Abstract
Background:
Regular physical activity (PA) is associated with a lower risk of several types of cancers. However, two-thirds of overweight/obese adults are not sufficiently active; this, in combination with the unfavorable effect of excess body weight, puts them at greater risk for cancer. One reason that these individuals do not engage in enough PA may be their lack of motivation to change their current behavior due to the perception of putting in effort for possible future gain without obvious short-term benefits. There is a need for innovative ways to help individuals recognize the immediate health benefits of PA and thus increase their motivation.
Methods:
This pilot intervention tested a PA education module that included a one-on-one counseling session highlighting the acute effects of PA on glucose patterns, followed by a 10-day self-monitoring period with a continuous glucose monitor (CGM) and a Fitbit. Participants rated the acceptability of the education module on a 5-point Likert scale and completed surveys assessing stages of change for motivational readiness.
Results:
Nineteen overweight/obese adults (84% female) completed the study. Participants gave high ratings to the counseling session for improving their PA-related knowledge (mean=4.22), increasing motivation (mean=4.29), and providing personally-relevant information (mean=4.35). The summary acceptability scores for self-monitoring period were 4.46 for CGM and 4.51 for Fitbit. Participants reported a significant decrease in the pre-contemplation stage and an increase in the action stage (p<.05).
Conclusions:
CGM is a feasible tool for PA interventions.
Impact:
Information from CGM could be used as biological-based feedback to motivate PA.
Keywords: Behavioral intervention, behavior change, exercise, obesity, mHealth
Introduction
Regular physical activity is associated with a reduced risk of many types of cancer, with the strongest evidence for endometrial cancer (>20% risk reduction) and breast and colorectal cancer (both >10% risk reduction) (1). However, nearly half of American adults do not meet the national guidelines for aerobic physical activity, which call for 30 minutes of moderate-intensity physical activity five days a week (2). The physical inactivity rate is even higher among overweight and obese (body mass index [BMI] ≥ 25 kg/m2) American adults (3–5); in combination with the negative impact of excess weight, further increases the cancer risk in this population (6,7). An estimated four in five American adults may be considered as overweight or obese in the next decade (8). Therefore, it is critical to promote regular physical activity in this population as a cancer prevention strategy.
Interventions that aim to increase physical activity levels often incorporate feedback as a behavior change strategy (9,10). Most feedback provided in physical activity interventions is performance based, which gives data about recorded activity levels or evaluates physical activity performance in relation to a set goal (11). The effects of feedback-based interventions on behavior change are highly variable (12,13). One reason that sedentary individuals are not physically active is that they are not sufficiently motivated to change their current behavior. According to the self-determination theory, individuals are more likely to engage in a behavior if they perceive it as personally important (14,15). Other popular theoretical constructs, such as perceived benefits and outcome expectancy, also address the importance of individuals’ beliefs about the positive outcomes of engaging in physical activity (16–18). However, one of the main challenges in motivating sedentary individuals to become more active is that physical activity is often characterized by immediate effort for possible future gain, with no obvious short-term benefit (19). Thus, there is a need to find innovative ways to help individuals recognize the more immediate health benefits of physical activity to enhance the feedback technique, optimize behavior change motivation, and subsequently increase physical activity levels.
Providing feedback on individuals’ biological indicators of health has been used to increase motivation and promote behavior change (20). Traditionally, such biological indices were obtained in a clinical setting (e.g., blood panel) at infrequent measurement points (e.g., annual physical examinations). However, recent advances in wearable sensor technology have made personal biological data available in real time (21); for example, continuous glucose monitors (CGMs), which measure glucose concentrations in the interstitial fluid in real time through a tiny sensor inserted under the skin, have been used by patients with type 1 diabetes to promote glycemic control (22). Given that physical activity has an acute impact on glucose patterns in both diabetic and nondiabetic populations (23–27), data from CGMs could be used for providing biological feedback that demonstrates the immediate benefits of physical activity.
In patients with type 2 diabetes, CGM-based physical activity counseling in intervention settings has been shown to increase physical activity (28,29). Incorporating CGMs into lifestyle interventions for non-diabetic individuals has also been explored (30,31). The results suggest that leveraging data from biosensors is highly promising. However, no studies have investigated the acceptability of CGM-incorporated physical activity intervention in overweight and obese adults. The goals of this pilot study, My Moves, were to (1) determine the acceptability of a physical activity intervention that incorporated the use of CGMs in sedentary overweight and obese adults without diabetes and (2) evaluate the changes in exercise motivation.
Materials and Methods
Study Design
The My Moves intervention included two in-person visits, with a 10-day self-monitoring period in between. During the first visit, participants completed the baseline assessment, received the study devices, and received a one-on-one physical activity education session. The 10-day self-monitoring period started the day after this first visit. At the second visit, participants returned the study devices, reviewed their data, and completed the post-intervention assessment and an exit interview. More details about each study procedure are given below. All study participants provided written informed consent. The study was approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center.
