Abstract
Traumatic brain injury (TBI) initiates many different signaling cascades throughout the brain that impact both pathophysiological and neuroprotective processes. Cellular mechanisms that can modulate these processes may play an important role in determining the nature and extent of the damage suffered after TBI, and therefore influence overall outcome after injury. MicroRNAs (miRNAs) comprise an important class of non-coding regulatory RNAs that provide an epigenetic mechanism for the regulation of protein expression levels of target genes. We report that miR-21 expression is significantly upregulated in the hippocampus after rodent TBI, with expression levels peaking by 3d post-injury and returning to near sham levels by 15d post-injury. In situ localization of miR-21 transcripts indicates widespread expression in normal brain, with a pronounced increase in expression after TBI evident throughout the cortex and hippocampus, including the dentate gyrus and CA3 cell layer. We used a combination of the miRanda, TargetScan, and PicTar prediction algorithms to identify 99 potential target genes that possess miR-21 binding sites within their 3′ untranslated regions. Analysis of these genes’ annotated Gene Ontology molecular function and biological process terms revealed an over representation of genes involved in enzyme linked receptor signaling, transcriptional regulation, and developmental processes. These results suggest that increased miR-21 expression in the hippocampus may influence multiple components of TBI pathophysiology.
Keywords: miR-21, Tiam1, TBI, apoptosis, ontology
Introduction
MicroRNAs (miRNAs) are small non-coding regulatory RNAs that inhibit gene expression at the post-transcriptional level (Fire et al., 1998). A recent Sanger miRBase release (v15, April 2010) contains 15,632 mature miRNA sequences identified in 133 species, with at least 1100 human miRNA sequences, and a comparable number (717 mouse, 387 rat) expressed in rodents (Griffiths-Jones et al., 2008). Up to 30% of messenger RNAs may be subject to miRNA regulation, and individual miRNAs are predicted to target up to several hundred genes (Lewis et al., 2005; Xie et al., 2005; Lim et al., 2005). Many highly regulated mRNAs contain multiple miRNA binding sites, often targeted by different miRNAs, which may enhance the effectiveness of regulation (Stark et al., 2005). However, highly regulated gene targets comprise only a small proportion of genes containing predicted miRNA binding sites. Therefore, miRNA-mediated regulation of most genes likely functions to fine tune expression level, rather than acting as an on-off switch (Bartel and Chen, 2004). Recently, comprehensive microarray, proteomic, and computational analyses empirically demonstrated the broad impact of a single miRNA on the regulation of hundreds of gene products (Baek et al., 2008; Selbach et al., 2008). MiRNAs accomplish this regulation of gene expression by mechanisms that are largely dependent on the degree of miRNA sequence match to the cognate binding site within the target mRNA (reviewed in (Valencia-Sanchez et al., 2006).
MiRNAs play a significant role in regulating a wide array of cellular and biological processes including growth, development, differentiation, proliferation and apoptosis (Kloosterman and Plasterk, 2006). Aberrant expression of miR-21 has been observed in many cancers and impacts numerous cellular process, including cell proliferation and apoptosis (Chan et al., 2005; Si et al., 2007; Ji et al., 2007). In glioblastomas, miR-21 antisense oligonucleotide transfection resulted in the downregulation of endogenous miR-21, activation of caspases, and increased apoptotic cell death (Chan et al., 2005; Corsten et al., 2007). Similarly, antisense-mediated suppression of miR-21 expression in cortical precursor cells leads to apoptotic cell death (Sathyan et al., 2007). Several direct or indirect gene targets of miR-21 have been identified, including many genes involved in apoptosis such as p21 RAS, TGFβ1, PTEN, PDCD4, TIMP-3, and BCL-2 (Bond et al., 2002; Meng et al., 2006; Si et al., 2007; Ji et al., 2007; Zavadil et al., 2007; Lu et al., 2008; Talotta et al., 2008). Taken together, these and other studies suggest that increased expression of miR-21 has a pro-survival function.
