METTL3 and METTL14.
To gain further insights into the organization of the MTC-PPIN, we used COMPLEAT to perform a protein-complex enrichment analysis and identified several protein complexes involved in posttranscriptional regulation and modification (Fig. 2B) (20). Physical interactions of those complexes with MTC were confirmed by coimmunoprecipitation (CoIP) (Fig. 2C and SI Appendix, Fig. S3). Surprisingly, we found that only METTL3 and METTL14, but not other MTC components, specifically associate with the CCT complex, a chaperonin complex that facilitates protein folding and complex assembly (Fig. 2 B and C and SI Appendix, Fig. S3) (21). To determine whether this interaction is direct, we performed GST pull-downs with in-vitro–translated GST-tagged CCT8 and TurboGFP-tagged METTL3 and METTL14. As shown in Fig. 2D, METTL3 and METTL14 directly interacted with CCT8 (Fig. 2D), revealing the direct interaction between MTC and CCT.
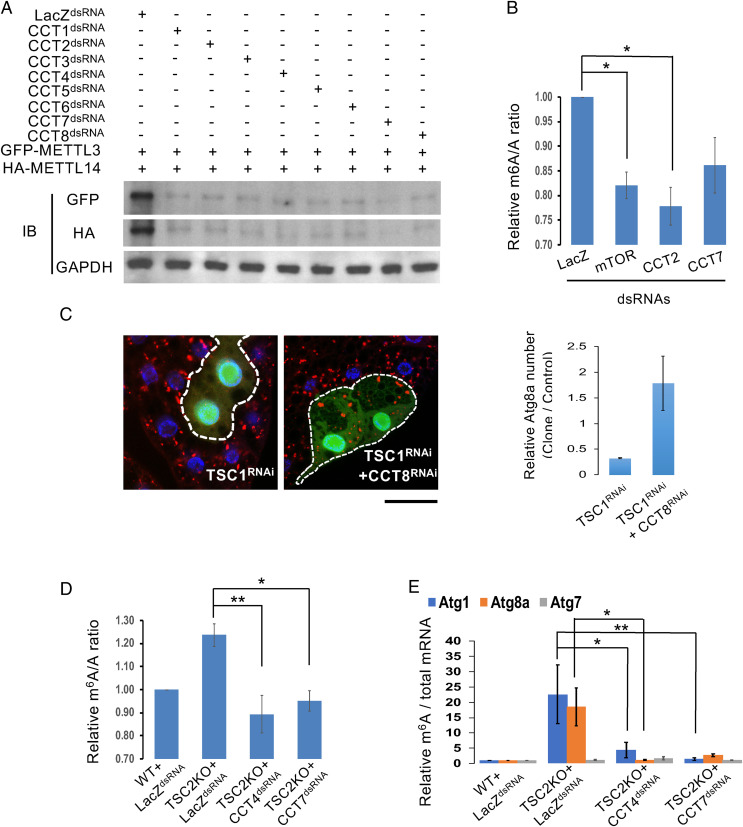
The interaction of the CCT complex with METTL3 and METTL14 raised the possibility that METTL3/14 may be substrates of chaperonin CCT complex and that CCT can help their folding and promote their protein stabilities. Indeed, we found that knockdown of CCT1-8 significantly reduced METTL3 and METTL14 protein levels, but not Nito, suggesting that CCT specifically stabilizes METTL3 and METTL14 proteins (Fig. 3A and SI Appendix, Fig. S4). Consistently, LC-MS analysis showed that m6A RNA levels are decreased in CCT2- or CCT7-depleted cells, similar to the effect of mTORC1 depletion (Fig. 3B). In conclusion, these results suggest that the CCT complex impacts METTL3 and METTL14 to enhance their protein stabilities and regulate m6A methylation.