Participants
Participants were recruited through public announcements (e.g., flyers, email listserv) around the Texas Medical Center in Houston, Texas. They included individuals who worked or lived in communities near the medical center, as well as patients and visitors at MD Anderson who did not have cancer or diabetes. Interested individuals were asked to contact the study team to obtain a more detailed description of the study, which included a brief introduction to the CGM. Individuals who remained interested after reading the study description were sent a screening questionnaire to determine their initial eligibility.
Eligible individuals were men and women aged 18 to 65 years who had a BMI ≥ 25 kg/m2; engaged in less than 150 minutes of moderate-intensity physical activity per week in the previous month; could speak, read, and write English; and had a mobile phone with daily internet access. Individuals were excluded if they reported being diagnosed with diabetes; reported using any medication known to affect glucose levels (e.g., corticosteroids, antidepressants, metformin); reported health issues that might limit unsupervised physical activity; worked overnight shifts; were pregnant or lactating; were already using a wearable activity tracker or CGM; or were unable or unwilling to use a CGM.
Individuals who passed the initial eligibility sceening were scheduled for an in-person visit with an overnight fast at MD Anderson. Their heights and weights were measured, and their fasting blood glucose levels were obtained using a commercially available glucometer. Individuals were deemed eligible for the study after their BMI status was confirmed and if their fasting blood glucose was < 125 mg/dL. The recruitment goal for this study was to enroll 20 participants. This sample size was chosen because of the pilot nature of the study and on the basis of previous feasibility and acceptability studies that tested technology-based tools (31,32).
Procedures
Upon study enrollment, participants first completed a set of surveys that assessed demographic information and exercise motivations. They were then given a Fitbit Alta HR (Fitbit Inc, San Francisco, California, USA) wristband to wear, which continuously tracks steps and activity levels with heart rate monitoring. Study staff assisted participants with downloading the Fitbit application to their mobile phones and setting up the device. Participants were instructed to wear the Fitbit at all times, including while showering and sleeping, and to regularly sync it with the phone application. After the introduction to Fitbit, study staff went over a physical activity education session with the participant, which is detailed in the next section.
Following the education session, participants were given a Freestyle Libre (Abbott Laboratories, Lake Bluff, Illinois, USA) CGM system, which consists of a sensor (measures at 5 mm height and 35 mm diameter, and weighs 5 grams) and a scanner (measures at 95 mm x 60 mm x 16 mm and weights 65 grams). Study staff helped participants to insert the sensor into the back of their upper arm. Upon activation, the sensor records interstitial glucose data every 15 minutes continuously for ten days without the need for finger-stick calibration. Therefore, there was no need to replace the sensor during the 10-day self-monitoring period. The sensor was designed to be water-proofed (i.e., can withstand immersion into 3 ft of water for up to 30 minutes). To ensure the sensor stayed in place, participants were advised to avoid bathing, swimming, or participating in other activities that would submerge the sensor under water for a long period of time. Study staff demonstrated how to access real-time glucose information using the reader.
The 10-day self-monitoring period started the following day as detailed in the next section. Participants came back for another in-person visit at MD Anderson on day 11 to return the study equipment, review their Fitbit and CGM data over the past 10 days with the study staff, discuss their self-monitoring experience, and complete another set of surveys and an exit interview. Participants received up to $100 for completing the study.
Physical Activity Education Session
This one-on-one session combined standard physical activity education materials (e.g., providing informational handouts and setting exercise goals) and CGM-based physical activity counseling that had originally been used in studies with diabetic patients (28,29). Study staff first provided an overview of the health benefits of physical activity, different types of physical activity, and general tips to become more active in daily life (i.e., the handout component of the education session). The short-term (e.g., decrease stress and improve energy), medium-term (e.g., decrease body weight and improve insulin sensitivity), and long-term health benefits (e.g., reduce the risk of certain cancers and improve quality of life) of physical activity were highlighted. Participants were also presented with a web-based glucose simulator (see Supplemental Figure 1; 33) that demonstrated acute physical activity (i.e., walking)-related glucose reduction. Next, study staff worked with the participants to calculate their targeted heart rate zone (sex and age adjusted) for light-, moderate-, and high-intensity physical activity. Finally, the staff worked with the participants to develop individualized exercise plans to help them to meet the goal of accumulating 150 minutes per week of moderate-intensity physical activity. The entire education session took about 30 minutes to complete.
Self-monitoring Period
The 10-day self-monitoring period started the day after the one-on-one physical activity education session. During this period, participants could check their accumulative steps and activity levels at any time through the Fitbit application. Participants were reminded about checking their heart rate reading from the Fitbit wristband while exercising to make sure that the activity reached moderate intensity. Participants’ real-time Fitbit data and syncing information were captured by Fitabase (Small Steps Labs LLC, San Diego, California, USA), a web-based platform that processes Fitbit data and generates reports.