Traumatic brain injury (TBI) is a major cause of death and disability among young adults. TBI triggers multiple molecular and cellular changes that underlie its pathophysiology and outcome, including apoptosis, neuron and glial dysfunction, and neurogenesis (Colicos et al., 1996; Raghupathi, 2004; Floyd and Lyeth, 2007; Sun et al., 2009). The apoptotic response involves caspase activation, and administration of caspase inhibitors can improve cognitive outcome after brain injury (Yakovlev and Faden, 2001). Despite its involvement in regulating cell survival, the involvement of miR-21 in TBI pathophysiology has not been investigated. We examined the expression of miR-21 in the rodent hippocampus following TBI. TBI significantly increases the expression of mature miR-21 in the ipsilateral hippocampus, with peak expression occurring 3d after injury and returning to near baseline levels by 15d post-injury. In situ hybridization analysis revealed enhanced miR-21 expression in cells throughout the hippocampus, with increased expression most prominent in the hippocampal neuronal cell layers as well as the cortex. Computational analysis of predicted miR-21 binding sites identified 99 putative target genes, including the previously validated target PDCD4, as well as novel targets including Tiam1 (Gabriely et al., 2008; Lu et al., 2008).
Materials and Methods
Materials:
Male Sprague-Dawley rats (250–300g) were purchased from Harlan (Indianapolis, IN). TaqMan® MicroRNA-21 RT-PCR assays and mirVana® miRNA isolation kits were purchased from Applied Biosystems (Foster City, CA). DIG-labeled antisense miRNA probes containing modified locked nucleic acids (LNA) were obtained from Exiqon (Woburn, MA). Antibodies directed against Tiam1, peli-1, and PCDC4 were purchased from Bethyl laboratories (Montgomery, TX), Abcam (Cambridge, MA), and Rockland (Gilbertsville, PA), respectively.
Brain injury:
Animal use was in accordance with NIH’s Guide for the Care and Use of Laboratory Animals. Surgical procedures were approved by The University of Texas Health Science Center at Houston Institutional Animal Care and Use Committee. Rats were deeply anesthetized with 5% isoflurane and a 1:1 N2O:O2 mixture, and anesthesia was maintained during surgery with 2% isoflurane and 1:1 N2O:O2. The animals’ head was kept horizontal during surgery with a stereotaxic frame. A midline incision was made and a 6 mm craniectomy was performed midway between the bregma and lambda with the medial edge of the craniectomy 1mm lateral to the midline. A unilateral brain injury consisting of a single impact (3.3 mm depth, 4 m/sec velocity, and 150 msec dwell time) at an angle of 10 degrees from the vertical plane was administered to rats using an electronic controlled cortical impact (CCI) device essentially as previously described (Dixon et al., 1991). Sham rats received only anesthesia and a midline incision. After injury or sham operation, the scalp was sutured, the animals allowed to recover from anesthesia, and then returned to their home cages.
Quantitative reverse transcriptase-PCR (qRT-PCR):
Total RNA was purified from ipsi- and contralateral hippocampal tissue harvested at 3hr, 24hr, 3d, or 15d after CCI or sham operation (n=6 animals/group for all time points except 3hr, which contained 5 animals) using the modified protocol recommended for the enrichment of small RNAs. Total RNA was eluted in 100 μl 10 mM Tris pH 8.0, 1 mM EDTA, and RNA concentration was determined by spectrophotometry. RNA integrity and concentration was then confirmed by denaturing gel electrophoresis. cDNA was generated from 1.0 μg total RNA in a 15 μl reaction containing 1X reverse transcriptase buffer, 3 μl miR-21 reverse transcription primers, 3.8 units RNase inhibitor, 1 mM dNTPs, and 50 units MultiScribe™ reverse transcriptase. The reverse transcription reactions were incubated for 10 min at 4°C, 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C. The synthesized cDNA was diluted 1:27 with water and assayed immediately. A reference curve was generated by combining equal amounts of RNA from each sham animal and the pooled RNA used as the template for the reverse transcription reaction. Each qRT-PCR reaction contained 10 μl 2X TaqMan Universal PCR mix, 1 μl 20X miR-21 primer mix, 1.33 μl diluted cDNA, and 7.67 μl H2O. The amplification reactions were performed in duplicate using an iCycler (Biorad, Hercules, CA) programmed for 1 cycle of 10 min at 95°C followed by 50 cycles of 15 sec at 95°C, 1 min at 60°C. The resulting data was analyzed using the iCycler iQ optical system software version 3.1 (Biorad, Hercules, CA). Changes in miR-21 expression level are presented as the difference in threshold cycle (Ct) using the formula: ΔCt = mean Ct(sham) − mean Ct(TBI) A positive difference indicates an increase in target abundance after TBI, while a negative difference indicates a decrease in target abundance. The ΔCt error is presented as standard deviation and was calculated using error propagation:
Non-radioactive in situ hybridization (ISH) using miRNA probes:
Tissue preparation and automated ISH for miRNAs were performed as previously described (Yaylaoglu et al., 2005; Redell et al., 2008c). Briefly, brains from sham or 3d post-TBI rats (N=1/time point) were embedded in OCT, and fresh frozen 25 μm sections cut with a cryostat (4 representative sections per brain spanning the dorsal hippocampus from approximately −3.0 mm to −4.0 mm Bregma). After paraformaldehyde fixation, acetylation, and dehydration, the slides were assembled into flow-through hybridization chambers and placed into in a Tecan Genesis 200 (Mannedorf, Switzerland) liquid-handling robot for automatic non-radioactive ISH processing using DIG-labeled antisense probes (230 nM) to miR-21 or a control scrambled sequence with no homology to known miRNAs. Hybridization and wash temperatures were adjusted to the theoretical melting temperatures of the DIG-labeled probes. Hybridized signal was detected by catalyzed reporter deposition (CARD) using biotinylated tyramide followed by colorimetric detection with avidin coupled to alkaline phosphatase (Carson et al., 2005; Yaylaoglu et al., 2005).