Fig. 3.
mTORC1-CCT–signaling pathway acts as a positive regulator of MTC in Drosophila. (A and B) Depletion of CCT complex reduces METTL3 and METTL14 protein levels as well as m6A RNA methylation. S2R+ cells were treated with dsRNAs against LacZ or CCT1-8. After 48 h, cells were transfected with GFP-METTL3 or HA-METTL14 and then subjected to immunoblotting (IB) with antibodies as indicated (A). Quantification of m6A in S2R+ cells treated with dsRNAs against LacZ, mTOR, CCT2, or CCT7. One-way ANOVA followed by Tukey’s multiple comparisons test was performed to identify significant differences. Data are expressed as mean ± SEM of three independent experiments; *P < 0.05 (B). (C) The CCT complex acts downstream of mTORC1. Clonal depletion of TSC1 in GFP-labeled cells suppressed the formation of mCherry-ATG8a puncta under starvation condition, compared to wild-type cells outside the circled dashed line. Coexpression of CCT8RNAi reversed the TSC1RNAi-induced effect. Fat-body cells were stained with DAPI. (Scale bar, 20 µm.) Quantification of the relative number of mCherry-ATG8a dots per cell. (D and E) mTORC1 activates m6A RNA methylation in a CCT-dependent manner. Quantification of m6A in wild-type or TSC2 KO S2R+ cells treated with dsRNAs against LacZ, CCT4, or CCT7. Compared with wild-type S2R+ cells, TSC2 KO cells showed enhanced m6A levels in their mRNAs while TSC2 KO cells treated with CCT dsRNAs had reduced m6A levels (D). Abundance of Atg transcripts among mRNA immunoprecipitated with anti-m6A antibody from S2R+ cells treated as indicated. m6A-modified Atg1 and Atg8a, but not Atg7 mRNAs, were increased in TSC2 KO cells, and knockdown of CCT4 or CCT7 in TSC2 KO cells reduced m6A-modified Atg1 and Atg8a (E). One-way ANOVA followed by Tukey’s multiple comparisons test was performed to identify significant differences. Data are expressed as mean ± SEM of three independent experiments; *P < 0.05; **P < 0.01.
The Effects of mTORC1 on m6A Modification and Autophagy Are Mediated by the CCT Complex.
Previous studies have reported that mTORC1 activates the CCT complex. A recent study in Drosophila provides in vivo evidence showing that the CCT complex is regulated by mTORC1 signaling (22). Moreover, p70 ribosomal S6 kinase (S6K), a downstream effector of mTORC1, phosphorylates CCT2, suggesting that mTORC1 plays critical roles in regulating the CCT complex (23). Thus, we investigated whether the CCT complex mediates the mTORC1-dependent regulation of autophagy and m6A methylation. As shown in Fig. 3C, TSC1-RNAi–induced mTORC1 activation inhibited autophagy upon starvation in fat body, while coexpression of CCT8-RNAi suppressed this effect (Fig. 3C). LC-MS and MeRIP assays further revealed that the TSC2 KO-enhanced global m6A and methylated Atg1 and Atg8a, but not Atg7, mRNA levels were significantly reduced by depletion of CCT4 or CCT7 (Fig. 3 D and E), suggesting that mTORC1-dependent m6A methylation and autophagy are mediated through CCT. Consistent with these results, depletion of the CCT complex alone was able to reduce METTL3 and METTL14 protein levels (SI Appendix, Fig. S5A), increase Atg1 and Atg8a transcripts (SI Appendix, Fig. S5B), and enhance ATG1 and ATG8 protein levels (SI Appendix, Fig. S5C), resulting in an induction of autophagy (SI Appendix, Figs. S1A and S5C). Thus, these results suggest that the CCT complex, which acts downstream of mTORC1, is a positive regulator of m6A RNA methylation and suppresses autophagy.
mTORC1-CCT-MTC Signaling Regulates Autophagy in Mammalian Cells.
