Significance
Apidaecin (Api) is a ribosome-targeting proline-rich antimicrobial peptide with a unique mechanism of action. Api’s activity against Gram-negative pathogens makes it an attractive candidate for developing new antibiotics. The approaches based on analyzing the antibacterial activity of chemically synthesized peptides fail to distinguish the effects upon cellular uptake from those affecting the on-target activity. By expressing a comprehensive library of api gene mutants in the bacterial cell and analyzing the library composition by next-generation sequencing, we identified in a single experiment the on-target activity of every single-amino-acid mutant. Location of the inactivating mutations defined the peptide’s pharmacophore, which is confined to five C-terminal residues. Identification of mutations preserving activity delineated the sequence space available for modifying the peptide’s antibiotic properties.
Keywords: ribosome, antibiotic, nascent peptide exit tunnel, translation termination, apidaecin
Abstract
Apidaecin (Api), an unmodified 18-amino-acid-long proline-rich antibacterial peptide produced by bees, has been recently described as a specific inhibitor of translation termination. It invades the nascent peptide exit tunnel of the postrelease ribosome and traps the release factors preventing their recycling. Api binds in the exit tunnel in an extended conformation that matches the placement of a nascent polypeptide and establishes multiple contacts with ribosomal RNA (rRNA) and ribosomal proteins. Which of these interactions are critical for Api’s activity is unknown. We addressed this problem by analyzing the activity of all possible single-amino-acid substitutions of the Api variants synthesized in the bacterial cell. By conditionally expressing the engineered api gene, we generated Api directly in the bacterial cytosol, thereby bypassing the need for importing the peptide from the medium. The endogenously expressed Api, as well as its N-terminally truncated mutants, retained the antibacterial properties and the mechanism of action of the native peptide. Taking advantage of the Api expression system and next-generation sequencing, we mapped in one experiment all the single-amino-acid substitutions that preserve or alleviate the on-target activity of the Api mutants. Analysis of the inactivating mutations made it possible to define the pharmacophore of Api involved in critical interactions with the ribosome, transfer RNA (tRNA), and release factors. We also identified the Api segment that tolerates a variety of amino acid substitutions; alterations in this segment could be used to improve the pharmacological properties of the antibacterial peptide.
The ribosome, which is responsible for the synthesis of all cellular proteins, is one of the main antibiotic targets in bacteria. The known antibiotics affect various steps of protein synthesis but the first specific inhibitor of translation termination, the antibacterial peptide apidaecin (Api) produced by honey bees, was only recently discovered (1).
Api belongs to a diverse group of proline-rich antimicrobial peptides (PrAMPs) (2, 3). It is imported into the bacterial cell by the action of the inner-membrane peptide transporter SbmA (4, 5). Once in the cytosol, Api inhibits protein synthesis by binding in the nascent peptide exit tunnel (NPET) of ribosomes that have reached the stop codons of the protein-coding sequences and are associated with the class 1 release factors 1 (RF1) or 2 (RF2) (1, 6, 7) (Fig. 1A). Because the nascent protein sterically hinders PrAMP binding, the ribosome becomes susceptible to Api only after peptidyl-tRNA (peptidyl-transfer RNA) hydrolysis has taken place and the newly made protein has been released from the ribosome. Api binds in the now-vacant NPET in the orientation that matches that of the growing protein chain with the N terminus extending down the tunnel and the C terminus approaching the peptidyl transferase center where it interacts with the A-site–bound RF1/2 and deacyl-tRNA located in the P site. As a result, Api traps the RF on the ribosome in the postrelease state (1, 8, 9) and locks the ribosome at the stop codon. Trapping of RFs triggers several downstream effects that contribute to inhibition of protein synthesis and growth arrest, including stop codon bypass and piling up of Api-free elongating ribosomes behind those stalled at the stop codons (10).
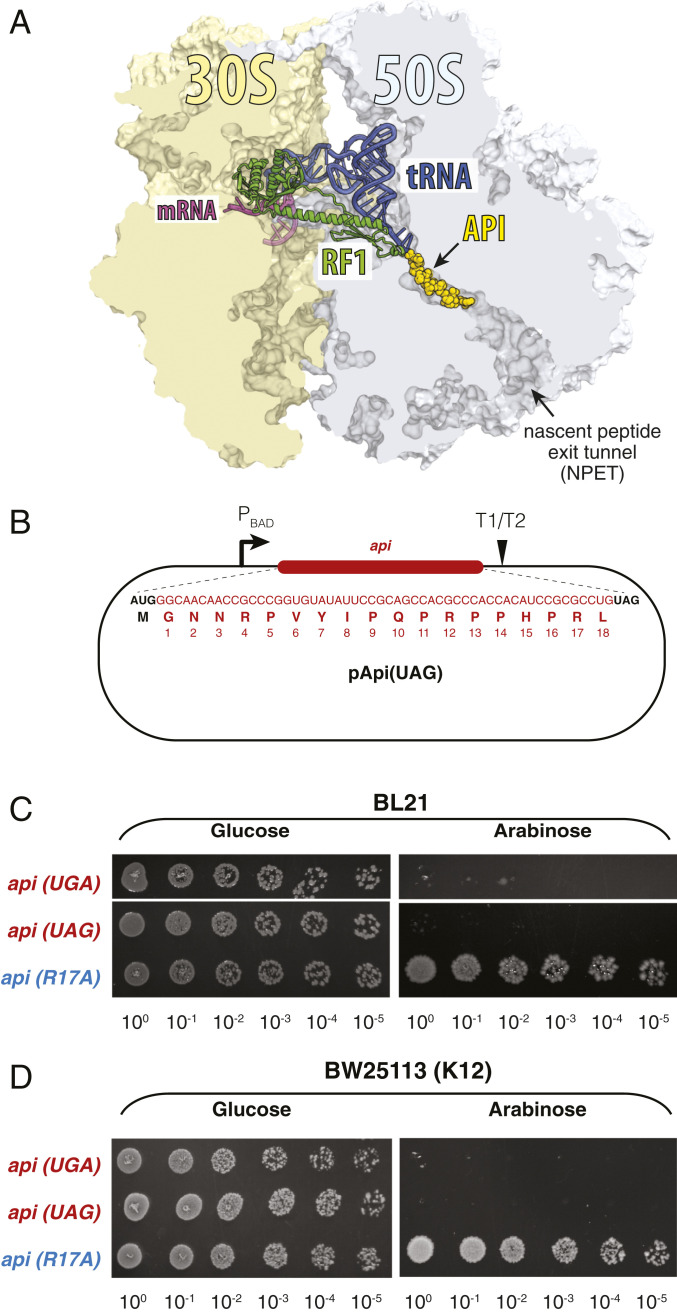
Fig. 1.
