Abstract
Genetics are known to be a significant risk factor for drug abuse. In human populations, the single nucleotide polymorphism (SNP) D398N in the gene CHRNA5 has been associated with addiction to nicotine, opioids, cocaine, and alcohol. In this paper, we review findings from studies in humans, rodent models, and cell lines and provide evidence that collectively suggests that the Chrna5 SNP broadly influences the response to drugs of abuse in a manner that is not substance-specific. This finding has important implications for our understanding of the role of the cholinergic system in reward and addiction vulnerability.
1. Introduction
Drug abuse liability is known to have a strong heritable component. Genetics account for as much as 75% of the risk for nicotine dependence (Kendler et al., 1999; Vink et al., 2005), and as much as 80% of the risk for cocaine and opioid dependence (Goldman et al., 2005). Human genome-wide association studies (GWAS) have identified common single nucleotide polymorphisms (SNPs) in a number of genes that are relevant to drug addiction. Among these, a SNP in the gene CHRNA5, which encodes the α5 subunit of the nicotinic acetylcholine receptor (nAChR), has been repeatedly associated with dependence to multiple substances. α5-containing nAChRs are located in regions of the cortex, striatum, hippocampus, thalamus, amygdala, and midbrain (Berrettini et al., 2008; Flora et al., 2000; Hsu et al., 2013; Wada et al., 1990) and thus are well-positioned to influence the expression of reward and addictive behaviors.
The SNP rs16969968 in CHRNA5 changes codon 398 from GAT encoding Aspartate (D) to AAT encoding Asparagine (N) (D398N). The prevalence of the minor allele (A, encoding asparagine) varies by ethnicity, with a ~35% frequency among European-origin populations but relatively uncommon frequency (~1–5%) among Asian and African populations (Bierut et al., 2008; M. D. Li et al., 2010; N. L. Saccone et al., 2009b). Overall, an estimated 28% of the worldwide population carry at least one copy of the minor ‘A’ allele (O’Neill et al., 2018).
The non-synonymous change from G to A has been shown to affect calcium permeability and concentration-response curves of receptor binding to a nicotine agonist in vitro (Bierut et al., 2008). The D398N SNP was first associated with risk for nicotine dependence over a decade ago (S. F. Saccone et al., 2007), and numerous studies have since corroborated this finding (L.-S. Chen et al., 2009; X. Chen et al., 2009; M. Liu et al., 2019; N. L. Saccone et al., 2009b; 2009a; Winterer et al., 2010). The minor ‘A’ allele has been linked to smoking heaviness (Bierut et al., 2008; Conlon and Bewick, 2011; J. Z. Liu et al., 2010; Perez-Morales et al., 2018; N. L. Saccone et al., 2010; Stevens et al., 2008) (Hallfors et al., 2013), pleasurable early smoking experiences (Sherva et al., 2008), delayed smoking cessation (L.-S. Chen et al., 2015) and greater enhancement in cognitive performance following nicotine exposure (Jensen et al., 2015).
Though some studies have found no association of the SNP with nicotine dependence (Amos et al., 2010; Erlich et al., 2010; Etter et al., 2009; Tomaz et al., 2018; Verde et al., 2011), the lack of association could be due to limitations such as small sample sizes, a study population with low variant frequency, a study population that has been ascertained for a different disease phenotype or a combination of these issues. Together, these studies demonstrate the importance of both quantity in GWAS and quality of phenotypic data, in order to understand the contribution of SNPs to complex diseases like drug dependence.
The association of the CHRNA5 SNP with other drugs of abuse has been less well-studied. For example, studies examining the influence of CHRNA5 D398N on alcohol phenotypes have been inconclusive. At least one study has identified an association of this SNP with decreased risk for symptoms of alcohol dependence (X. Chen et al., 2009) while others have found no association of the CHRNA5 SNP with alcohol dependence (Hallfors et al., 2013; M. Liu et al., 2019; Sherva et al., 2010; Wang et al., 2009). There is a strong correlation between smoking and alcohol abuse; comorbidity is higher in men compared to women and rates of comorbid smoking and alcohol use are highest among young smokers (Falk et al., 2006). However, in one of the largest GWAS studies of nicotine and alcohol phenotypes, the CHRNA5 SNP was associated with smoking, specifically cigarettes per day, but not with alcohol use (drinks per week) (M. Liu et al., 2019).
In addition to nicotine and alcohol dependence, human GWAS and candidate SNP studies have also linked CHRNA5 D398N to risk for dependence to opioids (Curtis et al., 2017; Erlich et al., 2010) and cocaine (Aroche et al., 2020; Grucza et al., 2008; Sherva et al., 2010). It should be noted that in several of these studies, individuals were co-dependent on more than one drug. Multiple substance dependence diagnoses might limit the ability to detect some associations and even obscure assessments of risk for various drug classes.
A limited number of studies have demonstrated that the direction of the association of CHRNA5 D398N with cocaine and alcohol is the opposite to that of nicotine and opioids; Specifically, for nicotine and opiate dependence, the minor ‘A’ allele is associated with risk. In contrast, for alcohol and cocaine dependence, the minor ‘A’ allele is protective (see section 2.4). One study has proposed that the opposite associations with nicotine and alcohol could reflect differences in the physiological effects of the two drugs; specifically, that a genetic predisposition to favor stimulant drugs could make an individual less inclined to prefer depressant drugs (X. Chen et al., 2009). However, considering subsequent evidence that the association with opioids is in the same direction as with nicotine and that the association with cocaine is in the same direction as alcohol, this seems unlikely. Another possibility is that the opposite associations reflect different mechanisms of action for these drugs—more specifically, direct and indirect dopaminergic mechanisms (Aroche et al., 2020; Grucza et al., 2008).
The mesolimbic dopamine neurons are part of a well-defined pathway involved in reward processing. This pathway consists primarily of dopamine fibers arising in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc). GABAergic interneurons in the VTA maintain a tonic inhibition over dopaminergic neurons, whereas glutamatergic inputs from the prefrontal cortex serve to excite these neurons (Johnson and North, 1992; Kalivas et al., 1989; Sesack and Pickel, 1992; Taber et al., 1995). Nicotinic receptors (nAChRs) are present throughout the brain on dopamine, GABA and glutamate containing neurons (Mansvelder and McGehee, 2002), and the relative amounts of receptors and subunit specificity can vary from cell to cell.
The activation of nAChR receptors specifically in the VTA is thought to underlie the rewarding effects of nicotine. Nicotine has been shown to activate and desensitize VTA dopamine cells (Calabresi et al., 1989; Pidoplichko et al., 1997). Thus, the presence of the CHRNA5 SNP, which results in reduced calcium signaling, could directly modulate dopamine release. In addition, reduced function of α5-containing nAChRs could attenuate nicotine-induced GABA transmission, thereby disinhibiting dopaminergic signaling, resulting in increased dopamine release (Mansvelder et al., 2002). Mu opioid receptors are expressed on GABAergic neurons in the VTA and activation of these receptors inhibits calcium conductance, leading to inhibition of GABA transmission and disinhibiting dopaminergic signaling, also resulting in increased dopamine release in the NAc (Matsui et al., 2014; Sugita et al., 1992). Reduced function of α5-containing nAChRs could further attenuate opioid-induced GABAergic transmission which may enhance increased dopamine release. The association of the CHRNA5 ‘A’ allele with risk for both nicotine and opioids may result from this common underlying neurochemical mechanism.
In contrast, cocaine, which binds directly to dopamine transporters to block reuptake of neurotransmitter, exerts its rewarding effect by increasing extracellular levels of dopamine in the NAc (Carlezon et al., 1995; Liao et al., 2000). Thus, in the presence of the CHRNA5 SNP, α5-containing nAChRs in the NAc could modulate downstream neural activity, reducing the impact of dopamine receptor activation and thereby serving a protective role (Grucza et al., 2008).
In the remainder of this review, we integrate findings from across studies in humans, animal models and cell lines that serve to elucidate the influence of CHRNA5 D398N on drug response and addiction vulnerability. First, we discuss human neuroimaging studies that corroborate the association of CHRNA5 D398N with substance abuse. We then discuss studies in rodent models that have identified specific behaviors that are impacted by the presence of this SNP. Third, we consider in vitro studies that promote a mechanistic understanding of the influence of this SNP on cellular processes that could ultimately impact behavior. Finally, we close with a discussion of recommendations for future work that may serve to fill gaps in our current understanding of how CHRNA5 D398N influences drug abuse liability.