Western blot:
Total protein lysates were prepared from ipsilateral hippocampal tissue (sham, 24hr, and 3d post-TBI, n=4/group) homogenized with 15 strokes of a glass-teflon homogenizer in lysis buffer [10 mM Tris-HCl (pH 7.4), 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 0.1 μM okadiac acid] supplemented with 2 μl/ml protease inhibitor cocktail (Roche, Indianapolis, IN). After sonication, total protein content was measured by bicinchoninic acid assay (Thermo Fischer Scientific, Rockford, IL) following the manufacturers’ recommended protocol, and the estimated protein concentration confirmed by an initial western blot against β-actin. The protein lysates were diluted into Laemmli buffer (1X final; (Laemmli, 1970), boiled 5 min, and stored at −80°C until needed. Blots were prepared by separating equal amounts (8 μg) of total protein on 4–12% SDS-PAGE gradient gels, transferred to a polyvinylidene fluoride membrane, and blocked overnight in Tris-buffered saline [10 mM Tris, 150 mM NaCl, pH 7.4] supplemented with 0.1% Tween-20 (TBS-T) and 5% (w/v) bovine serum albumin. Duplicate SDS-PAGE gels were run in parallel and stained with Sypro Ruby (BioRad, Hercules, CA) according to the manufacturers’ instructions to confirm equivalent loading. Blots were then incubated 2hr at room temperature with rabbit α-Tiam1 (30 ng/ml), rabbit α-peli-1 (1:3000), or rabbit α-PCDC4 (1.3 μg/ml) followed by 2hr at room temperature with alkaline phosphatase secondary antibodies (30 ng/ml). Immunoreactivity was detected using CPD-Star® chemiluminescent substrate and the densitometric signal from scanned film images quantified using ImageJ (NIH).
MicroRNA-21 target prediction and gene ontology analysis:
The predicted target gene set for miR-21 was assembled using a combination of the miRanda (August 2008 release, human data set) (John et al., 2004; Betel et al., 2008), TargetScan (release 4.2, rat data set) (Lewis et al., 2003) and PicTar (mouse data set) (Krek et al., 2005) algorithms. To be considered a viable target, a gene had to appear in at least 2 of the 3 data sets generated by the prediction algorithms.
To gain a high-level view of functions or processes that may be impacted after TBI by miR-21-mediated regulation of the predicted target genes’ expression, we used the Ontologizer statistical analysis tool to determine if any Gene Ontology (GO) terms associated with the gene targets were overrepresented compared to an expected genome-wide representation (Grossmann et al., 2007). A web-based version of Ontologizer was accessed through the project’s web site (http://compbio.charite.de/index.php/ontologizer2.html). The annotation and association files were accessed from the GO web site (http://www.geneontology.org/GO.current.annotations.shtml) and analyzed by the parent-child intersection method. GraphVis was used to generate a graphical representation of the overrepresented biological process and molecular function terms in relation to their parent GO terms.
Statistics:
Data obtained from western blot and qRT-PCR experiments were compared using one-way analysis of variance (ANOVA) to determine the overall significance level. The Holm-Sidak test for multiple comparisons was used as the post hoc test between different experimental groups. For data that did not pass the Kolmogorov-Smirnov normality test or Levene’s test for equal variance, a Kruskal-Walis one-way ANOVA on ranks was used to assess the overall significance level, and Dunn’s method for multiple comparisons was used for post hoc pair-wise comparisons. For western blot data, untransformed densitometric values obtained from scanned western blots were compared, and p-values ≤0.01 were considered to be significantly different. For qRT-PCR, threshold cycle values were compared, and p-values ≤0.01 were considered to be significantly different. A threshold value of p≤0.05, calculated using Bonferroni’s method with a correction for multiple comparisons, was used to determine the overrepresented GO terms.