Expression of the api gene is toxic for the cell. (A) Placement of Api in the exit tunnel of the bacterial ribosome (Protein Data Bank ID 5O2R) (1). The P-site tRNA and A-site–bound RF1 are shown. (B) Schematic of the plasmid pApi(UAG) for inducible expression of the Api1b peptide in E. coli. The same construct, but equipped with the UGA stop codon, is present in pApi(UGA) (not shown). (C and D) The effect of Api expression in bacterial cells. Spot test of BL21 (C) and BW25113 (D) strains carrying pApi(UAG) or pApi(UGA) plasmids. Cells expressing an inactive Api mutant (R17A) were used as a control.
Native apidaecins are synthesized by the insect as a prepropeptide, which is then secreted and processed to yield biologically active 18-amino-acid-long unmodified peptides GNNRPVYIPQPRPPHPRI/L (Ile18 in Api1a or Leu18 in Api1b) with attractive antibiotic properties (2, 3). Extensive studies of synthetic PrAMPs have shown that the pharmacological characteristics of Api can be further improved through amino acid substitutions or chemical modifications (11–16). One of the synthetic derivatives, Api137 (Gu-ONNRPVYIPRPRPPHPRL, where Gu is N,N,N′,N′-tethramethylguanidino and O is ornithine), which differs from the native Api1b at only two residues, exhibits notably improved antibacterial properties and serum stability (12), demonstrating that even minor modifications of the native Api could yield superior antibiotics. Yet, the direct effort for synthetically improving Api is intrinsically limited due to the difficulty of generating a sufficiently large number of derivatives as well as scalability problems.
A great variety of peptide antibiotics, including those that target the ribosome, are generated by nonribosomal peptide-synthetases or as ribosomally synthesized and posttranslationally modified peptides and contain multiple noncanonical amino acids, nonpeptide bond linkages, and/or elaborate chemical modifications (17–19). The intricate chemical features of these compounds make it difficult to manipulate the cell for producing new variants of the natural molecule with the goal of improving their properties and activity. In contrast, the native Api, as well as several other PrAMPs, are composed only of the canonical amino acids and can be produced in the cell directly by translation of the corresponding messenger RNA (mRNA). This “genetic nature” of Api presents unprecedented opportunities for generating the inhibitor directly in the target bacterial cell by translating the peptide-coding sequence in situ. Some pioneering efforts in this direction have already led to the synthesis of recombinant Api and a few other PrAMPs in engineered bacterial (20, 21) and yeast (22, 23) cells, and a few mutant PrAMPs with distinct properties have been identified (24–28). However, most of these attempts have been limited to only a few tested gene sequences and relied on the analysis of individual mutant genes. Furthermore, highly artificial constructs, where the Api sequence was appended to longer proteins, were usually employed because endogenously produced short peptides were deemed to be too unstable (17, 24).
The newly understood mode of Api action informs the improved design of endogenously expressed Api libraries. First, structural studies (1) suggest that the Api’s N terminus should tolerate modifications, including the addition of a formyl-methionine residue encoded in the AUG start codon required for the proper translation initiation of the api gene. Second, when the ribosome reaches the stop codon of the Api-coding open reading frame (ORF), the nascent Api is presumably positioned properly in the NPET to trap the associated RF1/2 molecule. Thus, in principle, Api could exert its inhibitory action even without leaving the NPET, where it remains impervious to cytoplasmic proteases, thereby alleviating the necessity of fusion designs. Finally, next-generation sequencing technologies afford analysis of a great variety of sequence variants in a single experiment.
Driven by these considerations, we exploited the genetic nature of Api to map the entire sequence space available for altering the PrAMP’s properties by introducing single-amino-acid substitutions. We show that in-vivo–expressed Api retains its inhibitory activity and interferes with cell growth via the same mechanism as the exogenously added PrAMP. Taking advantage of the expression system, we generated a comprehensive library of api genes encoding peptides with all possible single-amino-acid substitutions and assessed in a single experiment the ability of each of the variants to inhibit cell growth. Our study illuminates the advantages of using gene libraries for identifying biologically active peptides with ribosome-targeting activity.
Results
Expression of Unmodified Api1b Prevents Escherichia coli Growth.
Because previous efforts for expressing Api1b in bacteria involved its fusion to longer proteins or focused on minimizing its toxicity (24, 25, 27, 29), we first tested whether the native PrAMP generated endogenously in Escherichia coli cells would retain its antibacterial activity and mechanism of action. We engineered two plasmid constructs containing synthetic api genes, under the control of the arabinose-inducible PBAD promoter, starting with the initiator AUG codon, followed by the codon-optimized sequence specifying the 18 amino acids of Api1b and ending with either UAG (pApi-UAG) or UGA (pApi-UGA) stop codons, decoded by RF1 or RF2, respectively (Fig. 1B). E. coli cells (strain BL21) transformed with these plasmids readily formed colonies on agar plates supplemented with glucose, which represses transcription from the PBAD promoter, but were unable to grow on plates supplemented with the inducer arabinose (Fig. 1C). In contrast, a similar construct expressing the inactive mutant Api1b(R17A) (here and throughout we use the numbering corresponding to the residues of native Api1b), whose ability to trap RFs is likely impaired, was not deleterious (Fig. 1C).
Unlike the B-type strains, such as BL21, the E. coli K12 strains carry the amino-acid substitution A246T in RF2 (30, 31), which renders them more tolerant to Api action (1). Nevertheless, endogenously expressed Api was also toxic for the K12 strain BW25113 cells, although a higher concentration of arabinose was required to prevent cell growth (Fig. 1D). Altogether, these results show that, when expressed endogenously in E. coli, Api1b functions as an antimicrobial peptide, highly deleterious for cell growth.
Endogenously Expressed Api Inhibits Cell Growth by Targeting the Ribosome and Interfering with Translation Termination.