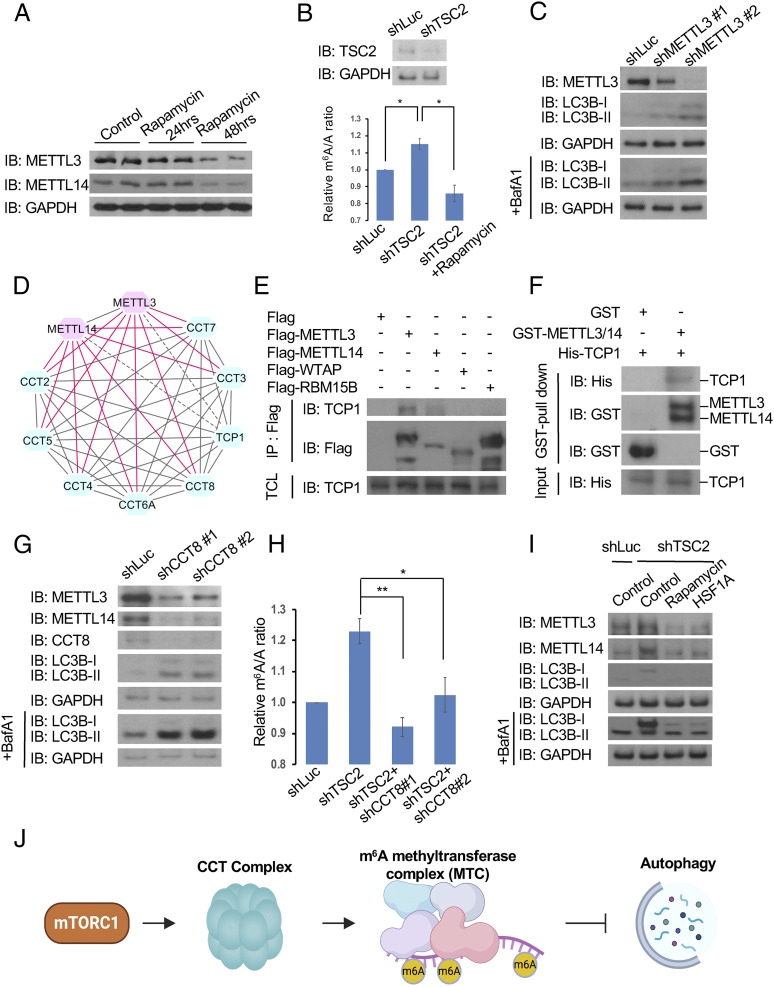
To determine whether the mTORC1-CCT-MTC axis is conserved in mammals, we treated MCF7 cells with rapamycin or transfected TSC2 short hairpin RNA (shRNA) to manipulate mTORC1 activity. Similar to previous results in Drosophila, rapamycin reduced both METTL3 and METTL14 protein levels (Fig. 4A). m6A levels were increased in TSC2-knocked down cells, an effect that was abrogated by rapamycin (Fig. 4B). Furthermore, depletion of human METTL3 increased the number of LC3B puncta as well as the conversion of cytosolic LC3B (LC3B-I) to the lipidated form (LC3B-II) (Fig. 4C and SI Appendix, Fig. S6A). The mCherry-EGFP-LC3B reporter assay also revealed increases in both autophagosomes (mCherry +; GFP + vesicles) and autolysosomes (mCherry +; GFP- vesicles) in METTL3-depleted cells, compared to control cells, suggesting that METTL3 knockdown increases autophagy flux (SI Appendix, Fig. S6B). Together, these results show that the regulation of MTC by mTORC1 to modulate autophagy is evolutionarily conserved.
Fig. 4.
