Significance
This study shows that the myeloid immune response in isolated rapid-eye-movement sleep behavior disorder (iRBD) patients involves both brain and periphery, and that these responses are related, supporting a cross-talk between the brain and the peripheral immune system. Furthermore, these immune events were correlated with dopaminergic neuronal changes, strongly supporting a role for the immune system in the neuronal process associated with iRBD and putatively with Parkinson’s disease. These findings support monocytes as biomarkers and potential targets. Future studies should address possible association between monocytic changes and disease progression in longitudinal cohorts and the use of monocytes as accessible prognostic markers of brain events in synucleinopathies.
Keywords: monocytes, TLR4, CD163, positron emission tomography, neuroinflammation
Abstract
Synucleinopathies are neurodegenerative diseases with both central and peripheral immune responses. However, whether the peripheral immune changes occur early in disease and their relation to brain events is yet unclear. Isolated rapid-eye-movement (REM) sleep behavior disorder (iRBD) can precede synucleinopathy-related parkinsonism and provides a prodromal phenotype to study early Parkinson's disease events. In this prospective case-control study, we describe monocytic markers in a cohort of iRBD patients that were associated with the brain-imaging markers of inflammation and neuronal dysfunction. Using 11C-PK11195 positron emission tomography (PET), we previously showed increased immune activation in the substantia nigra of iRBD patients, while 18F-DOPA PET detected reduced putaminal dopaminergic function. Here we describe that patients’ blood monocytic cells showed increased expression of CD11b, while HLA-DR expression was decreased compared to healthy controls. The iRBD patients had increased classical monocytes and mature natural killer cells. Remarkably, the levels of expression of Toll-like receptor 4 (TLR4) on blood monocytes in iRBD patients were positively correlated with nigral immune activation measured by 11C-PK11195 PET and negatively correlated with putaminal 18F-DOPA uptake; the opposite was seen for the percentage of CD163+ myeloid cells. This suggesting a deleterious role for TLR4 and, conversely, a protective one for the CD163 expression. We show an association between peripheral blood monocytes and brain immune and dopaminergic changes in a synucleinopathy-related disorder, thus suggesting a cross-talk among periphery and brain during the disease.
Parkinson’s disease (PD) is characterized by the loss of dopaminergic neurons in the substantia nigra compacta (SNc) and the intraneuronal aggregation of α-synuclein (a-syn) as Lewy bodies and neurites. Besides the neurodegenerative process, there is widespread inflammation in PD brains. Microgliosis has been observed in postmortem brains of PD patients, and positron emission tomography (PET) imaging with ligands targeting the translocator protein 18 kDa (TSPO) has shown immune activation in the SN, basal ganglia, and frontal cortex of PD patients in vivo (1). It has been suggested that the immune response is an early event and an active player promoting the a-syn aggregation and transmission that results in clinical symptoms (2). During recent years, the importance of peripheral pathology in PD has become recognized, and this is especially relevant regarding the immune response. PD patients show elevated levels of inflammation markers both in the cerebrospinal fluid (CSF) and serum (3). In addition, changes in blood immune cells have been described for monocytes and lymphocytes from PD patients (4, 5). These studies suggest a brain–periphery interaction that might have significant consequences on neuronal health. However, human data that support an association between immune cellular processes in periphery and brain disease events in synucleinopathies have not yet been described.
A-syn plays a central role in PD-related immune events, acting as a danger-associated molecular pattern, thus inducing inflammation by activating microglia and monocytes via Toll-like receptors (TLR) 2 and 4 among others (6). These cells have a dual action, clearing the extracellular a-syn aggregates, thus protecting neurons, but also promoting neurodegeneration by proinflammatory cytokine release (2). In addition, myeloid cells act as antigen-presenting cells, and through human leukocyte antigen DR isotype (HLA-DR), interact with T cells, initiating an adaptive immune response. Interestingly, single nucleotide polymorphisms (SNPs) of the HLA-DR gene are associated with a higher risk of developing PD (7). Thus, there are multiple mechanisms contributing to the immune component in PD, which involves both brain (microglia) and peripheral (monocytes) myeloid cells. However, it is still unclear how early in the disease immune activation occurs and how peripheral events relate to brain disease processes.
It is now known that isolated rapid-eye-movement sleep behavior disorder (iRBD) is a prodromal phenotype of synucleinopathy-related parkinsonism, including PD, dementia with Lewy bodies, and rarely multiple system atrophy (8). Therefore, iRBD provides a powerful tool for the study of early events in PD. iRBD patients have been shown to develop nonmotor symptoms reminiscent of PD, and show brain dysfunction on imaging that is also associated with PD pathology (9, 10). We have previously reported that a cohort of iRBD patients showed immune activation in the SN and subclinical decreases in striatal presynaptic dopaminergic function using 11C-PK11195 and 18F-DOPA PET, respectively (9). This supports the occurrence of early brain events in prodromal PD that involve both neuronal and immune processes. Here, we have analyzed the blood monocytic population in these iRBD patients and describe, early monocytic changes that resemble those seen in PD patients. In addition, we show that cellular immune markers on blood monocytes correlated strongly with loss of dopaminergic synapses function, with immune activation in the brain and with olfactory dysfunction. We show that the peripheral myeloid immune system is involved early in iRBD and that these peripheral events are associated with brain processes in these patients. Our data support a cross-talk between periphery and brain in synucleinopathies.
Materials and Methods
Cohort Description.
