Abstract
Bacteria, viruses, protozoa, and fungi establish a complex ecosystem in the gut. Like other microbiota, gut mycobiota plays an indispensable role in modulating intestinal physiology. Notably, the most striking characteristics of intestinal fungi are their extraintestinal functions. Here, we provide a comprehensive review of the importance of gut fungi in the regulation of intestinal, pulmonary, hepatic, renal, pancreatic, and brain functions, and we present possible opportunities for the application of gut mycobiota to alleviate/treat human diseases.
Video Abstract.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-021-01024-x.
Keywords: Intestinal mycobiota, Intestinal immunity, Gut-liver axis, Gut-lung axis, Gut-brain axis
Introduction
Intestinal tract is home to bacteria, archaea, fungi, viruses, and protozoa, which mutually interact with the host [1, 2]. In general, investigation of intestinal commensal-host symbiosis places emphasis on gut bacteria while neglecting gut fungi due to their lower abundance (0.01–0.1% of gut microbiome) [1, 3, 4]. For example, in the period of 2008–2018, there have been almost 100 times more peer-reviewed publications on microbiota than on mycobiota [5].
Commensal fungi exist in various body parts (e.g., oral cavity, gastrointestinal tract, and vagina) and sustain a relative stable colonization [6, 7]. Invasion by exogenous pathogenic fungi (e.g., inhaled environmental fungi) can disrupt the fungal homeostasis in the lungs; uncontrollable transfer of commensal fungi in immune incompetent individuals also leads to systemic disequilibrium [3, 8]. Thus, fungi such as Candida albicans [9], Zygomycetes, Fusarium, Acremonium, Trichosporon, and Penicillium marneffei [10] are associated with the occurrence of hospital-acquired and/or intercurrent fungal infections while the individuals are immunocompromised. In addition to causing infections, fungi affect the host immune responses to other stimuli both locally and distally [11, 12]. For example, recognition of β-1,2-mannans (a component of fungal cell wall) by galectin-3 [13] enhances toll-like receptor (TLR) 2- and TLR4-mediated immune responses in mouse primary splenocytes and human THP-1 cells [14, 15]. Thus, fungi are associated with the health and disease of the host. Notably, the effects of bacteria and fungi on the immune system are very similar, and intestinal bacteria could influence intestinal fungi; thus, the interaction between gut fungi and bacteria should not be neglected. For example, Escherichia coli super-infection promotes C. albicans virulence under certain circumstances [16], and commensal bacterial in the intestine of adult mice, especially Bacteroidetes and Firmicutes species, are major resisters for C. albicans colonization [17].
Recent evidence of a significant influence of gut mycobiota on host’s health has stimulated further research on gut mycobiota. Notably, despite relatively small number of gut fungi, they profoundly affect nutrition, metabolism, and immunity in the intestine [1, 2, 18–20]. Not only do intestinal fungi shape the functions of the gut, but they also affect the physiological functions of other crucial extraintestinal organs, such as the liver, lung, and brain [1]. Here, we summarize the current knowledge about gut mycobiota, including the methodologies for studying gut fungi, colonization, and composition of gut mycobiota, and the affecting factors. We also highlight the importance of gut fungi on the intestine and intestinal-associated distal targets, including gut-lung axis, gut-liver axis, gut-brain axis, and possibly gut-kidney axis and gut-pancreas axis. Multiple targets of gut fungi may offer new possibilities for diagnosis and treatment of various diseases.
Methodologies for studying gut fungi
There is still relatively poor understanding of the influence of gut mycobiota on host’s health and disease. One of main reason is that fungi have been traditionally investigated through culture-dependent methods, which largely limited the in-depth understanding of the fungal microbiota. Fortunately, the study of fungi populations benefited from deep-sequencing technologies and bioinformatics analysis developed for bacteria [e.g., next-generation sequencing (NGS)]. In this section, we briefly introduce the methodologies for studying gut mycobiome.
Culture-dependent methods
Traditionally, fungal diversity was assessed using culture-dependent methods, which highly depended on the culture medium. For example, Sabouraud dextrose agar (SDA) is a commonly used medium for fungi especially for filamentous fungi [21, 22], while blood agar (BA) and chocolate agar (CA) are used for fungi isolated from mycotic keratitis patients [21]. CHROMagarTM Candida medium has been used for Candida separation [23, 24], whereas ID fungi plate culture with matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) is suitable for pathogenic filamentous fungi [25]. Obviously, cultivation of fungi is the most direct method with visualization of fungal morphology and colony color. However, there are some limitations in its application. (1) During cultivation, the environmental fungi (e.g., Aspergillus) easily affect the precision of results, and even slight differences in culture process lead to the change in fungi morphology [25]. (2) It is difficult to distinguish specific species and genus of similarly looking fungi even by microscope, and some fungi cannot be cultured in the existing culture modes [26]. (3) Culture methods are very time-consuming and not adaptable to high-throughput analysis. Fortunately, some new culture-dependent approaches (e.g., culturomics or combination of culture and sequencing) are now used for fungus characterization [27].
Culture-independent methods
Recent advances in deep-sequencing technologies and bioinformatics analysis, which are based on the analysis of genomic DNA, have shed light on the complexity of the gut fungal communities. Interestingly, these methods could also be applied to a single fungus isolated from culture-dependent methods. Generally, many culture-independent methods have been developed, such as sequencing for 18s ribosomal DNA and internal transcribed spacer regions (ITS, 1 and 2) [28], denaturing gradient gel electrophoresis (DGGE) [7], amplified fragment length polymorphism fingerprinting (AFLP) [29], restriction fragment length polymorphoresis (RFLP), oligonucleotide fingerprinting of ribosomal RNA genes (OFRG) [30], and high-throughput sequence technology (HTST) [31]. Of note, the factors affecting fungi identification with culture-independent methods include DNA extraction (quality and quantity), primer selection (e.g., preferential ITS fragment), and bioinformatics pipelines (e.g., assignment algorithms) and databases (available or not) [32].
Overall, there is no consensus on the optimal methodology for characterizing gut mycobiome, and the available results vary depending on the chosen analytic method [33, 34], indicating that the methods selected for fungal analysis should be noted when comparing the results on intestinal fungi between the studies.
Colonization and composition of gut mycobiota
With the recent development of methodology, we have reached preliminary understanding of the colonization and composition of intestinal fungi. Gut colonization of mycobiota in humans has been established at 10 days after birth [35]. In the neonatal gut, the alpha diversity of fungi consistently increases from birth to 2 years of age, while the beta diversity reaches to the peak in a 10-day infant [35]. However, given the limitation of samples and methods for fungal detection, we cannot rule out the possibility that the colonization of gut fungi starts at the time of birth or even earlier (e.g., amniotic fluid exposure, umbilical substance exchange with mother [36]). Recently, fungi have been found in the first-pass meconium, suggesting that colonization of gut mycobiota starts at birth [37]. Nevertheless, due to the lack of direct evidence and low biomass of meconium, it is difficult to ignore the effects of environmental factors. New models that could mimic the entire pregnancy and advanced detection techniques are urgently needed for more convincing conclusions.
Similar with the gut bacteriota, there are temporal and spatial changes in the distribution and composition of gut mycobiota. It has been reported that in 10-day- to 3-month-old infants, Debaryomyces hansenii and Rhodotorula mucilaginosa are the most abundant fungi, while after 1–2 years, the composition of gut mycobiota changes, where S. cerevisiae becomes the most abundant fungus while the Candida spp. start to decrease [35]. Moreover, Aspergillus penicillioides is not detected in infants aged 10 days to 3 months, but could be detected in 1- to 2-year-old infants [35]. Intriguingly, Cystofilobasidium sp., Ascomycota sp., and Monographella sp. are only detected in 1- to 2-year-old offspring [35]. Afterwards, Ascomycota, Zygomycota, and Basidiomycota phyla dominate in healthy intestinal mycobiota [38], and Candida is the most prevalent and abundant fungi dwelling in gastrointestinal tract and other mucosal surfaces in humans and several other animals [2, 3, 39], while other genera include Pichia, Saccharomyces, Cladosporium, Malassezia, Aspergillus, Trichosporon, Penicillium, and Mucor [2]. Overall, the characterization of colonization and composition of intestinal fungi is still in its infancy.
Factors affecting the composition of gut mycobiota
Of note, similar like gut bacteria [36], gut fungi colonize neonatal gut instantly after birth, while the composition could be affected by delivery mode, gestational age at birth, infant feeding mode, maternal diet, environment, and host genetics (Table 1).
Table 1.
Factors affecting the composition of gut mycobiota
| Factors | Composition of gut mycobiota | References | ||
|---|---|---|---|---|
| Delivery method | Natural birth |
Fungi from mother’s genital tract ↑ Russulales ↑ |
[37, 40–42] | |
| C-section |
Fungi from maternal skin and surroundings ↑ Saccharomycetales ↑ |
|||
| Gestational age | Preterm infants |
Fungal diversity ↓ Saccharomycetales ↑ Candida ↑ |
[37] | |
| Term infants |
Polyporales ↑ Russulales ↑ Stereum ↑ Malassezia ↑ |
|||
| Environment | Mice from Jackson Laboratory’s & Services (JAX) | Basidiomycota ↑ | [18, 43] | |
| Mice from Weill Cornell Medicine (WCM-CE) | Ascomycota ↑ | |||
| SPF mice "rewilded" into the wild |
Candida ↑ Aspergillus ↑ |
|||
| Season | Spring |
Sclerotiniaceae ↑ Nectriaceae ↑ |
[19] | |
| Summer | Trichocomaceae ↑ | |||
| Autumn |
Wallemiaceae ↑ Hypocreaceae ↑ |
|||
| Winter | Devriesia ↑ | |||
| Diet and nutrition | Nutrition | Pistachio and almond |
Penicillium spp. ↓ Candida spp. ↓ |
[1, 27, 44–50] |
| Carbohydrate-rich diet | Candida ↑ | |||
| High-fat diet | S. cerevisiae ↓ | |||
| Protein-rich diet |
Methanobrevibacter ↓ Candida ↓ |
|||
| 2-hydroxyisocaproic acid (leucine derivative) |
Candida ↓ Aspergillus ↓ |
|||
| Microbial metabolites of nutrients | Short chain fatty acid (SCFAs) |
Aspergillus ↓ Metschnikowia ↓ |
||
| Acetate | Tomentella ↑ | |||
| Acetate and propionate |
Nephroma ↓ Taiwanofungus ↓ |
|||
| Butyrate and total SCFAs | Tomentella ↑ | |||
| Propionate | Loreleia ↑ | |||
| Gender | Female | Mycosphaerellaceae ↑ | [19] | |
| Male |
Ascomycota ↑ Tetraplosphaeriaceae ↑ |
|||
| Metabolic disorder | Obese | Yeast fungi ↑ | [27] | |
| Eutrophic | Filamentous fungi ↑ | |||
| Maternal antibiotic exposure | Saccharomycetales ↑ | [37] | ||
| Species | Gut Candida spp. only found in mammalian | [44, 49] | ||
| Chenghua, Yorkshire, and Tibetan pigs have different fungal abundance | ||||
“↑” indicates increase and “↓” indicates decrease
Delivery mode and maternal probiotic exposure
For neonates, the mother is one of the main factors affecting their gut fungal composition. Namely, healthy gut mycobiota colonization at birth is affected by delivery mode (vaginal versus cesarean) [40]. Naturally born infants are more likely to get fungi (e.g., Candida albicans) from maternal genital tract, whereas infants born after cesarean delivery are more likely to get fungi from maternal skin and surroundings [41]. Apart from the mode of delivery, gestational age and maternal probiotic exposure also determine gut mycobiota composition in infants [37]. However, in 298 pairs of mother-offspring subjects, Schei et al. reported that the type of delivery and/or use of infant antibiotic or maternal probiotic had little effect on the operational taxonomic units (OTUs) abundance of gut fungi [35]. This discrepancy may stem from the samples analyzed since they selected mother-offspring pairs who all participated in a single study.