2. Human neuroimaging studies
2.1. Circuitry implicated in human drug abuse phenotypes
Human functional neuroimaging (fMRI) studies of drug dependence have identified neural correlates of drug cue reactivity, dependence and withdrawal (Karoly et al., 2013; Sutherland et al., 2012; Yalachkov et al., 2012). Many of the regions implicated in these processes express α5-containing nAChRs, and their activity could be subject to modulation by CHRNA5 genotype. Resting-state fMRI studies have revealed altered connectivity strength between the ventral striatum, cortical, and subcortical regions in individuals with substance use disorders compared to age-matched controls (Kelly et al., 2011; J. Liu et al., 2009; Ma et al., 2010; Sutherland et al., 2012). While human neuroimaging studies cannot discern whether alterations in functional connectivity lead to addiction or result from chronic drug use, such studies that have provided insight into how CHRNA5 D398N mediates systems-level neural circuitry.
2.2. Neural correlates of smoking risk
The first neuroimaging study on CHRNA5 D398N found that resting-state functional connectivity between the dorsal ACC (dACC) and ventral striatum (VS) is moderated by CHRNA5 genotype, independent of smoking status (Hong et al., 2010). Across multiple ethnic backgrounds and both sexes, carriers of the ‘A’ allele at CHRNA5 D398N display reduced functional connectivity between these two brain regions (Hong et al., 2010). Given the important roles of the dACC and VS in cognitive control and reward, respectively, lower functional connectivity between these regions could be related to behavioral dysfunction associated with nicotine addiction (Hong et al., 2009). Thus, it follows that impaired dACC-VS connectivity could predispose ‘A’ allele carriers to smoking.
Sex-specific effects of CHRNA5 genotype on neural reactivity in smokers have also been reported. CHRNA5 genotype has been found to modulate reactivity to smoking cues in women smokers (Janes et al., 2012). Prior work suggests that smoking cues elicit stronger cravings in women than in men (Field and Duka, 2004; Waters et al., 2004). Janes et al. found that reactivity to smoking cues was lower amongst women with at least one copy of the ‘A’ allele as compared to women of the G/G genotype. The anterior cingulate cortex (ACC) and hippocampus are among the regions most frequently implicated in cue reactivity (Yalachkov et al., 2012). In these studies, cue reactivity was reduced in regions of the cortex, dorsal striatum, hippocampus and thalamus (Janes et al., 2012). This finding could reflect adverse effects of CHRNA5 D398N on the formation of hippocampal-dependent drug-cue memories via its inhibition of nicotine-induced intracellular calcium influx, thus leading to a weaker response to nicotine associated cues. A later study identified an association of the ‘A’ allele with smoking cessation success in response to pharmacological treatment among women, perhaps owing to lower levels of smoking cue reactivity (Tomaz et al., 2018).
CHRNA5 genotype has also been found to modestly impact gray matter volume of the ventromedial prefrontal cortex (vmPFC) in male and female adolescent smokers (Chaarani et al., 2019). An earlier study provided evidence that the CHRNA5 SNP is associated with greater enjoyment of early smoking experiences (Sherva et al., 2008), suggesting that it could be a risk factor for smoking initiation amongst adolescents. Chaarani et al. (2019) acquired structural MRI data from adolescents at 14 years of age, and again at 16 years of age. While reductions in vmPFC gray matter were observed in smokers of all genotypes, the greatest reduction was observed amongst carriers of the A/A genotype. Further, vmPFC volume in early adolescence (at 14 years of age) was not found to predict smoking status in later adolescence (at 16 years of age). Therefore, these findings suggest that vmPFC volume is not predictive of smoking behavior, but rather that even low levels of smoking can adversely impact brain maturation in a genotype-dependent manner (Chaarani et al., 2019). Reduced vmPFC volume in adolescents of the A/A genotype could further the risk of smoking later in life via adverse effects on executive functioning and inhibitory control (Goriounova and Mansvelder, 2012).
2.3. CHRNA5 modulates functional circuitry in opioid users
Although only one candidate SNP study has indicated that the CHRNA5 SNP is associated with opioid dependence (Erlich et al., 2010), a recent fMRI study provides evidence that it moderates functional neural circuitry amongst opioid users (Curtis et al., 2017). Curtis et al. (2017) determined that in opioid users of the CHRNA5 D398N G/G genotype, resting-state functional connectivity between the right habenula and left caudate is increased relative to ‘A’ allele carriers. Given the role of the habenula in encoding aversion, the authors speculate that greater opioid dependence amongst ‘A’ allele carriers could be driven by lower levels of negative reinforcement (Curtis et al., 2017). Lower functional connectivity of the ACh-rich habenula in humans could be relevant to phenotypes observed in Chrna5 null mice, including preference for high doses of drug that are normally aversive as well as attenuated withdrawal signs (Fowler et al., 2011; Jackson et al., 2008). The cholinergic system is known to be implicated in opiate dependence and withdrawal (Neugebauer et al., 2013), and it is plausible that altered habenula-striatum connectivity represents a common neurobiological substrate underlying the association of CHRNA5 D398N with increased risk for both nicotine and opioid dependence.
2.4. Association of CHRNA5 D398N with cocaine and alcohol dependence
Human candidate SNP studies have also identified associations of the minor ‘A’ allele at CHRNA5 D398N with protective effects for alcohol and cocaine dependence (Aroche et al., 2020; X. Chen et al., 2009; Forget et al., 2020; Grucza et al., 2008; Sherva et al., 2010). Specifically, Grucza et al. (2008) observed lower frequencies of the minor ‘A’ allele in cocaine-dependent individuals from two study populations of European descent; Sherva et al. (2010) observed the same association in a sample of European American and African American individuals (Grucza et al., 2008; Sherva et al., 2010). In line with these findings, Aroche et al. (2020) identified a protective effect of the ‘A’ allele for crack-cocaine addiction in European-descended patients (Aroche et al., 2020). Furthermore, the progression from initial cocaine use to disordered use appears to be slower amongst individuals carrying at least one copy of the ‘A’ allele (Forget et al., 2020).
Chen et al. (2009) identified an association of the ‘G’ allele at CHRNA5 D398N with symptoms of alcohol use disorder in individuals of European descent (X. Chen et al., 2009). However, this effect has not been replicated by other studies, (Hallfors et al., 2013; M. Liu et al., 2019; Sherva et al., 2010; Wang et al., 2009). Methodological differences represent one possible explanation for this discrepancy in findings. Chen et al. (2009) included in their study individuals who were “regular drinkers”, meaning that they consumed at least 5 drinks per month, but may not have met the DSM IV criteria for alcohol use disorder (X. Chen et al., 2009). The ‘A’ allele may therefore be associated with lower levels of alcohol consumption, but may not be protective against alcohol use disorder.
In summary, functional neuroimaging studies have identified genotype differences in regions of the cortex, thalamus and striatum relevant to drug abuse phenotypes. These studies have compared individuals carrying one or more copies of the minor ‘A’ allele with individuals carrying two copies of the major ‘G’ allele. To our knowledge, the association of CHRNA5 D398N with cocaine and alcohol dependence has not been explored in human neuroimaging studies. Further work in this area will be needed to advance our understanding of the bidirectional effects of CHRNA5 on substance abuse liability across multiple drug classes.
3. Studies in rodent models
3.1. Studies in the Chrna5 null mouse
Although human neuroimaging studies have provided significant insight into systems-level neural circuitry underlying the association of the CHRNA5 SNP with drug abuse liability, their ability to causally link this SNP to specific drug abuse phenotypes is limited. Thus, mechanistic studies in animal models are necessary. A Chrna5 knock-out mouse line has been useful for interrogating the function of the α5 nAChR subunit in animal models of drug abuse. Complete absence of the gene does not recapitulate the subtle difference in function associated with the SNP; however, as CHRNA5 D398N is associated with reduced α5 subunit function, deletion of this gene could approximate some effects of the ‘G’ to ‘A’ nucleotide substitution.
Chrna5 null mice are less sensitive to acute effects of nicotine; specifically, they are less susceptible to nicotine-induced seizures, hypolocomotion, and antinociception compared to WT mice (Jackson et al., 2010; Kedmi et al., 2004; Salas et al., 2003). These behavioral alterations could result from reduced nAChR function within the striatum, thalamus and hindbrain (Brown et al., 2007; Jackson et al., 2010). Chrna5 deletion has also been shown to alter nicotine reward, such that Chrna5 null mice develop conditioned place preference for high doses of nicotine that are not rewarding to WT mice (Jackson et al., 2010). During nicotine withdrawal, somatic signs and hyperalgesia are lower in Chrna5 null mice compared with WT, but anxiety-like behavior and conditioned place aversion are unaltered (Jackson et al., 2008; Salas et al., 2009). Thus, α5 subunits appear to play a role in the physical signs of withdrawal, but not withdrawal-associated negative affect.