Participants were also instructed to carry the CGM reader with them during most waking hours to access their glucose information in real time. Upon scanning the reader with the sensor, the reader displays the current glucose reading and a graph showing 8 hours of glucose history, with an arrow indicating the directional trend. Participants were instructed to obtain a glucose reading each morning when they woke up, each night before they went to sleep, and at least two other times throughout the day. Participants received four reminders each day through text messages for the scanning. The CGM reader records the date and time when the sensor is scanned. Participants were encouraged to observe how their daily glucose patterns were influenced by their behaviors (e.g., eating and exercising).
Measures
Acceptability
For the physical activity education session, participants rated each component (i.e., handout, web-based glucose simulator, targeted heart rate calculation, and exercise plan) in regards to knowledge (i.e., helped me better understand the health benefits of physical activity), motivation (i.e., increased my motivation to be more active), and relevance (i.e., the information was relevant to me). The response options were rated using a 5-point Likert scale, ranging from “strongly disagree” to “strongly agree.” For the self-monitoring period, a 10-item survey was used to assess the acceptability of wearing the Fitbit and the CGM, as in previous studies (31). The survey items focused on addressing the barriers and facilitators to the use of mHealth tools, such as convenience, value, and relevance. The response options were rated using a 5-point Likert scale, ranging from “strongly disagree” to “strongly agree.”
Exercise motivation
The Behavioral Regulation in Exercise Questionnaire-2 (BREQ-2) and the Exercise Stages of Change – Continuous Measure (URICA-E2) were used to assess exercise motivation. The BREQ-2 captured the stages of the self-determination continuum, including amotivation, external regulation, introjected regulation, identified regulation, and intrinsic regulation, reflecting constructs in the self-determination theory (34). The responses were rated on a 5-point Likert scale, ranging from “not true” to “very true.” The URICA-E2 captured readiness to change, as described in the transtheoretical model, in six stages: pre-contemplation (non-believer), pre-contemplation (believer), contemplation, preparation, action, and maintenance, with the assumption that physical activity behavior is typically a dynamic process that moves between different stages (35). The responses were rated using a 5-point Likert scale, ranging from “strongly disagree” to “strongly agree.”
Statistical Analysis