Regulation of autophagy by mTORC1-CCT-MTC signaling is conserved in mammals. (A–C) mTORC1-MTC signaling regulates autophagy in human cells. MCF7 cells treated with 20 nM rapamycin for 24 or 48 h were subjected to immunoblotting with antibodies as indicated (A). Quantifications of m6A relative to A in MCF7 cells stably infected with lentivirus expressing control (shLuc) or TSC2 shRNA (shTSC2) with or without 20 nM rapamycin and immunoblot analysis was performed to determine the level of TSC2 knockdown (B). MCF7 cells stably infected with lentivirus expressing control (shLuc) or METTL3 shRNAs in the presence or absence of the lysosomal inhibitor Bafilomycin A1 (BafA1) were subjected to immunoblotting with antibodies as indicated (C). (D and E) CCT complex physically interacts with METTL3 and METTL14 in mammalian cells. Recovered PPIs between CCT complex, METTL3, and METTL14 from the human MTC-PPIN. Gray edges indicate the known interactions. Red edges suggest new interactions while gray dashed edges indicate the insignificant interactions (SAINT score < 0.2) (D). HEK293T cells, transfected with plasmids as indicated, were subjected to immunoprecipitations with anti-Flag antibody. Immunoprecipitated proteins (IP) and total cell lysates (TCL) were analyzed by immunoblotting with antibodies as indicated (E). (F) Human METTL3 and METTL14 directly interact with TCP1. Recombinant human GST-METTL3, GST-METTL14, and His-TCP1 proteins were subjected to GST pull-down assay. Pull-down fractions and input were analyzed by immunoblotting with antibodies as indicated. (G) Depletion of CCT8 reduces METTL3 and METTL14 protein levels and induces autophagy. MCF7 cells stably infected with lentiviruses expressing control (shLuc) or CCT8 shRNA in the presence or absence of BafA1 were subjected to immunoblotting with antibodies as indicated. (H and I) Inhibition of the CCT complex suppresses the effects induced by mTORC1 hyperactivation in human cells. Quantification of m6A in MCF7 cells expressing shLuc, shTSC2, or shTSC2 along with shCCT8 (H). MCF7 cells stably expressing shLuc or shTSC2 were treated with dimethylsulfoxide (control), 20 nM rapamycin, 200 μM HSF1A, or 100 nM BafA1 for 48 h and subjected to immunoblotting with antibodies as indicated (I). (J) Model showing that mTORC1 activates CCT complex to assist METTL3 and METTL14 proteins in folding, in turn enhancing m6A RNA methylation, degrading Atg transcripts, and thus suppressing autophagy. Created with BioRender.com. One-way ANOVA test was performed followed by Tukey’s test. Measurements shown are mean ± SEM of triplicates; *P < 0.05; **P < 0.01.
Next, we examined the function of the human CCT complex in regulating MTC and autophagy. The physical interaction between the MTC and CCT complexes was observed in the human MTC-PPIN and confirmed in HEK293T cells (Fig. 4 D and E). A GST pull-down assay also revealed the direct interaction between human METTL3, METTL14, and TCP1 (also known as CCT1) (Fig. 4F). Depletion of CCT8 resulted in reduced METTL3 and METTL14 protein levels and increased autophagy (Fig. 4G and SI Appendix, Fig. S6 A and B), suggesting that the CCT complex can directly bind to and regulate METTL3 and METTL14 protein stabilities and their downstream functions. Furthermore, MCF7 cells expressing shTSC2 exhibited higher METTL3 and METTL14 protein levels, enhanced m6A levels, and reduced autophagy (Fig. 4 H and I). Depletion of CCT8 markedly suppressed an increase in m6A levels induced by mTORC1 activation (Fig. 4H). Similarly, HSF1A, an inhibitor of CCT (24), reversed the shTSC2-induced effects, including up-regulation of METTL3 and METTL14 and inhibition of autophagy (Fig. 4I). Therefore, these findings demonstrate that the mTORC1-CCT-MTC-autophagy axis is conserved between Drosophila and mammals (Fig. 4J).
Discussion
The role of mTORC1 signaling in RNA metabolism is just emerging. In this study, we demonstrate that the MTC acts as a downstream effector of mTORC1 to regulate m6A RNA methylation of Atg transcripts, inducing their degradation and thus suppressing autophagy. Furthermore, we identified the CCT complex as a link between mTORC1 and MTC. CCT downstream of mTORC1 signaling can stabilize METTL3 and METTL14 to up-regulate m6A levels and inhibit autophagy. Accordingly, depletion of either mTORC1, CCT, METTL3, or METTL14 compromises m6A RNA methylation and promotes autophagy. Importantly, the role of mTORC1-CCT-MTC signaling in regulating autophagy is conserved from Drosophila to mammals. Thus, our study discovered a function of mTORC1 in regulating m6A RNA methylation during autophagy (Fig. 4J).
mTORC1 Regulates m6A Methylation to Control mRNA Turnover and Autophagy.