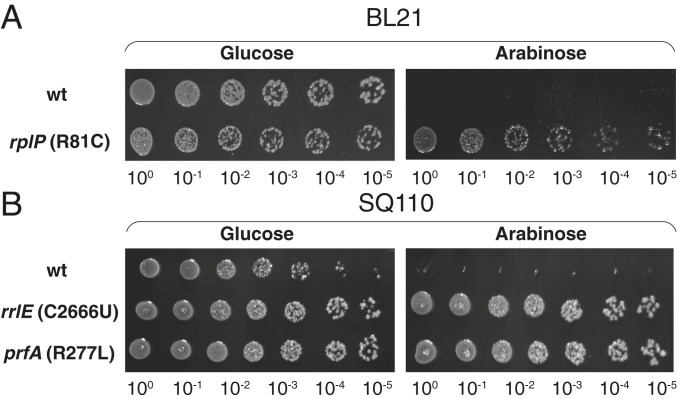
Because expression of a protein could be deleterious for a variety of reasons (32), we asked whether the endogenously produced Api interferes with ribosomal functions. We reasoned that if intracellularly expressed Api acts upon the terminating ribosome, its toxicity should be reduced by the same mutations that confer resistance to the exogenously added PrAMP. We isolated spontaneous Apir mutants by plating E. coli cells on Api137-containing agar plates. Two of the mutants were selected using the K12 strain SQ110 (33), well suited for isolating ribosomal RNA (rRNA) mutations because it carries a single rrn allele (34). One mutant indeed carried the C2666U substitution in the sarcin-ricin hairpin (H95) of 23S rRNA. H95 is critical for GTP hydrolysis by translation GTPases, including RF3 which promotes RF1 and RF2 recycling (9). It is possible that the isolated C2666U mutation, which was shown previously to reduce the accuracy of translation (35), decreases the residence time of RF1 and RF2 on the ribosome by accelerating RF3-catalyzed GTP hydrolysis. The other Apir mutant of the SQ110 strain had the G830T mutation in the prfA gene which resulted in the R277L substitution in the encoded RF1 protein. Similar to the previously characterized RF2 mutations (1), the new Apir mutation may facilitate the dissociation of RF1 from the ribosome-Api complex. Another isolated Apir mutant of the B-type BL21 strain carried a single-nucleotide alteration (G737A) in the prfB gene (SI Appendix, Tables S1 and S2), which resulted in the A246T amino-acid substitution in the encoded RF2, the same polymorphism that distinguishes the RF2 of the E. coli K12 strains from that of the B strains (36) and facilitates the factor turnover (1).
We transformed these newly isolated mutants, as well as several of the previously identified Apir strains (1) (SI Appendix, Table S1), with the pApi-UAG and pApi-UGA plasmids and tested their tolerance to the toxic effect of the endogenously expressed Api. Api-expressing cells with alterations in the 23S rRNA, the ribosomal protein uL16, or RF1/RF2 were able to form colonies at the inducer concentrations that abolished colony formation of the parental strains (Fig. 2 A and B and SI Appendix, Fig. S2), demonstrating that, similar to the exogenously added Api137, the endogenously expressed Api likely targets the ribosome and interferes with translation termination.
Fig. 2.
Apir mutants tolerate the endogenous expression of Api. (A and B) Spot test for WT or Apir mutant E. coli cells transformed with the pApi(UAG) plasmid. BL21 Apir cells contained a mutant (R81C) uL16 ribosomal protein encoded in the rplP gene (1) (A); SQ110 Apir mutants contained the C2666U mutation in the 23S rRNA gene rrlE or the R277L mutation in RF1 encoded in the prfA gene (B).
The In Vivo–Expressed N-Terminally Truncated Api Retains Its Antibacterial Activity.
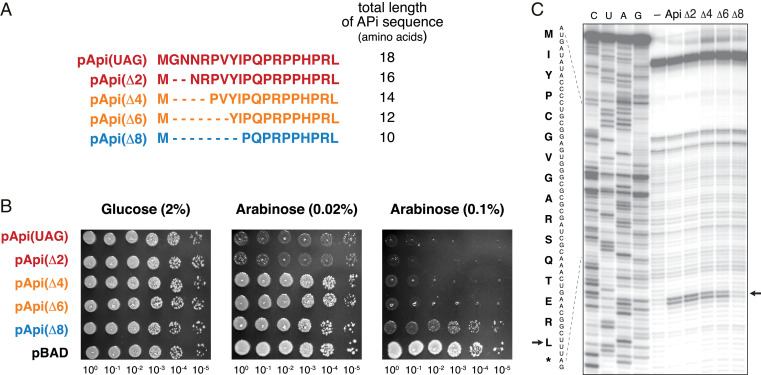
Phylogenetic, structural, and microbiological data have suggested that the N-terminal residues of Api are less critical for its activity than those of the C-terminal segment (1, 37–39). Hence, we asked whether endogenously expressed Api could be N-terminally truncated without losing its ability to inhibit cell growth. Accordingly, we truncated the api gene by deleting two, four, six, or eight codons of the coding sequence (Fig. 3A). The resulting constructs were expressed in the E. coli BL21 strain and cells were spotted on plates with varying concentrations of arabinose (Fig. 3B). Regardless of the inducer concentration, expression of Api lacking the first two amino acids was as toxic as the full-size version. Deletion of four or six amino acids slightly reduced the inhibitory activity of the PrAMP, allowing colony formation at 0.02% arabinose but preventing growth at a higher arabinose concentration (0.1%). Even though the deletion of eight amino acids caused a significant drop in the Api activity, the expression of this truncated peptide, encompassing only the 10 C-terminal amino acids of the native PrAMP, still caused some growth defects at a higher arabinose concentration (Fig. 3B).
Fig. 3.
Api variants with N-terminal truncations preserve their on-target activity. (A) The sequences of the truncated Api derivatives encoded in pApi(UAG) plasmids. The sequences are colored according to the toxicity level of the variant (as determined by the experiment shown in B): red: full activity; orange: moderate activity; blue: residual toxicity. (B) Growth of cells expressing truncated Api variants under no induction (2% glucose), mild induction (0.02% arabinose), or strong induction (0.1% arabinose) conditions. Cells with an empty pBAD vector were used as a control. (C) Toeprinting analysis of the ribosome arrest induced by the synthetic Api1b peptide (lane marked “Api”) or its N-terminally truncated derivatives (Δn); the lane marked "-" contains the control sample to which no inhibitor was added. The model ORF is derived from the E. coli gene yrbA (1). The sequences of the synthetic peptides are identical to those shown in A, except they lack the N-terminal methionine. The toeprint band corresponding to the ribosomes arrested with the stop codon in the A site is shown by a black arrow. The nucleotide sequence of the gene and the amino-acid sequence of the encoded protein are shown.