Seminal work by Fowler et al. (2011) demonstrated that mice lacking α5 nAChR subunits self-administer more nicotine than WT mice. While WT mice adjusted their responding to maintain a consistent level of nicotine intake across different doses, Chrna5 null mice maintained their level of responding and consumed greater amounts of nicotine as the dose increased (Fowler et al., 2011). Of interest, this altered behavioral phenotype could be reversed by virally expressing Chrna5 within the habenulo-interpeduncular tract of the Chrna5 null mice. This work provides evidence that the deletion of α5 nAChR subunits within the habenulo-interpeduncular pathway lessens the inhibitory influence of high doses of nicotine on activity within the brain’s reward circuitry.
In accordance with the findings of Fowler et al. (2011), a separate study found that not only do Chrna5 null mice consume significantly more nicotine than WT mice at high doses, but these mice also require a much higher dose to escalate their intake in a nicotine self-administration paradigm than do WT mice (Morel et al., 2014). Coinciding with these findings, Chrna5 null mice also require higher doses of nicotine than WT mice to elicit dopaminergic cell firing within the VTA. Of interest, this altered nicotine self-administration phenotype could be reversed by virally expressing WT but not SNP α5 subunits within VTA dopaminergic neurons (Morel et al., 2014). These findings highlight the critical role of α5-containing nAChRs on VTA dopaminergic neurons in nicotine reward and also demonstrate the functional impairment associated with the Chrna5 SNP. Collectively, these findings suggest that α5 subunits expressed within pathways implicated in both aversion (MHb-IPN) and reward (VTA-NAc) influence nicotine intake.
In mice, loss of the α5 nAChR subunit is also shown to potentiate ethanol-induced ataxia, hypothermia, hypnosis, and anxiolysis (Dawson et al., 2018; Santos et al., 2013). Ethanol conditioned place preference (CPP) is attenuated in α5 knock-out mice relative to wild type (WT), indicating that the rewarding properties of ethanol are reduced in these mice (Dawson et al., 2018). In light of this finding, one possible explanation for lower alcohol abuse liability amongst human ‘A’ allele carriers (X. Chen et al., 2009) could be that the drug is less reinforcing to these individuals.
While neither cocaine nor opioid reward have been assessed in Chrna5 null mice, the influence of Chrna5 expression on morphine withdrawal has been examined through experiments conducted in both the Chrna5 null mouse and in BXD recombinant mouse lines, which express varying levels of Chrna5 mRNA (Muldoon et al., 2014). A significant positive correlation between Chrna5 mRNA expression within the forebrain and midbrain and morphine withdrawal severity, as assessed by a defecation score, was observed in the BXD recombinant line. Thus, higher levels of Chrna5 mRNA expression are associated with greater withdrawal severity. In accordance with this finding, Chrna5 null mice showed significantly reduced total somatic signs of withdrawal relative to WT mice (Muldoon et al., 2014).
3.2. Studies in the Chrna5 SNP mouse
While gene deletion studies may approximate “loss of function” SNPs, it is critical to model human SNPs in mice to help clarify inconsistencies associated with human data. In addition, animal models expressing human SNPs allow for the determination of molecular mechanisms that mediate the behavioral consequences of these SNPs, and as such contribute to a better understanding of their significance in human disease. The impact of the Chrna5 SNP on behavior has been assessed in mice either by virally expressing the SNP in regions of the brain relevant to addiction (Frahm et al., 2011; Morel et al., 2014), or by genetically engineering endogenous expression of the SNP through homologous recombination (Koukouli et al., 2017; O’Neill et al., 2018; Sciaccaluga et al., 2015).
Studies in the Chrna5 SNP mouse have thus far focused on how this SNP affects sensitivity to nicotine and underlying neural circuitry. Koukouli et al. (2017) demonstrated that Chrna5 SNP mice show a reduction in the duration and frequency of putative pyramidal neuron activity within layers II/III of the prelimbic cortex, which is reversed by 1–2 weeks of chronic nicotine treatment. The authors propose that the observed effect of nicotine is due to the desensitization of nAChRs expressed by interneurons, which normally inhibit pyramidal neurons in this region (Koukouli et al., 2017). Pyramidal neurons within the mPFC serve an important function in cognitive and emotional control by integrating signals from the thalamus, hippocampus, basolateral amygdala and contralateral mPFC (Hoover and Vertes, 2007; Vertes, 2006). The findings of this study support the notion that individuals with the CHRNA5 SNP may smoke as a form of self-medication to compensate for impaired cognitive regulation (Koukouli et al., 2017).
nAChRs regulate brain maturation throughout development and are often transiently upregulated or show dynamic changes in subunit composition in regions undergoing major differentiation and synaptogenesis (Dwyer et al., 2009). Further, nicotine exposure directly effects the developing brain (Dwyer et al., 2008). To determine the impact of the Chrna5 SNP on the effects of developmental nicotine exposure, male and female Chrna5 G/G and A/A mice were treated with either nicotine or vehicle beginning prenatally and until weaning at 21 days of age via maternal nicotine drinking (O’Neill et al., 2018). A significant interaction of genotype and developmental nicotine exposure was observed on nicotine drinking behavior in adolescence, wherein nicotine-exposed mice with the Chrna5 variant consumed significantly more nicotine than their vehicle-treated counterparts, and nicotine-exposed Chrna5 G/G mice consumed significantly less nicotine than vehicle-treated G/G mice (O’Neill et al., 2018). A/A mice that were exposed to nicotine during early development consumed significantly more nicotine in adolescence than G/G mice. In contrast, there was no difference in nicotine consumption between vehicle-treated G/G and A/A mice. Further, nicotine-exposed Chrna5 A/A mice showed decreased expression of a3p4-type nAChRs in the habenula relative to G/G mice and decreased DA release from α4β2 and α4β2α5 nAChR-expressing cells within the striatum (O’Neill et al 2018).
3.3. Studies in the Chrna5 SNP rat
The impact of the Chrna5 SNP on nicotine, alcohol, cocaine and food self-administration has been investigated in a transgenic rat model, which was recently developed using zinc finger nuclease technology (Besson et al., 2019; Forget et al., 2018). Using this model, Forget et al. (2018) first examined the impact of the Chrna5 SNP and Chrna5 deletion on nicotine self-administration behavior and electrophysiological properties of VTA dopamine neurons. Although Chrna5 null rats did not acquire nicotine self-administration, rats with the Chrna5 A/A SNP consumed significantly greater amounts of nicotine than WT mice at the two highest doses tested. Rats with the Chrna5 A/A SNP also displayed a higher “break point” at the highest dose tested, indicating greater motivation to take drug, and stronger reinstatement in response to nicotine alone or nicotine and the cue presented together. Greater nicotine-induced reinstatement in the Chrna5 SNP rats was associated with greater neuronal activation in the medial portion of the lateral habenula and in the lateral hypothalamus, and lower neuronal activation in the IPN (Forget et al., 2018). Neuronal activation within the IPN, which is predominantly GABAergic, was inversely correlated with nicotine-induced reinstatement, suggesting an important role of Chrna5 in modulating the activity of GABAergic neurons. The absence of nicotine self-administration in Chrna5 null rats is inconsistent with what has previously been observed in Chrna5 null mice (Fowler et al., 2011), which may reflect differences in nicotine metabolism (Forget et al., 2018) or differences in the distribution of α5 subunit between mice and rats (Ishii et al., 2005).
In a subsequent investigation of ethanol self-administration, Chrna5 A/A rats required a higher dose of quinine to reduce their ethanol intake than did G/G rats, they self-administered more ethanol, and showed higher levels of reinstatement in response to ethanol and the cue together. Unlike G/G rats, Chrna5 A/A rats also showed increased activation in the anterior agranular insula in the relapse condition compared with the processing between the two genotypes (Besson et al., 2019). These findings contrast with the decreased ethanol reward phenotype observed in Chrna5 null mice in the CPP paradigm (Dawson et al., 2018), and with reduced alcohol abuse liability observed in human ‘A’ allele carriers (X. Chen et al., 2009).