A full description of our iRBD study population, PET imaging, and clinical assessments have been previously published (9, 10). Briefly, 21 patients with iRBD and 28 controls were recruited between March 23, 2015, and March 17, 2017. From these, 15 patients and 22 controls gave blood samples and thus were included in the present study (Table 1). Patients with polysomnography-confirmed iRBD according to established criteria (11) were recruited from tertiary sleep clinics at Aarhus University Hospital, Denmark and the Multidisciplinary Sleep Unit of the Hospital Clínic de Barcelona, Spain. Before inclusion, patients gave a full clinical history and were examined to exclude any neurological condition. Healthy controls (HCs) were recruited through newspaper advertisements. They were individuals with no motor or cognitive symptoms, a normal neurological examination, and a mean group age similar to the iRBD group. HCs were screened for the absence of RBD symptoms with a questionnaire (12) and by interviewing the individual and their bed partner. None of the participants were regular users of antiinflammatory drugs or were taking antidepressants at the time of this study.
Table 1.
Demographic and clinical characteristics
| HC (n = 22) | iRBD (n = 15) | P value | |
| Male/female | 22/0 | 12/3 | — |
| Age at visit (y) | 65 (5.5) | 64.4 (6.0) | 0.73 |
| Age at diagnosis (y) | N/A | 60.9 (6.0) | — |
| iRBD duration (y) | N/A | 3.45 (3.4) | — |
| UPDRS | N/A | 2.8 (2.0) | — |
| UPSIT | N/A | 19.9 (8.2) | — |
| MMSE | 29.1 (1.1) | 28.4 (1.5) | 0.12 |
| MoCA | 26.5 (2.6) | 26.3 (2.4) | 0.82 |
| NMSS | 10.7 (8.0) | 29.2 (17) | 0.0006*** |
| NMSS Quest | 2.7 (2.2) | 6.6 (4.1) | <0.0001**** |
| SCOPA (AUT) | 5.5 (3.6) | 15.5 (3.5) | <0.0001**** |
| PDSS2 | 6.8 (6.2) | 11.4 (9.4) | 0.0301* |
| 11C-PK11195 (n) | 10 | 15 | — |
| 18F-DOPA (n) | 9 | 15 | — |
| FACS (n) | 22 | 15 | — |
Demographic and clinical characteristics for the HC group and iRBD patients. MMSE, minimental state examination; MoCA, the Montreal cognitive assessment; PDDS2, Parkinson´s disease sleep scale-2; SCOPA (AUT), scales for outcomes in Parkinson´s disease (autonomic dysfunction); UPDRS, Unified Parkinson´s disease rating scale. UPSIT scores alone and divided into category, Data shown as mean with SD. Nonparametric unpaired Mann–Whitney for all, except unpaired t test for age and MoCA. n = number of individuals undergoing PET scanning with the 11C-PK11195 or 18F-DOPA tracer, or who provided blood for FACS (flow cytometry) examination of PBMCs. *P < 0.05, ***P < 0.001, ****P < 0.0001.
PET imaging was used to assess levels and distribution of immune activation and dopamine terminal function by measuring the binding potential (BPND) of 11C-PK11195 and influx constant (Ki) of 18F-DOPA, respectively (9, 10).
The study received approval from the local Ethics Committee at both centers: The Hospital Clinic of Barcelona Ethics Committee HCB/2015/0186 and the Science Ethical Committee of the Central Denmark Region 1-16-02-126-15. All participants gave written informed consent according to the Declaration of Helsinki before enrollment.
Peripheral Blood Mononuclear Cell Isolation.
Only participants whose blood samples were collected prior to PET scanning and processed during the same day were included: 15 iRBD and 22 HC subjects (Table 1). Blood samples (16 to 20 mL) were collected into EDTA tubes (Sarstedt), which were centrifuged, 400 × g for 10 min at room temperature. The supernatant (plasma) was removed and the tube was filled up with dilution media (2% heat-inactivated fetal bovine serum [HI-FBS, Gibco] in phosphate buffer saline [PBS, Biowest]) and mixed. The mixture was transferred to SepMate tubes (StemCell Technologies) containing density media (Lymphoprep, Fresenius Kabi) and centrifuged at 1,200 × g for 10 min with full brake. The top layer containing the enriched mononuclear cells was poured off into a 50-mL Falcon tube, which was filled up with dilution media before being centrifuged at 300 × g for 8 min at room temperature with full brake on. The supernatant was discarded and pellet resuspended in dilution media and centrifuged once again. The pellet was resuspended in 1 mL HI-FBS and cells counted on a MOXI Mini Automated cells counter (ORFLO). HI-FBS was added to the sample as to reach a concentration of 10 × 106 cell/mL. The cell suspension was then aliquoted into NuncCryovials and at a 1:1 ratio 20% DMSO (SigmaAldrich) in HI-FBS was added to a final concentration of 10% DMSO. The vials were frozen and stored at −80 °C.
Staining for Flow Cytometry Analysis.