Diet and nutrition
Diet is one of the determinants in shaping gut mycobiota composition. It has even been suggested that the source of fungi in the oral cavity and diet can explain all the fungi present in the feces of healthy subjects, suggesting the great influence of diet on intestinal fungal composition [51]. Interestingly, Western diet has a high risk of triggering metabolic syndrome due to high levels of fats and carbohydrates, and it has been shown to induce the alterations of intestinal fungal structure of human [52]. For example, diet with high amounts of fat decreases S. cerevisiae abundance in the gut of mice [1]. The abundance of Metschnikowia, Tomentella, and Loreleia correlates with short chain fat acids (SCFAs) in pigs [44], and SCFAs are negatively associated with the intestinal abundance of Aspergillus in human [45]. Moreover, carbohydrate-rich diet correlates positively with the abundance of gut Candida [46]. Likewise, diets with high levels of protein correlate negatively with the abundance of Methanobrevibacter and Candida in healthy volunteers [45]. Interestingly, S. cerevisiae have amino acid transporters, and some amino acids, such as gamma-aminobutyric acid (GABA) and citrulline, are important nitrogen sources for S. cerevisiae; thus, dietary amino acids probably have profound impact on gut mycobiota composition [53]. Furthermore, 2-hydroxyisocaproic acid, a metabolic by-product of leucine, is fungicidal at 72 mg/mL and fungistatic at a lower concentration, which could inhibit Candida hyphal formation [47]. Particular chemical compounds in human diet, such as phytochemicals from pistachio and almond, also negatively correlate with the abundance of Penicillium spp. and Candida spp. [48]. Taken together, it is important to examine whether diet control has potential to alleviate fungal infections. If this strategy works, it could be particularly beneficial for immunosuppressed patients to prevent and control secondary infections.
Other factors
Environmental fungi pass through the respiratory and gastrointestinal tract, and they can be a source of transitory colonization of gut mycobiota [5]. Facility environments also are capable of triggering changes in gut mycobiota. Interestingly, C57BL/6J mice acquired from Jackson Laboratory’s Mice & Services (JAX) and those bred at Weill Cornell Medicine (WCM-CE) differ in the composition of gut mycobiota and are dominated by Basidiomycota and Ascomycota, respectively [18]. Moreover, when SPF mice are “rewilded” into the wild condition, they experience a significant increase in intestinal fungi [43]. Season is another factor altering gut mycobiota, especially alpha diversity of fungi [19]. Gender and metabolic disorder (eutrophic, overweight, and obesity) shift gut mycobiota as well. For instance, female Tibetan macaques have different mycobiota compared with male Tibetan macaques [19]. Increased yeast is observed in obese human individuals, whereas eutrophic and overweight human individuals have more filamentous fungi [27]. Gut Candida spp. is found exclusively in mammalian species [49]. Therefore, to some extent, gut mycobiota probably varies by species or genotypes [44]. Collectively, gut mycobiota is affected by both internal and external factors (Table 1).
It should be noted that these factors are not independent. Namely, season and environment are associated with available food that mammals live on, especially for wild animals [19]. The environment also affects host’s exposure to the potential pathogenic or non-pathogenic microorganisms [43]. Moreover, different breeds always have different genetic background, not to mention food available to them [44]. Because offspring at 10 days and 3 months are almost exclusively breast-fed, their gut mycobiota are dominated by Debaryomyces hansenii from breast milk [35]. Consequently, the change in intestinal fungal communities from D. hansenii to S. cerevisiae has been observed to occur with the shift in diet from breastfeeding or formula milk to solid food [54]. Besides, with further growth, infants are subjected to diverse diets, exposed to complex environments and stimulus such as drugs and sexual hormones [27]. Therefore, these factors must be recognized in a systematic and interconnected manner, and it is not appropriate to overemphasize the unique roles of each factor alone.
Cross-talk between gut mycobiota and host immunity
Among the different kingdoms represented in the gut microbiota, the eubacteria and their effect on the host have been studied most extensively. Similar to gut bacteria, gut fungi are highly pleiotropic and regulate various physiological functions in the host. Among them, several fields deserve particular emphasis, such as modulation of host’s metabolism (e.g., purine metabolism) [55], control of aging and disease progression [40, 56], and, with particular relevance to this article, immune homeostasis [1, 57]. However, reports on intestinal mycobiota and host immunity are fragmented, and whether or how most intestinal commensal fungi interact with the host immune system is still the topic of investigation. The current understanding of the influence of intestinal mycobiota on the pathways and cellular networks of immune responses has been reviewed recently [1]; thus, here, we present a brief summary (Fig. 1).
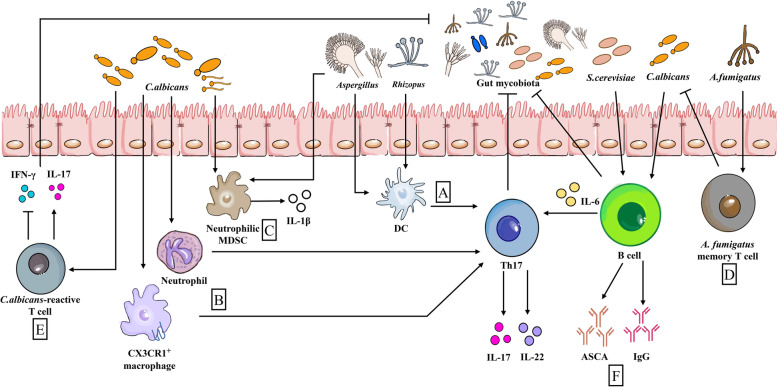
Fig. 1.
Influence of gut mycobiota on intestinal immunity. (a) When triggered by Aspergillus and Rhizopus, DCs promote Th17 responses. (b) CX3CR1+ macrophages and neutrophils are beneficial for early Candida control by inducing robust Th17 responses with production of high levels of IL-17 and IL-22. (c) Likewise, invading C. albicans and Aspergillus trigger neutrophilic MDSCs and production of IL-1β. (d) A. fumigatus memory T cells show cross-reactivity with C. albicans because they have a shared TCR sequence. (e) Likewise, C. albicans-reactive T cells cross-react with other gut fungi by producing IL-17. (f) ASCA and IgG (stimulated by C. albicans or S. cerevisiae) from B cells are able to anti-intestinal fungus. Besides, B cells also participate in anti-fungal immunity in an antibody-independent manner, and IL-6 from B cells enhances anti-fungal Th17 responses. DCs, Dendritic cells; MDSCs, myeloid-derived suppressor cells; IgG, Immunoglobulin G; TCR, T cell receptor; ASCA, Anti-Saccharomyces cerevisiae antibody. Arrows represent activation and horizontal lines represent suppression
The cell wall of some commensal fungi, such as S. cerevisiae and C. albicans, contains various ligands (e.g., β-glucans and chitin) for the receptors of innate immune cells (see review by Wheeler et al. [58] for more information about immunological recognition of fungus). Generally, as important pathogen recognition receptors (PRRs), C-type lectin receptors (CLRs) such as Dectin-1, -2, and Mincle are mainly expressed in dendritic cells (DCs) and macrophages to recognize commensal fungi [59]. Upon ligation of CLRs, nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPKs) are activated via CARD9-BCL10-MALT1 complex, which modulates pro-inflammatory cytokines and ROS production, finally restricting the growth of fungi [59]. Dectin-1 is also related to Syk recruitment and PKCδ activation [59]. Thus, lack of Dectin-1, Dectin-3, or CARD9 may result in dysbiosis of gut fungi [60]. Besides CLRs, TLRs function as direct recognizers of fungi; however, because of heterogeneous morphologies, fungi express different ligands and induce multitudinous immune responses [61]. For example, upon C. albicans colonization, TLR1−/− and TLR2−/− mice experience aggravated intestinal colitis, whereas in TLR6−/− mice C. albicans is nearly cleared [62].
Neutrophils could release nuclear DNA to form a network [named neutrophil extracellular traps (NET)] for trapping and killing extracellular pathogenic microorganisms [63]. Intriguingly, it has been found that the Syk-PKC-ROS cascade is activated by opsonized C. albicans through complement receptor 3 recognition, causing NADPH oxidase-dependent NET [64]. However, unopsonized C. albicans recognition by Dectin-2 activates Syk-Ca2+-PKCδ-PAD4 signaling pathway, triggering NADPH oxidase-independent NET [65]. Notably, PAD4 inhibition (GSK484), neutrophil elastase (NE) inhibition (Sivelestat), and/or Dectin-2 deficiency affect PAD4 activation, NE nuclear translocation, and histone H3-containing NET initiation result in increased renal fungal burden, suggesting that NET is involved in inhibiting remote fungal transmission [65]. Moreover, NET functions as a part of innate immune defense against fungal pathogens (e.g., Aspergillus spp. and Paracoccidioides spp.), which has been comprehensively discussed elsewhere [66].
Usually, intestinal bacteria induce the differentiation of tolerogenic DCs to induce Foxp3+ Treg cells [67]; however, invasion by opportunistic pathogenic fungi (e.g., Aspergillus or Rhizopus) induces DCs to promote Th17 responses [68]. There are various subtypes of macrophages, like CX3CR1+ and CD103+ macrophages [69]. After recognition of commensal and/or pathogenic mycobiota (e.g., Candida [70]) via CLRs, CX3CR+ macrophages participate in inducing Th17 responses to resist infection [71]. CX3CR1+ macrophages are needed for early Candida control by restraining caspase-dependent apoptosis and promoting Akt phosphorylation [71, 72]. In addition, a polymorphism mutation of CX3CR1 gene impairs generation of IgG in recognizing Ascomycota phylum (e.g., C. albicans, Pichia kudrazevii, S. cerevisiae, and Aspergillus amstellodamii), whereas recognition of flagellin remains unaffected [71], implying that CX3CR1+ macrophages have unique response modes to the gut fungi. The status of CD103+ macrophages in initiating anti-fungal responses needs further study. Of note, Candida tropicalis colonization drives CD103+ RALDH+ (retinaldehyde dehydrogenase) DCs migration and production of retinoic acid (RA) [73], which affects anti-inflammatory responses of intestinal macrophages, and establishes connection between intestinal fungi and peripheral immune system. Unfortunately, C. tropicalis is not common in the human gut [73, 74], so the role of CD103+ macrophages in human anti-fungal responses remains to be explored.
Gut fungi shape the fate decision of innate immune cells and also of T cells and B cells. Regardless of the form (yeast or hyphae), C. albicans uniquely stimulates robust Th17 responses [12]. Colonization of C. albicans drives robust fungal-reactive specific Th17 cells and circulating IL-17-reactive neutrophils [75], which was observed in mesenteric lymph nodes and colon [71]. Such immune response induced by Candida colonization blocks the invasion by exogenous Candida and reduces invasion by S. aureus [75]. Interestingly, memory T cells for A. fumigatus show cross-reactivity with C. albicans because they have a shared T cell receptor (TCR) sequence [12]. Furthermore, C. albicans cross-reactive T cells perform a variety of responses to other fungi [12]. B cells produce highly effective antibodies against the invading pathogenic fungi. For example, anti-Saccharomyces cerevisiae antibody (ASCA ) induced by S. cerevisiae or C. albicans is necessary for anti-intestinal fungal infection [71], especially IgG is probably involved in recognizing and resisting intestinal C. albicans. Interestingly, B cells also participate in anti-fungal immunity through antibody-independent manner by inducing antigen specific T cells and especially anti-fungal Th17 responses [76].