Descriptive statistics were generated for all variables, including the means and standard deviations for continuous variables and percentages for categorical variables. A summary score of acceptability was created for Fitbit and CGM by calculating the mean of the 10 survey items, as in previous studies (31). Two-tailed paired-sample t-tests were used to examine changes in exercise motivations before and after the intervention. A p-value of .05 or less was considered statistically significant. All statistical analyses were conducted using SPSS software (IBM Corp, Armonk, NY, USA) version 24.0.
Results
Recruitment and Participant Characteristics
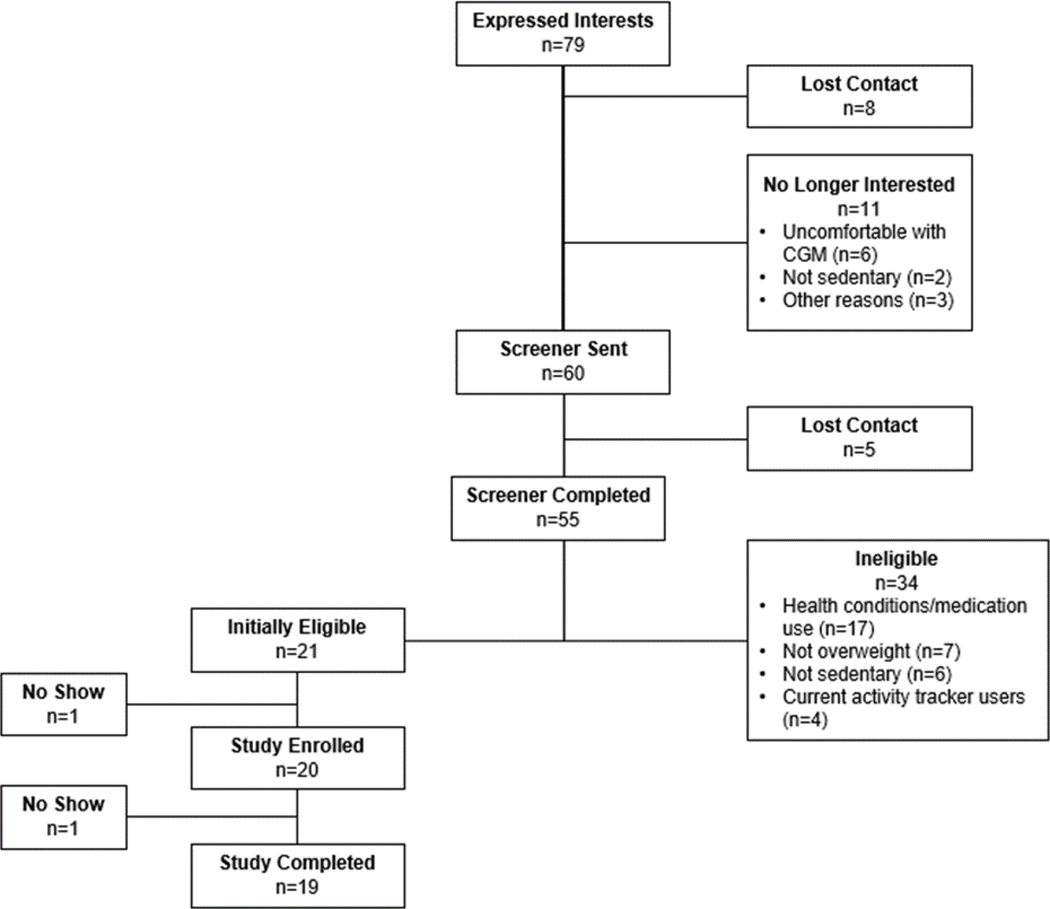
Figure 1 summarizes the flow of participants from recruitment to study completion. A total of 79 individuals expressed an interest in participating in the study. Eleven were no longer interested after reading more information about the study. Among these, six felt uncomfortable with the CGM aspect of the study (i.e., did not want to get poked with a sensor, did not want to wear something on their body), two thought they were too active, three were due to other reasons (e.g., scheduling issue). Of the remaining 68 individuals, eight could not be reached after three contact attempts. Thus, the eligibility screener was sent to 60 individuals. Five individuals did not complete the screener, with no response after three reminder attempts; 21 were eligible for the study on the basis of their screener answers. Of these, 19 completed the study and were included in the analysis.
Figure 1.
Flowchart of individuals from recruitment to the completion of the study.
Table 1 shows the demographic characteristics of the 19 participants. Participants’ average age was 42 years (SD = 8, range 26–55). Eighty-four percent (16/19) of the participants were female, 79% (15/19) were from a racial minority group, and 63% (12/19) were obese. Seventy-four percent (14/19) had an elevated fasting glucose (100–125 mg/dL) at their first visit.
Table 1.
Participant characteristics (n = 19)
| Characteristic | No. of participants (%)a |
|---|---|
| Mean age (SD), years | 41.5 (8.4) |
| Race/ethnicity | |
| African American | 9 (47.4) |
| Asian | 3 (15.8) |
| Hispanic white | 3 (15.8) |
| Non-Hispanic white | 3 (15.8) |
| Multiracial | 1 (5.3) |
| Weight category | |
| Overweight | 7 (36.8) |
| Class 1 obesity | 4 (21.1) |
| Class 2 obesity | 2 (10.5) |
| Class 3 obesity | 6 (31.6) |
| Education level | |
| High school or less | 3 (15.8) |
| Some college/technical training | 7 (36.8) |
| Bachelors degree | 4 (21.1) |
| Masters degree and above | 5 (26.3) |
| Marital status | |
| Single | 9 (47.4) |
| Married/living with significant other | 7 (36.8) |
| Divorced/separated/widowed | 3 (15.8.) |
| Employment status | |
| Employed (full-time) | 14 (73.7) |
| Not employed | 3 (15.8) |
| Other | 2 (10.5) |
SD, standard deviation.
All data are no. of patients (%) unless otherwise indicated.