Of interest, Besson et al. (2019) also compared food self-administration in Chrna5 A/A and G/G rats. Chrna5 A/A rats showed a trend towards greater motivation to self-administer sucrose, as well as increased resistance to extinction, and higher levels of reinstatement following food priming (Besson et al., 2019). Taken together, their findings in nicotine, ethanol and food self-administration paradigms may reflect alterations to reward pathways that generally impact the response to reinforcing stimuli.
Human studies have demonstrated that the ‘A’ allele may be protective against cocaine dependence (Aroche et al., 2020; Grucza et al., 2008; Sherva et al., 2010). In line with the results of these studies, Forget et al. (2020) demonstrated that the acquisition of cocaine self-administration is disrupted in Chrna5 SNP rats compared to WT rats. These rats did not increase their responding to maintain consistent levels of cocaine intake as the ratio schedule of reinforcement increased over the course of self-administration sessions. Interestingly, Forget et al. also observed potentiated relapse to cocaine-seeking in the Chrna5 null rat, which is accompanied by increased neuronal activation in the nucleus accumbens (Forget et al., 2020).
A critical mass of studies in humans link the CHRNA5 SNP with alterations in response to a variety of drugs of abuse. However, much of this data is contradictory and replication has been challenging. Further, environmental factors that are known to modulate disease phenotypes are difficult to control in any given population. Thus, the ability to interrogate the impact of this SNP in isolation and in a controlled manner is important for complete functional characterization of this genetic variant. Future studies using rodent models could serve as a valuable tool in determining the mechanisms underlying responses to other drugs of abuse and in developing personalized therapies based on genotype.
4. In vitro studies
The subunit composition of nAChRs varies across brain regions (Le Novère et al., 2002). α5 subunits are almost exclusively incorporated into the α4β2 class of receptors in hippocampus, striatum, cerebral cortex and thalamus (Mao et al., 2008). Studies in rodent models suggest that the Chrna5 SNP influences the brain’s reward circuitry, and this finding is consistent with in vitro studies that have demonstrated a critical role for α5 nAChR subunits in the function of dopaminergic (DA) neurons. The α5 nAChR subunits are expressed within most DA neurons in the ventral tegmental area (VTA) and substantia nigra (SN) (Klink et al., 2001) that project to the striatum. The critical role of the cholinergic system in regulating dopamine release within the striatum is further evidenced by overlap of cholinergic and dopaminergic fibers in the striatum (Zhou et al., 2001) and by the influence of nicotine on dopaminergic release within the striatum via nAChRs (Rice and Cragg, 2004).
In vitro studies in HEK293 cell lines have demonstrated that CHRNA5 D398N compromises the function, but not the expression, of α4β2α5 and α3β4α5 nAChRs (Bierut et al., 2008; Tammimaki et al., 2012). α4β2α5 receptors are located on the terminals of DA neurons within the striatum, where they modulate DA release in response to nicotine (Salminen et al., 2004; Zoli et al., 2002). α3β4-type nAChRs are located in the habenulo-interpeduncular pathway (Grady et al., 2009) where they may associate with α5 subunits; α3β4α5 nAChRs are also expressed peripherally in ganglion neurons (Conroy and Berg, 1995; David et al., 2010). In α4β2α5 nAChRs, expression of CHRNA5 398N reduces the maximal calcium response to agonist (Bierut et al., 2008). A later study showed that the presence of the variant reduces calcium permeability and increases desensitization (Kuryatov et al., 2011).
Following activation, nAChRs desensitize and recover from desensitization with kinetics that depend on receptor composition (Fenster et al., 1997; Vibat et al., 1995). Studies on the impact of CHRNA5 D398N on desensitization of α3β4α5 nAChRs have reported mixed findings. Li et al. (2011) observed no effect of the 398N variant on channel activation by agonists or desensitization of α3β4α5 nAChRs (P. Li et al., 2011). The absence of an effect on desensitization is in agreement with the findings of Kuryatov et al., who additionally found no effect of the variant on calcium permeability of α3β4α5 nAChRs (Kuryatov et al., 2011). However, Tammimäki et al. determined that the presence of the variant attenuates the intracellular calcium response of α3β4α5 nAChRs to nicotine, acetylcholine (ACh), and varenicline, and increases the EC50 for nicotine (Tammimaki et al., 2012). The variant did not impact desensitization of agonist-induced calcium influx in α3β4α5 nAChRs. However, the variant was found to prevent the increase in desensitization observed in D398 cells following an increase in the concentration of external calcium, as measured via whole-cell electrophysiology. (Tammimaki et al., 2012).
Studies in cultured VTA neurons indicate that modulation of intracellular calcium may be the mechanism by which Chrna5 D398N impacts the function of midbrain DA neurons. The Chrna5 398N variant reduced the proportion of neurons exhibiting nicotine-evoked calcium increases relative to Chrna5 D398 (Sciaccaluga et al., 2015). Human DA neuron cultures derived from induced pluripotent stem cells show slightly greater spontaneous action potential firing frequency and increased spontaneous postsynaptic current frequency and amplitude in the presence of the 398N variant (Oni et al., 2016). Thus, CHRNA5 398N appears to confer greater excitability to DA neurons expressed within the brain’s reward circuitry.
nAChR upregulation may be another mechanism by which nicotine dependence is maintained. The upregulation observed following chronic exposure to nicotine (Govind et al., 2009; Schwartz and Kellar, 1983) appears to largely involve α4β2-type receptors (Flores et al., 1992; Nguyen et al., 2003) but this increased receptor density is not observed when the α5 subunit is incorporated (Mao et al., 2008). Thus, the presence of α5 subunits, in addition to influencing calcium influx, alter receptor regulation by nicotine as well. Recently, C57BL/6 mice engineered to express Chrna5 D398N via CRISPR/cas9 genome editing (Brynildsen et al., 2017) were chronically exposed to nicotine for 14 days via osmotic minipumps. Consistent with what was found in rats (Mao et al., 2008), α4β2α5 receptors were not upregulated in mice. Of interest, the presence of Chrna5 D398N variant did not influence receptor regulation (R.P. Yasuda and K.J. Kellar, personal communication). This study demonstrates that neither variant influences nicotine-induced upregulation.
5. Summary and recommendations for future work
In summary, human, animal, and in vitro studies provide evidence to suggest that the CHRNA5 SNP broadly influences substance dependence liability. In the remaining sections, we encapsulate findings from across these studies and provide recommendations for future work that would further our understanding of this genetic variant and potentially inform treatment.
5.1. Integrating findings from across scales to understand the impact of the CHRNA5 SNP on substance abuse liability
Collectively, studies on the influence of CHRNA5 D398N on addiction-relevant phenotypes indicate that this SNP impacts the function of circuitry implicated in distinct addiction-relevant processes. The SNP is expressed within regions of the brain implicated in drug reward (VTA, SN and striatum), aversion (MHb-IPN pathway), attention (mPFC) and cue memory (hippocampus). Evidence for the behavioral influence of the SNP is demonstrated by studies in humans and rodent models, and in vitro studies have provided insight into the underlying cellular and molecular mechanisms.