However, we are still lacking sufficient data about the influences of intestinal mycobiota on other immune cells, such as natural killer (NK) cells, mast cells, and innate lymphoid cells (ILCs), as well as on non-immune cells, such as intestinal epithelial cells. Intestinal epithelial cells are vital barriers [77–79], and the first and essential step in Candida infection involves adherence to epithelial cells, followed by invasion of this barrier primarily by active penetration [80, 81]. Although it has been recently reported that distal colon “balloon-like” protrusions (BLPs)+ macrophages could prevent the absorption of fungal toxins, protecting the barrier integrity [82], the influence of intestinal mycobiota on the cellular composition of intestine needs further investigation. In particular, some metabolites (e.g., indoles) from intestinal microbiota highly shape the cell fate of intestinal cells [83, 84]. More importantly, at present, the research on the influence of intestinal mycobiota to host immunity is still in its early stages. Studying gut mycobiota as a collective unit contributes to a better understanding of its deep role in the host, such as the significance of change in their composition and colonization over time, and the possibility of improving host immunity through external intervention to artificially regulate the intestinal fungal community.
Besides the direct recognition by host cells, intestinal mycobiota produces some pleiotropic substances, including candidalysin and farnesol, to regulate host immune responses (Table 2). Candidalysin, firstly detected in 2016 from Candida albicans [111], affects protective immune responses and systematic infection [98, 99]. Interestingly, candidalysin utilizes NLRP3 inflammasome-dependent cytolysis to evade phagocytic clearance [100, 101]. In addition, it is positively associated with severity of ethanol-associated hepatitis [102]. Farnesol, one of the Candida-secreted quorum-sensing molecules (QSMs) [112], acts as vital virulence factor to impair the ability of immature DCs (iDC) to induce T cell differentiation and expression of pro-inflammatory cytokines, thereby, affecting pro-inflammatory and Th1 responses [85]. Notably, fungi also secrete prostaglandins (PGs) or convert exogenous arachidonic acid (AA) into PGs [103–105] to affect the functions of phagocytes, which contributes to continuous colonization of C. albicans [103, 106–108]. Moreover, fungal oxylipins are vital factors in modulating immune responses [109, 110].
Table 2.
Fungi-derived compounds and their functions
| Fungi-derived compounds | Functions | References | |
|---|---|---|---|
| Quorum-sensing molecules | Farnesol |
Filamentous growth and formation ↓ C. albicans biofilm formation ↓ |
[85–91] |
|
Drug-resistance ↓ Synergy with fluconazole, amphotericin B or micafungin, anti-fungal AMP peptidomimetics, chitosan (CS), and cysteine protease metacaspase | |||
| Tyrosol | C. albicans biofilm formation ↓ | ||
| Fungal secondary metabolites |
Source of antibiotics and immunosuppressant drugs Hepatotoxicity and/or nephrotoxicity |
[54, 92, 93] | |
| Fungi-derived extractives | (1,3)/(1,6)-β-glucan | Obesity property ↓ | [94–97] |
| Pyronepolyene C-glucoside iso-D8646-2-6 | Influenza A virus (H1N1) infection ↓ | ||
| Xanthones | Virus infection (H1N1, simplex virus types 1 and 2) ↓ | ||
| Some mangrove-associated or soil-associated fungus-derived compounds | Virus infection ↓ | ||
| Other compounds | Candidalysin |
Systematic infection ↑ Phagocytic clearance ↓ Severity of ethanol-associated hepatitis ↑ |
[98–110] |
| Prostaglandins E2 (PGE2) |
Biofilm formation ↑ Yeast to hyphae transition ↑ C. albicans clearance by phagocytes ↓ |
||
| Oxylipins | Modulating immune responses | ||
“↑” indicates increase and “↓” indicates decrease
In addition to the aforementioned fungal components and fungal metabolites, extracts, and secretions, secondary metabolites are also responsible for influencing host homeostasis (Table 2) [113]. Various clusters of genes for production of secondary metabolites indicate that fungi operate complex pathways to produce secondary metabolites, which have huge structural and functional diversity (see a review by Macheleidt et al. [92]). Notably, fungal secondary metabolites have limitless potential in fungal diseases and synthetic biology; they are also prolific sources of antibiotics (e.g., penicillins and cephalosporins) and immunosuppressant drugs (reviewed in [93]) for their ability in targeting or interfering with fungi and/or bacteria. However, some of the fungal secondary metabolites, including aflatoxin and citritin, have strong hepatotoxicity and/or nephrotoxicity [54].
Gut mycobiota in intestinal and extraintestinal diseases
As Wheeler et al. determined that disturbance of intestinal fungi with prolonged anti-fungal treatment in mice deteriorated colitis and even worsens allergic airway disease [114], imbalance in the gut mycobiota composition may be associated with intestinal and also extraintestinal diseases. Although the complete intestinal targets for gut fungi have not been determined completely, it is clear that gut fungi affect more than the intestinal tract and lungs. We review the related investigations and propose several possible fungal targets: intestinal tract, lung, liver, kidney, pancreas, and brain. Within each niche, dysbiosis of gut mycobiota may be related to the progression of certain diseases (Table 3). Whenever available, we also highlight the major findings on the potential mechanisms by which gut mycobiota affects disease status.
Table 3.
Association of enteral and parenteral diseases with gut mycobiota
| Targets | Diseases | Related fungi | Reference | |
|---|---|---|---|---|
| Intestinal tract | Inflammatory bowel disease (IBD) |
Basidiomycota ↑ Ascomycota ↓ Candida ↑ S. cerevisiae ↓ |
[115–118] | |
| Celiac disease | Candida↑ | [119, 120] | ||
| Colon cancer | C. tropicalis ↑ | [121, 122] | ||
| Extra-intertinal tract | Lung |
Fluconazole induced Allergic airway disease (AAD) |
Candida ↓ Aspergillus ↑ Wallemia ↑ Epicoccum ↑ |
[11, 114] |
| Pulmonary infection | Histoplasma capsulatum | [123] | ||
| Liver | Cirrhosis | Fungal detection ↑ | [124] | |
| Kidney (possible) | Sepsis | C. albicans | [125, 126] | |
| Pancreas | Pancreatic ductal adenocarcinoma (PDA) | Malassezia ↑ | [127] | |
| Brain | Multiple sclerosis (MS) | Candida | [128] | |
| Schizophrenia (SCs) | Chaetomium ↑ | [129] | ||
“↑” indicates increase and “↓” indicates decrease
Gut mycobiota and intestinal diseases
Specifically, healthy gut mycobiota is dominated by several abundant commensal fungi in various species (e.g., Candida tropicalis and S. cerevisiae in C57BL/6 mice [130], and Saccharomyces, Malassezia, and Candida in humans [6, 74]). Notably, gut mycobiome could be the reservoir of opportunistic pathogens, although some commensal fungi (e.g., Candida albicans) are recognized as the true symbionts [3, 8, 39, 131–133]. Inflammatory bowel disease (IBD), including ulcerative disease (UC) and Crohn’s disease (CD), is associated with dysbiosis of gut mycobiota. Fecal samples from children with IBD show lower fungal diversity compared with healthy children, while Candida genus is twofold more abundant in IBD samples (72.9%) than in healthy controls (32.9%) [115]. These results are in agreement with previous reports of UC- and CD-associated intestinal colonization by C. albicans in human [116, 117]. Moreover, C. albicans increases in IBD flares compared with remissions [118]. Recently, Limon et al. have reported that Malassezia restricta, the common skin resident fungus, is linked to the pathogenesis of CD, especially in patients harboring the IBD-linked polymorphism in CARD9 (S12N), and that the colonization of M. restricta exacerbates disease severity in DSS-induced colitis in murine models [134]. This study suggests that, besides well-known C. albicans, other identified fungi can be involved in the development of IBD, and that genetic factors, especially CARD9 polymorphisms, are important in defining the inflammatory responses to colonization. Also, the ratio of fungi-to-bacteria diversity increases in CD samples, suggesting the intestinal environment of CD patients is probably more suitable for fungal colonization [114, 118, 135]. Collectively, the alterations in gut environment during IBD are associated with changes in various fungi and bacteria and induce changes in fungal-bacterial relationship [118, 136]. However, the ultimate destiny of host IBD is not only related to the bacterial and/or fungal communities that affect intestinal homeostasis, but the host immunity plays a decisive role [71, 137]. More importantly, the aforementioned results focused on the characterization of fungal communities in CD and/or a mixed UC and CD patients or experimental models; further independent studies about the variation of gut fungal communities in UC patients or experimental models are needed. Interestingly, S. cerevisiae is a beneficial fungus that has capacity to ameliorate gastroenteritis [3, 138], mitigate adherent-invasive Escherichia coli (AIEC)-induced colitis in CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6) -expressing mice [139], and relieve abdominal pain in irritable bowel syndrome (IBS) in human subjects [140]. Therefore, the modulation of intestinal mycobiota may be a potential target for treating IBD. The cause-effect relationship between intestinal commensal fungi and IBD should be further investigated. Moreover, it is still unclear whether gut fungi affect IBD progression by interacting with gut bacteria.
In addition to IBD, celiac disease (CeD) has been suggested to be associated with disorders of intestinal mycobiota. The typical sign of CeD is reversible mucosal atrophy of the small-bowel, and atypical clinical symptoms often result in a missed diagnosis of CeD, which leads to increased disease severity [141]. Candida is related to the pathogenesis of CeD [119]. Specifically, the association between C. albicans and CeD began with the hypothesis that C. albicans virulence factor-hyphal wall protein 1 (HWP1), which is identical or highly homologous with the CeD associated-α and γ gliadin of T cell antigen epitopes, and serves as a transglutaminase (TG) substrate, assisting in the production of autoreactive antibodies. Therefore, C. albicans may be one of the underlying reasons for CeD [119]. Later, by using antigen-antibody reaction and microchip analysis, both CeD group and C. albicans infection (CI) group expressed high anti-HWP1, anti-gliadin antibody and anti-transglutaminase (anti-TG) IgA antibody, but CeD had a higher reaction to HMP1 [142]. However, more evidence is needed to establish the involvement of C. albicans in CeD.
Moreover, colon cancer is also associated with dysbiosis of intestinal fungi, especially a significant increase of C. tropicalis in CARD9−/− mice [121]. Few studies have also demonstrated the link between IBS and gut mycobiota. Symptoms of IBS (e.g., diarrhea) are related to Candida species overgrowth in patients receiving antibiotic therapy or triggered by Candida products [143, 144]. However, more studies should be conducted to reveal the causal relationship between gut mycobiota and those intestinal diseases.
As discussed above, the mucosal immune system may mediate the influence of intestinal fungi on the pathogenesis of intestinal diseases [145, 146]. Especially, intestinal epithelial cells, their resident immune cells [62, 71, 147, 148], the mesenteric lymphatic system [149], cytokines [12], antibodies [71], and the aforementioned fungal metabolites, all may play significant roles in the pathogenesis of intestinal diseases. Notably, the direct or indirect effects of gut mycobiota on intestinal diseases also stimulate further investigations about the mycobiota composition and means of modulating its diversity.