Peripheral blood mononuclear cells (PBMCs) were stained for CD14, CD16, HLA-DR, CD11b, CD192 (CCR2), TLR4, CD163, CD56, and TLR2 (CD282) using a 10-color flow cytometry panel. PBMCs were thawed in 10 mL preheated RPMI 1640 Glutamax (Gibco) with 1% penicillin/streptomycin, washed, and counted on a MOXI Mini Automated cells counter (ORFLO). One million cells were seeded and live/dead stained using 0.1 μL LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Invitrogen, Thermo Fisher Scientific) in 80 μL DPBS (BioWest), washed, and subsequently blocked in 100 μL 10% heat-inactivated human AB serum (H4522 Sigma) in DPBS (Biowest). Cells were stained using: 3 μL mouse IgG2b K anti-human CD14-BV421 Mϕp9 (BD Horizon), 2.5 μL Mouse IgG2a K anti-human HLA-DR-BV650 L243 (BioLegend), 1.25 μL mouse IgG1 K anti-human CD11b-SuperBright780 ICRF44 (eBioscienceTM Thermo Fischer Scientific), 5 μL mouse IgG2a K anti-human CD192 (CCR2)-FITC K036C2 (BioLegend), 10 μL mouse IgG2a anti-human TLR4-PerCP 610015 (Novus, R&D systems), 1 µL Mouse IgG1 anti-human CD163-R-PE MAC2-158 (IQProducts), 2 μL Mouse IgG1 anti-human CD56-ECD N901 (Beckman Coulter), 2.5 μL Mouse IgG1 anti-human CD16-PC7 3G8 (Beckman Coulter), and 2 μL recombinant human IgG1 anti-TLR2 (CD282)-APC REA109 (MACS Miltenyi Biotec) with 50 μL Brilliant Stain Buffer (BD Horizon) in a total volume of 100 μL DPBS with 1% Albumin fraction V, from bovine serum (Merck). After additional washing, the cells were fixed in 0.9% formaldehyde (Sigma-Aldrich) in DPBS and left to incubate 15 min and centrifuged. The pellet was resuspended in DPBS sealed and stored cold and dark until analysis the following day on LSRFortessa (BD Bioscience). The compensation matrix was calculated using single stains on OneComp eBeads (Thermo Fischer Scientific) and ArC Armine reactive compensation bead kit (Invitrogen) for ABs and live/dead stain, respectably. Cytometer voltage settings were confirmed using SPHERO rainbow calibration particles (BD Bioscience). The cells were analyzed with FlowJo v10.
Flow Cytometry Gating Strategy.
Debris and nonsinglets were excluded based on forward and side-scatter parameters and dead cells were excluded using a live/dead marker. CD56, a marker for natural killer (NK) cells and TLR2, pan-monocytic marker, expressed by monocytes (Mo) and dendritic cells (DCs), were used to separate the monocytic (TLR2+) and NK cells (TLR2−/CD56dim/bright) from others (13).The separation between CD56 dim and bright was based on CD16 expression differences (by heat map) (SI Appendix, Figs. S1 and S2). CD14 and CD16 were used to separate subpopulations: CD14++/CD16− classical, CD14++/CD16+ intermediate, and CD14low/CD16++ nonclassical monocytes; and CD14low/CD16− unclassified monocytes corresponding to DCs (14). Fluorescence minus one control samples were used for correct placement of gates.
Statistical Analysis.
GraphPad Prism-8 was used for statistical analyses (α 5%). Outliers were identified by Grubb’s test. Normality was tested with the D’Agostino–Pearson test. Group differences were examined with an unpaired t test or Mann–Whitney U test (nonparametric). Correlations were interrogated using Pearson or Spearman (nonparametric) statistics and confirmed using age and disease-duration as covariates by simple linear and multiple regression analysis using JMP-14.
Results
iRBD Patients Show Nonmotor Symptoms.
In this prospective case-control study, we recruited 21 iRBD and 28 age-matched HC (see full cohort description in refs. 9 and 10). Six patients and six HC of the original cohort did not have blood samples taken at the time of PET scanning and were excluded from the analysis. The present study, therefore, included 15 iRBD patients and 22 HC. All 15 iRBD patients had both 11C-PK11195 and 18F-DOPA PET, whereas to minimize exposition to ionizing radiation, HC had either 11C-PK11195 PET (n = 10) or 18F-DOPA PET (n = 9). In both cohorts, males were overrepresented. The iRBD group had significantly higher ratings on the nonmotor symptoms scale (NMSS) and questionnaire (NMSS quest), and scales for outcomes in PD autonomic dysfunction (Table 1).
iRBD Patients Have Increased Nigral Immune Activation and Decreased Dopaminergic Putaminal Transmission.
The binding potential (BPND) values of 11C-PK11195 and influx constant (Ki) of 18F-DOPA of these 15 patients were compared with those of the 19 HC (10 for 11C-PK11195 and 9 for 18F-DOPA). As previously shown (9), in this smaller cohort of iRBD patients, we found significantly higher BPND of the 11C-PK11195 ligand in the left SN, while on the right SN we observed a trend (P = 0.06) toward increase compared to HC (Table 2), a lateralization reported before in the larger cohort of 20 patients. As before, the 18F-DOPA mean Ki was significantly reduced in both sides of the putamen in iRBD patients compared to the HC group (9).
Table 2.
Binding potential values
| Area/ligand | Hemisphere | HC | iRBD | P value |
| S.Nigra 11C-PK11195 | L | −0.0027 (0.09) [10] | 0.1328 (0.15) [15] | 0.013* |
| R | 0.0186 (0.13) [10] | 0.1327 (0.19) [15] | 0.062 | |
| Putamen 11C-PK11195 | L | 0.0687 (0.13) [10] | 0.1161(0.19) [15] | 0.36 |
| R | 0.096 (0.13) [10] | 0.1403 (0.17) [15] | 0.40 | |
| Caudatus 11C-PK11195 | L | −0.219 (0.19) [10] | −0.1491 (0.15) [15] | 0.16 |
| R | −0.177 (0.14) [10] | −0.1491 (0.16) [15] | 0.33 | |
| Putamen 18F-DOPA | L | 0.013 (0.0008) [9] | 0.0103 (0.0017) [15] | 0.0002*** |
| R | 0.013 (0.0006) [9] | 0.0104 (0.0016) [15] | 0.0002*** | |
| Caudatus 18F-DOPA | L | 0.0114 (0.0009) [9] | 0.0104 (0.0017) [15] | 0.13 |
| R | 0.0113 (0.001) [9] | 0.0105 (0.0015) [15] | 0.11 |
BPND of 11C-PK11195 and influx constants (Ki) of 18F-DOPA for iRBD patients and the control population who provided blood samples (9). Left (L), right (R) SN. Data are mean with (SD) [n]. P value obtained by t test or Mann–Whitney U test (when normality was not achieved), n = number of individuals undergoing PET scanning. *P < 0.05, ***P < 0.0005.
iRBD Patients Showed an Elevated Frequency of Mature NK Cells and Blood Monocytes Expressing CD11b.