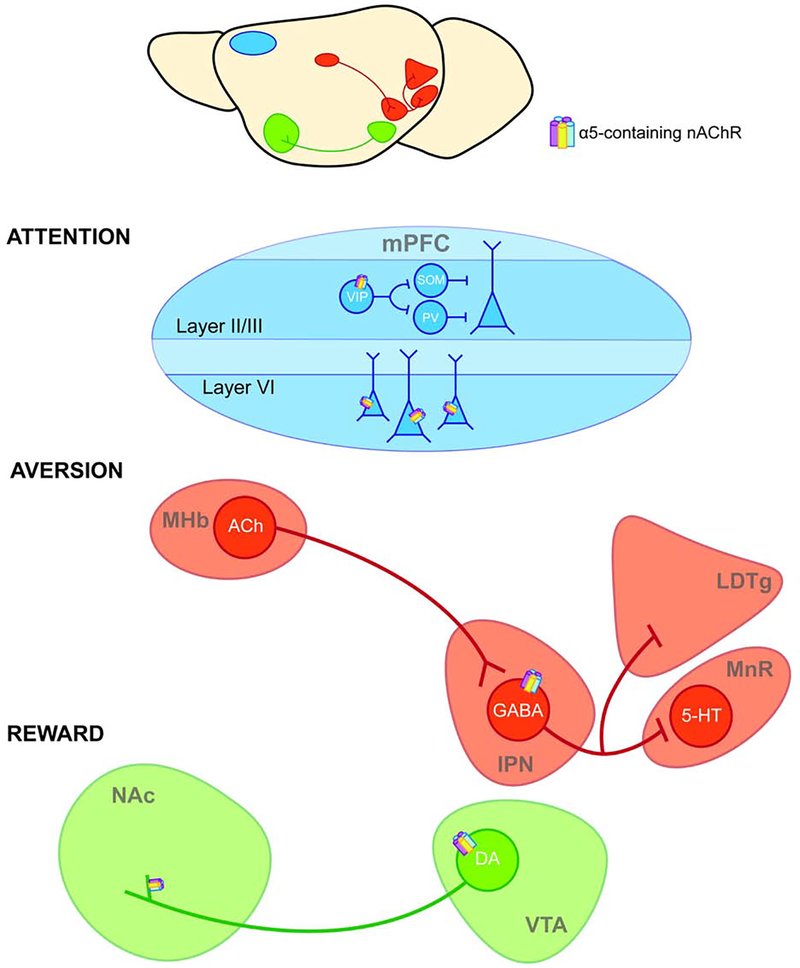
In the cortex, α5-containing nAChRs are expressed within the mPFC, where they play a critical function in the development of cortical neurons and in attentional processes (Bailey et al., 2012; 2010). In the absence of α5 subunits, the normal developmental pruning of layer VI mPFC neurons does not occur, and apical dendrites are thus extended in adulthood. This altered neuronal morphology could change the input received by layer VI mPFC neurons, and thus impact feedback to the thalamus (Bailey et al., 2012). Further, excitation of layer VI mPFC pyramidal neurons is dependent on the expression of α5 subunits, and Chrna5 null mice exhibit disrupted attentional performance in the 5-choice serial reaction time test (Bailey et al., 2010). Within layer II/III of the mPFC, Chrna5 D398N inhibits the activity of α5 subunit-expressing vasoactive intestinal polypeptide (VIP) interneurons (Figure 1), resulting in behavioral deficits associated with cognitive impairment (Koukouli et al., 2017).These findings may be related to the reduced functional connectivity between the dACC and striatum observed in human carriers of the 398N variant (Hong et al., 2010), which suggests a reduction in the functional association between circuitry implicated in attention and reward.
Figure 1. CHRNA5 D398N alters the function of α5 nAChR subunits within addiction-relevant circuitry.
CHRNA5 D398N impacts the function α5 nAChR subunits that are expressed within neural circuits implicated in attention (mPFC), aversion (MHb-IPN), and reward (VTA-NAc). In layer II/III of the mPFC, α5-containing nAChRs are expressed on vasoactive intestinal polypeptide (VIP) interneurons that inhibit somatostatin- (SOM) and parvalbumin- (PV) interneurons; these interneurons inhibit pyramidal cells (Pi et al., 2013). In layer VI, α5-containing nAChRs are expressed on pyramidal neurons (Bailey et al., 2012). α5 subunits are also located on GABAergic neurons in the IPN, where they mediate aversion via inhibitory projections to the mesopontine raphe (MnR) and laterodorsal tegmental area (LDTg) (Hsu et al., 2013; Morton et al., 2018; Quina et al., 2017; Wolfman et al., 2018), and on the cell bodies and terminals of DA neurons that project from the VTA to the NAc (Salminen et al., 2004; Zoli et al., 2002), where they mediate reward.
Identifying the precise location of α5 subunits within the MHb-IPN pathway has been challenging. Due to co-localization of CHRNA5, CHRNA3 and CHRNB4 within the genome, expression of α5 subunits was initially presumed to overlap with expression of α3 and β4 subunits in the MHb. However, recent studies have interrogated α5 subunit expression by measuring mRNA or electrophysiological responses and demonstrated little to no expression of α5 subunits within the MHb (Hsu et al., 2013; Morton et al., 2018). α5-containing nAChRs are located primarily within the rostral subnucleus of the IPN, on GABAergic neurons that project to the mesopontine raphe and dorsal tegmental area (Hsu et al., 2013; Morton et al., 2018; Quina et al., 2017; Wolfman et al., 2018) (Figure 1). These GABAergic neurons may influence reinstatement to nicotine seeking and avoidance behaviors (Forget et al., 2018; Wolfman et al., 2018). To our knowledge, no studies have yet investigated the impact of the Chrna5 SNP on the IPN-mesopontine raphe pathway and relevant phenotypes. Further work in this area would be informative with respect to the potential modulating role of the Chrna5 SNP on the serotonergic system.
In the mesolimbic dopamine pathway, α5-containing nAChRs are located on dopaminergic cell bodies in the VTA, and on terminals in the NAc, where they modulate DA release (Figure 1). Expression of the CHRNA5 398N variant appears to impact the excitability of VTA DA neurons (Oni et al., 2016), which could be relevant to increased nicotine intake observed in mice lacking α5 subunit expression (Morel et al., 2014). Altered connectivity between the habenula and striatum in opioid users with the 398N variant (Curtis et al., 2017) provides evidence for an imbalance in reward- and aversion-encoding circuitry in the presence of the SNP.
In summary, the CHRNA5 SNP appears to alter the function of circuitry that underlies distinct addiction-relevant phenotypes. Further studies in vitro and in animal models will be needed to clarify the functional impact of CHRNA5 D398N within other regions of the brain that are implicated in substance use disorders, including the hippocampus and amygdala.
5.2. Recommendations for future study
5.2.1. Sex differences
The investigation of sex-specific effects of the CHRNA5 SNP on substance abuse liability represents a gap in the current literature. Although human studies have identified sex-specific effects of the influence of CHRNA5 on neural reactivity to smoking cues and smoking cessation (Janes et al., 2012; Tomaz et al., 2018), and sex-specific effects of Chrna5 deletion on anxiety-like behavior have been identified in mice (Gangitano et al., 2009), as yet, only one study in rodent models bearing the Chrna5 SNP has included females (O’Neill et al., 2018). Therefore, little is known about neuronal mechanisms underlying sex differences observed in humans. Future studies should include both males and females in order to identify potential sex × genotype interactions on addiction-relevant behaviors, which could have important treatment implications.
5.2.2. Interactions of CHRNA5 D398N with other genetic variants
Gene targeting in mice has revolutionized our approach to studying gene function in mammals. During the past decade, thousands of null, hypomorphic and conditional alleles have been constructed. With the advent of rapid genome editing technologies, such as the CRISPR/Cas9 system, we are entering a new era for the interrogation of SNP function in animal models. Modeling human SNPs in mice allows for the determination of molecular mechanisms that mediate the behavioral consequences of these SNPs in human disease and can contribute to the development of more effective treatments. Yet, even for GWAS-identified human genetic variants that have been recapitulated in mice, it remains unclear how genetic background contributes to altered cellular and behavioral responses.
Genetic variation is known to influence addiction vulnerability. Genome-wide association studies have identified multiple loci associated with addictive behaviors. However, GWA studies for addiction typically test for associations between increasing additive genotype dosage at SNPs and phenotype one variant at a time. GWAS scans of SNP x SNP for the detection of non-additive pairwise effects (i.e., “interactions”) require further stringency for discovery (e.g., P < 10–11), due to multiple testing considerations. In the past, the small size of human genetic studies in addiction research has hindered our ability to identify SNP × SNP effects. A recent GWAS of 1.2 million participants provides sufficient power for the detection of polygenic associations with nicotine and alcohol abuse (M. Liu et al., 2019). In addition, animal models could greatly facilitate the study of genetic interactions. For example, introducing risk SNPs such as Chrna5 D398N directly on the background of genetically diverse strains would allow one to quickly ascertain both additive and synergistic effects between multiple loci.
5.2.3. Relevance of CHRNA5 D398N to other neuropsychiatric disorders
There is some evidence for the association of CHRNA5 D398N with other disorders that are frequently co-morbid with substance use disorders such as schizophrenia and post-traumatic stress disorder (PTSD) (Boscarino et al., 2011; Hong et al., 2011). Hong et al. (2011) demonstrated that the minor ‘A’ allele is also associated with schizophrenia, and Koukouli et al. (2017) determined that mice expressing Chrna5 398N exhibit behaviors characteristic of schizophrenia, including compromised social ability and disrupted sensorimotor gating (Hong et al., 2011; Koukouli et al., 2017). Boscarino et al. (2011) found that CHRNA5 398N genotype is more common in PTSD cases compared to controls (Boscarino et al., 2011).