To determine whether the growth inhibition by the shortened Api peptides is mediated by their action upon the ribosome, we chemically synthesized the peptides and tested them in a cell-free translation system. As revealed by toeprinting analysis, which reports the position of arrested ribosomes on mRNA (40), the peptides with the deletion of two, four, and six N-terminal amino acids efficiently stalled the ribosome at the stop codon of a model ORF (Fig. 3C). In good agreement with the in vivo data, the truncated peptides containing less than 12 of the C-terminal amino acids of the original Api were notably less prone to arrest translation. The combination of in vivo and in vitro results show that several of the N-terminal amino acids of Api are dispensable for its on-target activity and, while the rest of the N-terminal segment does modulate to some extent the PrAMP’s activity, its main pharmacophore is confined to the 12 C-terminal residues.
Mapping the Sequence Space of Single-Point Mutations That Preserve Api Activity.
A number of synthetic Api variants have been previously chemically synthesized and tested in microbiological assays (11, 12, 14, 41). Some of the derivatives showed improved activity against specific bacterial strains whereas the activity of others was dramatically reduced, providing initial insights into the amino acid positions that are amenable to alterations. However, the MIC-based assays commonly used in those experiments are unable to distinguish whether the alteration affects the on-target activity of Api or its import into the bacterial cell. In contrast, expressing functionally active Api within the cell bypasses the import hurdle, making it possible to directly address the PrAMP’s on-target activity.
In order to assess the inhibitory activity of every single-amino-acid Api mutant, we prepared a comprehensive library of api genes with single-codon substitutions, introduced it into E. coli, and tested the antibacterial activity of the endogenously expressed Api variants. The gene library was prepared on the basis of the pApiΔ2-UAG plasmid encoding the Api peptide truncated by two N-terminal amino acids, whose in vivo activity was indistinguishable from that of the full-size PrAMP (Fig. 3). Each plasmid in the library carried an apiΔ2 gene wherein every single codon (except the initiation codon) was replaced with NNK sequences that can encode either 1 of the 20 amino acids or a UAG stop codon. Approximately 50,000 transformants were plated onto agar plates supplemented with either glucose or arabinose. While all library clones should be able to form colonies on the noninducing (glucose) plate, only the clones that express inactive Api variants would grow on the inducing (arabinose) plate. Deep sequencing of the api genes in the pooled clones washed off the glucose or arabinose plates, and computing the difference in the abundance of individual variants allowed for cataloging of the amino-acid substitutions that either preserve or abolish the ability of Api to inhibit cell growth (Fig. 4). The results of the api gene library screening in the BL21 and BW25513 strains, despite containing different RF2 variants, were highly convergent (Fig. 4 A and B), indicating that the toxic Api mutants identified in a more sensitive B strain retain their activity in a more Api-tolerant K12 strain.
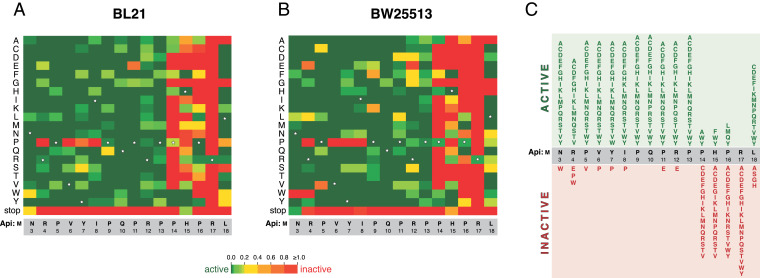
Fig. 4.
The effects of single-amino-acid substitutions on the activity of endogenously expressed Api(Δ2) in (A) BL21 or (B) BW25113 E. coli strains. The activity plots were computed as the ratio of the fraction of each mutant in the library of clones formed on inducing (arabinose) or noninducing (glucose) plates. The green rectangles indicate the mutations that preserve the activity of the PrAMP; the red rectangles show the inactivating mutations. For the intermediate effects, refer to the heatmap scale. Rectangles corresponding to the amino acid residues of the WT Api are indicated by stars. (C) Summary diagram for the effects of the mutations. A substitution was considered to render an active variant (green) when it caused reduction of the occurrence of the mutant clone on the arabinose plate by at least two-fold in at least one of the two strains tested.
The distribution of the mutations that preserved Api’s activity showed a striking bias: the majority of substitutions within the 11 N-terminal amino acids (N3 to P13) yielded Api variants which readily inhibited cell growth (Fig. 4 A–C). Only a few mutations within the N-terminal segment perturbed Api’s activity, with the most notable changes being the replacement of amino acids at positions 4, 6, 7, and 8 with a proline residue (Fig. 4 A and B).
The tolerance of Api to amino acid substitutions within the N-terminal segment is contrasted by the clustering of inactivating mutations within C-terminal residues. Only a few mutations of P14, H15, and P16 preserved Api activity, favoring substitutions with aromatic amino acids at positions 14 and 15. All substitutions of R17 inactivated the PrAMP, underscoring the critical role of this residue in interactions with RFs (1) (Fig. 1). The C-terminal L18 residue was more tolerant of mutations (Fig. 4 A–C), and the majority of substitutions preserved the activity of the endogenously expressed Api derivatives.
The importance of Api’s C-terminal segment for biological activity that follows from the analysis of our comprehensive mutant gene library is consistent with the results of previous studies that showed that specific mutational or chemical alterations of this region of Api yielded less-active variants (14, 39).
Endogenously Active Api Variants Inhibit Translation Termination.