mTORC1 inhibition suppresses protein translation but also affects gene expression at different levels. Here, we identify an epitranscriptomic mechanism by which mTORC1 activates m6A RNA methylation to promote Atg mRNA turnover and inhibits autophagy. This m6A-mediated mRNA degradation represents a layer of gene regulation by mTORC1. Moreover, as mTORC1 activity regulates global m6A levels, it is likely that the MTC also mediates additional physiological functions of mTORC1. We noted that depletion of METTL3, METTL14, or CCT8 cannot fully rescue TSC1-induced effects. Although these results could be caused by partial RNAi knockdown, they may also indicate that other pathways contribute to mTORC1 regulation of autophagy. Indeed, studies have reported that mTORC1 suppresses autophagy through modulation of transcription factors, RNA-processing complexes, and mRNA degradation machinery, further highlighting that mTORC1 utilizes multiple RNA biogenesis processes to control autophagy (6, 17, 25, 26).
Interspecies MTC-PPINS.
The catalytic core components of the MTC, METTL3/METTL14, have a substrate sequence specificity for a DRA*CH motif (D = G/A/U, R = G/A, A* = methylated adenosine, H = A/U/C) (27). However, only a subset of consensus sites across the mRNA transcriptome are methylated. Thus, it has been speculated that other factors in the MTC specify METTL3/METTL14 methylation patterns. Our proteomic results combined with biochemical validation in both Drosophila and mammalian cells identified multiple splicing factors that interact with known MTC components. Future work will be needed to confirm whether these factors are directly involved in the regulation of m6A methylation and how they coordinate with the m6A machinery to affect RNA processing. It will also be interesting to investigate whether mTORC1 controls other regulators of RNA m6A methylation, in addition to METTL3 and METTL14. Moreover, our proteomics data revealed that multiple components of E3 ubiquitin ligase complex interact with MTC, suggesting that they may be involved in ubiquitination of MTC. Ubiquitination of METTL3 has also been observed, but its function and related E3 ubiquitin ligases remain unclear (28).
mTORC1 Activates CCT Complex Transcriptionally and Posttranslationally to Stabilize METTL3 and METTL14.
Previous genetic analyses showed that the CCT complex functions downstream of mTORC1 and that mTORC1 positively regulates the transcriptional levels of the CCT complex (22). Another study identified CCT2 as a substrate of S6 kinase, a downstream effector of mTOR, in mammalian cells (23), suggesting that both transcriptional and posttranslational regulations contribute to CCT complex activation by mTORC1. However, the phosphorylation site (Ser-260) of mammalian CCT2 is not conserved in Drosophila and how this phosphorylation modulates CCT function is not clear. Multiple phosphorylation sites have been detected in CCT components (29, 30). Interestingly, our previous study showed that CCT8 was phosphorylated following insulin stimulation (31), suggesting that other phosphorylation sites are involved in mTORC1-regulated CCT activation. Future studies are needed to comprehensively map the phosphorylation sites on CCT components and investigate their physiological roles.
The CCT complex is a highly conserved complex that assists the folding of about 10% of the eukaryotic proteome (21). The interactions of the CCT complex with METTL3 and METTL14 were observed in a previous study using AP/MS in human cells (32). Consistently, our genetic and biochemical data further confirmed their interactions and characterized the functions of CCT in stabilizing METTL3 and METTL14 and controlling m6A RNA methylation. Our findings thus further expand the impact of the CCT complex on RNA metabolism.
Multiple studies have reported that CCT complex protein levels dramatically increase in autophagy mutants (33, 34), proposing that CCT is one of the substrates of autophagy. Future studies will be needed to test whether autophagy is able to degrade the CCT complex and whether autophagy feedback inhibits CCT.