Expression of α5 subunits in attention circuitry (mPFC) could influence the risk for other disorders that are associated with impaired attentional processes, such as ADHD. α5-containing nAChRs are also expressed on GABAergic neurons within the IPN that project to serotonergic nuclei. The presence of the CHRNA5 398N variant could thus limit the inhibition of nAChR agonist-induced serotonin release (Morton et al., 2018). These data suggest that this SNP could also influence depressive phenotypes by modulation of the serotonergic system. Further investigation into neuropsychiatric conditions associated with CHRNA5 D398N could be useful to inform treatment.
6. Conclusion
Human GWAS and clinical studies suggest that the CHRNA5 SNP may be relevant to addiction treatment as well as vulnerability. While the SNP is associated with lower overall rates of smoking cessation (M. Liu et al., 2019), one study has indicated that women carriers of the ‘A’ allele who attempt to quit with the aid of pharmacotherapies may have higher success rates (Tomaz et al., 2018). With respect to therapeutic interventions, it has also been suggested that individuals who carry the CHRNA5 SNP may be amenable to treatment by ‘positive allosteric modulators’ (PAMs) (Kuryatov et al., 2011; Maskos, 2020). These small molecules could restore the loss of function associated with CHRNA5 D398N by specifically targeting α5-containing nAChRs. As yet, however, there is no behavioral evidence demonstrating the treatment efficacy of PAMs amongst carriers of the CHRNA5 SNP. Because nAChRs containing α5 subunits reside in brain regions that also modulate neural responses to drugs like opioids, cocaine and alcohol, the SNP may be relevant in identifying therapies for addiction to these other substances as well. Further studies in vitro and in animal models will allow detailed investigations into the mechanisms underlying the influence of the SNP on treatment response, which will be necessary to make progress toward personalized medicine.
Clinical findings documenting familial patterns of drug use and dependence and GWAS have identified a number of genes and allelic variants that contribute to the risk for substance use disorders. However, understanding how these allelic variants, particularly single nucleotide polymorphisms (SNPs), contribute to addiction is complicated by the fact that each variant contributes to only a small fraction of the phenotype. Further, environmental factors that are known to modulate disease phenotypes are difficult to control in any given population. Thus, the ability to interrogate addiction-relevant SNPs in isolation and in a controlled manner is critical. Here we review the impact of the CHRNA5 D398N SNP across species and scales. The ability to integrate findings in human, animal and cellular systems provides an optimal framework to not only investigate the pathology associated with addiction, but to facilitate the development and delivery of new treatments.
Highlights.
Up to 28% of the worldwide population carry at least one copy of CHRNA5 D398N
D398N has been linked to risk for dependence to nicotine, opioids, cocaine, alcohol
In humans, resting-state functional connectivity is moderated by CHRNA5 genotype
Animal models expressing the CHRNA5 SNP show alterations in reward pathways
Acknowledgments
Funding: This work was supported by NIH grants R01-DA041180 (J.A.B.) and T32-DA028874 (J.K.B.). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amos CI, Gorlov IP, Dong Q, Wu X, Zhang H, Lu EY, Scheet P, Greisinger AJ, Mills GB, Spitz MR, 2010. Nicotinic acetylcholine receptor region on chromosome 15q25 and lung cancer risk among African Americans: a case-control study. J. Natl. Cancer Inst. 102, 1199–1205. doi: 10.1093/jnci/djq232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroche AP, Rovaris DL, Grevet EH, Stolf AR, Sanvicente-Vieira B, Kessler FHP, Diemen, von L, Grassi-Oliveira R, Bau CHD, Schuch JB, 2020. Association of CHRNA5 Gene Variants with Crack Cocaine Addiction. Neuromolecular Med. 7, 248–7. doi: 10.1007/s12017-020-08596-1 [DOI] [PubMed] [Google Scholar]
- Bailey CDC, Alves NC, Nashmi R, De Biasi M, Lambe EK, 2012. Nicotinic α5 subunits drive developmental changes in the activation and morphology of prefrontal cortex layer VI neurons. Biol. Psychiatry 71, 120–128. doi: 10.1016/j.biopsych.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CDC, De Biasi M, Fletcher PJ, Lambe EK, 2010. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 30, 9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V, 2008. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 13, 368–373. doi: 10.1038/sj.mp.4002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Forget B, Correia C, Blanco R, Maskos U, 2019. Profound alteration in reward processing due to a human polymorphism in CHRNA5: a role in alcohol dependence and feeding behavior. Neuropsychopharmacology 44, 1906–1916. doi: 10.1038/s41386-019-0462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger J, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM, 2008. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165, 1163–1171. doi: 10.1176/appi.ajp.2008.07111711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Rukstalis M, Stewart WF, 2011. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res 188, 173–174. doi: 10.1016/j.psychres.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RWB, Collins AC, Lindstrom JM, Whiteaker P, 2007. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J. Neurochem. 103, 204–215. doi: 10.1111/j.1471-4159.2007.04700.x [DOI] [PubMed] [Google Scholar]
- Brynildsen JK, Perez EE, De Biasi M, Blendy JA, 2017. A common SNP in Chrna5 attenuates dopamine release from the nucleus accumbens in response to morphine, in:. Presented at the Society for Neuroscience Annual Meeting, Washington, DC. [Google Scholar]
- Calabresi P, Lacey MG, North RA, 1989. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br. J. Pharmacol. 98, 135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Devine DP, Wise RA, 1995. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl.) 122, 194–197. doi: 10.1007/BF02246095 [DOI] [PubMed] [Google Scholar]
- Chaarani B, Kan K-J, Mackey S, Spechler PA, Potter A, Orr C, D’Alberto N, Hudson KE, Banaschewski T, Bokde ALW, Bromberg U, Büchel C, Cattrell A, Conrod PJ, Desrivieres S, Flor H, Frouin V, Gallinat J, Gowland P, Heinz A, Ittermann B, Martinot J-L, Nees F, Papadopoulos-Orfanos D, Paus T, Poustka L, Smolka MN, Walter H, Whelan R, Higgins ST, Schumann G, Althoff RR, Stein EA, Garavan H, IMAGEN Consortium, 2019. Low Smoking Exposure, the Adolescent Brain, and the Modulating Role of CHRNA5 Polymorphisms. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 672–679. doi: 10.1016/j.bpsc.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Hung RJ, Baker T, Horton A, Culverhouse R, Saccone N, Cheng I, Deng B, Han Y, Hansen HM, Horsman J, Kim C, Lutz S, Rosenberger A, Aben KK, Andrew AS, Breslau N, Chang S-C, Dieffenbach AK, Dienemann H, Frederiksen B, Han J, Hatsukami DK, Johnson EO, Pande M, Wrensch MR, McLaughlin J, Skaug V, van der Heijden HF, Wampfler J, Wenzlaff A, Woll P, Zienolddiny S, Bickeböller H, Brenner H, Duell EJ, Haugen A, Heinrich J, Hokanson JE, Hunter DJ, Kiemeney LA, Lazarus P, Le Marchand L, Liu G, Mayordomo J, Risch A, Schwartz AG, Teare D, Wu X, Wiencke JK, Yang P, Zhang Z-F, Spitz MR, Kraft P, Amos CI, Bierut LJ, 2015. CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis--a meta-analysis. J. Natl. Cancer Inst. 107, 24. doi: 10.1093/jnci/djv100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Johnson EO, Breslau N, Hatsukami D, Saccone NL, Grucza RA, Wang JC, Hinrichs AL, Fox L, Goate AM, Rice JP, Bierut LJ, 2009. Interplay of Genetic Risk Factors and Parent Monitoring in Risk for Nicotine Dependence. Addiction 104, 1731–1740. doi: 10.1111/j.1360-0443.2009.02697.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An S-S, Hettema JM, Aggen SH, Neale MC, Kendler KS, 2009. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 926–933. doi: 10.1002/ajmg.b.30919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon MS, Bewick MA, 2011. Single nucleotide polymorphisms in CHRNA5 rs16969968, CHRNA3 rs578776, and LOC123688 rs8034191 are associated with heaviness of smoking in women in Northeastern Ontario, Canada. Nicotine Tob. Res. 13, 1076–1083. doi: 10.1093/ntr/ntr140 [DOI] [PubMed] [Google Scholar]
- Conroy WG, Berg DK, 1995. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J. Biol. Chem. 270, 4424–4431. doi: 10.1074/jbc.270.9.4424 [DOI] [PubMed] [Google Scholar]
- Curtis K, Viswanath H, Velasquez KM, Molfese DL, Harding MJ, Aramayo E, Baldwin PR, Ambrosi E, Madan A, Patriquin M, Frueh BC, Fowler JC, Kosten TR, Nielsen DA, Salas R, 2017. Increased habenular connectivity in opioid users is associated with an α5 subunit nicotinic receptor genetic variant. Am J Addict 26, 751–759. doi: 10.1111/ajad.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P, 2010. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur. J. Neurosci. 31, 978–993. doi: 10.1111/j.1460-9568.2010.07133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Wolstenholme JT, Roni MA, Campbell VC, Jackson A, Slater C, Bagdas D, Perez EE, Bettinger JC, De Biasi M, Miles MF, Damaj MI, 2018. Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice. Neuropharmacology 138, 341–348. doi: 10.1016/j.neuropharm.2018.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM, 2008. Nicotine and brain development. Birth Defects Res C Embryo Today 84, 30–44. doi: 10.1002/bdrc.20118 [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM, 2009. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 122, 125–139. doi: 10.1016/j.pharmthera.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, Linda Kao WH, Gerhard GS, Stewart WF, Boscarino JA, 2010. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum. Genet. 128, 491–499. doi: 10.1007/s00439-010-0876-6 [DOI] [PubMed] [Google Scholar]