Gut-brain axis and gut mycobiota
Evidence of a plausible gut microbiota-brain axis mainly includes the following: (1) gut microbiota affects the brain via vagus nerve, cytokines, and their metabolic products such as tryptophan, GABA, and acetylcholine; (2) hypothalamus-pituitary-adrenal (HPA) axis plays a core role in communication of gut microbiota and the brain; and (3) gut-brain-microbiota axis provides a new treatment target for depression, autism, and Parkinson disease [150–155]. A recent study revealed that mycobiota regulates expression of genes in the kynurenine pathway and associated micro-RNAs (miRNAs) in the hippocampus in a sex-depend manner [156], suggesting a possible gut mycobiota-brain axis [5]. Candida kefyr (also termed as Candida pseudotropicalis) is beneficial to alleviate experimental autoimmune encephalomyelitis in C57BL/6 mice [58, 157]. In multiple sclerosis (MS) patients, Candida spp. is detected in peripheral blood and in the cerebrospinal fluid [128]. In a cross-sectional study in 10 patients with schizophrenia (SCs) and 16 healthy controls (HCs), the composition and alpha diversity of gut mycobiota changed in SCs, showing enrichment of Chaetomium in SCs [129]. The aforementioned findings indicate that Candida species might have a significant role in mediating gut mycobiome-brain interaction. As a result, gut mycobiota may hold a significant position in mental diseases (Fig. 2). Although the cross-sectional study had a limited sample size, it emphasized that gut fungi may contribute to the progression of mental diseases. However, we still need to reveal the underlying mechanisms by which gut mycobiota affects the brain, and whether gut fungi communicate through neurotransmitters. Moreover, there is still a clear gap (e.g., various sequencing assays) in the scientific literature regarding fungal involvement in neurological diseases, and some approaches like metagenomics should be employed.
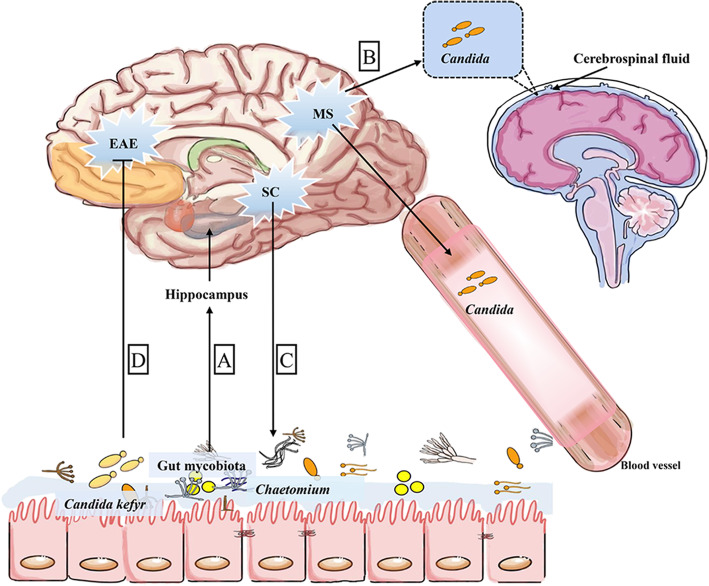
Fig. 2.
Potential mechanism of gut mycrobiota in gut-brain axis. (a) Gut mycobiota modulates the expression of genes in kynurenine pathway and associated micro-RNAs (miRNAs) in the hippocampus in a sex-dependent manner. (b) In MS, high Candida spp. burden is detected in peripheral blood and cerebrospinal fluid. (c) Patients with SC have a changed gut mycobiota alpha diversity and higher abundance of Chaetomium. (d) Candida kefyr supplement in gut is beneficial to alleviate EAE. MS, multiple sclerosis; SC, schizophrenia; EAE, experimental autoimmune encephalomyelitis. Arrows represent activation and horizontal lines represent suppression
Gut-lung axis and gut mycobiota
Asthma is a typical chronic allergic airway disease (AAD) regarded as Th2-related disease. According to eosinophil and neutrophil proportion, asthma has been divided into different inflammatory subtypes [158]. In addition to Th2 cells, Th1, Th9, Th17, NKT, CD8+ T, and Treg cells are also involved in different types of asthma [159–163]. Many components of fungal cell walls are known to be allergens for asthma, so it is not surprising that fungal disorders are linked to asthma [164, 165]. Notably, the dysbiosis of intestinal fungi caused by oral administration of fluconazole deteriorates lung AAD induced by house dust mite (HDM) [11]. In mice without gut mycobiota, fluconazole treatment had no influence on AAD, indicating that experimentally induced AAD is associated with dysbiosis of gut mycobiota. The underlying mechanism is that fluconazole-induced dysbiosis of gut mycobiota affects the macrophage function, and neutrophil and eosinophil infiltration [11], and Syk-mediated CX3CR+ macrophage activation promotes Th2 cell-related responses in the lung, thus participating in the aggravation of AAD [11]. Antimycotics may trigger the expansion of some filamentous fungi (e.g., Wallemia mellicola, Aspergillus amstelodami, and Epicoccum nigrum) to deteriorate AAD through supporting type 2-associated immune responses in the lung [114]. Except for antimycotic-induced gut fungal dysbiosis, antibiotic-induced bacterial disruption also causes Candida spp. overgrowth and Candida-derived PGE2 overproduction to promote M2 polarization of alveolar macrophages, thus aggravating allergic airway inflammation [105]. Moreover, in asthma, fungal dysbiosis is more significant than bacterial, indicating that fungal disturbance may become ideal marker for asthma detection [164, 166].
Cystic fibrosis (CF) may be also linked to fungal infections (Candida spp. are persistent colonizers) [149]. Intestinal colonization of C. albicans or S. cerevisiae is sufficient to overturn lethal phenomenon caused by influenza A virus [167], which primarily invades the respiratory epithelium cells [168]. Gut immune cells may directly deliver signals about dysbiosis of intestinal mycobiota to the lung [123] (Fig. 3). Moreover, gut microbiota migration potential to the lung has been shown [169]. Consequently, it remains to clarify whether migration of immune cells or the direct migration of gut fungi is related to the gut-lung axis. Also, it is interesting to know whether dysbiosis of gut fungi has different effects on different subtypes of asthma. Furthermore, we also found that fluconazole-induced dysbiosis of gut mycobiota exacerbates the infectious pneumonia (unpublished data), but it is still unknown whether lung neutrophils and/or macrophages mediate the above process. Most intriguingly, the influence of intestinal fungi on commensal fungi in the respiratory tract, and the involvement of respiratory tract fungi on the intestinal fungi-mediated gut-lung axis remain to uncover. Collectively, gut commensal fungi-primed immune cells may be recruited to the lung and may serve as an underappreciated factor affecting the pathogenesis of inflammatory airway diseases (asthma in particular) involved in gut fungal dysbiosis.
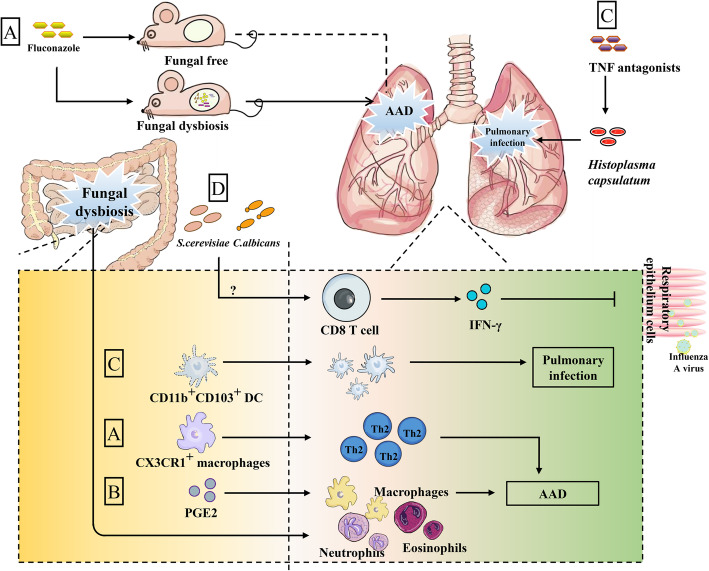
Fig. 3.
Potential mechanism of gut mycrobiota in gut-lung axis. (a) Intestinal fungal dysbiosis caused by fluconazole is sufficient to deteriorate lung AAD induced by house dust mite (HDM) but has no influence in mice without gut mycobiota. Fluconazole-induced dysbiosis of gut mycobiota stimulates intestinal CX3CR+ macrophages, leading to Th2 amplification accompanied with lung infiltration by macrophages, neutrophils, and eosinophils. (b) Also, gut fungus-induced PGE2 promotes M2 polarization of alveolar macrophages to aggravate AAD. (c) TNF antagonists enhance the susceptibility of Histoplasma capsulatum-induced pulmonary infection, during which intestinal specific CD11b+CD103+ DCs migrate and augment in the lung to enhance pulmonary infection. (d) Intestinal colonization by C. albicans and S. cerevisiae triggers viral-specific CD8 T cells in the lung (with unknown reasons) and production of IFN-γ, ultimately, preventing the influenza virus from invading the respiratory epithelial cells. AAD, allergic airway diseases; PGE2, Prostaglandin E2; TNF, tumor necrosis factor; DCs, dendritic cells. Arrows represent activation and horizontal lines represent suppression
Gut-liver axis and gut mycobiota
The liver is an important detoxification organ and participates in defense responses to gut-derived dangers, known as “gut-liver-axis” [170, 171]. Disruption of intestinal microbiota is closely associated with liver diseases such as steatohepatitis and cirrhosis [170, 172]. For instance, after oral administration of ochratoxin A (OTA, a mycotoxin) for ducklings, the diversity of cecum microbiota is reduced, the abundance of LPS-producting Bacteroidetes is increased in both cecum and liver, ultimately, OTA promoted liver inflammation through TLR4-Myd88 pathway [172]. Gut mycobiota may be involved in liver diseases through gut-liver-axis. Namely, mycobiota diversity is increased in primary sclerosing cholangitis patients compare with healthy controls [173]. Patients with liver cirrhosis have high abundance of fungi in the duodenum [124], and alcohol abuse-induced cirrhosis is related to Candida overgrowth and has higher serum S. cerevisiae IgG antibodies [174]. Notably, kefir is a beneficial therapy for anti-alcoholic fatty liver diseases by targeting gut mycobiota [175]. Furthermore, feeding mice with ethanol increases the total intestinal fungal burden, translocation of fungal cell products (e.g., 1,3-β-glucan), and hepatocyte damage [174]. Patients with alcoholic hepatitis show higher percentage of individuals carrying genes for candidalysin (a metabolite from C. albicans), and candidalysin treatment exacerbates ethanol-induced liver disease in mice [176]. Thus, gut-liver axis might exist for gut mycobiota, and it probably has great potential for treating liver disease (Fig. 4). Nevertheless, there are still challenges in the field of gut fungi in liver diseases. The existence of the causal relationship between gut fungi and liver diseases needs further compelling investigations. Also, further studies should explore whether the specific fungi isolated from the gut determine the pathogenesis of liver diseases.
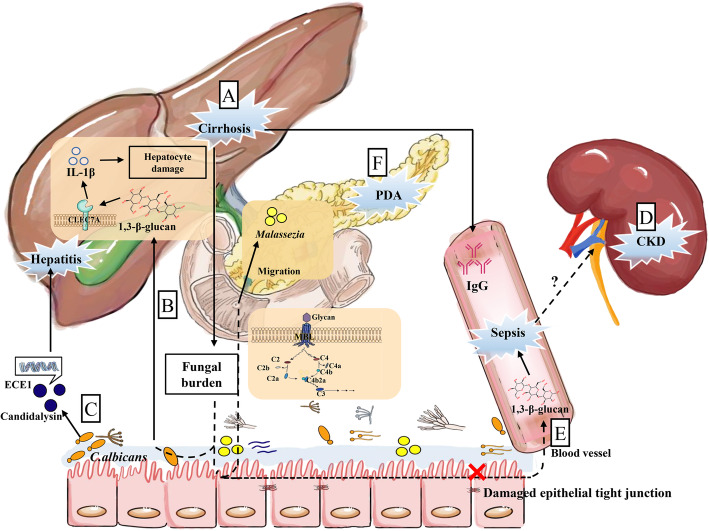
Fig. 4.
