Significance
Urinary tract infections (UTIs) are the second most common bacterial infections. Caring for UTI patients is very difficult and often perplexing. We typically view our immune system as an adaptive line of defense that becomes more responsive to specific pathogens after episodes of infection. However, the opposite is seen in these patients, in whom each infection actually increases the risk of a subsequent infection. Our group recently identified the underlying basis as a highly Th2-biased response in bladder that inhibits Th1-mediated bacterial clearance. To resolve this situation, we investigated whether immunizing mice intravesically with bacterial antigens combined with a Th1-skewing adjuvant (CpG) would evoke a more balanced and protective immune response.
Keywords: bladder, UTI, Th1, vaccine
Abstract
Given the high frequency of urinary tract infections (UTIs) and their recurrence, there is keen interest in developing effective UTI vaccines. Currently, most vaccine studies, including those in humans, involve parenteral vaccination aimed at evoking and sustaining elevated levels of systemic antibody directed at the uropathogens. In view of recent reports of aberrant Th2-biased bladder immune responses to infection, we hypothesized that immunizing mice intravesically with antigens from uropathogenic Escherichia coli (UPEC) combined with a Th1-skewing adjuvant could correct this defect and promote protection against UTIs. Here we report that compared with mice immunized subcutaneously with this vaccine combination, intravesically immunized mice were markedly more protected from UTIs because of their distinctive ability to recruit Th1 cells into the bladder. This mode of vaccination was effective even in mice that experienced multiple UTIs and displayed pronounced aberrant bladder immune responses. Thus, intravesical vaccination with one or more UPEC antigens to induce bladder Th1 responses represents a superior strategy to combat UTIs, especially in UTI-prone subjects.
Urinary tract infections (UTIs) are one of the most common bacterial infections and are mostly found to afflict women. Indeed, as many as 50% of women will experience at least one UTI during their lifetime (1–3). These infections mainly afflict adult females but also occur in children and elderly persons (1–3). Along with their high incidence rate, UTIs have markedly higher recurrence rates (ranging from 27% to 44%) (1–6) compared with recurrence rates of infections at other body sites (7–13). UTIs are typically initiated when certain gut-derived bacteria, such as uropathogenic Escherichia coli (UPEC), reach the bladder through the urethra and proliferate in urine, followed by invasion into bladder epithelial cells (BECs). Studies in experimental UTI models have revealed that a small population of quiescent UPEC can persist in BECs for months (14–16), even after resolution of an acute infection, which provides a nidus for future infection.
Although antibiotic treatment is usually highly effective in resolving acute UTIs, it is not effective in preventing new infections or recurrence of previous infections (3, 17). Therefore, for many decades, much attention was focused on developing an efficacious vaccine in animal models to evoke long-term protective humoral immunity against UTI pathogens (17–19). Several UPEC antigens were found to be immunogenic, evoking strong antibody responses that significantly reduced colonization of the bladder and kidneys following bacterial challenge (20–25). One of the more efficacious vaccine candidates revealed in these studies is FimH (20–22), a determinant of bacterial binding to BECs expressed not only by E. coli, but also by many other species of enterobacteria (20). Despite the promise of vaccines and initiation of several human trials using various bacterial components as vaccine antigens (17–19), an effective UTI vaccine remains unavailable. One reason for this appears to be the lack of effective adjuvants to boost immunogenicity of bacterial antigens to levels that are protective against UTI (17–19). Another possibility could be that vaccines delivered via needles at parenteral sites might not evoke adequate antibody production in the bladder (14, 26).
We reasoned that to develop an effective UTI vaccine, it is important to take into consideration the nature of immune responses evoked in the bladder to bacterial infections, as well as the subset of subjects most likely to receive a UTI vaccine. A recent study in mice has revealed several unique aspects of bladder immunity (7). For example, following infection, the bladder’s immune response was observed to evoke only a limited T helper type 1 (Th1) response, which is essential for bacterial clearance. Instead, the immune response was heavily skewed toward Th2 immune responses, which are largely directed at bladder tissue repair (7, 27). This could explain the bacterial persistence following bladder infection and the enhanced chances for recurrence (7). Additionally, the magnitude of Th2-biased immune responses in the bladder increased with each infection, such that mice subjected to multiple UTIs were greatly compromised in their capacity to clear infection compared with their naive counterparts (7). Taken together, these studies revealed a significant defect in the cellular adaptive immune response of the bladder to infections, which increased with each infection.
Since the candidates for vaccination against UTIs most likely are individuals prone to recurrent UTIs, conceivably these individuals would gain limited benefit from traditional vaccination, as they already have been primed to evoke a strong Th2 immune response.
As it is now possible to reprogram adaptive immune responses to vaccines by using Th1- or Th2-polarizing adjuvants (28–31), we hypothesized that it may be possible to boost bacteria-clearing Th1 immune responses in the bladder of subjects already primed to evoke Th2 responses. Such a vaccine would not only protect against bladder colonization, but also eliminate residual bacteria that persist following infection. To maximize the local impact of the evoked immune responses and to ensure that the activated Th1 cells will localize to the bladder, we reasoned that the vaccination site should be the bladder. Here we describe the studies we embarked on to validate these notions with the goal of identifying an effective vaccination formulation and the site to overcome the limitations of bladder adaptive immune responses to UTIs.
Results
Local Vaccination with Th1-Polarizing Adjuvant Can Evoke Bladder Th1 Responses Promoting Bacterial Clearance.