Results
Increased miR-21 expression is observed in the ipsilateral hippocampus after injury:
We previously examined changes in the ipsilateral hippocampal expression levels of 444 validated rodent miRNAs at 3hr and 24hr after cortical impact injury (Redell et al., 2008b). One of the miRNAs that exhibited elevated expression in the ipsilateral hippocampus after injury was miR-21 (Fig. 1A). Using qRT-PCR, we broadened our microarray findings by extending the post-injury time frame examined to 15d, as well as examining both ipsilateral and contralateral hippocampal miR-21 expression levels. In the ipsilateral hippocampus, elevated miR-21 expression peaked at 3d post-injury, and was back to near sham levels by 15d post-injury (1-way ANOVA, F(4,23)=22.386; p<0.001; Fig. 1B). No statistically significant changes in miR-21 levels were detected in the injured versus sham contralateral hippocampus at any of the time points examined (Kruskal-Walis 1-way ANOVA on ranks, H=6.974; p=0.137; Fig. 1C).
Figure 1.
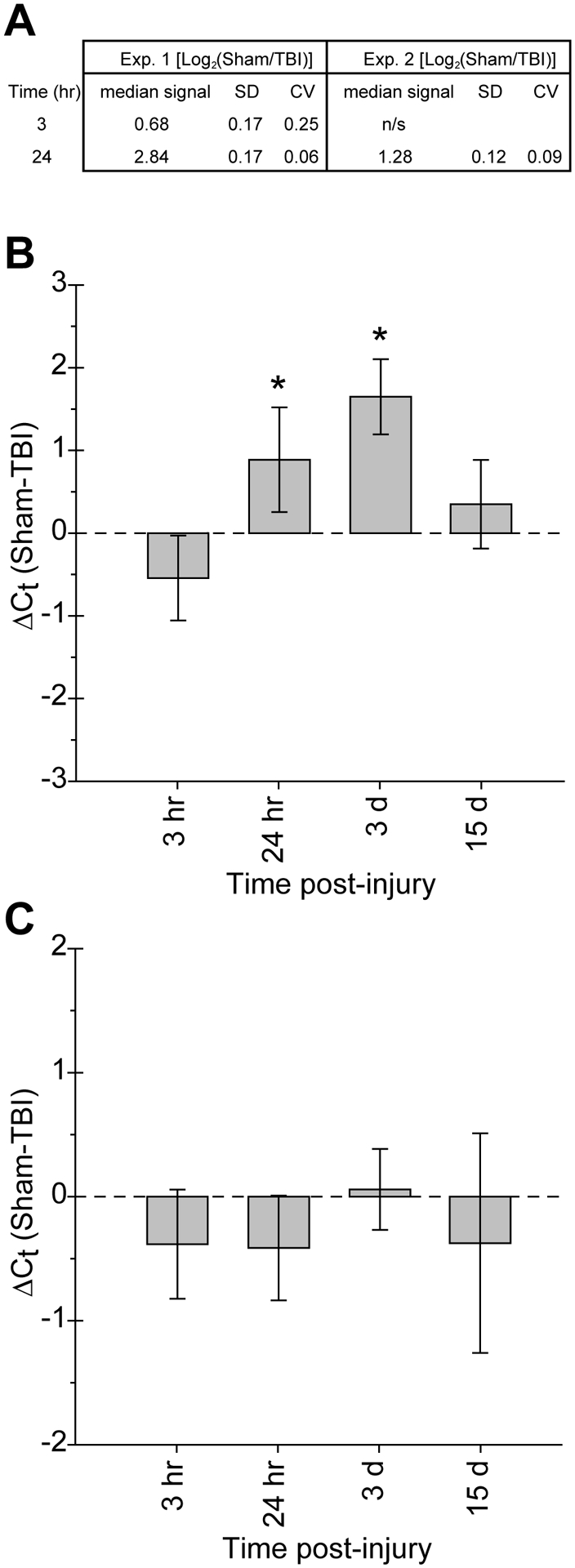
MicroRNA-21 expression is altered in the hippocampus after TBI. A) Summary of miR-21 expression altered after TBI as detected by miRNA microarray analysis (Redell et al., 2008a). B) Changes in miR-21 expression level detected by qRT-PCR analysis of total RNA isolated from ipsilateral hippocampal tissue harvested at the indicated times after injury. C) qRT-PCR analysis of changes in miR-21 expression in the contralateral hippocampus at the indicated times after injury. Data is presented as the mean±SD of the difference in threshold cycle as compared to sham animals (ΔCt = CtSham - CtTBI). Group sizes for sham, 3hr, 24hr, and 3d post-TBI were n=6, 5, 6, and 6, respectively. Asterisks (*) indicate p≤0.01 determined by 1-way ANOVA or Kruskal-Walis ANOVA on ranks; SD= standard deviation; CV= coefficient of variation.