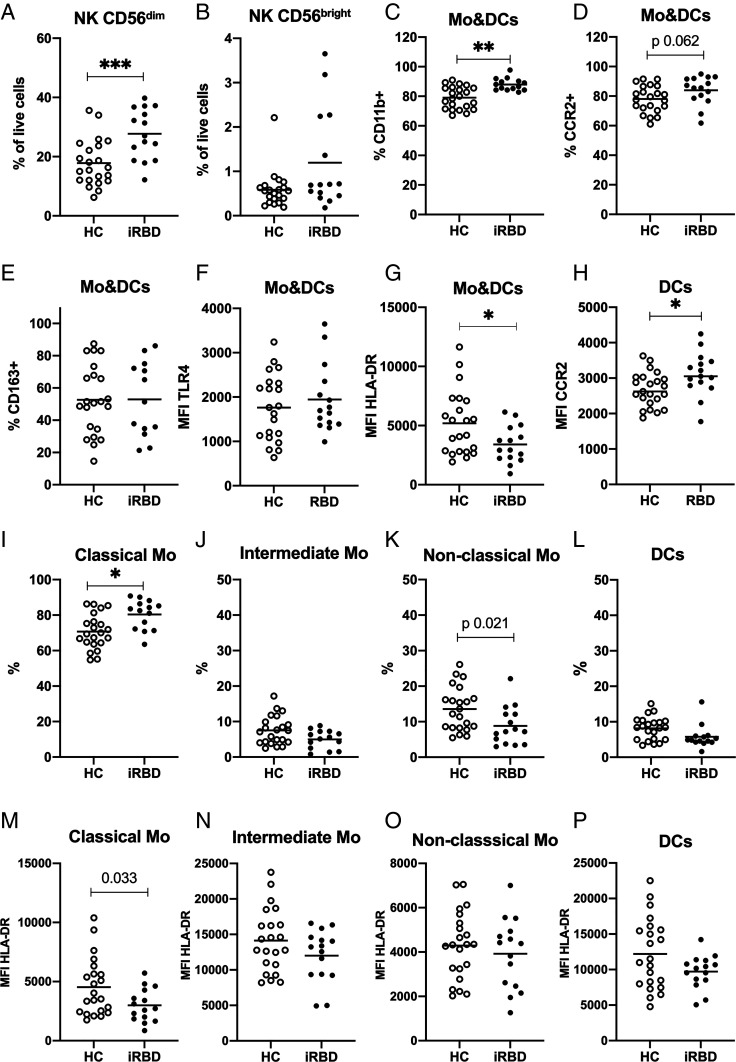
To characterize the peripheral immune changes, PBMCs were isolated and analyzed by flow cytometry designed to address changes on Mos, DCs, and innate lymphoid NK cells (see gating in SI Appendix, Figs. S1 and S2). We observed no changes in the percentage of precursor NK cells (TLR2−/CD56bright) between groups, but the percentage of mature NK cells (TLR2−/CD56dim) was significantly increased in the iRBD group (Fig. 1 A and B). The elevation in NK cells in the cohort was excluded to be due to neuroendocrine tumors, based on follow-up data from clinical records.
Fig. 1.
Flow cytometry analysis of PBMCs from HCs and iRBD patients. PBMCs in blood from iRBD patients and HCs were analyzed based on TLR2 and CD56 expression. NK cells were identified as TLR2−/CD56+ and divided into (A) CD56 dim mature NK cells and (B) CD56 bright precursor NK cells. TLR2 was used as a pan marker for Mo and DCs and subtyped based on CD14, CD16, and HLA-DR expression. Mo&DCs are shown with respect to the percentage expressing the following surface markers: (C) CD11b (D) CCR2, and (E) CD163; and their MFI of (F) TLR4 and (G) HLA-DR. (H) MFI of CCR2 on DCs. Percentage of the subpopulations in Mo&DCs population: (I) classical monocytes (CD14++/CD16−), (J) intermediate monocytes (CD14++/CD16+), (K) nonclassical monocytes (CD14low/CD16++), and (L) DCs (CD14low/CD16−). The MFI of HLA-DR shown for the Mo&DCs subpopulations: (M) classical Mo, (N) intermediate Mo, (O) nonclassical Mo, and (P) DCs. Lines show means. Mann–Whitney test for B and C, and unpaired t test for A and D–P. *P < 0.05, **P < 0.01, ***P < 0.001. Bonferroni correction (P value set to 0.0125) was applied for I–L and M–P.
No difference between the percentage of total monocytic cells (Mo&DCs, TLR2+) was observed between the groups (HC 22.18 ± 11.53 vs. iRBD 20.71 ± 8.22). Next, we evaluated the surface expression of immune molecules, previously associated with PD and a-syn, in the total monocytic population (TLR2+). We observed a higher percentage of Mo&DCs expressing CD11b in the iRBD group, and a trend (P = 0.062) toward an increased percentage of cells expressing CCR2 (Fig. 1 C and D). HLA-DR expression on the total Mo&DCs population was decreased in iRBD patients (Fig. 1G). The percentage of cells expressing CD163 (Fig. 1E) and levels of TLR4 expression (Fig. 1F) on Mo&DCs were variable in both groups and no significant mean differences were found.
iRBD Patients Have Increased Classical Monocytes and Decreased Expression of HLA-DR.