In view of the finding that the bladder’s natural adaptive immune response to infection is Th2-biased, which inhibits Th1-mediated bacterial clearance capacity (7, 27), we reasoned we could modify this response through vaccination. We hypothesized that bacteria-specific immune responses with enhanced bacteria-clearing capacity could be evoked by immunizing mice with bacterial antigens accompanied by a known Th1-polarizing adjuvant. Since vaccine-enhanced Th1 responses would be most effective when localized in the bladder, a logical site for immunization would be in the bladder. We undertook the present studies using interferon-gamma (IFNγ) reporter (Great) mice (7, 32, 33), because regular quantification of Th1 cells in the wild-type (WT) mouse bladder is challenging, as their numbers are relatively small. Great mice carry an internal ribosomal entry site (IRES)-yellow fluorescent protein (YFP) cassette tagged at the 3′ end of the endogenous ifng gene, the prototype Th1 marker (7, 32, 33). Groups of mice were immunized intravesically (through the urethra) with a lysate of uropathogenic E. coli (UPEC) strain J96 (7, 14, 34) suspended either in phosphate-buffered saline (PBS) or in PBS containing increasing amounts of CpG oligodeoxynucleotide (ODN), a Th1-polarizing adjuvant (SI Appendix, Fig. S1). We used UPEC lysates as vaccine antigens here because bacterial lysates have proven protective against UTIs when administered at urogenital sites such as the vagina in clinical studies (23, 35). Each mouse group was boosted with its appropriate vaccine formulation on days 7 and 14 (7). On day 21, all the immunized mouse groups were intravesically challenged with UPEC J96. Three days later, when acute bladder inflammation had subsided (7), bladders were collected for flow cytometry analysis of Th1 cells (CD3+CD4+IFNγ+). As a control for systemic effects of intravesical immunization, we quantitated Th1 cells in the spleens of each of the mice. WT C57BL/6J mice infected with UPEC J96 were used as negative control for YFP gating. The gating strategy is shown in SI Appendix, Fig. S2. We observed that the presence of CpG ODN as the adjuvant significantly enhanced bladder Th1 responses in a dose-dependent manner up to 10 μg of CpG, evoking a two-fold increase over the response evoked by the lysate alone (Fig. 1A). In contrast, there was a minimal increase in the Th1 response in the spleen (Fig. 1A). These findings support our hypothesis that –intravesical vaccination with bacterial antigens accompanied by a Th1-skewing adjuvant can evoke strong Th1 responses in the bladder.
Fig. 1.
Intravesical vaccination with bacterial lysates and CpG induced strong local Th1 responses in bladder. (A) Naïve Great mice were intravesically vaccinated with J96 lysates and different amounts of CpG three times, then challenged with UPEC J96. Bladders and spleens were collected for flow cytometry analysis on day 3 after challenge. WT C57BL/6J mice infected with J96 were used as controls for YFP gating. (B) Naïve WT mice were intravesically vaccinated with UPEC J96 lysates and combined with increasing amount of CpG three times, then intravesically challenged with UPEC J96. Bladders were collected to determine bacterial load on day 3 postchallenge. (C) Naïve WT mice were intravesically vaccinated three times with PBS, UPEC J96 lysates, or UPEC J96 lysates combined with 10 μg of CpG, then intravesically challenged with UPEC J96. Bladders were collected to determine bacterial load on day 14 after challenge. Each data point represents one mouse. Data are shown as mean ± SD. Data were analyzed by an ordinary one-way ANOVA with Tukey’s multiple comparison post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
To test whether this mode of vaccination promotes bacterial clearance in the bladder following infection, we repeated the above vaccination study in WT C57BL/6J mice. At 3 d after UPEC J96 challenge, we quantitated bacterial loads in the mouse bladders. We found that mice vaccinated with a formulation composed of bacterial lysate and increasing doses of CpG significantly reduced bladder bacterial loads compared with PBS-vaccinated or lysate-alone–vaccinated mice (Fig. 1B). Also, vaccination with CpG alone did not have any significant effect on bacterial clearance (SI Appendix, Fig. S3).
Since UPEC can persist in bladders within BECs even after apparent resolution of infection (14–16), we investigated if given more time, the recruited Th1 cells in the bladder would further reduce residual bacteria. For this, we repeated the previous study but this time assessed bacterial numbers on day 14 postchallenge. We found that 50% of mice immunized with UPEC lysates and the adjuvant CpG completely cleared their bladder bacteria, which is in sharp contrast to PBS-immunized or lysate-alone–immunized mouse groups, which continued to harbor appreciable numbers of bladder bacteria (Fig. 1C). Thus, the recruitment of bacteria-specific Th1 cells to the bladder following intravesical vaccination is highly effective in promoting bacterial clearance during infection, as well as after the infection has seemingly resolved.
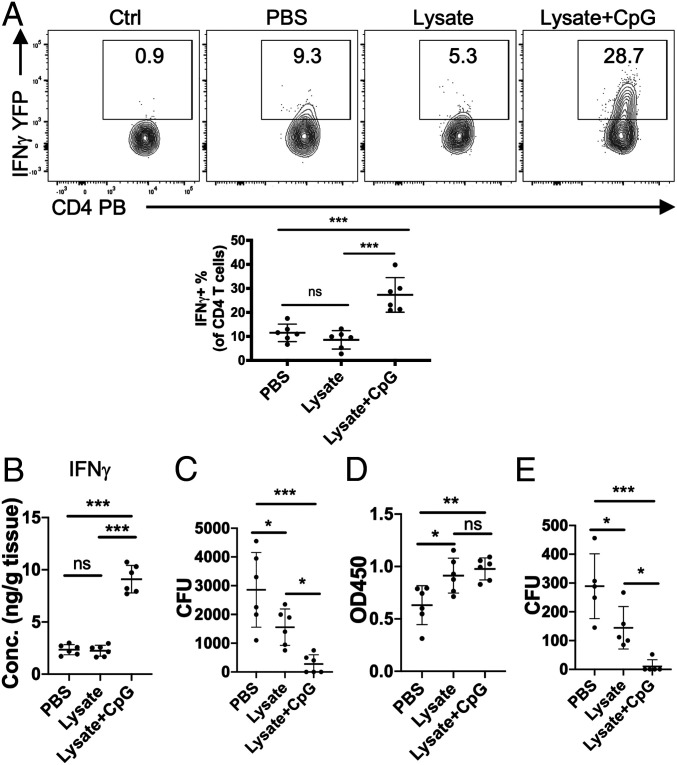
Bacterial Clearance following Local Bladder Vaccination Is Mainly Mediated by Th1 Activity rather than IgG Antibody Responses.