In situ localization of miR-21 transcripts after TBI:
The increase in mature miR-21 expression level detected in the hippocampus after TBI could result from up regulating expression in previously expressing cell populations, activating expression in new populations of resident cells, and/or the infiltration of non-resident cells after injury. To determine if the increased miR-21 expression after TBI detected by qRT-PCR analysis is associated with altered spatial distribution, we examined the localization of miR-21 transcripts in sham and injured brains by in situ hybridization with a locked nucleic acid (LNA)-containing antisense oligonucleotide probe (Kloosterman et al., 2006). As negative controls, we used no probe or a scrambled LNA probe with no homology to known miRNAs to establish the signal level attributable to background hybridization. Representative sham brain sections hybridized with the scrambled probe (Fig. 2A), or secondary detection antibodies only (not shown), yielded little to no detectable signal in sham animals. Injury did not appreciably affect the level of background hybridization signal (Fig. 2H). Using the antisense probe, miR-21-specific hybridization signal was detectable in the dentate gyrus and CA1-CA3 subfields of sham sections (Fig. 2B, C–E). Low-level labeling of cells throughout presumed blood vessels can be seen in the hippocampal fissure of sham animals (Fig. 2F). Hybridization signal in the cortical cell layers (Fig. 2G), and thalamus (not shown) was also evident. In agreement with the qRT-PCR results, there was a qualitative increase in hippocampal miR-21 hybridization signal intensity observed in 3d post-TBI versus sham tissue (compare Fig 2B and 2I). Elevated miR-21 labeling was evident throughout the hippocampus at 3d post-injury, including the CA3 cell layer (Fig 2D, K) and dentate gryus (Fig. 2E, L), while minimal changes were observed in the CA1 (Fig. 2C, J). In addition, presumed vessels located in the hippocampal fissure (Fig. 2F, M), as well as vessel-like profiles in the cortex and thalamus (not shown), exhibited increased miR-21 hybridization signal. MicroRNA-21 labeling intensity was also markedly increased in cortical cells (Fig. 2G, N).
Figure 2.
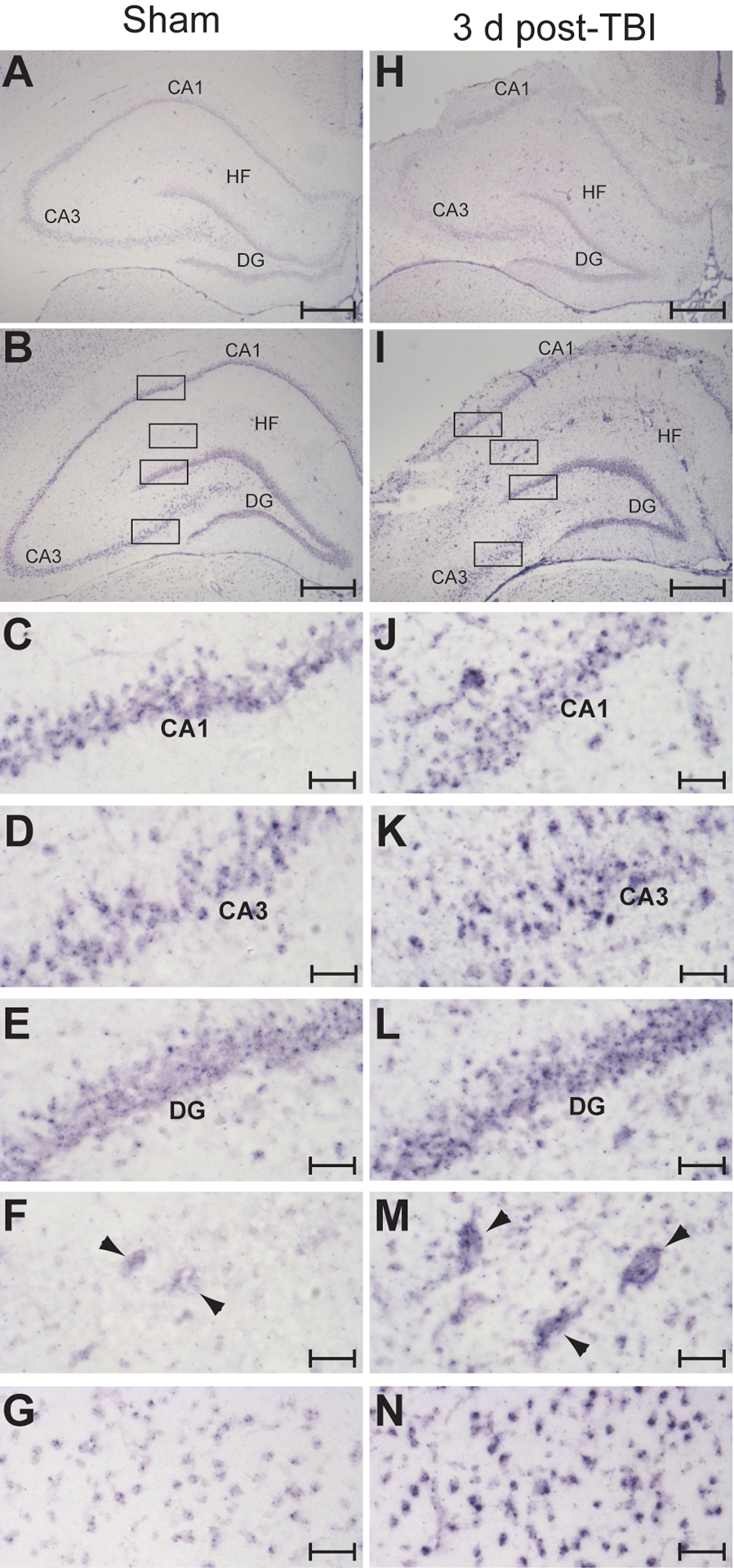