Potential mechanism of gut mycrobiota in gut-liver axis, pancreatic diseases, and gut-kidney axis. (a) Patients with liver cirrhosis have a high gut fungal burden and high serum IgG. (b) The increased gut fungal burden comes with translocation of 1,3-β-D-glucan (BG). Then, BG is recognized by C-type lectin-like receptor CLEC7A to induce IL-1β production, aggravating hepatocyte damage. (c) Intestinal colonized C. albicans secrete candidalysin encoded by ECE1 gene, which is involved in exacerbation of the disease in patients with alcoholic hepatitis. (d) Mice with CKD show the change in gut microbiota and damaged epithelial tight junctions, leading to leakage of bacterial or fungal products. (e) Gut leakage may promote migration of BG in the serum to aggravate sepsis. (f) PDA tumor is highly enriched with Malassezia spp. in the pancreas, which results from the migration of gut fungi through the direct link between these two organs via the sphincter of Oddi. Besides, glycan of fungal cells wall-MBL-complement cascade pathway plays an important role in PDA. ECE1, extent of cell elongation 1; BG, 1,3-β-D-glucan; CKD, chronic kidney diseases; PDA, pancreatic ductal adenocarcinoma. Arrows represent activation and horizontal lines represent suppression
Gut-kidney axis and gut mycobiota
Gut-kidney axis may be a distal target of gut microbiota [177]. Noteworthy, the change of gut microbiota in mice with chronic kidney disease (CKD) is associated with the damaged epithelial tight junctions [178], and bacterial products might leak through intestinal barrier to activate immune responses [177]. Similarly, Candida colonization in the gut of patients in intensive-care unit is a susceptibility factor for candidaemia [125]. C. albicans-administration in mice might worsen sepsis because of higher serum 1,3-β-D-glucan (BG), altered intestinal microbiota, and production of inflammatory factors [126]. Both studies suggested that intestinal colonization of C. albicans may be related to sepsis, and gut leakage may promote migration of BG to aggravate disease process. In ICR mice with Candida-disseminated infection, the kidneys have the highest fungal burden [179], suggesting that they may play an important role in circulating fungal infection, but the mechanism remains to be explored. Unfortunately, abundant changes of certain fungi in renal diseases and the presumed BG leakage cannot really explain the existence of the fungal gut-kidney axis. Likewise, investigations of gut mycobiota imbalance direct the pathogenesis of renal diseases are quite limited, thus the fungal gut-kidney axis is speculated to be feasible (Fig. 4). It is worth exploring whether there are other links between gut mycobiota and kidney.
Gut-pancreas axis and gut mycobiota
Pancreatic beta cells are associated with the pathogenesis of type 1 diabetes mellitus (T1DM) [180–183]. Intriguingly, patients with T1DM and type 2 diabetes mellitus (T2DM) have higher colonization of C. albicans compared with healthy controls [184, 185]. Patients with T1DM even have higher diversity of fungal species [186]. These findings indicate that gut fungi may be involved in the pathogenesis of diabetes. However, the evidence about the direct relationship between gut fungi and function of pancreatic beta cells is still limited. Moreover, intestinal commensal bacteria-derived Nod1 ligands (acting as signal molecules) are required for insulin trafficking in pancreatic beta cells [187]. Thus, it would be very interesting to explore whether gut fungi-derived molecules have implications for the function of pancreatic beta cells.
Additionally, in the patients who have pancreatic ductal adenocarcinoma (PDA), bacteria and fungi are markedly increased in pancreas [127, 188]. Malassezia spp. is highly enriched in PDA in human and mouse, and gut fungi migrate to the pancreas possibly via the sphincter of Oddi [127, 188]. Specifically, mannose-binding lectin (MBL)-deleted (Mbl−/−) mice have mild tumor pathogenesis, while recombinant C3a (rC3a) increases pancreatic tumor volume, suggesting that glycan of fungal cell wall-MBL-complement cascade pathway plays an important role in pancreatic diseases (Fig. 4) [127]. However, it is unclear whether dysbiosis of gut mycobiota is the cause of oncogenic progression or just the consequence, and revealing fungal profile for PDA is necessary; therefore, we only propose that Oddi and/or MBL complement cascades might serve as a link in the gut-pancreas axis. Collectively, similar like the gut-kidney axis, the research about fungi-pancreas interaction is still in its early phase.
Conclusion remarks
Although we generally put more attention on gut bacteriota, it is noteworthy that gut mycobiota also has plenty of potential functions. With the development of technologies for fungal detection, we begin to understand that fungal communities are established immediately after or even before birth. However, it is still unknown how humans become colonized by fungi and whether it is affected by the environment and/or any other factors. Since the establishment of early fungal colonization impacts on later disease status, more direct and continuous research approaches are needed to understand the community of early fungi. The involvement of intestinal fungi in intestinal diseases or diseases in other organs may provide a new window to develop new therapeutic strategies for diseases and provide novel targets for diagnosis. For example, celiac disease usually cannot be diagnosed in a timely manner due to the lack of clinical symptoms, but Candida may be involved in the pathogenesis of celiac disease; therefore, if we can establish a direct link between Candida and celiac disease, Candida could become a diagnostic marker for celiac disease. Nevertheless, more investigations are needed to establish the causality between gut mycobiota and intestinal or extraintestinal diseases. The scientific community eagerly needs well-designed experiments with large number of individuals to compare the alteration of intestinal fungi between patients and healthy controls, and advanced technologies (e.g., metagenomics, metabolomics, and culturomics) to illustrate the underlying mechanisms about how intestinal fungi shape the pathogenesis of diseases. Except for fungus itself, the compounds of gut fungi may have great potential in disease treatments. For example, farnesol is helpful in combinatorial treatments to prevent drug-resistance. Some fungal extracts have antiviral properties, so utilization of fungal compounds might provide possible breakthrough to overcome incurable viral diseases. Previous studies focused mostly on the function of Candida, but it should be borne in mind that Candida is not the only member of gut fungi and it may not be even the most influential fungus in some diseases. For example, there is no significant difference in Candida abundance between patients with schizophrenia and healthy controls [129]. In a word, there is also a long way to find particular fungi uniquely relevant to particular disease, which would qualify as novel marker for diagnosis.
Acknowledgements
Not applicable
Abbreviations
- AA
Arachidonic acid
- AAD
Allergic airway diseases
- AFLP
Amplified fragment length polymorphism fingerprinting
- ASCA
Anti-Saccharomyces Cerevisiae Antibody
- BA
Blood agar
- BG
1,3-β-D-glucan
- BLPs
“Balloon-like” protrusions
- CD
Crohn’s disease
- CEACAM6
Carcinoembryonic antigen-related cell adhesion molecule 6
- CeD
Celiac disease
- CF
Cystic fibrosis
- CI
C. albicans infection
- CKD
Chronic kidney disease
- CLRs
C-type lectin receptors
- DCs
Dendritic cells
- DGGE
Denaturing gradient gel electrophoresis
- EAE
Experimental autoimmune encephalomyelitis
- ECE1
Extent of cell elongation 1
- GABA
Gamma-aminobutyric acid
- HDM
House dust mite
- HPA
Hypothalamus-pituitary-adrenal
- HTST
High-throughput sequence technology
- HWP1
Hyphal wall protein 1
- IBS
Irritable bowel syndrome
- iDC
Immature DCs
- IgG
Immunoglobulin G
- ILCs
Innate lymphoid cells
- ITS
Internal transcribed spacer regions
- MAPKs
Mitogen-activated protein kinase
- MALDI-TOF MS
Matrix-assisted laser desorption ionization time of flight mass spectrometry
- MBL
Mannose-binding lectin
- MDSCs
Myeloid-derived suppressor cells
- MS
Multiple sclerosis
- miRNAs
Micro-RNAs
- NE
Neutrophil elastase
- NET
Neutrophil extracellular traps
- NF-κB
Nuclear factor-kappa B
- NK
Natural killer
- OFRG
Oligonucleotide fingerprinting of ribosomal RNA genes
- OTU
Operational taxonomic units
- SCs
Schizophrenia patients
- PDA
Pancreatic ductal adenocarcinoma
- PGs
Prostaglandins
- PGE2
Prostaglandin E2
- PRRs
Pathogen recognition receptors
- QSMs
Quorum-sensing molecules
- RALDH+
Retinaldehyde dehydrogenase
- rC3a
Recombinant C3a
- RFLP
Restriction fragment length polymorphoresis
- SCs
Schizophrenia
- SCFAs
Short chain fat acids
- SDA
Sabouraud dextrose agar
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TCR
T cell receptor
- TG
Transglutaminase
- TNF
Tumor necrosis factor
- UC
Ulcerative disease
Authors’ contributions
XW, YX, and WR designed and wrote the review article. XW, YX, and CZ revised the review article. FH helped with designing figures and finding relevant literatures. WR approved the final manuscript. The author(s) read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31922079, 31872365) and Guangdong Basic and Applied Basic Research Foundation (2019B1515210002).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyan Wu and Yaoyao Xia contributed equally to this work.
Contributor Information
Xiaoyan Wu, Email: xiaoyanwu2020@126.com.
Yaoyao Xia, Email: yaoyaoxia2018@126.com.
Fang He, Email: hefang2017@sina.com.
Congrui Zhu, Email: congruiz@ksu.edu.
Wenkai Ren, Email: renwenkai19@scau.edu.cn.