Although our data show that bacterial clearance is correlated with the presence of recruited Th1 cells in the bladder, vaccination typically evokes both cellular adaptive responses and humoral responses. Therefore, we sought to examine how much of the bacterial clearance could be strictly ascribed to Th1 responses. We intravesically vaccinated WT and IFNγ−/− mice, which are deficient in Th1 responses (7), with the vaccine formulation composed of either PBS or UPEC lysate plus CpG as before. After bacterial challenge, we assessed bacterial loads in the bladders of the various mouse groups on day 3. We found that in mice immunized with PBS, bacterial clearance in IFNγ−/− mice was comparable to that seen in WT mice. However, in mice immunized with UPEC lysate and CpG, bacterial clearance in WT mice were markedly more efficacious compared with IFNγ−/− mutant mice (Fig. 2A). At the same time, we examined serum levels of UPEC J96-specific IgG in all of the immunized mice groups and found that the degree of enhancement of UPEC-specific IgG levels seen in IFNγ−/− mice were comparable to the one observed in WT mice (Fig. 2B). Together, their IgG levels were significantly higher than the minimal levels seen in the two groups of PBS- immunized mice (Fig. 2B). Thus, clearance of bacteria in the two groups of immunized mice correlated with the bladder Th1 response rather than with the circulating IgG response.
Fig. 2.
Th1 bladder responses are more critical for bacterial clearance than antibody-dependent responses with intravesical vaccination. (A) Intravesical vaccination with UPEC J96 lysates combined with 10 μg of CpG followed by UPEC J96 challenge were performed in WT and IFNγ−/− mice. Bladders were collected to determine bacterial load on day 3 postchallenge. (B) Serum from A was collected to determine the concentration of UPEC J96-specific IgG by ELISA. (C) Naïve WT mice were intravesically vaccinated three times with FimH combined with 10 μg of flagellin or 10 μg of CpG. Mice treated with PBS or FimH alone served as controls. Then all mice were challenged with UPEC J96. The concentration of IFNγ in bladder lysates was measured on day 3 postchallenge by ELISA. (D) Naïve WT mice were intravesically vaccinated three times with FimH with 10 μg of flagellin or 10 μg of CpG. Mice treated with PBS or FimH alone served as controls. Then all mice were challenged with UPEC J96. Bladders were collected to determine bacterial load on day 3 postchallenge. (E) Serum from D was collected to determine the concentration of FimH-specific IgG by ELISA. Each data point represents one mouse. Data are shown as mean ± SD. Data were analyzed by an ordinary two-way ANOVA (A and B) or one-way ANOVA (C–E) with Tukey’s multiple comparison post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
To further support the notion that specifically boosting bladder Th1 responses is an effective strategy for bladder bacterial clearance, we examined whether combining two distinct type 1 polarizing adjuvants in the vaccination formulation would evoke more enhanced bacterial clearance compared with the use of just a single adjuvant, CpG (36). We vaccinated the Th1 reporter Great mice with bacterial lysate along with CpG or a combination of CpG and interleukin (IL)-12, another widely used Th1-skewing adjuvant (37). We used PBS-vaccinated and bacteria lysate-alone–vaccinated Great mice as negative controls. As an additional comparison, we included Great mice vaccinated with bacterial lysate combined with papain, a notable Th2-skewing adjuvant (38, 39). Thereafter, all the immunized mouse groups were challenged with UPEC J96, and their respective Th1 responses and bacteria burden was quantitated 3 d later. We found that Great mice vaccinated with bacteria lysate and a combination of CpG and IL-12 evoked higher levels of both Th1 responses and bacterial clearance compared with mice vaccinated with CpG only (SI Appendix, Fig. S4 A and B). Interestingly, immunization of Great mice with bacterial lysate and the Th2-skewing adjuvant papain failed to evoke any significant changes to either Th1 responses or bacterial clearance compared with mice immunized with bacterial lysates alone (SI Appendix, Fig. S4 A and B).
Since Th1 and Th2 responses have been reported to mutually inhibit each other (7, 40–43), we investigated whether vaccination using type 1 polarizing adjuvants would impair valuable Th2 responses during bladder infection. We examined Th2 responses in IL-4 reporter (4get) mice, in which the IRES-enhanced green fluorescent protein (eGFP) sequence was tagged at the 3′ end of endogenous Il4 gene, the prototype Th2 marker (7, 32, 44), following vaccination and bacterial challenge. Although not statistically significant, we found that 4get mice vaccinated with UPEC J96 lysate coadministered with papain trended toward evoking an enhanced Th2 response (SI Appendix, Fig. S4C). Interestingly, when 4get mice were immunized with bacterial lysate and the adjuvant combination of CpG and IL-12, they showed no reduction in Th2 responses compared with mice vaccinated with PBS or with lysate alone (SI Appendix, Fig. S4C). Of note, naïve mice can harbor large numbers of memory Th2 cells primed by microbiota that cross-react with UPEC (7). Conceivably, vaccination with the adjuvant combination CpG and IL-12 promotes induction of new bladder Th1 cells from naïve CD4 T cells but does not eliminate already present memory Th2 cells.