We then analyzed the different Mo&DC subpopulations based on their CD14 and CD16 expression, as previously established (15). Since the four defined subpopulations intercorrelate, Bonferroni correction was applied (α 1.25%), also when HLA-DR was analyzed in each population since this marker was used for the gating strategy (SI Appendix, Fig. S2). The percentage of classical monocytes (CD14++/CD16−) was significantly increased in iRBD patients (Fig. 1I). This was associated with decrease in the nonclassical monocytes (CD14low/CD16++, P = 0.021 did not stand Bonferroni-correction) (Fig. 1K), and a nonsignificant decrease in the other two populations: intermediate monocytes (CD14++/CD16+) (Fig. 1J) and DCs (CD14low/CD16−) (Fig. 1L).
The observed decreased HLA-DR expression on the Mo&DC population in iRBD (Fig. 1G) was mainly driven by changes in the classical monocytes (P = 0.033, did not stand Bonferroni-correction) (Fig. 1M), while nonsignificant decreases were seen for the other three subpopulations: intermediate, nonclassical, and DCs (Fig. 1 N–P). In addition, the DCs in iRBD patients showed higher surface expression of CCR2 (median fluorescence intensity, MFI) than HC (Fig. 1H).
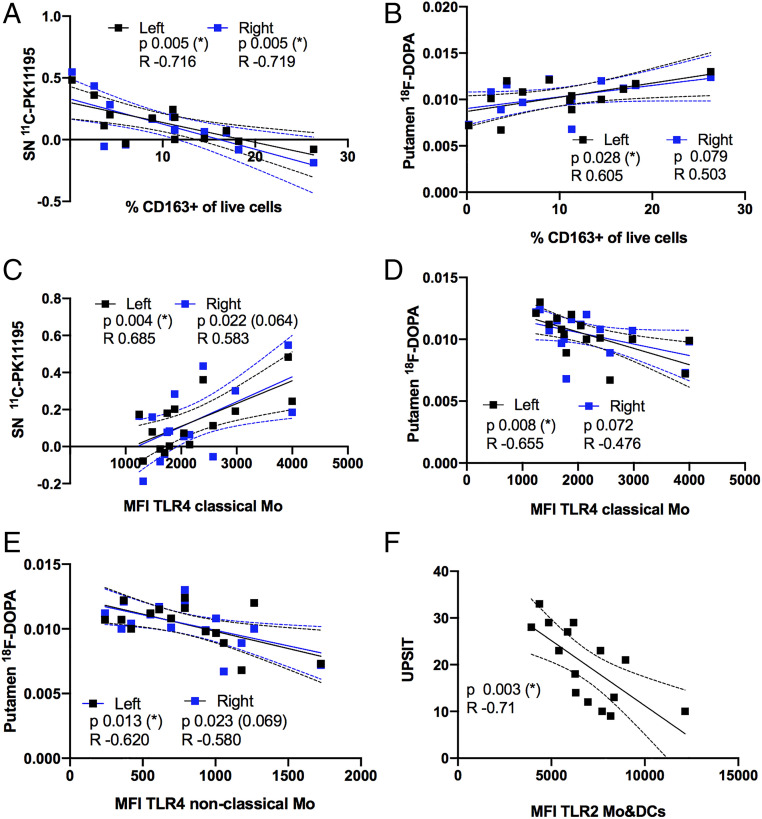
CD163 and TLR4 Levels on Blood Myeloid Cells Were Correlated with PET Findings in iRBD Brains.
To correlate peripheral immune events with PET CNS observations, we compared flow cytometry data with the SN BPND of 11C-PK11195 and putamen Ki of 18F-DOPA. No significant associations were seen for the HC group; however, we observed significant associations when interrogating iRBD data. CD163 is a receptor exclusively expressed by monocytic cells and classically associated with M2-monocytes (16, 17). In iRBD, the percentage of cells expressing CD163 in the total live cell population was inversely correlated with the 11C-PK11195 signal on left and right SN (Fig. 2A). 18F-DOPA Ki values were positively correlated with the percentage of CD163+ cells. This was significant for the left putamen and a trend (P = 0.08) for the right putamen (Fig. 2B). Therefore, elevated CD163+ cell frequency correlated with lower immune activation in SN and higher putamen dopamine storage in iRBD patients. This was also true after correcting for age and disease duration covariance by multiple regression analysis (SI Appendix, Table S1).
Fig. 2.
Surface expression of monocytic markers and PET data or UPSIT score in iRBD patients. Each patients’ PBMC parameter of choice (x axis) was paired with its PET or UPSIT score (y axis), all points plotted (n = 15 in all, except A and B, n = 13 [two outliers by Grubb’s test]) and analyzed for correlation. (A–D) Graphs show individual patients paired values and regression lines with 95 confidence interval (discontinued) lines for the correlation between surface markers in monocytes and BPND of 11C-PK11195 in the SN and influx constant (Ki) of 18F-DOPA in putamen. Due to lateralization for each ligand, left (black) and right (blue) hemisphere PET data were correlated separately to the patients’ immune marker: (A) %CD163+ of live cells versus BPND of 11C- PK11195 in the SN. (B) %CD163+ of live cells versus Ki of 18F-DOPA in the putamen. (C) MFI of TLR4 on classical Mo versus BPND of 11C-PK11195 in the SN. MFI of TLR4 on (D) classical Mo and (E) nonclassical Mo versus Ki of 18F-DOPA in the putamen. (F) Graphs show individual patients paired values and regression lines with 95 confidence interval lines for the correlation of TLR2 MFI on Mo&DCs with UPSIT scores (n = 15). Pearson correlation analysis P and R values are shown. Associations were tested for covariates by multiple regression analysis (SI Appendix, Tables S1–S3), An asterisk (*) indicates significance remained after correction *P < 0.05, otherwise corrected P value is indicated in brackets (P).