- Etter J-F, Hoda J-C, Perroud N, Munafo M, Buresi C, Duret C, Neidhart E, Malafosse A, Bertrand D, 2009. Association of genes coding for the alpha-4, alpha-5, beta-2 and beta-3 subunits of nicotinic receptors with cigarette smoking and nicotine dependence. Addict Behav 34, 772–775. doi: 10.1016/j.addbeh.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H-Y, Hiller-Sturmhofel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29, 162–171. [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA, 1997. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J. Neurosci. 17, 5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Duka T, 2004. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol. Biochem. Behav. 78, 647–652. doi: 10.1016/j.pbb.2004.03.026 [DOI] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, Fornasari D, 2000. Neuronal and extraneuronal expression and regulation of the human alpha5 nicotinic receptor subunit gene. J. Neurochem. 75, 18–27. doi: 10.1046/j.1471-4159.2000.0750018.x [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ, 1992. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol. Pharmacol. 41, 31–37. [PubMed] [Google Scholar]
- Forget B, Icick R, Robert J, Correia C, Prevost MS, Gielen M, Corringer P-J, Bellivier F, Vorspan F, Besson M, Maskos U, 2020. Alterations in nicotinic receptor alpha5 subunit gene differentially impact early and later stages of cocaine addiction: a translational study in transgenic rats and patients. Prog. Neurobiol. 101898. doi: 10.1016/j.pneurobio.2020.101898 [DOI] [PubMed] [Google Scholar]
- Forget B, Scholze P, Langa F, Morel C, Pons S, Mondoloni S, Besson M, Durand-de Cuttoli R, Hay A, Tricoire L, Lambolez B, Mourot A, Faure P, Maskos U, 2018. A Human Polymorphism in CHRNA5 Is Linked to Relapse to Nicotine Seeking in Transgenic Rats. Curr. Biol. 28, 3244–3253.e7. doi: 10.1016/j.cub.2018.08.044 [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. doi: 10.1038/nature09797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine J-F, Tsetlin V, Maskos U, Ibañez-Tallon I, 2011. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70, 522–535. doi: 10.1016/j.neuron.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M, 2009. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 8, 398–406. doi: 10.1111/j.1601-183X.2009.00476.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F, 2005. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 6, 521–532. doi: 10.1038/nrg1635 [DOI] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD, 2012. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb Perspect Med 2, a012120–a012120. doi: 10.1101/cshperspect.a012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN, 2009. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 78, 756–765. doi: 10.1016/j.bcp.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C, 2009. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 29, 2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ, 2008. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol. Psychiatry 64, 922–929. doi: 10.1016/j.biopsych.2008.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hällfors J, Loukola A, Pitkäniemi J, Broms U, Männistö S, Salomaa V, Heliövaara M, Lehtimäki T, Raitakari O, Madden PA, Heath AC, Montgomery GW, Martin NG, Korhonen T, Kaprio J, 2013. Scrutiny of the CHRNA5-CHRNA3-CHRNB4 smoking behavior locus reveals a novel association with alcohol use in a Finnish population based study. Int J Mol Epidemiol Genet 4, 109–119. [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA, 2009. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch. Gen. Psychiatry 66, 431–441. doi: 10.1001/archgenpsychiatry.2009.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA, 2010. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. U.S.A. 107, 13509–13514. doi: 10.1073/pnas.1004745107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, Stein ES, Thaker GK, 2011. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav. 10, 530–535. doi: 10.1111/j.1601-183X.2011.00689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP, 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212, 149–179. doi: 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hsu Y-WA, Tempest L, Quina LA, Wei AD, Zeng H, Turner EE, 2013. Medial habenula output circuit mediated by α5 nicotinic receptor-expressing GABAergic neurons in the interpeduncular nucleus. J. Neurosci. 33, 18022–18035. doi: 10.1523/JNEUROSCI.2927-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K, 2005. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J. Comp. Neurol. 493, 241–260. doi: 10.1002/cne.20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI, 2010. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 334, 137–146. doi: 10.1124/jpet.110.165738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 325, 302–312. doi: 10.1124/jpet.107.132977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Smoller JW, David SP, Frederick BD, Haddad S, Basu A, Fava M, Evins AE, Kaufman MJ, 2012. Association between CHRNA5 genetic variation at rs16969968 and brain reactivity to smoking images in nicotine dependent women. Drug Alcohol Depend 120, 7–13. doi: 10.1016/j.drugalcdep.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M, 2015. A CHRNA5 Smoking Risk Variant Decreases the Aversive Effects of Nicotine in Humans. Neuropsychopharmacology 40, 2813–2821. doi: 10.1038/npp.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA, 1992. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Barrow J, 1989. Regulation of the mesocorticolimbic dopamine system by glutamic acid receptor subtypes. J. Pharmacol. Exp. Ther. 251, 378–387. [PubMed] [Google Scholar]
- Karoly HC, Harlaar N, Hutchison KE, 2013. Substance use disorders: a theory-driven approach to the integration of genetics and neuroimaging. Ann. N. Y. Acad. Sci. 1282, 71–91. doi: 10.1111/nyas.12074 [DOI] [PubMed] [Google Scholar]
- Kedmi M, Beaudet AL, Orr-Urtreger A, 2004. Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures. Physiol. Genomics 17, 221–229. doi: 10.1152/physiolgenomics.00202.2003 [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo X-N, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP, 2011. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry 69, 684–692. doi: 10.1016/j.biopsych.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA, 1999. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med 29, 299–308. doi: 10.1017/s0033291798008022 [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove D’Exaerde A, Zoli M, Changeux JP, 2001. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 21, 1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukouli F, Rooy M, Tziotis D, Sailor KA, O’Neill HC, Levenga J, Witte M, Nilges M, Changeux J-P, Hoeffer CA, Stitzel JA, Gutkin BS, DiGregorio DA, Maskos U, 2017. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat. Med. 23, 347–354. doi: 10.1038/nm.4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J, 2011. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol. Pharmacol. 79, 119–125. doi: 10.1124/mol.110.066357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère N, Corringer P-J, Changeux J-P, 2002. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456. doi: 10.1002/neu.10153 [DOI] [PubMed] [Google Scholar]
- Li MD, Yoon D, Lee J-Y, Han B-G, Niu T, Payne TJ, Ma JZ, Park T, 2010. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS ONE 5, e12183. doi: 10.1371/journal.pone.0012183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, McCollum M, Bracamontes J, Steinbach JH, Akk G, 2011. Functional characterization of the α5(Asn398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor. Mol. Pharmacol. 80, 818–827. doi: 10.1124/mol.111.073841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao RM, Chang YH, Wang SH, Lan CH, 2000. Distinct accumbal subareas are involved in place conditioning of amphetamine and cocaine. Life Sci. 67, 2033–2043. doi: 10.1016/s0024-3205(00)00789-x [DOI] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, Deneen, von KM, Liu Y, Gold MS, 2009. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci. Lett. 460, 72–77. doi: 10.1016/j.neulet.2009.05.038 [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJF, Barroso I, Khaw K-T, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann H-E, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schäfer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St Clair D, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Völzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson CA, Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, Thomson W, Eyre S, Barton A, Wellcome Trust Case Control Consortium, Mooser V, Francks C, Marchini J, 2010. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 42, 436–440. doi: 10.1038/ng.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X, 23andMe Research Team, HUNT All-In Psychiatry, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga J-J, Huang H, Jang S-K, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orru V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S, 2019. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51, 237–244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, Xu H-S, Fu X-M, Hu X, Zhang D-R, 2010. Addiction related alteration in resting-state brain connectivity. Neuroimage 49, 738–744. doi: 10.1016/j.neuroimage.2009.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS, 2002. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919. doi: 10.1016/s0896-6273(02)00625-6 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS, 2002. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 53, 606–617. doi: 10.1002/neu.10148 [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ, 2008. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J. Neurochem. 104, 446–456. doi: 10.1111/j.1471-4159.2007.05011.x [DOI] [PubMed] [Google Scholar]