Next, we sought to validate our strategy of intravesical vaccination using Th1-polarizing adjuvants by switching vaccine antigens from a mixture of UPEC antigens to a single antigen, FimH, a widely studied UPEC vaccine antigen (20–22). Since some previous studies using FimH as the vaccine antigen used flagellin as the adjuvant (22, 45), we included flagellin as a separate adjuvant in our comparative studies. Unlike the adjuvants that we used previously, flagellin is capable of evoking a balanced Th1 and Th2 responses (22, 45, 46). We intravesically vaccinated groups of WT mice with PBS, FimH alone, FimH with flagellin, or FimH with CpG on days 0, 7, and 14. Then all the mice were challenged with UPEC J96. At 3 d after the challenge, we examined bladder lysates for IFNγ levels and bacteria burden. We found that of all the vaccines, only the formulation composed of FimH and CpG evoked enhanced IFNγ levels in the bladder and maximum clearance of bacterial load (Fig. 2 C and D). Of note, mice immunized with FimH and CpG evoked comparable levels of circulating FimH-specific IgG as the FimH vaccine coadministered with flagellin (Fig. 2E). Taken together, the Th1, but not the Th2, responses to vaccines are effective in bladder bacterial clearance. Furthermore, Th1-polarizing adjuvants are still effective even when administered with a single UPEC vaccine antigen.
Bladder Vaccination Induces Stronger Th1 Responses and Superior Bacterial Clearance Compared with Subcutaneous Vaccination.
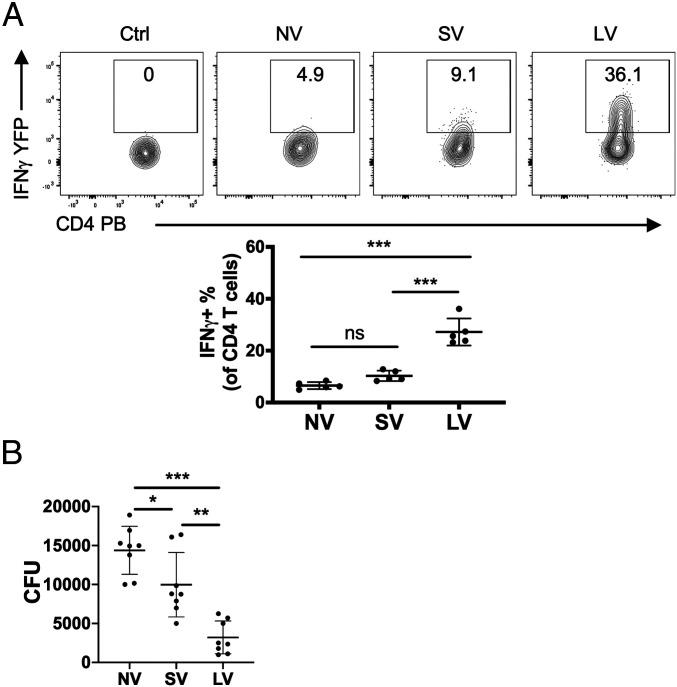
For the aforementioned studies, we used a bladder vaccination strategy rather than a conventional route of vaccination, because we believed that local vaccination would be more effective in inducing strong Th1 responses in the bladder. To validate this notion, we compared bladder Th1 responses to a vaccine composed of FimH and CpG in Great mice following local vaccination (LV) or subcutaneous vaccination (SV), a common route of UTI vaccine administration (22, 25). We observed that LV mice evoked a significantly stronger bladder Th1 response than either SV or control unvaccinated (NV) Great mice (Fig. 3A). We investigated whether LV also induces better bacteria clearance than SV by comparing bladder bacterial loads in the above three groups of WT mice following vaccination and bacterial challenge. We found that the bacterial loads were significantly lower in SV and LV mice than in control NV mice, but, more importantly, the bacterial load was markedly lower in LV mice than in SV mice (Fig. 3B). Thus, although both vaccination routes conferred protection, local immunization evoked greater numbers of Th1 cells in the bladder and was markedly more protective against bladder infection than the common subcutaneous route of immunization.
Fig. 3.
Local vaccination induced a stronger Th1 response and better bacterial clearance than subcutaneous vaccination. (A) Naïve Great mice were intravesically vaccinated (LV) or subcutaneously vaccinated (SV) three times with FimH and 10 μg of CpG, then challenged with UPEC J96. Unvaccinated naïve Great mice (NV) were also challenged with UPEC J96 as a control. Bladders and spleens were collected for flow cytometry analysis on day 3 postchallenge. WT C57BL/6J mice infected with J96 served as controls for YFP gating. (B) Naïve WT mice were LV or SV three times with FimH and 10 μg of CpG, then challenged with UPEC J96. Unvaccinated naïve WT mice (NV) were also challenged with UPEC J96 as a control. Bladders were collected to determine bacterial load on day 3 postchallenge. Each data point represents one mouse. Data are shown as mean ± SD. Data were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparison post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
Vaccination with CpG Can Induce Protective Th1 Responses Even in Mice Heavily Programmed to Evoke a Strong Th2 Response.
Recent studies have revealed that mice subjected to multiple UTIs are heavily programmed to evoke a powerful Th2 response with limited capacity to clear infections (7, 27). This condition is closely reminiscent of subjects prone to recurrent UTIs, in whom each infectious bout predisposes to another infection (1–6). All our above studies demonstrating the efficacy of Th1-polarizing vaccine were undertaken in naïve mice. However, in view of the fact that the subpopulation most likely to receive a UTI vaccine is individuals with a history of recurrent UTIs, we are interested in investigating whether our vaccine formulation would be efficacious in mice whose immune system has already been polarized toward a strong Th2 response.