References
- 1.Li XV, Leonardi I, Iliev ID. Gut mycobiota in immunity and inflammatory disease. Immunity. 2019;50(6):1365–1379. doi: 10.1016/j.immuni.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Chen D, Yu B, He J, Zheng P, Mao X, Yu J, Luo J, Tian G, Huang Z, et al. Fungi in gastrointestinal tracts of human and mice: from community to functions. Microbial Ecol. 2017;75(4):821–829. doi: 10.1007/s00248-017-1105-9. [DOI] [PubMed] [Google Scholar]
- 3.Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21(7):334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes JD, Bernstein CN, Tremlett H, Van Domselaar G, Knox NC. A fungal world: could the gut mycobiome be involved in neurological disease? Front Microbiol. 2018;9:3249. doi: 10.3389/fmicb.2018.03249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017;5(1):153. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limon JJ, Skalski JH, Underhill DM. Commensal fungi in health and disease. Cell Host Microbe. 2017;22(2):156–165. doi: 10.1016/j.chom.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesner DL, Klein BS. Lung epithelium: barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma. Curr Opin Microbiol. 2017;40:8–13. doi: 10.1016/j.mib.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan J. Global trends in candidemia: review of reports from 1995-2005. Curr Infect Dis Rep. 2005;7(6):429–439. doi: 10.1007/s11908-005-0044-7. [DOI] [PubMed] [Google Scholar]
- 10.Groll AH, Walsh TJ. Uncommon opportunistic fungi: new nosocomial threats. Clin Microbiol Infect. 2001;7(Suppl 2):8–24. doi: 10.1111/j.1469-0691.2001.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, et al. Response to fungal dysbiosis by gut-resident CX3CR1(+) mononuclear phagocytes aggravates allergic airway disease. Cell Host Microbe. 2018;24(6):847–856 e844. doi: 10.1016/j.chom.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, Rohmel J, Eschenhagen P, Grehn C, Seidel K, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. 2019;176(6):1340–1355 e1315. doi: 10.1016/j.cell.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol (Baltimore, Md : 1950) 2006;177(7):4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu FT, Springall T, Xu D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol (Baltimore, Md : 1950) 2008;181(4):2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 15.Tamai R, Kiyoura Y. Heat-killed Candida albicans augments synthetic bacterial component-induced proinflammatory cytokine production. Folia Microbiol (Praha) 2019;64(4):555–566. doi: 10.1007/s12223-019-00679-2. [DOI] [PubMed] [Google Scholar]
- 16.Akagawa G, Abe S, Yamaguchi H. Mortality of Candida albicans-infected mice is facilitated by superinfection of Escherichia coli or administration of its lipopolysaccharide. J Infect Dis. 1995;171(6):1539–1544. doi: 10.1093/infdis/171.6.1539. [DOI] [PubMed] [Google Scholar]
- 17.Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21(7):808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doron I, Leonardi I, Iliev ID. Profound mycobiome differences between segregated mouse colonies do not influence Th17 responses to a newly introduced gut fungal commensal. Fungal Genet Biol. 2019;127:45–49. doi: 10.1016/j.fgb.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun B, Gu Z, Wang X, Huffman MA, Garber PA, Sheeran LK, Zhang D, Zhu Y, Xia DP, Li JH. Season, age, and sex affect the fecal mycobiota of free-ranging Tibetan macaques (Macaca thibetana) Am J Primatol. 2018;80(7):e22880. doi: 10.1002/ajp.22880. [DOI] [PubMed] [Google Scholar]
- 20.Angleró-Rodríguez YI, Talyuli OAC, Blumberg BJ, Kang S, Demby C, Shields A, Carlson J, Jupatanakul N, Dimopoulos G. An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. eLife. 2017;6. [DOI] [PMC free article] [PubMed]
- 21.Reddy AK, Brahmaiah U, Narayen N, Reddy RK, Reddy RK, Chitta M, Prasad S, Swarup R, Mohiuddin SM, Reddy M, et al. Is blood agar an alternative to sabouraud dextrose agar for the isolation of fungi in patients with mycotic keratitis. Int Ophthalmol. 2013;33(3):251–254. doi: 10.1007/s10792-012-9683-5. [DOI] [PubMed] [Google Scholar]
- 22.Odds FC. Sabouraud('s) agar. J Med Vet Mycol. 1991;29(6):355–359. doi: 10.1080/02681219180000581. [DOI] [PubMed] [Google Scholar]
- 23.Sarmadian H, Hazbavi Y, Didehdar M, Ghannadzadeh MJ, Hajihossein R, Khosravi M, Ghasemikhah R. Fungal and parasitic contamination of indoor public swimming pools in Arak, Iran. J Egypt Public Health Assoc. 2020;95(1):8. doi: 10.1186/s42506-020-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasanvand S, Azadegan Qomi H, Kord M, Didehdar M. Molecular epidemiology and in vitro antifungal susceptibility of Candida isolates from women with vulvovaginal candidiasis in northern cities of Khuzestan Province, Iran. Jundishapur J Microbiol. 2017;10(8).
- 25.Robert MG, Romero C, Dard C, Garnaud C, Cognet O, Girard T, Rasamoelina T, Cornet M, Maubon D. Evaluation of ID fungi plates medium for identification of molds by MALDI biotyper. J Clin Microbiol. 2020;58(5). [DOI] [PMC free article] [PubMed]
- 26.Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CH, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA. Identification and nomenclature of the genus Penicillium. Stud Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borges FM, de Paula TO, Sarmiento MRA, de Oliveira MG, Pereira MLM, Toledo IV, Nascimento TC, Ferreira-Machado AB, Silva VL, Diniz CG. Fungal diversity of human gut microbiota among eutrophic, overweight, and obese individuals based on aerobic culture-dependent approach. Curr Microbiol. 2018;75(6):726–735. doi: 10.1007/s00284-018-1438-8. [DOI] [PubMed] [Google Scholar]
- 28.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A. 2012;109(16):6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel TGP, Slabbers L, de Jong C, Melchers WJG, Hagen F, Verweij PE, Merkus P, Meis JF. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients - a Dutch, multicentre study. J Cyst Fibros. 2019;18(2):221–226. doi: 10.1016/j.jcf.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Scupham AJ, Presley LL, Wei B, Bent E, Griffith N, McPherson M, Zhu F, Oluwadara O, Rao N, Braun J, et al. Abundant and diverse fungal microbiota in the murine intestine. Appl Environ Microbiol. 2006;72(1):793–801. doi: 10.1128/AEM.72.1.793-801.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard ML, Sokol H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2019;16(6):331–345. doi: 10.1038/s41575-019-0121-2. [DOI] [PubMed] [Google Scholar]
- 33.Huseyin CE, Rubio RC, O'Sullivan O, Cotter PD, Scanlan PD. The fungal frontier: a comparative analysis of methods used in the study of the human gut mycobiome. Front Microbiol. 2017;8:1432. doi: 10.3389/fmicb.2017.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickes BL, Wiederhold NP. Molecular diagnostics in medical mycology. Nat Commun. 2018;9(1):5135. doi: 10.1038/s41467-018-07556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schei K, Avershina E, Oien T, Rudi K, Follestad T, Salamati S, Odegard RA. Early gut mycobiota and mother-offspring transfer. Microbiome. 2017;5(1):107. doi: 10.1186/s40168-017-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):e00036–e00017. doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willis KA, Purvis JH, Myers ED, Aziz MM, Karabayir I, Gomes CK, Peters BM, Akbilgic O, Talati AJ, Pierre JF. Fungi form interkingdom microbial communities in the primordial human gut that develop with gestational age. FASEB J. 2019;33(11):12825–12837. doi: 10.1096/fj.201901436RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Li J, An Y, Li P, Xiong W, Li J, Yan D, Wang M, Zhong G. Chitooligosaccharides prevents the development of colitis-associated colorectal cancer by modulating the intestinal microbiota and mycobiota. Front Microbiol. 2019;10:2101. doi: 10.3389/fmicb.2019.02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohm L, Torsin S, Tint SH, Eckstein MT, Ludwig T, Perez JC. The yeast form of the fungus Candida albicans promotes persistence in the gut of gnotobiotic mice. PLoS Pathog. 2017;13(10):e1006699. doi: 10.1371/journal.ppat.1006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones L, Kumar J, Mistry A, Sankar Chittoor Mana T, Perry G, Reddy VP, Obrenovich M. The transformative possibilities of the microbiota and mycobiota for health, disease, aging, and technological innovation. Biomedicines. 2019;7(2). [DOI] [PMC free article] [PubMed]
- 41.Ward TL, Knights D, Gale CA. Infant fungal communities: current knowledge and research opportunities. BMC Med. 2017;15(1). [DOI] [PMC free article] [PubMed]
- 42.Sam QH, Chang MW, Chai LY. The Fungal Mycobiome and Its Interaction with gut bacteria in the host. Int J Mol Sci. 2017;18(2). [DOI] [PMC free article] [PubMed]
- 43.Yeung F, Chen YH, Lin JD, Leung JM, McCauley C, Devlin JC, Hansen C, Cronkite A, Stephens Z, Drake-Dunn C, et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe. 2020;27(5):809–822 e806. doi: 10.1016/j.chom.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Chen D, Yu B, He J, Huang Z, Mao X, Zheng P, Yu J, Luo J, Tian G, et al. The fungal community and its interaction with the concentration of short-chain fatty acids in the faeces of Chenghua, Yorkshire and Tibetan pigs. Microb Biotechnol. 2019. [DOI] [PMC free article] [PubMed]
- 45.Pan C, Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS one. 2013;8(6):e66019. doi: 10.1371/journal.pone.0066294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam S, Zuo T, Ho M, Chan FKL, Chan PKS, Ng SC. Review article: fungal alterations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;50(11-12):1159–1171. doi: 10.1111/apt.15523. [DOI] [PubMed] [Google Scholar]
- 47.Sakko M, Moore C, Novak-Frazer L, Rautemaa V, Sorsa T, Hietala P, Jarvinen A, Bowyer P, Tjaderhane L, Rautemaa R. 2-hydroxyisocaproic acid is fungicidal for Candida and Aspergillus species. Mycoses. 2014;57(4):214–221. doi: 10.1111/myc.12145. [DOI] [PubMed] [Google Scholar]
- 48.Ukhanova M, Wang X, Baer DJ, Novotny JA, Fredborg M, Mai V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br J Nutr. 2014;111(12):2146–2152. doi: 10.1017/S0007114514000385. [DOI] [PubMed] [Google Scholar]
- 49.Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2016;8(3):352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilotta AJ, Cong Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: implication in precision medicine. Precis Clin Med. 2019;2(2):110–119. doi: 10.1093/pcmedi/pbz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auchtung TA, Fofanova TY, Stewart CJ, Nash AK, Wong MC, Gesell JR, Auchtung JM, Ajami NJ, Petrosino JF. Investigating colonization of the healthy adult gastrointestinal tract by fungi. mSphere. 2018;3(2). [DOI] [PMC free article] [PubMed]
- 52.Suhr MJ, Banjara N, Hallen-Adams HE. Sequence-based methods for detecting and evaluating the human gut mycobiome. Lett Appl Microbiol. 2016;62(3):209–215. doi: 10.1111/lam.12539. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi F, Van't Klooster JS, Ruiz SJ, Poolman B. Regulation of amino acid transport in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2019;83(4). [DOI] [PMC free article] [PubMed]
- 54.Fiers WD, Leonardi I, Iliev ID. From birth and throughout life: fungal microbiota in nutrition and metabolic health. Annu Rev Nutr. 2020;40:323–343. doi: 10.1146/annurev-nutr-013120-043659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiaro TR, Soto R, Zac Stephens W, Kubinak JL, Petersen C, Gogokhia L, Bell R, Delgado JC, Cox J, Voth W, et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci Transl Med. 2017;9(380). [DOI] [PMC free article] [PubMed]
- 56.Mukherjee PK, Sendid B, Hoarau G, Colombel JF, Poulain D, Ghannoum MA. Mycobiota in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2015;12(2):77–87. doi: 10.1038/nrgastro.2014.188. [DOI] [PubMed] [Google Scholar]
- 57.Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017;17(10):635–646. doi: 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheeler ML, Limon JJ, Underhill DM. Immunity to commensal fungi: detente and disease. Annu Rev Pathol. 2017;12:359–385. doi: 10.1146/annurev-pathol-052016-100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roth S, Ruland J. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 2013;34(6):243–250. doi: 10.1016/j.it.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Gaffen SL, Wang T, Pan D, Zhou Z, You Y, Jiang C, Zhao X, Lin X. Dectin-3 deficiency promotes colitis development due to impaired antifungal innate immune responses in the gut. PLOS Pathogens. 2016;12(6):e1005662. doi: 10.1371/journal.ppat.1005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gil ML, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front Biosci (Landmark edition) 2009;14:570–582. doi: 10.2741/3263. [DOI] [PubMed] [Google Scholar]