Acceptability
Physical activity education session
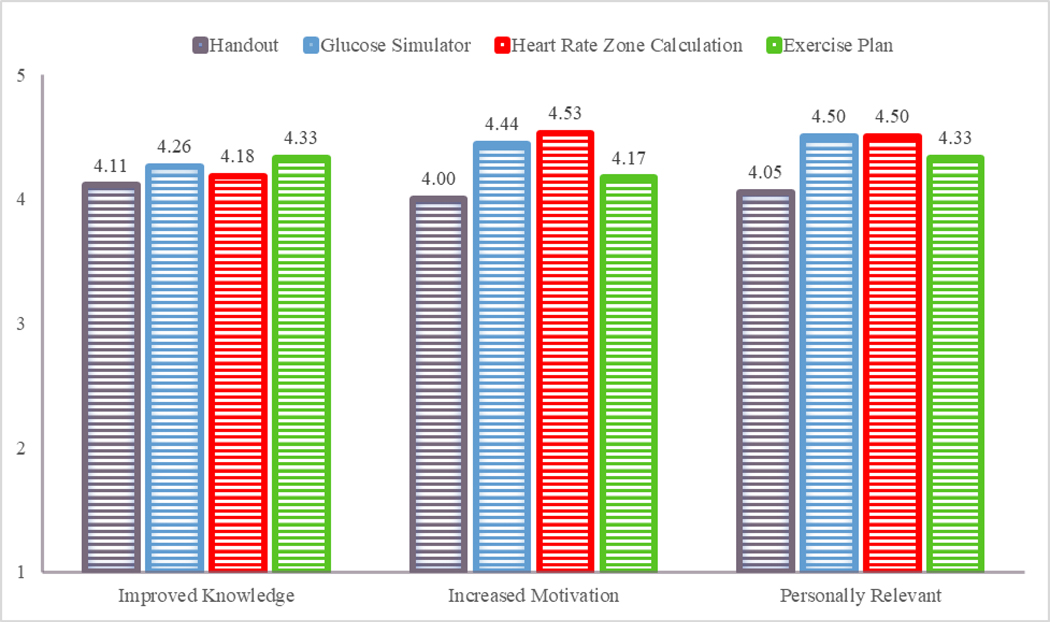
Overall, most participants rated each of the education session components favorably. For the informational handout component, 78.9% (15/19) agreed that it improved their knowledge, and 84.2% (16/19) agreed that it was motivating and personally relevant. For the web-based glucose simulator component, all agreed that it improved their knowledge, and 89.5% (17/19) agreed that it was motivating and personally relevant. For the heart rate zone calculation component, 84.2% (16/19) agreed that it improved their knowledge, 94.7% (18/19) agreed that it was motivating, and 89.5% (17/19) agreed that it was personally relevant. For the exercise plan component, 89.5% (17/19) agreed that it improved their knowledge, 78.9% (15/19) agreed that it was motivating and personally relevant. Figure 2 shows the mean rating score for each education session component.
Figure 2.
Participants’ feedback about each component of the one-on-one physical activity education session. All ratings were on a 5-point Likert scale ranging from “strongly disagree” (1) to “strongly agree” (5).
Self-monitoring period
Participants did not report any problems wearing the Fitbit or the CGM device during the self-monitoring period. All participants wore the Fitbit for the entire 10-day period and kept the device synced with the server through the Fitbit application (on the basis of information provided by Fitabase). Sixty-eight percent (13/19) of the participants reported checking their Fitbit application at least once a day. For the first two participants in the study, the CGM sensor fell off within the first 24 hours. New sensors were then placed with extra adhesive tape to secure them in place. All subsequent sensors stayed in place and collected data for the entire 10-day period. Participants, on average, scanned the sensor with the reader six times a day (SD = 2.4, range = 3–10). Participants followed the specified scanning schedule on average 8 days of the 10-day period (SD = 2.2, range = 1–10).
For the Fitbit device, all participants agreed with statements regarding usability, convenience, value, motivating, confidence, recommendability, and likability. Ninety-five percent (18/19) agreed with the statement regarding relevance, and 32% (6/19) expressed concerns about privacy. For the CGM, all participants agreed with statements regarding usability and confidence; 95% (18/19) with convenience, value, relevance, and recommendability; 84% (16/19) with motivating; 90% (17/19) with likability; and 26% (5/19) expressed concern about privacy. Table 2 shows the average score for each acceptability item. Overall, the Fitbit had an acceptability score of 4.51 (SD = 0.43), and the CGM had an acceptability score of 4.46 (SD = 0.53).
Table 2.
Participants’ experiences with the wearable sensors during a 10-day self-monitoring period (n = 19)
| Acceptability survey item | Mean Likert ratinga (SD) | |
|---|---|---|
| Fitbit | CGM | |
| Usability: this tool is easy to use and user friendly | 4.63 (0.50) | 4.68 (0.48) |
| Convenience: this tool is convenient for me to use in my everyday life | 4.58 (0.51) | 4.47 (0.77) |
| Value: this tool is useful and beneficial | 4.68 (0.48) | 4.63 (0.76) |
| Relevance: this tool provides information that is of interest to me | 4.63 (0.60) | 4.63 (0.60) |
| Motivating: I am motivated to use this tool to track my daily behaviors | 4.68 (0.48) | 4.42 (0.90) |
| Tech support: there is adequate availability and quality of professional assistance throughout use of this tool | 4.53 (0.62) | 4.41 (0.80) |
| Confidence: I feel confident that I use this tool correctly | 4.63 (0.50) | 4.63 (0.50) |
| Privacy: I am concerned about my privacy when using this tool | 2.47 (1.65) | 2.39 (1.58) |
| Recommendability: I would recommend this tool to my friends and family | 4.68 (0.48) | 4.63 (0.76) |
| Likeability: I like using this tool | 4.53 (0.51) | 4.42 (0.69) |
SD, standard deviation.