We previously established that mice subjected to three consecutive UTIs exhibited Th2 immune responses and symptoms typically associated with patients prone to recurrent UTIs, including impaired bladder function and the inability to totally clear bladder bacteria (7). To test whether immunization of this population of mice will enhance their capacity to clear challenge bacteria, we subjected Great mice to three consecutive UTIs and then vaccinated them as before, with PBS, UPEC lysate alone, or lysate with CpG. Thereafter, the mice were challenged by UPEC J96 infection. At 3 d after the challenge, we examined the bladder immune cells using flow cytometry. Thrice-infected WT C57BL/6J mice were used as a negative control for YFP gating. We found that Great mice vaccinated with bacterial lysates and CpG exhibited a markedly enhanced Th1 response compared with the PBS group or bacterial lysate-alone group (Fig. 4A). Next, we repeated this study in WT C57BL/6J mice and then sought to assess the corresponding IFNγ levels and bacterial loads in bladder lysates. The results were consistent with those obtained from Great mice, showing that mice vaccinated with UPEC J96 lysate along with CpG had significantly higher IFNγ levels and were better able to clear bacteria compared with mice vaccinated with PBS vaccination or with lysate alone (Fig. 4 B and C). We also measured the levels of UPEC-specific IgG in the serum of these mice and found that the CpG did not further enhance IgG production compared with repeatedly infected mice with lysate-alone vaccination (Fig. 4D). We previously found improved bladder bacterial clearance on day 14 postinfection compared with day 3 (Fig. 1C). Here we examined if given more time, enhanced bacterial clearance could be achieved in multiple infected mice following immunization. Although both mice immunized with UPEC lysate alone or lysate with CpG harbored a significantly reduced bacterial load compared with PBS immunized mice on day 14, up to 80% of mice immunized with bacterial lysate and CpG were totally cleared of bladder bacteria (Fig. 4E). Thus, intravesical vaccination with Th1-polarizing adjuvant has the potential to promote complete bacterial clearance in UTI-prone bladders.
Fig. 4.
Intravesical vaccination with bacterial lysate and CpG of repeatedly infected mice induced strong bladder Th1 responses that significantly improved bacterial clearance on bacterial challenge. (A) Naïve Great mice were intravesically infected by UPEC J96 three times, then vaccinated three times with PBS, UPEC J96 lysates alone, or UPEC J96 lysates with 10 μg of CpG. These immunized mice were then challenged once again with UPEC J96. Bladders were collected for flow cytometry analysis on day 3 postchallenge. WT C57BL/6J mice infected with UPEC J96 served as controls for YFP gating. (B) Naïve WT mice were intravesically infected with UPEC J96 three times, then vaccinated three times with PBS, UPEC J96 lysates alone, or UPEC J96 lysates with 10 μg of CpG. These mice were once again challenged with UPEC J96. The concentration of IFNγ in bladder lysates was measured on day 3 postchallenge by ELISA. (C) Naïve WT mice were intravesically infected with UPEC J96 three times, then vaccinated three times with PBS, UPEC J96 lysates alone, or UPEC J96 lysates with 10 μg of CpG. These mice were once again challenged with UPEC J96. Bladders were collected to determine bacterial load on day 3 postchallenge. (D) Serum from C was collected to determine the concentration of UPEC J96-specific IgG by ELISA. (E) Naïve WT mice were intravesically infected with UPEC J96 three times, then vaccinated three times with PBS, UPEC J96 lysates alone, or UPEC J96 lysates with 10 μg of CpG. These mice were once again challenged with UPEC J96. Bladders were collected to determine bacterial load on day 14 postchallenge. Each data point represents one mouse. Data are shown as mean ± SD. Data were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparison post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
Discussion
There is growing evidence that even healthy humans and mice harbor basal levels of UPEC-reactive antibodies in their circulation (47), likely evoked by the endogenous microflora, of which E. coli is a prominent component (7, 48–52). Regardless, healthy mice are not protected from UTIs on intravesical challenge, possibly because of the low levels of circulating UPEC-reactive antibody. When levels of UPEC-specific antibody levels in circulation were greatly elevated in mice, following parenteral vaccination, appreciable protection from UTIs was observed (17–25). Much of this protection was attributed to the capacity of antibodies to bind UPEC, preventing its invasion into bladder epithelial cells. However, it is also notable that a residual population of bladder bacteria that resisted clearance consistently remained, presumably because the bacteria had already gained entry into bladder cells (14–16). Although there have been several attempts at intramuscular vaccination against UPEC in humans, these were abandoned due to their insufficient efficacy (17–19, 53). Thus, currently there is no effective vaccine to combat UTIs.
Here we examined a novel strategy to evoke protective UPEC-directed immunity in the bladder through local vaccination combined with the use of Th1-skewing adjuvants. This strategy is informed by our recent finding from bladder infections in mice revealing that although Th1 immune responses were highly efficacious in clearing bacteria, the natural bladder response was highly skewed toward a Th2 response (7). This Th2 response mediates regeneration of the superficial bladder epithelium, which massively exfoliates during the acute phase of UTIs, and it is even more pronounced in mice that have experienced multiple UTIs (7). Since Th1 and Th2 responses mutually inhibit each other, a consequence of a powerful Th2 response is that the bacteria-clearing Th1 response is suppressed in hosts that have experienced multiple UTIs, making them even more susceptible to reinfections. To rectify this anomalous response, we sought to boost and redirect the bladder immune response by incorporating one or more Th1-polarizing adjuvants in the vaccine formulation. To ensure that the Th1 response was targeting the bladder, we administered the vaccine intravesically with a formulation composed of either UPEC lysates or a prominent UPEC antigen and the Th1-skewing adjuvant CpG. Although intravesical administration of bacillus Calmette–Guérin is a standard immunotherapy for bladder cancer patients (54–58), intravesical bladder immunization is an overlooked mode of UTI immunization. In this study, bladder immunization was found to be superior to subcutaneous immunization in reducing bacterial burden in the bladder. The superior bacterial clearance directly correlated with a sharp increase in bladder Th1 (CD3+CD4+IFNγ+) cells, highlighting the crucial role of these immune cells in clearing bacteria harboring in bladder epithelial cells.