- 62.Choteau L, Vancraeyneste H, Le Roy D, Dubuquoy L, Romani L, Jouault T, Poulain D, Sendid B, Calandra T, Roger T, et al. Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination. Gut Pathog. 2017;9:9. doi: 10.1186/s13099-017-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 64.Kenno S, Perito S, Mosci P, Vecchiarelli A, Monari C. Autophagy and reactive oxygen species are rnvolved in neutrophil extracellular traps release induced by C. albicans morphotypes. Front Microbiol. 2016;7:879. doi: 10.3389/fmicb.2016.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu SY, Weng CL, Jheng MJ, Kan HW, Hsieh ST, Liu FT, Wu-Hsieh BA. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019;15(11):e1008096. doi: 10.1371/journal.ppat.1008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban CF, Nett JE. Neutrophil extracellular traps in fungal infection. Semin Cell Dev Biol. 2019;89:47–57. doi: 10.1016/j.semcdb.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58(11):1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 68.Rizzetto L, Kuka M, De Filippo C, Cambi A, Netea MG, Beltrame L, Napolitani G, Torcia MG, D'Oro U, Cavalieri D. Differential IL-17 production and mannan recognition contribute to fungal pathogenicity and commensalism. J Immunol (Baltimore, Md : 1950) 2010;184(8):4258–4268. doi: 10.4049/jimmunol.0902972. [DOI] [PubMed] [Google Scholar]
- 69.Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10(6):415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Casanova JL, Puel A. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol. 2018;11(3):581–589. doi: 10.1038/mi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science. 2018;395(6372):232–236. doi: 10.1126/science.aao1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123(12):5035–5051. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Li J, Zheng W, Zhao G, Zhang H, Wang X, Guo Y, Qin C, Shi Y. Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity. 2016;44(2):330–342. doi: 10.1016/j.immuni.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PloS one. 2013;8(6):e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao TY, Ang WXG, Jiang TT, Huang FS, Andersen H, Kinder JM, Pham G, Burg AR, Ruff B, Gonzalez T, et al. Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe. 2019;25(3):404–417 e406. doi: 10.1016/j.chom.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li R, Rezk A, Li H, Gommerman JL, Prat A, Bar-Or A. Antibody-independent function of human B cells contributes to antifungal T cell responses. J Immunol. 2017;198(8):3245–3254. doi: 10.4049/jimmunol.1601572. [DOI] [PubMed] [Google Scholar]
- 77.Mowat AM. To respond or not to respond — a personal perspective of intestinal tolerance. Nat Rev Immunol. 2018;18(6):405–415. doi: 10.1038/s41577-018-0002-x. [DOI] [PubMed] [Google Scholar]
- 78.Pearce SC, Al-Jawadi A, Kishida K, Yu S, Hu M, Fritzky LF, Edelblum KL, Gao N, Ferraris RP. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018;16(1):19. doi: 10.1186/s12915-018-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(6):603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheppard DC, Filler SG. Host cell invasion by medically important fungi. Cold Spring Harbor Perspect Med. 2014;5(1):a019687. doi: 10.1101/cshperspect.a019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruere C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12(2):248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 82.Chikina AS, Nadalin F, Maurin M, San-Roman M, Thomas-Bonafos T, Li XV, Lameiras S, Baulande S, Henri S, Malissen B, et al. Macrophages maintain epithelium integrity by limiting fungal product absorption. Cell. 2020;183(2):411–428.e416. doi: 10.1016/j.cell.2020.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powell DN, Swimm A, Sonowal R, Bretin A, Gewirtz AT, Jones RM, Kalman D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc Natl Acad Sci U S A. 2020;117(35):21519–21526. doi: 10.1073/pnas.2003004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott SA, Fu J, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2020;117(32):19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leonhardt I, Spielberg S, Weber M, Albrecht-Eckardt D, Bläss M, Claus R, Barz D, Scherlach K, Hertweck C, Löffler J, et al. The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity. mBio. 2015;6(2). [DOI] [PMC free article] [PubMed]
- 86.Rodriguez Lopez AL, Lee MR, Wang NB, Dunn KK, Sanchez H, Raman N, Andes DR, Lynn DM, Palecek SP. Small-molecule morphogenesis modulators enhance the ability of 14-helical beta-peptides to prevent Candida albicans biofilm formation. Antimicrob Agents Chemother. 2019:63(9). [DOI] [PMC free article] [PubMed]
- 87.Sebaa S, Boucherit-Otmani Z, Courtois P. Effects of tyrosol and farnesol on Candida albicans biofilm. Mol Med Rep. 2019;19(4):3201–3209. doi: 10.3892/mmr.2019.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cordeiro RA, Pereira LMG, de Sousa JK, Serpa R, Andrade ARC, Portela FVM, Evangelista AJJ, Sales JA, Aguiar ALR, Mendes PBL, et al. Farnesol inhibits planktonic cells and antifungal-tolerant biofilms of Trichosporon asahii and Trichosporon inkin. Med Mycol. 2019;57(8):1038–1045. doi: 10.1093/mmy/myy160. [DOI] [PubMed] [Google Scholar]
- 89.Yu L-h, Wei X, Ma M, Chen X-j, Xu S-b. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob Agents Chemother. 2012;56(2):770–775. doi: 10.1128/AAC.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katragkou A, McCarthy M, Alexander EL, Antachopoulos C, Meletiadis J, Jabra-Rizk MA, Petraitis V, Roilides E, Walsh TJ. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J Antimicrob Chemother. 2014;70(2):470–478. doi: 10.1093/jac/dku374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leger T, Garcia C, Camadro JM. The Metacaspase (Mca1p) Restricts O-glycosylation during farnesol-induced apoptosis in Candida albicans. Mol Cell Proteomics. 2016;15(7):2308–2323. doi: 10.1074/mcp.M116.059378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macheleidt J, Mattern DJ, Fischer J, Netzker T, Weber J, Schroeckh V, Valiante V, Brakhage AA. Regulation and role of fungal secondary metabolites. Ann Rev Genet. 2016;50(1):371–392. doi: 10.1146/annurev-genet-120215-035203. [DOI] [PubMed] [Google Scholar]
- 93.Bills GF, Gloer JB. Biologically active secondary metabolites from the fungi. Microbiol Spectr. 2016;4(6). [DOI] [PubMed]
- 94.Muthuramalingam K, Singh V, Choi C, Choi SI, Kim YM, Unno T, Cho M. Dietary intervention using (1,3)/(1,6)-beta-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. Eur J Nutr. 2019. [DOI] [PubMed]
- 95.Peng J, Jiao J, Li J, Wang W, Gu Q, Zhu T, Li D. Pyronepolyene C-glucosides with NF-κB inhibitory and anti-influenza A viral (H1N1) activities from the sponge-associated fungus Epicoccum sp. JJY40. Bioorg Med Chem Lett. 2012;22(9):3188–3190. doi: 10.1016/j.bmcl.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 96.Kang HH, Zhang HB, Zhong MJ, Ma LY, Liu DS, Liu WZ, Ren H. Potential antiviral xanthones from a coastal saline soil fungus Aspergillus iizukae. Mar Drugs. 2018;16(11). [DOI] [PMC free article] [PubMed]
- 97.Luo X, Yang J, Chen F, Lin X, Chen C, Zhou X, Liu S, Liu Y. Structurally diverse polyketides from the mangrove-derived fungus Diaporthe sp. SCSIO 41011 with their anti-influenza A virus activities. Front Chem. 2018;6:282. doi: 10.3389/fchem.2018.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ho J, Yang X, Nikou SA, Kichik N, Donkin A, Ponde NO, Richardson JP, Gratacap RL, Archambault LS, Zwirner CP, et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10(1):2297. doi: 10.1038/s41467-019-09915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naglik JR, Gaffen SL, Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol. 2019;52:100–109. doi: 10.1016/j.mib.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasper L, Konig A, Koenig PA, Gresnigt MS, Westman J, Drummond RA, Lionakis MS, Gross O, Ruland J, Naglik JR, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9(1):4260. doi: 10.1038/s41467-018-06607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rogiers O, Frising UC, Kucharikova S, Jabra-Rizk MA, van Loo G, Van Dijck P, Wullaert A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. MBio. 2019;10(1). [DOI] [PMC free article] [PubMed]
- 102.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, Mogavero S, Bosques-Padilla F, Abraldes JG, et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2019. [DOI] [PMC free article] [PubMed]
- 103.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69(5):2957–2963. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noverr MC, Toews GB, Huffnagle GB. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect Immun. 2002;70(1):400–402. doi: 10.1128/IAI.70.1.400-402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Nunez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE(2) Cell Host Microbe. 2014;15(1):95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duffin R, O'Connor RA, Crittenden S, Forster T, Yu C, Zheng X, Smyth D, Robb CT, Rossi F, Skouras C, et al. Prostaglandin E(2) constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science. 2016;351(6279):1333–1338. doi: 10.1126/science.aad9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan TG, Lim YS, Tan A, Leong R, Pavelka N. Fungal symbionts produce prostaglandin E2 to promote their intestinal colonization. Front Cell Infect Microbiol. 2019;9:359. doi: 10.3389/fcimb.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alem MA, Douglas LJ. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother. 2004;48(1):41–47. doi: 10.1128/AAC.48.1.41-47.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brodhun F, Feussner I. Oxylipins in fungi. FEBS J. 2011;278(7):1047–1063. doi: 10.1111/j.1742-4658.2011.08027.x. [DOI] [PubMed] [Google Scholar]
- 110.Fischer GJ, Keller NP. Production of cross-kingdom oxylipins by pathogenic fungi: an update on their role in development and pathogenicity. J Microbiol (Seoul, Korea) 2016;54(3):254–264. doi: 10.1007/s12275-016-5620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67(7):2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mogilnicka I, Ufnal M. Gut mycobiota and fungal metabolites in human homeostasis. Curr Drug Targets. 2019;20(2):232–240. doi: 10.2174/1389450119666180724125020. [DOI] [PubMed] [Google Scholar]
- 114.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19(6):865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chehoud C, Albenberg LG, Judge C, Hoffmann C, Grunberg S, Bittinger K, Baldassano RN, Lewis JD, Bushman FD, Wu GD. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1948–1956. doi: 10.1097/MIB.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Standaert-Vitse A, Sendid B, Joossens M, Francois N, Vandewalle-El Khoury P, Branche J, Van Kruiningen H, Jouault T, Rutgeerts P, Gower-Rousseau C, et al. Candida albicans colonization and ASCA in familial Crohn's disease. Am J Gastroenterol. 2009;104(7):1745–1753. doi: 10.1038/ajg.2009.225. [DOI] [PubMed] [Google Scholar]
- 117.Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14(4):386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nieuwenhuizen WF, Pieters RH, Knippels LM, Jansen MC, Koppelman SJ. Is Candida albicans a trigger in the onset of coeliac disease? Lancet (London, England) 2003;361(9375):2152–2154. doi: 10.1016/S0140-6736(03)13695-1. [DOI] [PubMed] [Google Scholar]
- 120.Renga G, Bellet MM, Stincardini C, Pariano M, Oikonomou V, Villella VR, Brancorsini S, Clerici C, Romani L, Costantini C. To be or not to be a pathogen: Candida albicans and celiac disease. Front Immunol. 2019;10:2844. doi: 10.3389/fimmu.2019.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, Jiang C, Zhao X, Hou Y, Hung MC, et al. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity. 2018;49(3):504–514.e504. doi: 10.1016/j.immuni.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K, Makarewicz W. Fungal hut microbiota dysbiosis and its role in colorectal, oral, and pancreatic carcinogenesis. Cancers. 2020;12(5). [DOI] [PMC free article] [PubMed]