The Likert scale used in the ratings was 1=strongly disagree, 2=disagree, 3=neither agree nor disagree, 4=agree, 5=strongly agree
Exercise Motivation
Table 3 shows the changes in exercise motivation before and after the study period. Overall, there was no significant change in self-determination motivation. However, there was a trend towards a decrease in amotivation and an increase in intrinsic regulation (p ≤ .10). In general, participants scored higher for the contemplation and preparation stages compared to the other stages, suggesting that most of them were considering a change in their physical activity behavior. After the study period, the score decreased significantly for the two pre-contemplation stages (p < .01) and increased significantly for the action stage (p = .01).
Table 3.
Changes in exercise motivations before (visit 1) and after (visit 2) the study period.
| Motivational Scale | Mean Likert rating (SD) |
Change (mean, SD) | p-valuea | |
|---|---|---|---|---|
| Visit 1 | Visit 2 | |||
| Self-determination Motivationb | ||||
| Amotivation | 0.50 (0.52) | 0.24 (0.57) | −0.26 (0.59) | .066 |
| External Regulation | 0.80 (0.69) | 0.57 (0.73) | −0.24 (0.69) | .155 |
| Introjected Regulation | 1.65 (1.09) | 1.63 (1.33) | −0.02 (0.69) | .913 |
| Identified Regulation | 2.96 (0.72) | 3.16 (0.91) | 0.19 (0.72) | .260 |
| Intrinsic Regulation | 2.25 (0.95) | 2.61 (0.99) | 0.36 (0.89) | .101 |
| Stages of Changec | ||||
| Precontemplation (Non-believers) | 1.92 (0.59) | 1.45 (0.55) | −0.47 (0.53) | .001 |
| Precontemplation (Believers) | 2.59 (0.94) | 2.18 (0.96) | −0.41 (0.60) | .009 |
| Contemplation | 3.74 (0.84) | 3.83 (0.63) | 0.09 (0.80) | .622 |
| Preparation | 3.04 (0.90) | 3.11 (1.06) | 0.07 (0.88) | .749 |
| Action | 2.70 (1.00) | 3.38 (1.05) | 0.68 (1.08) | .013 |
| Maintenance | 2.63 (1.06) | 2.90 (1.10) | 0.28 (0.98) | .233 |
SD, standard deviation.
Significance test using the two-tailed paired-sample t test.
The Behavioral Regulation in Exercise Questionnaire-2 (BREQ-2). The Likert scale used in the ratings ranged from not true (0) to very true (4).
Discussion
The current study represents the first physical activity intervention to incorporate the use of CGM in sedentary overweight and obese adults. We found that there was high acceptability of a physical activity education module with CGM, which includes a one-on-one counseling session followed by a 10-day free-living self-monitoring period. For the one-on-one counseling session, the web-based glucose simulator, which introduced the acute impact of physical activity on glucose patterns, was well received by the participants, suggesting that individuals without diabetes can understand and find value in glucose-based biological information. For the self-monitoring period, the acceptability rating of the CGM was comparable to that of the Fitbit wristband and was higher than that found in a previous observational study that used CGM in healthy individuals (31). One reason for this higher acceptability could be that the CGM model used in the present study did not require daily finger-stick calibration, as the older model did. During the recruitment phase, six individuals (8% of all those who expressed an interest in the study) declinded to participate in the study because they felt uncomfortable with the CGM device. As CGM technology advances and the idea of biosensors becomes more acceptable in society, we expect that the acceptability of CGM will only further improve. For example, the latest model of the Freestyle Libre no longer requires a separate reader to scan the sensor; users have the option of using their smartphone to retrieve the information.
Although the overall acceptability of using the CGM and Fitbit wearable devices was high, the rating for privacy was considerably lower than was that of all other acceptability survey items. Twenty-six percent (5/19) of the participants rated all items favorably (i.e., either agree or strongly agree) except for the privacy question for both CGM and Fitbit. To use the Fitbit application, all users are required to sign up for a Fitbit account. To ensure privacy, we created a study email account for each participant. No personal information was associated with this account. The CGM reader does not require users to input any personal information. However, if a smartphone application is used to replace the reader in future studies, a user account will be required. In that case, we recommended the use of a study email account instead of participants’ personal email accounts. Future studies should also further explore the participants’ privacy about wearable devices, such as whether they worry about privacy issues with the device manufacturers or with the research team, and whether they worry about their personal information being leaked or about the inappropriate use of the data collected by the devices. Identifying these issues will help the research team to better address them in future studies.