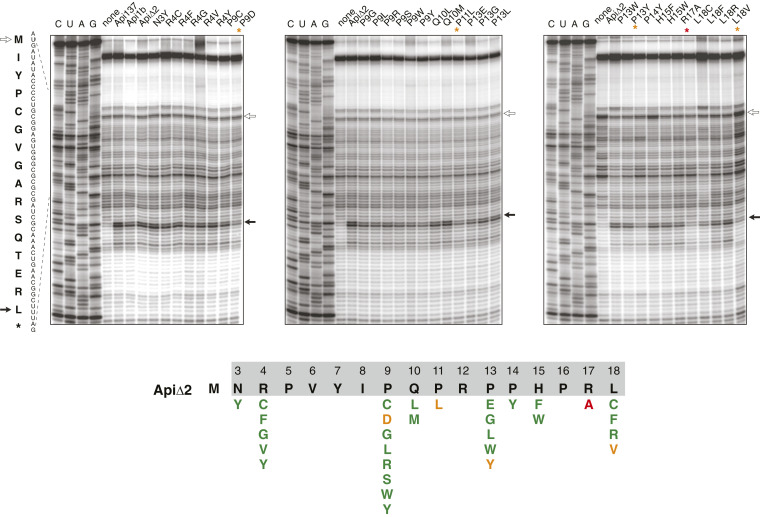
To determine whether the cellularly synthesized toxic Api mutants identified by our screening prevent bacterial growth by interfering with translation termination, we chemically synthesized 29 of them and tested their effect upon cell-free translation by toeprinting (Fig. 5). Synthetic native Api1b, its truncated ApiΔ2 variant, and Api137 readily inhibited translation termination by arresting ribosomes at the stop codon of a model ORF. In contrast, the negative control peptide ApiΔ2(R17A) was unable to cause termination arrest. Four of the selected peptides (with the P9D, P11L P13Y, and L18V substitutions) also showed somewhat reduced activity compared to the control (Fig. 5). However, the rest of the selected synthetic Api variants arrested the ribosome at the stop codon as efficiently as the unmodified ApiΔ2 at the tested concentration (50 µM). This result demonstrates that the majority of the identified Api mutants with prominent antibacterial activity retain their ability to inhibit translation termination.
Fig. 5.
Toeprinting analysis of the effect of synthetic derivatives of Api(Δ2) on ribosome arrest at the stop codon of a model ORF. Api137, Api1b, and Api(Δ2) were used as positive controls. The R17A mutant was used as a negative control (lanes marked with a red asterisk). The toeprint bands corresponding to ribosomes at the initiation and stop codons are indicated by open and solid arrows, respectively. The lanes with the samples where translation termination arrest was significantly reduced compared to the controls, as judged by the low intensity of the toeprint band of the ribosomes stalled at the stop codon, are indicated by orange asterisks. The gels represent one of two independent experiments which produced highly convergent results. The results are summarized on the diagram below the gels where mutations that preserved the activity in the cell-free translation system are shown in green and those that reduced the activity are shown in orange or red.
Cellular Uptake Affects the Antibacterial Action of Functionally Active Api Variants.
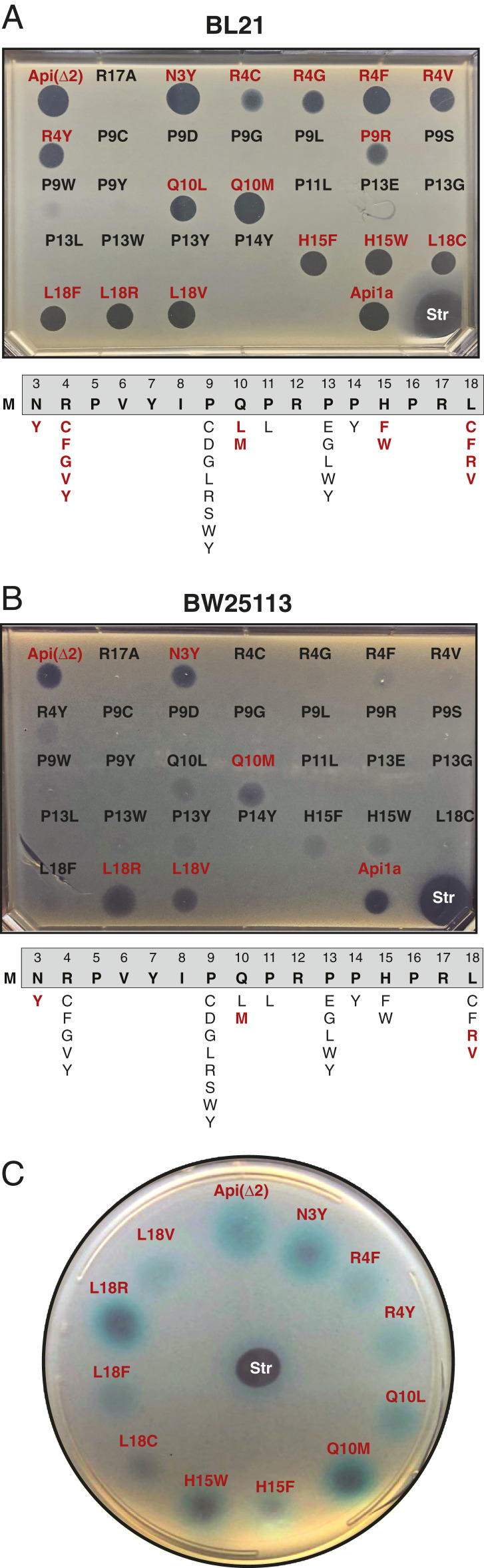
We proceeded to determine the ability of the synthetic Api variants to inhibit cell growth using the drop diffusion assay. A zone of no growth around the PrAMP drop placed on the lawn of the tester cells revealed the antibacterial activity of a subset of the peptides (Fig. 6A). Because the agar-diffusion properties of the Api mutants are likely affected by their structures, we disregarded the diameter of the clearing zone and classified the tested peptides into two major categories: those which produced a well-defined zone of inhibition and those which failed to do so. Of the 29 synthetic mutant peptides, most of which readily arrested the ribosome at the stop codon in the cell-free reaction (Fig. 5), 15 exhibited antibacterial properties, whereas 14 were unable to inhibit growth of the tester E. coli BL21 strain. It is noteworthy that most of the alterations of the proline residues (P9, P11, P13, and P14) prevented the peptides from inhibiting growth, whereas only some of these mutations affected the on-target activity (Figs. 5 and 6), indicating that these residues may play important roles in both the uptake of Api and its on-target activity. As expected, the K12-type strain was notably more resistant to the synthetic Api derivatives (Fig. 6B). The peptides active against the BW25113 strain represented a subset of those inhibitory to the BL21 cells with several peptides inhibiting growth exclusively of the BL21 strain (e.g., all the R4 substitutions and the L18C/L18F mutants). This result indicates that, by introducing variations in the Api sequence, it is possible to modulate its activity in a strain-specific manner.