TLR4 has previously been shown to interact with a-syn, which will lead to uptake and clearance of the protein, as well as to the proinflammatory immune activation (18). Interestingly, we showed that surface expression levels (MFI) of TLR4 on classical monocytes in the iRBD patients correlated positively with 11C-PK11195 BPND on the left SN and a similar trend was seen in the right side (Fig. 2C), linking TLR4 expression on peripheral classical monocytes with higher immune activation in the brain of iRBD patients. Moreover, TLR4 expression was also associated with the 18F-DOPA signal in iRBD. Surface TLR4 expression levels on classical and nonclassical monocytes were negatively correlated with putamen 18F-DOPA levels on the left side, with a similar trend in the right side (Fig. 2 D and E). Therefore, higher expression levels of TLR4 on monocytes were related to impaired dopaminergic neurotransmission in the putamen.
The Surface Expression of TLR2 on Mo&DCs Correlates with Olfactory Function.
Since the iRBD patients did not exhibit substantial motor or cognitive symptoms, but showed significant clinical nonmotor phenotypes we investigated whether they were associated with the immune molecules analyzed. Surface expression of the immune receptor and a-syn interactor TLR2 (19) on Mo&DCs showed a negative correlation with University of Pennsylvania smell identification tests (UPSIT) scores (Fig. 2F). Thus, higher TLR2 surface expression was associated with lower olfactory function in iRBD patients.
Discussion
This study shows innate immune changes in peripheral blood monocytes in iRBD and that monocytic immune markers correlate with in vivo brain immune and neuronal changes detected with PET.
Epidemiological, clinical, and genetic studies all support a role for immune factors in PD (2), with monocytes as essential contributors (20). However, it is still unknown how early the peripheral immune events take place and if they are drivers of PD onset and how they contribute to brain changes. The discovery of iRBD as a prodromal phenotype of PD gives us an opportunity to study early factors in PD development. With this in mind, we analyzed the immune blood cells of a small group of iRBD patients and HC and related the expression of surface immune molecules to the PET imaging data obtained for the cohort (9). Our data showed an increase in blood classical monocytes in iRBD patients. In addition, we observed an increased percentage of monocytes expressing CD11b and a similar trend for CCR2 on iRBD monocytes, while the HLA-DR expression was lower compared to HCs. Remarkably, we found that expression of immune monocyte markers in blood were significantly correlated with PET findings of nigral inflammation and putamen dopamine deficit. In particular, we observed that a higher percentage of CD163+ myeloid cells in the blood was related to lower levels of immune activation and preserved dopaminergic transmission. Conversely, the expression level of TLR4 on monocytes was associated with inflammation levels in the SN and lower putamen terminal uptake of 18F-DOPA. Our data showed early changes in the monocytic population in iRBD and suggest that monocytic markers are associated with stimulation of, or protection against, activation of the brain immune cells and reduction of dopaminergic function. This supports a role for the peripheral immune system in the early stages of synucleinopathy. Moreover, the monocytic TLR2 expression correlated with ratings of olfaction in iRBD patients supporting their phenotypic relevance.
Microgliosis in PD brains is associated with a-syn pathology and a-syn in turn is an initiator of immune activation (6). Our previous work with this iRBD cohort suggests that brain immune activation is an early event in synucleinopathies (9). Evidence suggests that not only brain immune cells, but also peripheral immune cells are involved in the PD inflammatory process. These central and peripheral processes will affect each other in response to the neurodegenerative event, to finally influence neuronal fate. Cellular and functional changes in blood monocytes have previously been reported in PD patients (5, 21). We show here that, as reported before in PD, iRBD patients’ blood also contains increased classical monocytes, in accordance with being a prodromal PD phenotype (5, 22). Classical monocytes infiltrate inflamed tissue and differentiate into macrophages and DCs, and they are crucial for tissue inflammation and its resolution (23). Accordingly, iRBD patients showed a higher percentage of monocytes expressing CD11b and CCR2, proteins related to adhesion and migration of leukocytes into tissue. The integrin CD11b interacts with extracellular matrix components and endothelial cells, thus allowing monocyte migration (24). CD11b also interacts with a-syn and mediates the a-syn chemoattractant capacity (25). The chemokine receptor CCR2 was essential for infiltration of monocytes into brain in a PD transgenic a-syn mouse model, where they contributed to neuronal death (26). We observed an increased CCR2 expression on DCs and elevated percentage (trend) of CCR2+ myeloid cells in iRBD blood, in agreement with recent data reported for PD patients’ monocytes (27). CCR2’s ligand, CCL2, is increased in PD patients’ blood (22) and differences in CCL2 levels in serum or CSF are associated with different PD clinical subtypes (28). Thus, changes in the expression levels of CD11b and CCR2 in iRBD supports a role for these proteins in the neurodegenerative process in synucleinopathies. Interestingly, CCL2 can also recruit NK cells (29), which are found in PD brain (30) and increased in PD blood (31). The iRBD patients had increased mature NK cells, which constitute those of higher CD16 expression and that possess cytotoxic capacity (29). Interestingly, it is suggested that NK cells might have a protective role by clearing a-syn (30). Altogether, our data suggest an early innate immune response with an increase on myeloid compartments able to infiltrate the brain with the aim to resolve the inflammatory event.
SNPs in the HLA-DR region associate with late-onset idiopathic PD (7), and this functionally translates to increased HLA-DR expression on PBMCs isolated from SNP-carrying PD patients (32). However, studies of idiopathic PD blood monocytes found no changes in HLA-DR expression (5, 33). We observed here a decrease in HLA-DR expression by iRBD monocytic cells. This could be associated with the recently reported increase of interleukin (IL)-10 in iRBD patients’ serum (34), given that HLA-DR expression is down-regulated by IL-10 (35). Alternatively, it could also be related to the increase of monocyte precursors and higher monocytic proliferative capacity seen in PD patients (36), which we have shown in vitro gave rise to a higher percentage of newly generated HLA-DR− monocytes (21). Interestingly, both findings were more relevant in the early stages of PD. Thus, the HLA-DR decrease in iRBD described here might be related to the monocytic proliferation and expansion of the classical population, an event especially relevant in the early stages of synucleinopathies.