Results from this study show promising effects on participants’ exercise motivation, especially in helping them transition from the pre-contemplation stage of behavioral change (i.e., not intending to make changes) to the action stage (i.e., actively engaging in the new behavior). In the self-determination continuum, there was a trend towards decreasing amotivation and increasing intrinsic motivation to exercise. Research on exercise motivation shows the importance of autonomous (identified and intrinsic) regulation in promoting physical activity; the identified regulation is predictive of initial or short-term adoption of exercise, and the intrinsic motivation is predictive of long-term exercise adherence (36). Future intervention studies could find ways to package the information from CGM (i.e., the immediate impact of physical activity on glucose patterns) with other constructs of behavioral change strategies (e.g., building self-efficacy in exercising, overcoming perceived barriers in exercising) to develop a more comprehensive approach to adopting and maintaining an active lifestyle.
In the current pilot study, we tested the CGM-featured education session and self-monitoring period in a non-clinical setting. Moving forward, this type of intervention has high potential for delivery in clinical settings. For example, future studies could test the feasibility of implementing the intervention in primary care clinics, targeting the prediabetic population – a group that might be particularly motivated to change their behavior on the basis of their glucose information. Most (74%) of the participants in the current study thought that the 10-day monitoring period was about the right duration for them to learn the acute benefits of physical activity, and 79% of the participants expressed the desire to repeat wearing the devices (in 6–12 months) to determine whether their glucose patterns had changed. Therefore, finding ways to incorporate the intermittent use of CGM along with other wearable devices such as Fitbit into regular clinical encounters (e.g., annual physical examinations) will enable participants to recognize the implications of their behavior changes for long-term health outcomes.
The current study has several limitations, given its pilot structure. First, since it was a one-group study design, it is unclear how many of the positive changes in exercise motivation were attributable to the CGM-related component. Second, given the short duration of the study, it is unclear how long the increase in motivation will last or whether it will lead to actual changes in behavior. Third, most of the participants were female. It is unclear whether the results would be generalizable to males. Nevertheless, our pilot study included a diverse sample in terms of race/ethnicity (79% from a racial minority group), education level (53% without a bachelor’s degree), and weight status (42% at moderate-to-high risk obesity; BMI ≥ 30). Our data provide preliminary evidence that CGM could be a viable tool in different subgroups and has the potentials to be integrated into tailored interventions targeting some of these groups. Further, although we did not collect information about participants’ family history of diabetes, several participants mentioned that their immediate family members had diabetes during the exit interview. Future CGM-featured studies could consider targeting this specific group as they might be more motivated by glucose-related information.
In summary, the current study demonstrated a novel strategy to motivate sedentary overweight and obese adults to participate in physical activity using a biological sensor – CGM. CGM represents one type of wearable sensors that frequently assesses a biomarker, which is the foundation for providing just-in-time feedback (37). Since a sedentary lifestyle and excess weight are putting an increasing number of individuals at greater risk for various types of cancer, it is imperative to find innovative solutions and develop scalable strategies to promote regular physical activity in the overweight and obese population and thus reduce the overall burden of cancer.
Supplementary Material
Acknowledgments
The authors would like to acknowledge additional supports for this project from the Assessment, Intervention and Measurement (AIM) Shared Resource and the Center for Energy Balance in Cancer Prevention and Survivorship at the University of Texas MD Anderson Cancer Center. The authors would also like to thank the Department of Scientific Publications at MD Anderson for editing this manuscript.
Financial support: Y. Liao was supported by a faculty fellowship from the Duncan Family Institute for Cancer Prevention and Risk Assessment at The University of Texas MD Anderson Cancer Center. K. M. Basen-Engquist and S. M. Schembre was supported by the National Cancer Institute of the National Institutes of Health under award number R21CA215415. This research was also supported by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (NCI Grant P30 CA016672). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 2016;176(6):816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zenko Z, Willis EA, White DA. Proportion of adults meeting the 2018 physical activity guidelines for Americans according to accelerometers. Front Public Health 2019;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young DR, Jerome GJ, Chen C, Laferriere D, Vollmer WM. Patterns of physical activity among overweight and obese adults. Prev Chronic Dis 2009;6(3):A90. [PMC free article] [PubMed] [Google Scholar]
- 4.Spees CK, Scott JM, Taylor CA. (2012). Differences in amounts and types of physical activity by obesity status in US adults. Am J Health Behav 2012;36(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med 2014;127(8):717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 2011;13(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obesity 2013;2013:291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity 2008;16(10):2323–30. [DOI] [PubMed] [Google Scholar]