Fig. 6.
Antimicrobial activity of Api and its mutants determined by the drop-diffusion assay. (A and B) Activity of the synthetic Api(Δ2) derivatives was determined by the clearing zone around the drop of 2 µL of a 2-mM peptide solution placed onto a lawn of BL21 (A) or BW25113 (B) E. coli cells. The mutants exhibiting inhibitory activity are indicated in red. (C) The Api variants with antimicrobial activity cause stop codon readthrough. The peptides able to inhibit cell growth (as determined in A and B) were tested on the reporter SQ171 E. coli strain whose mutant lacZ gene contains an inactivating premature stop codon (1). The blue halos on the cells plated in the presence of IPTG (isopropyl β-d-1-thiogalactopyranoside) (to induce the expression of lacZ) and X-Gal (to detect β-galactosidase activity) indicate readthrough of the premature stop codon induced by the Api derivatives. The antibiotic streptomycin (Str) was used as a positive control of stop-codon readthrough activity.
To further verify that exogenously added synthetic Api variants can sequester the RFs in the cell, we used our previously developed stop codon readthrough assay (1) based on the use of K12-type E. coli cells (strain SQ171) expressing the lacZ gene that carries the single-nucleotide C2035T mutation that generates a premature stop codon UAG (Fig. 6C). In these cells, synthesis of the functional β-galactosidase depends on Api-mediated depletion of the available RF1, leading to the premature stop codon bypass. When the Api variant peptides capable of inhibiting cell growth were spotted on the lawn of reporter cells on the indicator plate, a blue halo of β-galactosidase–expressing cells appeared around the peptide spots indicating that the Api mutants act by sequestering RFs.
Discussion
In this study we explored the activity of Api endogenously expressed in E. coli cells and defined the sequence space available for modifying and possibly expanding the antibiotic properties of this PrAMP. We demonstrated that endogenously produced Api not only retains its antibacterial activity, but also that, similar to the native peptide, inhibits bacterial growth by interfering with translation termination. Therefore, we screened a comprehensive library of mutant genes and, in a sole experiment, mapped all the active and inactive Api variants carrying single-amino-acid substitutions. Expressing mutant peptides directly in the cell made it possible to bypass the uptake issues and distill the effect of mutations specifically upon the ability of the PrAMPs to inhibit translation.
The N-terminal truncations, as well as mutations of the amino acids belonging to the N-terminal segment, preserve the general inhibitory activity of the endogenously expressed peptides (Figs. 3 and 4). However, the N-terminal segment may modulate the activity of Api because several substitutions, in particular those which introduce additional proline residues at positions 4 to 8, reduce the toxicity of the endogenously expressed variants (Fig. 4). These mutations could perturb the proper positioning of the Api mutants within its binding site in the NPET, affect the diffusion of the peptides through the tunnel, or possibly affect translation of the api gene. Nevertheless, the malleability of Api’s N-terminal segment opens the possibility to modify this region to alter the properties of Api, such as its cellular uptake, stability, or spectrum of action.
Our comprehensive mutational data strongly argue that the pharmacophore of Api is confined to its five C-terminal amino acids where most of the mutations abolish the ability of the endogenously expressed PrAMP to inhibit cell growth. While significantly expanding the previous observations that alterations of the C-terminal residues often inactivate the peptide (14, 25, 26, 39), our findings reveal that the reason for the activity loss is the inability of the Api mutants to functionally interact with the target. The critical importance of Api’s five C-terminal residues revealed by our findings rationalizes the observations obtained in previous structural studies (1, 42). Every mutation of the penultimate R17 residue, which directly interacts with the ribosome-bound RF (Fig. 4 and SI Appendix, Fig. S1), abolishes the activity of Api. This observation corroborates the importance of the interaction between the arginine side chain and the critical and conserved glutamine residue of the RF (SI Appendix, Fig. S1). The proper placement and orientation of R17 likely relies on the specific contacts of the preceding three amino acids (P14, H15, and P16) with 23S rRNA nucleotides that form the peptidyl transferase-proximal segment of the NPET (SI Appendix, Fig. S1). The proline residues of this sequence form van der Waals interactions with 23S rRNA residues: P16 with U2506 and P14 with A2059 and A2503; mutations of the latter two rRNA nucleotides confer resistance to Api137 (1), as well as to the endogenously expressed Api (SI Appendix, Table S3). The interactions of P14 with the ribosome apparently can be mimicked by the aromatic amino acids because substitution of this proline with tryptophan, tyrosine, and, to a lesser extent, phenylalanine sustain the PrAMP’s activity (Fig. 4). The side chain of H15 stacks upon the A2505 base (SI Appendix, Fig. S1). This interaction is apparently important for the binding and action of Api because replacing H15 with stacking-prone aromatic amino acids (F, W, Y) preserves the activity of the mutants (Fig. 4 A–C). The identity of Api’s C-terminal residue seems less critical (Fig. 4 A–C): most of the substitutions here are tolerated except for amino acids with the short side chain (A, S, G). This result is compatible with the structural data because it is the carboxyl group of the main chain rather than the side chain of the C-terminal residue of Api that interacts with the 3′ ribose hydroxyl of the P-site–bound deacylated tRNA (1, 42) (SI Appendix, Fig. S1). Taken together, our data argue that the pharmacophore of Api is P/Φ-H/Φ-P-R-X, where Φ is an aromatic amino acid and X is almost any amino acid except those with the short side chain.
Our library screening findings show the vastness of the sequence space available for modulating Api’s activity because an unexpectedly large number of functionally active Api derivatives were identified among the single-amino-acid mutants. Furthermore, allowing multiple mutations in the api gene would likely dramatically increase the assortment of active mutants. Conceivably, some of the Api variants could show improved activity against the ribosomes of distinct bacterial species, may serve as better substrates for the uptake transporters of specific pathogens, or may even bypass the necessity of the transporter altogether, thereby evading one of the main mechanisms of resistance to PrAMPs (13, 14).
Materials and Methods
Cloning the Api Gene into pBAD18 Vector.