- 123.Tweedle JL, Deepe GS, Bäumler AJ. Tumor necrosis factor alpha antagonism reveals a gut/lung axis that amplifies regulatory T cells in a pulmonary fungal infection. Infect Immun. 2018;86(6). [DOI] [PMC free article] [PubMed]
- 124.Krohn S, Zeller K, Bohm S, Chatzinotas A, Harms H, Hartmann J, Heidtmann A, Herber A, Kaiser T, Treuheit M, et al. Molecular quantification and differentiation of Candida species in biological specimens of patients with liver cirrhosis. PloS one. 2018;13(6):e0197319. doi: 10.1371/journal.pone.0197319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vardakas KZ, Michalopoulos A, Kiriakidou KG, Siampli EP, Samonis G, Falagas ME. Candidaemia: incidence, risk factors, characteristics and outcomes in immunocompetent critically ill patients. Clin Microbiol Infect. 2009;15(3):289–292. doi: 10.1111/j.1469-0691.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 126.Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Finkelman M, Worasilchai N, Chindamporn A, Palaga T, Tumwasorn S, Leelahavanichkul A. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1-->3)-beta-D-glucan. PloS one. 2017;12(7):e0181439. doi: 10.1371/journal.pone.0181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574(7777):264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pisa D, Alonso R, Jimenez-Jimenez FJ, Carrasco L. Fungal infection in cerebrospinal fluid from some patients with multiple sclerosis. Eur J Clin Microbiol Infect Dis. 2013;32(6):795–801. doi: 10.1007/s10096-012-1810-8. [DOI] [PubMed] [Google Scholar]
- 129.Zhang X, Pan LY, Zhang Z, Zhou YY, Jiang HY, Ruan B. Analysis of gut mycobiota in first-episode, drug-naive Chinese patients with schizophrenia: a pilot study. Behav Brain Res. 2019;112374. [DOI] [PubMed]
- 130.Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science (New York, NY) 2012;336(6086):1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fiers WD, Gao IH, Iliev ID. Gut mycobiota under scrutiny: fungal symbionts or environmental transients? Curr Opin Microbiol. 2019;50:79–86. doi: 10.1016/j.mib.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Richardson MD. Opportunistic and pathogenic fungi. J Antimicrob Chemother. 1991;28(Suppl A):1–11. doi: 10.1093/jac/28.suppl_A.1. [DOI] [PubMed] [Google Scholar]
- 133.Odds FC. Candida infections: an overview. Crit Rev Microbiol. 1987;15(1):1–5. doi: 10.3109/10408418709104444. [DOI] [PubMed] [Google Scholar]
- 134.Limon JJ, Tang J, Li D, Wolf AJ, Michelsen KS, Funari V, Gargus M, Nguyen C, Sharma P, Maymi VI, et al. Malassezia is associated with Crohn's disease and exacerbates colitis in mouse models. Cell Host Microbe. 2019;25(3):377–388.e376. doi: 10.1016/j.chom.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kumamoto CA. The fungal mycobiota: small numbers, large impacts. Cell Host Microbe. 2016;19(6):750–751. doi: 10.1016/j.chom.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 136.Basmaciyan L, Bon F, Paradis T, Lapaquette P, Dalle F. Candida albicans interactions with the host: crossing the intestinal epithelial barrier. Tissue Barriers. 2019;7(2):1612661. doi: 10.1080/21688370.2019.1612661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leonardi I, Li X, Iliev ID. Macrophage interactions with fungi and bacteria in inflammatory bowel disease. Curr Opin Gastroenterol. 2018;34(6):392–397. doi: 10.1097/MOG.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hatoum R, Labrie S, Fliss I. Antimicrobial and probiotic properties of yeasts: from fundamental to novel applications. Front Microbiol. 2012;3:421. doi: 10.3389/fmicb.2012.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sivignon A, de Vallee A, Barnich N, Denizot J, Darcha C, Pignede G, Vandekerckove P, Darfeuille-Michaud A. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn's disease. Inflamm Bowel Dis. 2015;21(2):276–286. doi: 10.1097/MIB.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 140.Gayathri R, Aruna T, Malar S, Shilpa B, Dhanasekar KR. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Int J Colorectal Dis. 2019. [DOI] [PubMed]
- 141.Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet (London, England) 2018;391(10115):70–81. doi: 10.1016/S0140-6736(17)31796-8. [DOI] [PubMed] [Google Scholar]
- 142.Aaron L, Torsten M. Candida albicans in celiac disease: a wolf in sheep's clothing. Autoimmun Rev. 2020;19(9):102621. doi: 10.1016/j.autrev.2020.102621. [DOI] [PubMed] [Google Scholar]
- 143.Zwolinska-Wcislo M, Brzozowski T, Budak A, Kwiecien S, Sliwowski Z, Drozdowicz D, Trojanowska D, Rudnicka-Sosin L, Mach T, Konturek SJ, et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009;60(1):107–118. [PubMed] [Google Scholar]
- 144.Di Vito M, Bellardi MG, Sanguinetti M, Mondello F, Girolamo A, Barbanti L, Garzoli S, Sabatino M, Ragno R, Vitali A, et al. Potent in vitro activity of Citrus aurantium essential oil and Vitis vinifera hydrolate against gut yeast isolates from irritable bowel syndrome patients-the right mix for potential therapeutic use. Nutrients. 2020;12(5). [DOI] [PMC free article] [PubMed]
- 145.Limon JJ, Kershaw KM, Underhill DM. Mucosal immune responses to fungi and the implications for inflammatory bowel disease. Curr Opin Gastroenterol. 2018;34(6):398–403. doi: 10.1097/MOG.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 146.Huseyin CE, O'Toole PW, Cotter PD, Scanlan PD. Forgotten fungi-the gut mycobiome in human health and disease. FEMS Microbiol Rev. 2017;41(4):479–511. doi: 10.1093/femsre/fuw047. [DOI] [PubMed] [Google Scholar]
- 147.Morel PA, Butterfield LH. Dendritic cell control of immune responses. Front Immunol. 2015;6:42. doi: 10.3389/fimmu.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asano K, Takahashi N, Ushiki M, Monya M, Aihara F, Kuboki E, Moriyama S, Iida M, Kitamura H, Qiu CH, et al. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun. 2015;6:7802. doi: 10.1038/ncomms8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 151.Vagnerova K, Vodicka M, Hermanova P, Ergang P, Srutkova D, Klusonova P, Balounova K, Hudcovic T, Pacha J. Interactions between gut microbiota and acute restraint stress in peripheral structures of the hypothalamic-pituitary-adrenal axis and the intestine of male mice. Front Immunol. 2019;10:2655. doi: 10.3389/fimmu.2019.02655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pusceddu MM, Barboza M, Keogh CE, Schneider M, Stokes P, Sladek JA, Kim HJD, Torres-Fuentes C, Goldfild LR, Gillis SE, et al. Nod-like receptors are critical for gut-brain axis signalling in mice. J Physiol. 2019. [DOI] [PMC free article] [PubMed]
- 153.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 154.Hoban AE, Stilling RM, Moloney GM, Moloney RD, Shanahan F, Dinan TG, Cryan JF, Clarke G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome. 2017;5(1):102. doi: 10.1186/s40168-017-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leprun PMB, Clarke G. The gut microbiome and pharmacology: a prescription for therapeutic targeting of the gut-brain axis. Curr Opin Pharmacol. 2019;49:17–23. doi: 10.1016/j.coph.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 156.Moloney GM, O'Leary OF, Salvo-Romero E, Desbonnet L, Shanahan F, Dinan TG, Clarke G, Cryan JF. Microbial regulation of hippocampal miRNA expression: implications for transcription of kynurenine pathway enzymes. Behav Brain Res. 2017;334:50–54. doi: 10.1016/j.bbr.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 157.Takata K, Tomita T, Okuno T, Kinoshita M, Koda T, Honorat JA, Takei M, Hagihara K, Sugimoto T, Mochizuki H, et al. Dietary yeasts reduce inflammation in central nerve system via microflora. Ann Clin Transl Neurol. 2015;2(1):56–66. doi: 10.1002/acn3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology (Carlton, Vic) 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 159.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–1204 e1192. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28(1):42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 161.Essilfie AT, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, Gibson PG, Hansbro PM. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7(10):e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bedoya SK, Lam B, Lau K, Larkin J., 3rd Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. doi: 10.1155/2013/986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10(12):838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52(2):241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol (Baltimore, Md : 1950) 2011;187(5):2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, Way SS. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017;22(6):809–816 e804. doi: 10.1016/j.chom.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85(2):85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 169.Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, Huffnagle GB. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amornphimoltham P, Yuen PST, Star RA, Leelahavanichkul A. Gut leakage of fungal-derived inflammatory mediators: part of a gut-liver-kidney axis in bacterial sepsis. Dig Dis Sci. 2019;64(9):2416–2428. doi: 10.1007/s10620-019-05581-y. [DOI] [PubMed] [Google Scholar]
- 172.Wang W, Zhai S, Xia Y, Wang H, Ruan D, Zhou T, Zhu Y, Zhang H, Zhang M, Ye H, et al. Ochratoxin A induces liver inflammation: involvement of intestinal microbiota. Microbiome. 2019;7(1):151. doi: 10.1186/s40168-019-0761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lemoinne S, Kemgang A, Ben Belkacem K, Straube M, Jegou S, Corpechot C, Chazouillères O, Housset C, Sokol H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut. 2020;69(1):92–102. doi: 10.1136/gutjnl-2018-317791. [DOI] [PubMed] [Google Scholar]
- 174.Yang AM, Inamine T, Hochrath K, Chen P, Wang L, Llorente C, Bluemel S, Hartmann P, Xu J, Koyama Y, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim DH, Kim H, Jeong D, Kang IB, Chon JW, Kim HS, Song KY, Seo KH. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: targeted and untargeted community analysis with correlation of biomarkers. J Nutr Biochem. 2017;44:35–43. doi: 10.1016/j.jnutbio.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 176.Chu H, Duan Y, Lang S, Jiang L, Wang Y, Llorente C, Liu J, Mogavero S, Bosques-Padilla F, Abraldes JG, et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J Hepatol. 2020;72(3):391–400. doi: 10.1016/j.jhep.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Noel S, Martina-Lingua MN, Bandapalle S, Pluznick J, Hamad AR, Peterson DA, Rabb H. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract. 2014;127(1-4):139–143. doi: 10.1159/000363209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 179.Fakhim H, Vaezi A, Dannaoui E, Chowdhary A, Nasiry D, Faeli L, Meis JF, Badali H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61(6):377–382. doi: 10.1111/myc.12754. [DOI] [PubMed] [Google Scholar]
- 180.Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus--why the β cells? Nat Rev Endocrinol. 2016;12(5):263–273. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349–362. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 182.Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13(11):712–720. doi: 10.1038/nrneph.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 184.Soyucen E, Gulcan A, Aktuglu-Zeybek AC, Onal H, Kiykim E, Aydin A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int. 2014;56(3):336–343. doi: 10.1111/ped.12243. [DOI] [PubMed] [Google Scholar]
- 185.Gosiewski T, Salamon D, Szopa M, Sroka A, Malecki MT, Bulanda M. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes - a pilot study. Gut Pathogens. 2014;6(1):43. doi: 10.1186/s13099-014-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kowalewska B, Zorena K, Szmigiero-Kawko M, Wąż P, Myśliwiec M. Higher diversity in fungal species discriminates children with type 1 diabetes mellitus from healthy control. Patient Prefer Adherence. 2016;10:591–599. doi: 10.2147/PPA.S97852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Q, Pan Y, Zeng B, Zheng X, Wang H, Shen X, Li H, Jiang Q, Zhao J, Meng ZX, et al. Intestinal lysozyme liberates Nod1 ligands from microbes to direct insulin trafficking in pancreatic beta cells. Cell Res. 2019;29(7):516–532. doi: 10.1038/s41422-019-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.