- 9.Olander EK, Fletcher H, Williams S, Atkinson L, Turner A, French DP. What are the most effective techniques in changing obese individuals’ physical activity self-efficacy and behaviour: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 2013;10(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter SJ, Sheats JL, King AC. The use of behavior change techniques and theory in technologies for cardiovascular disease prevention and treatment in adults: a comprehensive review. Prog Cardiovasc Dis 2016;58(6):605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology 2008;27(3):379–87. [DOI] [PubMed] [Google Scholar]
- 12.Kluger AN. DeNisi A. The effects of feedback interventions on performance: A historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychol Bull 1996;119(2):254–84. [Google Scholar]
- 13.Kramer JN. Kowatsch T. Using feedback to promote physical activity: The role of the feedback sign. J Med Internet Res 2017;19(6):e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deci EL, Ryan RM. The” what” and” why” of goal pursuits: Human needs and the self-determination of behavior. Psychol Inquiry 2000;11(4):227–68. [Google Scholar]
- 15.Hartmann C, Dohle S, Siegrist M. A self-determination theory approach to adults’ healthy body weight motivation: A longitudinal study focusing on food choices and recreational physical activity. Psychol Health 2015;30(8):924–48. [DOI] [PubMed] [Google Scholar]
- 16.Bauman AE, Sallis JF, Dzewaltowski DA, Owen N. Toward a better understanding of the influences on physical activity: the role of determinants, correlates, causal variables, mediators, moderators, and confounders. Am J Prev Med 2002;23(2):5–14. [DOI] [PubMed] [Google Scholar]
- 17.Plotnikoff RC, Costigan SA, Karunamuni N, Lubans DR. (2013). Social cognitive theories used to explain physical activity behavior in adolescents: a systematic review and meta-analysis. Prev Med 2013;56(5):245–53. [DOI] [PubMed] [Google Scholar]
- 18.Biddle SJ, Mutrie N. Psychology of physical activity: Determinants, well-being and interventions. Routledge;2007. [Google Scholar]
- 19.Orji R, Vassileva J, Mandryk R. Towards an effective health interventions design: An extension of the health belief model. Online J Public Health Inform 2012;4(3):ojphi.v4i3.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure JB. Are biomarkers useful treatment aids for promoting health behavior change?: An empirical review. Am J Prev Med 2002;22(3):200–7. [DOI] [PubMed] [Google Scholar]
- 21.Corrie SR, Coffey JW, Islam J, Markey KA, Kendall MAF. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015;140(13):4350–64. [DOI] [PubMed] [Google Scholar]
- 22.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaishi T, Imaeda K, Tanaka T, Moritani T, Hayashi T. A short bout of stair climbing–descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Appl Physiol Nutr Metabo 2011;37(1):193–6. [DOI] [PubMed] [Google Scholar]
- 24.Manohar C, Levine JA, Nandy DK, Saad A, Dalla Man C, McCrady-Spitzer SK, et al. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care 2012;35(12):2493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little JP, Jung ME, Wright AE, Wright W, Manders RJ. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Physiol Nutr Metabo 2014;39(7):835–41. [DOI] [PubMed] [Google Scholar]
- 26.DiPietro L, Gribok A, Stevens MS, Hamm LF, Rumpler W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013;36(10):3262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crespo NC, Mullane SL, Zeigler ZS, Buman MP, Gaesser GA. Effects of standing and light-intensity walking and cycling on 24-h glucose. Med Sci Sports Exerc 2016;48(12):2503–11. [DOI] [PubMed] [Google Scholar]
- 28.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2008;80(3):371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen N, Whittemore R, Melkus G. A continuous glucose monitoring and problem-solving intervention to change physical activity behavior in women with type 2 diabetes: a pilot study. Diabetes Technol Ther 2011;13(11):1091–9. [DOI] [PubMed] [Google Scholar]
- 30.Kingsnorth AP, Whelan ME, Sanders JP, Sherar LB, Esliger DW. Using digital health technologies to understand the association between movement behaviors and interstitial glucose: exploratory analysis. JMIR mHealth uHealth 2018;6(5):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Schembre SM. Acceptability of continuous glucose monitoring in free-living healthy individuals: Implications for the use of wearable biosensors in diet and physical activity research. JMIR mHealth uHealth, 2018;6(10):e11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Y, Goldberg D, Dahlke DV, Ory MG, Cargill JS, Coughlin R, et al. Testing usability and acceptability of a web application to promote physical activity (iCanFit) among older adults. JMIR Hum Factors 2014;1(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson B, Yingling L, Bednarchuk A, Janamatti A, Oakley-Girvan I, Allen N. An interactive simulation to change outcome expectancies and intentions in adults with type 2 diabetes: within-subjects experiment. JMIR Diabetes 2018;3(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markland D, Tobin VA modification to the behavioural regulation in exercise questionnaire to include an assessment of amotivation. J Sport Exerc Psychol 2004;26(2):191–6. [Google Scholar]
- 35.Lerdal A, Moe B, Digre E, Harding T, Kristensen F, Grov EK, et al. Stages of Change–Continuous Measure (URICA‐E2): psychometrics of a Norwegian version. J Adv Nurs 2009;65(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act 2012;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schembre SM, Liao Y, Robertson MC, Dunton GF, Kerr J, Haffey ME, et al. Just-in-time feedback in diet and physical activity interventions: systematic review and practical design framework. J Med Internet Res 2018;20(3):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.