The Apis mellifera DNA sequence coding for the mature active unit of Apidaecin1b (GNNRPVYIPQPRPPHPRL) was codon-optimized for expression in E. coli using the Vectorbuilder platform (SI Appendix, Fig. S3A). The Api-encoding sequence, along with the added start and stop codons, a Shine–Dalgarno sequence, and flanking SacI and XbaI restriction sites, was synthesized by four-primer crossover PCR reactions. Individual samples contained a combination of three common primers (SacI-RBS-Api-fwd, pBAD-Api-fwd, and pBAD-Api-rev) and one of the reaction-specific reverse primers (Api-UAG-Xba1-rev, Api-UGA-Xba1-rev) (see primer sequences in SI Appendix, Table S3). The resulting product was introduced by Gibson assembly into the pBAD18 vector digested with SacI and XbaI enzymes to yield pApi(UAG) and pApi(UGA) plasmids. Transformants were selected on lysogeny broth (LB) agar plates supplemented with 30 μg/mL chloramphenicol (Chl) and 2% glucose.
To generate the R17A variant of the pApi plasmids, the same procedure was used except in the PCR ApiR17A-UAG-Xba1-rev or ApiR17-UGA-Xba1-rev primers (SI Appendix, Table S3) were used instead of the Api-UAG-Xba1-rev and Api-UGA-Xba1-rev primers, respectively.
Construction of pApi Plasmids Expressing N-Terminally Truncated Api Peptides.
The pBAD18 plasmids carrying the truncated variants of the api gene were prepared following the approach described above for the full-size gene but using the universal reverse primer Api-UAG-Xba1-rev and one of the direct primers ApiΔ2fwd, ApiΔ4fwd, ApiΔ6fwd, or ApiΔ8fwd (SI Appendix, Table S3) to introduce two, four, six, or eight codon deletions after the start codon of the api gene, respectively.
Testing Toxicity of Endogenously Expressed Api.
Plasmids expressing wild-type (WT) pApi(UAG), pApi(UGA), or inactive R17A-Api mutant were transformed into either WT or Apir E.coli strains (BL21, BW25113, or SQ110). The transformants were grown overnight in LB medium supplemented with 30 μg/mL Chl and 2% glucose. The cultures were diluted 100-fold into fresh LB containing 30 μg/mL Chl and 0.2% glucose and grown at 37 °C until OD600 reached ∼0.7. The cultures were then diluted to an OD600 of 0.1, and 10-fold serial dilutions were prepared. The dilutions (3 µL) were spotted on LB/agar plates with 30 μg/mL Chl supplemented with either 2% glucose or varying concentrations of arabinose. The plates were incubated at 37 °C for 16 to 24 h and photographed.
Mutant Library Construction.
Sixteen separate 122-nucleotide-long DNA ultramers were procured from Integrated DNA Technologies, Inc. (IDT); each ultramer had one codon of the api(Δ2) gene replaced with the NNK sequence (SI Appendix, Fig. S3B). The ultramers were individually PCR-amplified using the primers pBAD-Api-fwd and pBAD-Api-rev (SI Appendix, Table S3) to render them double-stranded and were cloned into the SacI/XbaI sites of pBAD18 via Gibson Assembly. The libraries were transformed into XL1-Blue cells and plated at high density on LB/agar plates supplemented with 30 µg/mL of Chl. The resulting clones were washed off the plates, the total plasmid of each individual codon library was then extracted without regrowing cells, and the pooled plasmids from each library were mixed in equimolar amounts to generate the final plasmid library of api mutant genes.
The pooled plasmid library was transformed into E. coli BL21 or BW25113 cells and plated on large LB/agar plates containing Chl (30 µg/mL) and either glucose (1%) or arabinose (0.1% and 1% for the BL21 and BW25113 strains, respectively) yielding ∼7 × 105 clones per condition (>100-fold coverage counting the possible number of single-amino-acid mutants). The concentrations of arabinose used in the selection experiment corresponded to those required for moderate or strong inhibition of the growth of cells transformed with the original pApi(UAG) plasmid.
The clones were washed off the plates and, after addition of 15% (vol/vol) glycerol, stored at –80 °C.
Library Screening.
Plasmids from the colonies washed off the glucose- or arabinose-containing plates were isolated, and the api genes were PCR-amplified (18 cycles) using Api-lib-seq-fwd and api-lib-seq-rev primers (SI Appendix, Table S3) and sequenced on the Illumina MiSeq sequencer at the DNA sequencing facility of University of Illinois, Chicago.
For the BL21 library, 157,153 reads were obtained for the uninduced condition and 129,231 reads for the high-arabinose condition. For the BW25113 library, the corresponding numbers of reads were 173,948 and 127,637.
Additional materials and methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Yury Polikanov (University of Illinois, Chicago) for helping with the structure analysis and preparation of some figures and the members of A.S.M/N.V.-L. laboratory for helpful discussions. This work was supported by NSF Grant MCB 1951406 (to N.V.-L. and A.S.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026465118/-/DCSupplemental.
Data Availability
The next-generation sequencing data are available as Dataset S1 and the variant-count tables are available as Dataset S2. The plasmdis generated in this study are available from the corresponding authors upon request.