- Maskos U, 2020. The nicotinic receptor alpha5 coding polymorphism rs16969968 as a major target in disease: Functional dissection and remaining challenges. J. Neurochem. 154, 241–250. doi: 10.1111/jnc.14989 [DOI] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT, 2014. Separate GABA Afferents to Dopamine Neurons Mediate Acute Action of Opioids, Development of Tolerance, and Expression of Withdrawal. Neuron 82, 1346–1356. doi: 10.1016/j.neuron.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W, Maskos U, Faure P, 2014. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 19, 930–936. doi: 10.1038/mp.2013.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton G, Nasirova N, Sparks DW, Brodsky M, Sivakumaran S, Lambe EK, Turner EE, 2018. Chrna5-expressing neurons in the interpeduncular nucleus mediate aversion primed by prior stimulation or nicotine exposure. J. Neurosci. 38, 6900–6920. doi: 10.1523/JNEUROSCI.0023-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, McIntosh JM, Dierssen M, Miles MF, Chen X, De Biasi M, Damaj MI, 2014. The α3β4* nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies. Br. J. Pharmacol. 171, 3845–3857. doi: 10.1111/bph.12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Einstein EB, Lopez MB, McClure-Begley TD, Mineur YS, Picciotto MR, 2013. Morphine dependence and withdrawal induced changes in cholinergic signaling. Pharmacol. Biochem. Behav. 109, 77–83. doi: 10.1016/j.pbb.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC, 2003. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J. Pharmacol. Exp. Ther. 307, 1090–1097. doi: 10.1124/jpet.103.056408 [DOI] [PubMed] [Google Scholar]
- O’Neill HC, Wageman CR, Sherman SE, Grady SR, Marks MJ, Stitzel JA, 2018. The interaction of the Chrna5 D398N variant with developmental nicotine exposure. Genes Brain Behav. 17, e12474. doi: 10.1111/gbb.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni EN, Halikere A, Li G, Toro-Ramos AJ, Swerdel MR, Verpeut JL, Moore JC, Bello NT, Bierut LJ, Goate A, Tischfield JA, Pang ZP, Hart RP, 2016. Increased nicotine response in iPSC-derived human neurons carrying the CHRNA5 N398 allele. Sci Rep 6, 34341–11. doi: 10.1038/srep34341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Morales R, González-Zamora A, González-Delgado MF, Calleros Rincón EY, Olivas Calderón EH, Martínez-Ramírez OC, Rubio J, 2018. CHRNA3 rs1051730 and CHRNA5 rs16969968 polymorphisms are associated with heavy smoking, lung cancer, and chronic obstructive pulmonary disease in a mexican population. Ann. Hum. Genet. 82, 415–424. doi: 10.1111/ahg.12264 [DOI] [PubMed] [Google Scholar]
- Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A, 2013. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. doi: 10.1038/nature12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA, 1997. Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390, 401–404. doi: 10.1038/37120 [DOI] [PubMed] [Google Scholar]
- Quina LA, Harris J, Zeng H, Turner EE, 2017. Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. J. Comp. Neurol. 525, 2632–2656. doi: 10.1002/cne.24221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, 2004. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 7, 583–584. doi: 10.1038/nn1244 [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An T-H, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliövaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang B-Z, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PAF, Nöthen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ, 2010. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 6, e1001053. doi: 10.1371/journal.pgen.1001053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PAF, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ, 2009a. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 453–466. doi: 10.1002/ajmg.b.30828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ, 2009b. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 69, 6848–6856. doi: 10.1158/0008-5472.CAN-09-0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ, 2007. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 16, 36–49. doi: 10.1093/hmg/ddl438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M, 2003. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 63, 1059–1066. doi: 10.1124/mol.63.5.1059 [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M, 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 29, 3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR, 2004. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol. Pharmacol. 65, 1526–1535. doi: 10.1124/mol.65.6.1526 [DOI] [PubMed] [Google Scholar]
- Santos N, Chatterjee S, Henry A, Holgate J, Bartlett SE, 2013. The α5 neuronal nicotinic acetylcholine receptor subunit plays an important role in the sedative effects of ethanol but does not modulate consumption in mice. Alcohol. Clin. Exp. Res. 37, 655–662. doi: 10.1111/acer.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ, 1983. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220, 214–216. doi: 10.1126/science.6828889 [DOI] [PubMed] [Google Scholar]
- Sciaccaluga M, Moriconi C, Martinello K, Catalano M, Bermudez I, Stitzel JA, Maskos U, Fucile S, 2015. Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 29, 3389–3398. doi: 10.1096/fj.14-268102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM, 1992. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J. Comp. Neurol. 320, 145–160. doi: 10.1002/cne.903200202 [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J, 2010. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology 35, 1921–1931. doi: 10.1038/npp.2010.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, Bierut LJ, Neuman RJ, Pomerleau OF, 2008. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with “pleasurable buzz” during early experimentation with smoking. Addiction 103, 1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE, 2008. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol. Biomarkers Prev. 17, 3517–3525. doi: 10.1158/1055-9965.EPI-08-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA, 1992. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci. Lett. 134, 207–211. doi: 10.1016/0304-3940(92)90518-C [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA, 2012. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62, 2281–2295. doi: 10.1016/j.neuroimage.2012.01.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC, 1995. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J. Neurochem. 65, 1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x [DOI] [PubMed] [Google Scholar]
- Tammimaki A, Herder P, Li P, Esch C, Laughlin JR, Akk G, Stitzel JA, 2012. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharmacology 63, 1002–1011. doi: 10.1016/j.neuropharm.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz PRX, Santos JR, Scholz J, Abe TO, Gaya PV, Negrño AB, Krieger JE, Pereira AC, Santos PCJL, 2018. Cholinergic receptor nicotinic alpha 5 subunit polymorphisms are associated with smoking cessation success in women. BMC Med. Genet. 19, 55–8. doi: 10.1186/s12881-018-0571-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde Z, Santiago C, Rodríguez González-Moro JM, de Lucas Ramos P, López Martín S, Bandrés F, Lucia A, Gómez-Gallego F, 2011. “Smoking genes”: a genetic association study. PLoS ONE 6, e26668. doi: 10.1371/journal.pone.0026668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142, 1–20. doi: 10.1016/j.neuroscience.2006.06.027 [DOI] [PubMed] [Google Scholar]
- Vibat CR, Lasalde JA, McNamee MG, Ochoa EL, 1995. Differential desensitization properties of rat neuronal nicotinic acetylcholine receptor subunit combinations expressed in Xenopus laevis oocytes. Cell. Mol. Neurobiol. 15, 411–425. doi: 10.1007/BF02071877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI, 2005. Heritability of smoking initiation and nicotine dependence. Behav. Genet. 35, 397–406. doi: 10.1007/s10519-004-1327-8 [DOI] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW, 1990. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res. 526, 45–53. doi: 10.1016/0006-8993(90)90248-a [DOI] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM, 2009. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol. Psychiatry 14, 501–510. doi: 10.1038/mp.2008.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH, 2004. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol 72, 1136–1143. doi: 10.1037/0022-006X.72.6.1136 [DOI] [PubMed] [Google Scholar]
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Müller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Moller H-J, Gieger C, Wichmann H-E, Illig T, Dahmen N, Rujescu D, 2010. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1448–1458. doi: 10.1002/ajmg.b.31126 [DOI] [PubMed] [Google Scholar]
- Wolfman SL, Gill DF, Bogdanic F, Long K, Al-Hasani R, McCall JG, Bruchas MR, McGehee DS, 2018. Nicotine aversion is mediated by GABAergic interpeduncular nucleus inputs to laterodorsal tegmentum. Nat Commun 9, 2710. doi: 10.1038/s41467-018-04654-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ, 2012. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev 36, 825–835. doi: 10.1016/j.neubiorev.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA, 2001. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 4, 1224–1229. doi: 10.1038/nn769 [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C, 2002. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J. Neurosci. 22, 8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]