It is noteworthy that we observed comparable bladder protection when using UPEC lysates and FimH as vaccine antigens, perhaps indicating that the particular vaccine antigen used was not as important as the nature of the adjuvant in our vaccination strategy. Nevertheless, it would be interesting to use previously studied UPEC antigens (23–25) to validate our studies. It is pertinent to point out that FimH has been shown to be expressed by a wide variety of Enterobactericeae (20), and thus this vaccine antigen is likely to be broadly protective against a wide range of UPEC and other FimH-expressing enterobacteria infecting the bladder. Even though Enterobactericeae spp. is a major component of endogenous microflora of the gut, it is unlikely that the immune responses evoked by bladder immunization will impact them, as we have not observed significant Th1 recruitment into sites other than the bladder (Fig. 1A). In the current study, most of the assessment of bacterial burden was undertaken on day 3 postinfection. However, when bacterial burden was assessed on day 14 postchallenge, complete bacterial clearance was achieved in up to 50% to 80% of the immunized mice (Figs. 1C and 4E). Conceivably, with further optimization of the Th1-adjuvant doses/combinations and increased incubation time, complete eradication of bladder bacteria could be consistently achieved. This capacity of Th1-skewing adjuvants to promote bladder bacterial clearance was observed even in mice that had experienced multiple UTIs and that typically evoke markedly diminished Th1 immunity. This finding is highly relevant, as a prime candidates for receipt of a UTI vaccine are individuals with a history of recurrent UTIs (1–6).
A potential concern regarding our vaccination strategy is that the strong Th1 responses evoked in the bladder could in turn suppress local Th2 responses that are critical for uroepithelial repair. However, assessment of the Th2 responses of immunized mice revealed no inhibition of Th2 responses. There are two possible reasons why the Th2 responses are not suppressed. First, as mentioned above, the endogenous microbiota in healthy mice is capable of priming Th2 cells engaged in bladder epithelial repair. These Th2 cells could be memory cells not easily affected by vaccination, as the CpG vaccination might induce new Th1 cells from naïve CD4 T cells but might not affect existing memory Th2 cells. Second, recent studies have revealed that the Th2 microenvironment in the bladder is overwhelming, with antigen-presenting cells constitutively expressing the type 2 ligand OX40L even without stimulus from UPEC (7). Therefore, our Th1-skewing vaccination may not be powerful enough to completely suppress the Th2 signals in the bladder microenvironment.
Finally, in view of our observations, it may be necessary and urgent to carefully reconsider the adjuvants currently being used in mouse UTI models and in human clinical trials, as the current focus is to maximize circulating bacteria-specific antibody production while overlooking the potentially critical contribution of bladder T cell responses, especially of the Th1 type.
Methods
Mice.
C57BL/6J mice (000664) were purchased from The Jackson Laboratory. IFNγ−/− mice (002287) were obtained from The Jackson Laboratory and bred in the animal facility of Duke University. IL-4 reporter mice (4get) and IFNγ reporter mice (Great) were graciously provided by Richard Locksley, University of California San Francisco, and were bred to C57BL/6J mice for more than 10 generations. All mice were housed under specific pathogen-free (SPF) condition in the animal facility of Duke University, and 8- to 10-wk-old female mice were used for all the experiments. All mouse experiments were performed in accordance with protocols approved by the Duke University Animal Care and Use Committee.
Bacterial Strain.
Clinical uropathogenic E. coli isolate strain J96 was used for infection in the mouse UTI model (7, 14, 34). The bacteria were statically grown in Luria–Bertani broth overnight prior to infecting the mice.
Vaccination and Mouse UTI Model.
Uropathogenic J96 lysates were prepared by heating at >60 °C for 30 min (59, 60) (SI Appendix, Fig. S1). Mice were given pentobarbital sodium (Oak Pharmaceuticals) i.p. for anesthesia, then lysates from 1 × 108 UPEC strain J96 combined with different amounts of adjuvants (as indicated in the figure legends) in 50 μL of PBS were slowly introduced into their bladders through a 2-cm catheter inserted through the urethra. Vaccine was administered three times at 7-d intervals. In some experiments (as indicated in the figure legends), 10 μg of FimH (ab236920; Abcam) was also used with 10 μg of CpG (tlrl-1585-1; InvivoGen), and the procedure was the same as with vaccination with J96 lysate. The systemic vaccination procedure was modified from previous research (22, 25). In brief, 10 μg of FimH and 10 μg of CpG were administered subcutaneously into mice three times at 7-d intervals. UTI models were modified from previous research (7, 14). In brief, at 7 d after the final vaccination, 1 × 108 J96 UPEC was intravesically introduced into mouse bladders in the same way as in the vaccination procedure. At 3 d after infection, samples were collected for analysis. For recurrent UTIs, bladder infection was intravesically induced three times at 7-d intervals. At 7 d after the final infection, vaccination and subsequent bacteria challenge were initiated.
Bacteria Load Assessment.
The bladders were collected at different time point postinfection as indicated in the figure legends and homogenized in 0.1% Triton X-100 (Sigma-Aldrich) using zirconia silica beads for three cycles of 1.5 min each in an automatic homogenizer. Lysate underwent series dilution in PBS and was plated on Difco MacConkey agar plates. After overnight incubation in 37 °C, colony-forming units (CFU) were counted. The limit of bacterial detection in this study was 10 CFU.
Flow Cytometry Analysis.