References
- 1.Florin T., et al., An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol. 24, 752–757 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casteels P., Ampe C., Jacobs F., Vaeck M., Tempst P., Apidaecins: Antibacterial peptides from honeybees. EMBO J. 8, 2387–2391 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graf M., Wilson D. N., Intracellular antimicrobial peptides targeting the protein synthesis machinery. Adv. Exp. Med. Biol. 1117, 73–89 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Mattiuzzo M., et al., Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 66, 151–163 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Krizsan A., Knappe D., Hoffmann R., Influence of the yjiL-mdtM gene cluster on the antibacterial activity of proline-rich antimicrobial peptides overcoming Escherichia coli resistance induced by the missing SbmA transporter system. Antimicrob. Agents Chemother. 59, 5992–5998 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krizsan A., et al., Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. Int. Ed. Engl. 53, 12236–12239 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Mardirossian M., et al., The host antimicrobial peptide Bac71-35 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 21, 1639–1647 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Graf M., et al., Visualization of translation termination intermediates trapped by the Apidaecin 137 peptide during RF3-mediated recycling of RF1. Nat. Commun. 9, 3053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adio S., et al., Dynamics of ribosomes and release factors during translation termination in E. coli. eLife 7, e34252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangano K., et al., Genome-wide effects of the antimicrobial peptide apidaecin on translation termination in bacteria. eLife 9, e62655 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czihal P., et al., Api88 is a novel antibacterial designer peptide to treat systemic infections with multidrug-resistant Gram-negative pathogens. ACS Chem. Biol. 7, 1281–1291 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Berthold N., et al., Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by gram-negative pathogens. Antimicrob. Agents Chemother. 57, 402–409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostorhazi E., Nemes-Nikodem É., Knappe D., Hoffmann R., In vivo activity of optimized apidaecin and oncocin peptides against a multiresistant, KPC-producing Klebsiella pneumoniae strain. Protein Pept. Lett. 21, 368–373 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Bluhm M. E. C., Knappe D., Hoffmann R., Structure-activity relationship study using peptide arrays to optimize Api137 for an increased antimicrobial activity against Pseudomonas aeruginosa. Eur. J. Med. Chem. 103, 574–582 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Goldbach T., Knappe D., Reinsdorf C., Berg T., Hoffmann R., Ribosomal binding and antibacterial activity of ethylene glycol-bridged apidaecin Api137 and oncocin Onc112 conjugates. J. Pept. Sci. 22, 592–599 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R., Knappe D., Wende E., Ostorházi E., Hoffmann R., In vivo efficacy and pharmacokinetics of optimized apidaecin analogs. Front Chem. 5, 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fosgerau K., Hoffmann T., Peptide therapeutics: Current status and future directions. Drug Discov. Today 20, 122–128 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Tracanna V., de Jong A., Medema M. H., Kuipers O. P., Mining prokaryotes for antimicrobial compounds: From diversity to function. FEMS Microbiol. Rev. 41, 417–429 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Polikanov Y. S., Aleksashin N. A., Beckert B., Wilson D. N., The mechanisms of action of ribosome-targeting peptide antibiotics. Front. Mol. Biosci. 5, 48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi S., Maeno M., Momose H., Extracellular production system of heterologous peptide driven by a secretory protease inhibitor of Streptomyces. Appl. Microbiol. Biotechnol. 36, 749–753 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Maeno M., Taguchi S., Momose H., Production of antibacterial peptide ‘apidaecin’ using the secretory expression system of Streptomyces. Biosci. Biotechnol. Biochem. 57, 1206–1207 (1993). [DOI] [PubMed] [Google Scholar]
- 22.Chen X., et al., High-level heterologous production and Functional Secretion by recombinant Pichia pastoris of the shortest proline-rich antibacterial honeybee peptide Apidaecin. Sci. Rep. 7, 14543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J., et al., Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synth. Biol. 7, 896–902 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Taguchi S., Nakagawa K., Maeno M., Momose H., In vivo monitoring system for structure-function relationship analysis of the antibacterial peptide apidaecin. Appl. Environ. Microbiol. 60, 3566–3572 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguchi S., Ozaki A., Nakagawa K., Momose H., Functional mapping of amino acid residues responsible for the antibacterial action of apidaecin. Appl. Environ. Microbiol. 62, 4652–4655 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taguchi S., Mita K., Ichinohe K., Hashimoto S., Targeted engineering of the antibacterial peptide apidaecin, based on an in vivo monitoring assay system. Appl. Environ. Microbiol. 75, 1460–1464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuru E., et al., Release factor inhibiting antimicrobial peptides improve nonstandard amino acid incorporation in wild-type bacterial cells. ACS Chem. Biol. 15, 1852–1861 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Muthunayake N. S., et al., Expression and in vivo characterization of the antimicrobial peptide oncocin and variants binding to ribosomes. Biochemistry 59, 3380–3391 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Mo Q., et al., Expression and purification of antimicrobial peptide AP2 using SUMO fusion partner technology in Escherichia coli. Lett. Appl. Microbiol. 67, 606–613 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Dreyfus M., Heurgué-Hamard V., Termination troubles in Escherichia coli K12. Mol. Microbiol. 79, 288–291 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Uno M., Ito K., Nakamura Y., Functional specificity of amino acid at position 246 in the tRNA mimicry domain of bacterial release factor 2. Biochimie 78, 935–943 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Vázquez-Laslop N., Lee H., Neyfakh A. A., Increased persistence in Escherichia coli caused by controlled expression of toxins or other unrelated proteins. J. Bacteriol. 188, 3494–3497 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan S., Skovgaard O., McLaughlin R. E., Buurman E. T., Squires C. L., Markerless Escherichia coli rrn deletion strains for genetic determination of ribosomal binding sites. G3 (Bethesda) 5, 2555–2557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orelle C., et al., Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob. Agents Chemother. 57, 5994–6004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor M., Dahlberg A. E., The influence of base identity and base pairing on the function of the alpha-sarcin loop of 23S rRNA. Nucleic Acids Res. 24, 2701–2705 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinçbas-Renqvist V., et al., A post-translational modification in the GGQ motif of RF2 from Escherichia coli stimulates termination of translation. EMBO J. 19, 6900–6907 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casteels P., et al., Biodiversity of apidaecin-type peptide antibiotics. Prospects of manipulating the antibacterial spectrum and combating acquired resistance. J. Biol. Chem. 269, 26107–26115 (1994). [PubMed] [Google Scholar]
- 38.Dutta R. C., Nagpal S., Salunke D. M., Functional mapping of apidaecin through secondary structure correlation. Int. J. Biochem. Cell Biol. 40, 1005–1015 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Czihal P., Hoffmann R., Mapping of apidaecin regions relevant for antimicrobial activity and bacterial internalization. Int. J. Pept. Res. Ther. 15, 157–164 (2009). [Google Scholar]
- 40.Orelle C., et al., Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 41, e144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai P. K., Tresnak D. T., Hackel B. J., Identification and elucidation of proline-rich antimicrobial peptides with enhanced potency and delivery. Biotechnol. Bioeng. 116, 2439–2450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan K.-H., et al., Mechanism of ribosome rescue by alternative ribosome-rescue factor B. Nat. Commun. 11, 4106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The next-generation sequencing data are available as Dataset S1 and the variant-count tables are available as Dataset S2. The plasmdis generated in this study are available from the corresponding authors upon request.