Materials and Methods
Details on the fly strains, plasmids, and antibodies used in this study, as well as methods used for antibody staining, RNA interference, immunoprecipitation, RT-PCR, and analyzing m6A levels, can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jean-Yves Roignant, Dr. Matthias Soller, the Vienna Drosophila Resource Center, and the Bloomington Drosophila Stock Center for fly stocks and the Drosophila RNAi Screening Center (Harvard Medical School) for plate-reader equipment and complementary DNA clones (FlyBi ORFeome Collection, NIH Grant 2P40OD010949). We thank Mian Li for his help on CoIP and mammalian cell experiments and members of the N.P. laboratory for critical comments on the manuscript. This work was supported by the NIH (R01 AR057352 and 5P01CA120964), Department of Defense (DOD) (W81XWH1810659), the Starr Consortium (I11-0015), and a start-up fund from the Duke-NUS Medical School (R-913-200-171-263) (to H.-W.T.). H.-W.T. is supported by the Human Frontier Science Program and the Postdoctoral Research Abroad Program, sponsored by Ministry of Science and Technology, Taiwan. N.P. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021945118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Saxton R. A., Sabatini D. M., mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Manning B. D., Toker A., AKT/PKB signaling: Navigating the network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara K., et al., Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 273, 14484–14494 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Puente C., Hendrickson R. C., Jiang X., Nutrient-regulated phosphorylation of ATG13 inhibits starvation-induced autophagy. J. Biol. Chem. 291, 6026–6035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee G., et al., Post-transcriptional regulation of de novo lipogenesis by mTORC1-S6K1-SRPK2 signaling. Cell 171, 1545–1558.e1518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang H. W., et al., The TORC1-regulated CPA complex rewires an RNA processing network to drive autophagy and metabolic reprogramming. Cell Metab. 27, 1040–1054.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu G., et al., A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat. Cell Biol. 17, 930–942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer K. D., et al., Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia G., et al., N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H., Wei J., He C., Where, when, and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lence T., Soller M., Roignant J. Y., A fly view on the roles and mechanisms of the m6A mRNA modification and its players. RNA Biol. 14, 1232–1240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng S., Wu Y. T., Chen B., Zhou J., Shen H. M., Impaired autophagy due to constitutive mTOR activation sensitizes TSC2-null cells to cell death under stress. Autophagy 7, 1173–1186 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Mauthe M., et al., Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14, 1435–1455 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin S., et al., m6A RNA modification controls autophagy through upregulating ULK1 protein abundance. Cell Res. 28, 955–957 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., et al., m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan L., et al., The m6A pathway facilitates sex determination in Drosophila. Nat. Commun. 8, 15737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laplante M., Sabatini D. M., Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 126, 1713–1719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H., et al., SAINT: Probabilistic scoring of affinity purification-mass spectrometry data. Nat. Methods 8, 70–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edupuganti R. R., et al., N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol. 24, 870–878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinayagam A., et al., Protein complex-based analysis framework for high-throughput data sets. Sci. Signal. 6, rs5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yam A. Y., et al., Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 15, 1255–1262 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim A. R., Choi K. W., TRiC/CCT chaperonins are essential for organ growth by interacting with insulin/TOR signaling in Drosophila. Oncogene 38, 4739–4754 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe Y., et al., p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J. Biol. Chem. 284, 14939–14948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neef D. W., et al., A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 9, 955–966 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Nunez R. T., et al., Modulation of nonsense mediated decay by rapamycin. Nucleic Acids Res. 45, 3448–3459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martina J. A., Chen Y., Gucek M., Puertollano R., MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linder B., et al., Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y., et al., SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 46, 5195–5208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma K., et al., Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 8, 1583–1594 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Sacco F., et al., Glucose-regulated and drug-perturbed phosphoproteome reveals molecular mechanisms controlling insulin secretion. Nat. Commun. 7, 13250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinayagam A., et al., An integrative analysis of the InR/PI3K/Akt network identifies the dynamic response to insulin signaling. Cell Rep. 16, 3062–3074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue Y., et al., VIRMA mediates preferential m6A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 4, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T., Shen S., Qu J., Ghaemmaghami S., Global analysis of cellular protein flux quantifies the selectivity of basal autophagy. Cell Rep. 14, 2426–2439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeger K., et al., Cornification of nail keratinocytes requires autophagy for bulk degradation of intracellular proteins while sparing components of the cytoskeleton. Apoptosis 24, 62–73 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y., et al., Molecular interaction search tool (MIST): An integrated resource for mining gene and protein interaction data. Nucleic Acids Res. 46, D567–D574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J., Tang H. W., Li J., Perrimon N., Yan D., Xio is a component of the Drosophila sex determination pathway and RNA N6-methyladenosine methyltransferase complex. Proc. Natl. Acad. Sci. U.S.A. 115, 3674–3679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.