Bladders and spleens were collected at different time points postinfection as indicated in the figure legends. Single-cell suspensions were prepared as described previously (7), and light was avoided during the entire process. Bladders were digested with 1 mg/mL collagenase (C7657; Sigma-Aldrich) and 200 μg/mL DNase I (DN25; Sigma-Aldrich) in RPMI 1640 medium for 1 h at 37 °C. Spleens were smashed to produce single-cell suspensions. ACK lysis buffer was used to lyse red blood cells. Then samples were filtered through a 70-μm cell strainer in FACS buffer (3% heat- inactivated fetal bovine serum and 5 mM EDTA in PBS). Samples were blocked with 1% anti-mouse CD16/CD32 (BD Biosciences), 5% normal mouse serum, and 5% normal rat serum in FACS buffer for 15 min at 4 °C. Then surface staining was performed with 7-AAD (BD Biosciences) and the following antibodies: APC-Cy7 conjugated to anti-mouse CD8a (53-6.7; BioLegend), and PE conjugated to anti-mouse Gr1 (RB6-8C5), PECy7 conjugated to anti-mouse CD3e (145-2C11), Pacific Blue conjugated to anti-mouse CD4 (RM4-5), BV510 conjugated to anti-mouse NK-1.1 (PK136), APC-Cy7 conjugated to anti-mouse CD45 (30-F11), and BV510 conjugated to anti-mouse I-A/I-E (M5/114.15.2) (all from BD Biosciences). All the samples were pregated by size and granularity based on forward scatter and side scatter to select single cells and then gated on 7-AAD–negative cells. Data were collected with the FACSCanto flow cytometry system (BD Biosciences) and analyzed with FlowJo software.
Enzyme-Linked Immunosorbent Assay.
Bladder and serum were collected at different time points postinfection, as indicated in the figure legends. Then an enzyme-linked immunosorbent assay (ELISA) was performed based on previous studies (7, 14, 22, 45). For IFNγ measurement, bladders were homogenized using zirconia silica beads for three cycles of 1.5 min each in an automatic homogenizer, and ELISA was performed using an IFNγ ELISA kit (XEL485; R&D Systems) following the manufacturer’s instructions. Concentrations were determined by a standard curve. For IgG measurements, 96-well plates were coated overnight at 4 °C with lysate of 107 J96 or 1 μg FimH (Abcam) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3); blocked with carbonate buffer, 3% nonfat dry milk, and 0.1% Kathon for 2 h at room temperature; and then incubated at 4 °C overnight with serum samples diluted in complete sample diluent (PBS, 1% BSA, 1% nonfat dry milk, 0.05% Tween 20, and 0.1% Kathon). Plates were washed four times with wash buffer (PBS, 0.05% Tween 20, and 0.1% Kathon), after which HRP-conjugated mouse-IgG detection antibodies (Bio-Rad) diluted in secondary antibody diluent (PBS, 0.05% BSA, 0.05% Tween 20, and 0.1% Kathon) was added. Plates were incubated at room temperature for 2 h, washed four times with wash buffer, and then incubated with 3,3′,5,5′-tetramethylbenzidine for 30 min, after which the reaction was stopped by sulfuric acid. Data were collected in a Synergy H1 Microplate Reader using Gen5 version 2.06 software (BioTek).
Statistics.
Statistical analyses were performed using GraphPad Prism v.8.4.1. A two-tailed unpaired t test was used for comparisons between two groups, and ordinary ANOVA with a post hoc test corrected for multiple comparisons was used for comparisons among more than two groups. Each experiment was repeated independently two to three times with similar results. Detailed information is in provided in the figure legends. P < 0.05 was considered statistically significant. Posttest P values were as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Material
Acknowledgments
We thank R. Locksley for providing the reporter strains and the Flow Cytometry Shared Resource of the Duke Cancer Institute for their assistance with flow cytometry analyses. Support was provided by NIH Grants R01 DK121032 and R01 DK121969 (to S.N.A.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026461118/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Foxman B., Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North Am. 28, 1–13 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Al-Badr A., Al-Shaikh G., Recurrent urinary tract infections management in women: A review. Sultan Qaboos Univ. Med. J. 13, 359–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores-Mireles A. L., Walker J. N., Caparon M., Hultgren S. J., Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooton T. M., Recurrent urinary tract infection in women. Int. J. Antimicrob. Agents 17, 259–268 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Foxman B., Recurring urinary tract infection: Incidence and risk factors. Am. J. Public Health 80, 331–333 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikäheimo R., et al., Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 22, 91–99 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Wu J., et al., A highly polarized TH2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat. Immunol. 21, 671–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye M. G., Fox M. J., Bartlett J. G., Braman S. S., Glassroth J., The clinical spectrum of Staphylococcus aureus pulmonary infection. Chest 97, 788–792 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Créixems M., et al., Recurrent pneumococcal bacteremia. A warning of immunodeficiency. Arch. Intern. Med. 156, 1429–1434 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Hedlund J., Kalin M., Örtqvist A., Recurrence of pneumonia in middle-aged and elderly adults after hospital-treated pneumonia: Aetiology and predisposing conditions. Scand. J. Infect. Dis. 29, 387–392 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Niv Y., Hazazi R., Helicobacter pylori recurrence in developed and developing countries: Meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter 13, 56–61 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Zar F. A., Bakkanagari S. R., Moorthi K. M., Davis M. B., A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45, 302–307 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Borody T. J., et al., Recurrence of duodenal ulcer and Campylobacter pylori infection after eradication. Med. J. Aust. 151, 431–435 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Chan C. Y., St. John A. L., Abraham S. N., Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38, 349–359 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvey M. A., Schilling J. D., Hultgren S. J., Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69, 4572–4579 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Miao Y., Abraham S. N., The multiple antibacterial activities of the bladder epithelium. Ann. Transl. Med. 5, 35 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivick K. E., Mobley H. L., Waging war against uropathogenic Escherichia coli: Winning back the urinary tract. Infect. Immun. 78, 568–585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brumbaugh A. R., Mobley H. L., Preventing urinary tract infection: Progress toward an effective Escherichia coli vaccine. Expert Rev. Vaccines 11, 663–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein R. D., Hultgren S. J., Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 18, 211–226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham S. N., Sun D., Dale J. B., Beachey E. H., Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 336, 682–684 (1988). [DOI] [PubMed] [Google Scholar]