We found a strong significant correlation of two immune markers in monocytes, CD163 and TLR4, with brain PET findings. We have previously shown that peripheral CD163+ cells infiltrate the brain in a rat PD model and that CD163-targeted dexamethasone therapy decreased neuronal death in the model (37). Here we show that a higher percentage of CD163+ cells in blood is related to lower immune activation and preserved dopaminergic terminals in iRBD patients, thus suggesting a protective role for the CD163+ population. CD163 is highly expressed by classical monocytes (16), it has long been used as a marker for M2 monocytes/macrophages and it is associated with antiinflammatory cells and immunosuppressive mechanisms (17). Thus, these CD163+ cells could have a role in suppression of inflammation occurring in iRBD patients and preserving neuronal integrity.
PD patients’ blood monocytes show increased expression of TLR4 and TLR2, both binders of a-syn and inflammatory mediators (5, 22). We observed no changes in the expression levels of these proteins on monocytes in iRBD. However, we found that peripheral levels of these TLR markers correlated with 11C-PK11195 and 18F-DOPA PET data and olfaction-related clinical scores (UPSIT). The TLR4 expression levels on monocytes increased while the dopaminergic terminal function decreased and immune activation in SN increased. This suggests a role for TLR4 in the neurodegenerative and inflammatory disease processes. Increased expression of TLR4 has been found in the SN and caudate-putamen and also in the intestinal mucosa of PD patients (reviewed in ref. 38). Notably, TLR4 also seems to mediate the clearance of a-syn, which may be protective (39). However, a prolonged presence of oligomeric a-syn leads to TLR4 sensitization and a proinflammatory neurotoxic profile (40). Our data suggest that the TLR4 contributes to the inflammatory process that parallels or causes the neurodegeneration of dopaminergic SN neurons in iRBD. In addition, we also showed a correlation between TLR2 expression and UPSIT scores, associating this molecular pattern receptor with worsening of this phenotypic manifestation. TLR2 is increased in the brain and on monocytes of PD patients and it interacts with neuron-released oligomeric a-syn, which results in a proinflammatory neurotoxic activation (19), thus TLR2 increase might promote disease.
In conclusion, our study shows that iRBD patients exhibit an altered blood myeloid compartment with an increased expression of proteins associated with monocyte adhesion and infiltration, decreased HLA-DR expression, and an increase of classical monocytes. This is compatible with a monocytic population involved in the resolution of an inflammatory process. The expression of CD163 and TLR4 on blood monocytes were associated with changes in immune activation and dopaminergic function in the iRBD brain, together suggesting a beneficial role for CD163 expressing monocytic cells in maintaining brain health, while indicating a deleterious function for TLR4 expression on blood immune cells. Moreover, the expression of TLR2 was correlated with manifestation of olfactory dysfunction, an early nonmotor symptom of prodromal PD. The robust findings in this case-control study should be considered with caution due to the small cohort sample size and the lack of longitudinal data. Although replication in additional cohort is necessary, our data grant solid support to suggest that the myeloid immune process in iRBD patients seems to involve both brain and periphery and that these processes are related. Therefore, supporting the presence of a cross-talk between the brain and the peripheral immune system. Furthermore, these immune events were associated with dopaminergic neuronal changes, suggesting a role for the immune system in the neuronal process associated with iRBD and putatively with synucleinopathy-related parkinsonism.
The unique opportunity of associating monocytic data with PET imaging data in an iRBD cohort, as described here, encourage us to propose that the brain–periphery interaction is an early disease event. However, this case-control study is limited by the small number of participants, and further studies in bigger cohorts using the same methodology are needed to support our observations. Moreover, longitudinal analysis of monocytic response, brain changes, and clinical progression in iRBD might clarify the relevance of the changes observed.
Supplementary Material
Acknowledgments
We thank all study participants; Filip Kirov and Pia Ring-Nielsen (Department of Neurology, Viborg Region Hospital, Denmark) for contacting patients; Dr. Mikkel C. Gjelstrup (Department of Biomedicine, Aarhus University) for advice on the flow cytometry panel; Rainer Hinz (University of Manchester, England), laboratory technician Gitte U. Toft (Department of Biomedicine, Aarhus University), as well as biomedical laboratory scientists and radiochemists at the Department of Nuclear Medicine and PET, Aarhus University Hospital, Denmark, for technical assistance; and the study coordinator, Anne Sofie Møller Andersen (Danish Neuroscience Centre, Aarhus University, Denmark), for invaluable help. Flow cytometry was performed at the FACS Core Facility, Aarhus University, Denmark. Funding was provided by the Aarhus University Research Foundation (IDEAS, Neurodin), the Danish Council for Independent Research, the Lundbeck Foundation, the Jascha Foundation, the Instituto de Salud Carlos III, and the Swiss National Foundation.
Footnotes
Competing interest statement: K.Ø. reports grants from The Danish Parkinson Association, The Danish Council for Independent Research, and the Lundbeck Foundation, and personal fees from Medtronic, UCB, Fertin Pharma, and AbbVie. D.J.B. reports grants from The Danish Council for Independent Research, the Lundbeck Foundation, The Danish Parkinson Association, European Union FP7 programme, and Alzheimer Research UK, and personal fees from GE Healthcare and Plexxikon. E.T. reports grants from The Michael J Fox Foundation and The Instituto de Salud Carlos III. N.P. reports grants from The Danish Council for Independent Research. M.R.-R. receives research support from NovoNordisk, Desiree og Niels Ydes Fond, The Danish Parkinson Association, The Danish Council for Independent Research, The Michael J Fox Foundation, and Aarhus University Research Foundation.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020858118/-/DCSupplemental.