- 21.Langermann S., et al., Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181, 774–778 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Asadi Karam M. R., Oloomi M., Mahdavi M., Habibi M., Bouzari S., Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 31, 1210–1216 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Uehling D. T., Hopkins W. J., James L. J., Balish E., Vaginal immunization of monkeys against urinary tract infection with a multi-strain vaccine. J. Urol. 151, 214–216 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Mike L. A., Smith S. N., Sumner C. A., Eaton K. A., Mobley H. L., Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc. Natl. Acad. Sci. U.S.A. 113, 13468–13473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsyth V. S., et al., Optimization of an experimental vaccine to prevent Escherichia coli urinary tract infection. MBio 11, e00555-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham S. N., Miao Y., The nature of immune responses to urinary tract infections. Nat. Rev. Immunol. 15, 655–663 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Wang K. E., Zhou X. L., Zhou J., Ye C. H., Preoperative Th1/Th2 and related cytokines: Prediction value in postoperative febrile UTI after ureteroscopy in patients with ureteral calculi. Adv. Clin. Exp. Med. 28, 125–132 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D. M., CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 10, 499–511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribes S., et al., Intraperitoneal prophylaxis with CpG oligodeoxynucleotides protects neutropenic mice against intracerebral Escherichia coli K1 infection. J. Neuroinflammation 11, 14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St John A. L., Ang W. X. G., Rathore A. P. S., Abraham S. N., Reprograming immunity to food allergens. J. Allergy Clin. Immunol. 141, 1936–1939.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macatonia S. E., et al., Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154, 5071–5079 (1995). [PubMed] [Google Scholar]
- 32.Reinhardt R. L., Liang H. E., Locksley R. M., Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinhardt R. L., et al., A novel model for IFN-γ-mediated autoinflammatory syndromes. J. Immunol. 194, 2358–2368 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Normark S., et al., Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect. Immun. 41, 942–949 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam G., Khera R., Vaginal mucosal vaccine for recurrent urinary tract infections. Indian J. Urol. 23, 335–336 (2007). [PMC free article] [PubMed] [Google Scholar]
- 36.Mount A., et al., Combination of adjuvants: The future of vaccine design. Expert Rev. Vaccines 12, 733–746 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Wynn T. A., et al., An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376, 594–596 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Kumamoto Y., et al., CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 39, 733–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen D., Forrest L., Kepler T. B., Parker I., Cahalan M. D., Selective and site-specific mobilization of dermal dendritic cells and Langerhans cells by Th1- and Th2-polarizing adjuvants. Proc. Natl. Acad. Sci. U.S.A. 107, 8334–8339 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J., Paul W. E., CD4 T cells: Fates, functions, and faults. Blood 112, 1557–1569 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fields P. E., Kim S. T., Flavell R. A., Cutting edge: Changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J. Immunol. 169, 647–650 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Bashyam H., Th1/Th2 cross-regulation and the discovery of IL-10. J. Exp. Med. 204, 237 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukens J. R., Anand P. K., Adapt(ed) to repair—TH 2 immune responses in the bladder promote recurrent infections. Nat. Immunol. 21, 597–599 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Mohrs M., Shinkai K., Mohrs K., Locksley R. M., Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Karam M. R., Oloomi M., Mahdavi M., Habibi M., Bouzari S., Assessment of immune responses of the flagellin (FliC) fused to FimH adhesin of uropathogenic Escherichia coli. Mol. Immunol. 54, 32–39 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Bobat S., et al., Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur. J. Immunol. 41, 1606–1618 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Sarkissian C. A., Alteri C. J., Mobley H. L. T., UTI patients have pre-existing antigen-specific antibody titers against UTI vaccine antigens. Vaccine 37, 4937–4946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hegazy A. N.et al., Oxford IBD Cohort Investigators , Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153, 1320–1337.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farber D. L., Yudanin N. A., Restifo N. P., Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14, 24–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honda K., Littman D. R., The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Brubaker L., Wolfe A., The urinary microbiota: A paradigm shift for bladder disorders? Curr. Opin. Obstet. Gynecol. 28, 407–412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antunes-Lopes T., et al., The role of urinary microbiota in lower urinary tract dysfunction: A systematic review. Eur. Urol. Focus 6, 361–369 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Langermann S., Ballou W. R. Jr, Vaccination utilizing the FimCH complex as a strategy to prevent Escherichia coli urinary tract infections. J. Infect. Dis. 183 (suppl. 1), S84–S86 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Biot C., et al., Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci. Transl. Med. 4, 137ra72 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Derré L., et al., Intravesical bacillus Calmette–Guerin combined with a cancer vaccine increases local T-cell responses in non-muscle-invasive bladder cancer patients. Clin. Cancer Res. 23, 717–725 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Kates M., et al., Intravesical BCG induces CD4+ T-cell expansion in an immune competent model of bladder cancer. Cancer Immunol. Res. 5, 594–603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redelman-Sidi G., Glickman M. S., Bochner B. H., The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat. Rev. Urol. 11, 153–162 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Domingos-Pereira S., et al., Intravesical Ty21a vaccine promotes dendritic cells and T cell-mediated tumor regression in the MB49 bladder cancer model. Cancer Immunol. Res. 7, 621–629 (2019). [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization , Water sanitation hygiene. Boil water: Technical brief. https://www.who.int/water_sanitation_health/publications/boiling-water/en/. Accessed 31 January 2015.
- 60.Mocé-Llivina L., Muniesa M., Pimenta-Vale H., Lucena F., Jofre J., Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol. 69, 1452–1456 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.