Data Availability
The data supporting the findings of this study are available within the article and SI Appendix. Full access to protected pseudonymized clinical, imaging, and biological data will be granted to collaborating scientists who are willing to complete an “Data Access Agreement” and an “Data Access Application form” and in accordance with General Data Protection Regulation.
References
- 1.Gerhard A., et al., In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21, 404–412 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Tansey M. G., Romero-Ramos M., Immune system responses in Parkinson’s disease: Early and dynamic. Eur. J. Neurosci. 49, 364–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin X. Y., Zhang S. P., Cao C., Loh Y. P., Cheng Y., Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kustrimovic N., et al., Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J. Neuroinflammation 15, 205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijeyekoon R. S., et al., Peripheral innate immune and bacterial signals relate to clinical heterogeneity in Parkinson’s disease. Brain Behav. Immun. 87, 473–488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira S. A., Romero-Ramos M., Microglia response during Parkinson’s disease: Alpha-synuclein intervention. Front. Cell. Neurosci. 12, 247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamza T. H., et al., Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 42, 781–785 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauvilliers Y., et al., REM sleep behaviour disorder. Nat. Rev. Dis. Primers 4, 19 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Stokholm M. G., et al., Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A case-control study. Lancet Neurol. 16, 789–796 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Stokholm M. G., et al., Extrastriatal monoaminergic dysfunction and enhanced microglial activation in idiopathic rapid eye movement sleep behaviour disorder. Neurobiol. Dis. 115, 9–16 (2018). [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Sleep Medicine , International Classification of Sleep Disorders (American Academy of Sleep Medicine, Darien, IL, 3rd Ed., 2014). [Google Scholar]
- 12.Stiasny-Kolster K., et al., The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord. 22, 2386–2393 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Shirk E. N., Kral B. G., Gama L., Toll-like receptor 2bright cells identify circulating monocytes in human and non-human primates. Cytometry A 91, 364–371 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn C. M., et al., Activation of Toll-like receptor 2 (TLR2) induces interleukin-6 trans-signaling. Sci. Rep. 9, 7306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L., Blood monocytes and their subsets: Established features and open questions. Front. Immunol. 6, 423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian Z., et al., Deciphering human macrophage development at single-cell resolution. Nature 582, 571–576 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Fischer-Riepe L., et al., CD163 expression defines specific, IRF8-dependent, immune-modulatory macrophages in the bone marrow. J. Allergy Clin. Immunol. 146, 1137–1151 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Fellner L., et al., Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C., et al., Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4, 1562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y. I., Wong G., Humphrey J., Raj T., Prioritizing Parkinson’s disease genes using population-scale transcriptomic data. Nat. Commun. 10, 994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nissen S. K., et al., Alterations in blood monocyte functions in Parkinson’s disease. Mov. Disord. 34, 1711–1721 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Grozdanov V., et al., Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 128, 651–663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong K. L., et al., Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118, e16–e31 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Bednarczyk M., Stege H., Grabbe S., Bros M., β2 integrins-multi-functional leukocyte receptors in health and disease. Int. J. Mol. Sci. 21, 1402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., et al., α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 112, E1926–E1935 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms A. S., et al., Peripheral monocyte entry is required for alpha-synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp. Neurol. 300, 179–187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A. M., et al., Mitochondrial dysfunction and increased glycolysis in prodromal and early Parkinson’s blood cells. Mov. Disord. 33, 1580–1590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockmann K., et al., Inflammatory profile discriminates clinical subtypes in LRRK2-associated Parkinson’s disease. Eur. J. Neurol. 24, 427-e6 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Earls R. H., Lee J. K., The role of natural killer cells in Parkinson’s disease. Exp. Mol. Med. 52, 1517–1525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earls R. H., et al., NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proc. Natl. Acad. Sci. U.S.A. 117, 1762–1771 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niwa F., Kuriyama N., Nakagawa M., Imanishi J., Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr. Gerontol. Int. 12, 102–107 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Kannarkat G. T., et al., Common genetic variant association with altered HLA expression, synergy with pyrethroid exposure, and risk for Parkinson’s disease: An observational and case-control study. NPJ Parkinsons Dis. 1, 15002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiszer U., Mix E., Fredrikson S., Kostulas V., Link H., Parkinson’s disease and immunological abnormalities: Increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol. Scand. 90, 160–166 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Kim R., et al., Peripheral blood inflammatory cytokines in idiopathic REM sleep behavior disorder. Mov. Disord. 34, 1739–1744 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Thibodeau J., et al., Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur. J. Immunol. 38, 1225–1230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funk N., et al., Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov. Disord. 28, 392–395 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Tentillier N., et al., Anti-inflammatory modulation of Microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson’s disease Model. J. Neurosci. 36, 9375–9390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouli A., Horne C. B., Williams-Gray C. H., Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav. Immun. 81, 41–51 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Choi I., Seegobin S. P., Liang D., Yue Z., Synucleinphagy: A microglial “community cleanup program” for neuroprotection. Autophagy 16, 1718–1720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes C. D., et al., Picomolar concentrations of oligomeric alpha-synuclein sensitizes TLR4 to play an initiating role in Parkinson’s disease pathogenesis. Acta Neuropathol. 137, 103–120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and SI Appendix. Full access to protected pseudonymized clinical, imaging, and biological data will be granted to collaborating scientists who are willing to complete an “Data Access Agreement” and an “Data Access Application form” and in accordance with General Data Protection Regulation.