Increased miR-21 expression is observed in cells throughout the brain after injury. Representative in situ images taken from a sham (A-G) and a 3d post-TBI (H-N) brain showing increased miR-21 expression after injury. A scrambled miRNA probe with no similarity to known miRNAs was used to assess the level of non-specific hybridization in sham (A) and 3d post-TBI (H) brain tissue. Injury did not substantially alter background hybridization signal. Hybridization using a miR-21 antisense probe revealed increased signal intensity at 3d post-injury (I) relative to sham (B) throughout the hippocampus, including in the dentate gyrus, and CA1-CA3 subfields, as well as in cells located outside of the neuronal cell layers. The boxed regions of B and I are shown at higher magnification in the following panels: CA1 (C,J); CA3 (D, K); dentate gyrus (E, L); hippocampal fissure (F, M); cortex (G, N). Scale bars in A, B, H and I represent 500 μm; scale bars in C-G and J-N represent 50 μm. Arrowheads indicate presumed vessels in the hippocampal fissure. DG, dentate gyrus; CA1-CA3, Cornu Ammonis 1–3; HF, hippocampal fissure.
MicroRNA-21 target gene prediction and ontology analysis:
To identify likely miR-21 target genes, we used a combination of the results from three predictive algorithms: miRanda, PicTar, and TargetScan (Krek et al., 2005; Lewis et al., 2005; Betel et al., 2008). First, the miR-21 target genes retrieved from the miRanda database (2228 elements) were filtered for redundant binding sites, and multiple site interactions were compressed into a single gene entry. Predicted binding sites located within hypothetical open reading frame genes, unclassified gene products, or gene sequences associated with discontinued records (http://www.ncbi.nlm.nih.gov/sites/entrez; Entrez Gene database), were ignored. The resulting filtered target list (998 genes containing 1123 predicted sites) was then ranked by binding site alignment and conservation scores, and genes in the top 15% retained for further evaluation. The retrieved PicTar data set contained 112 mouse gene interactions comprising a total of 121 miR-21 binding sites. TargetScan identified a total of 192 conserved and 22 poorly conserved miR-21 binding sites from 186 rat genes. In total, these predictive computational algorithms generated a data set containing 328 different target genes with 521 predicted miR-21 binding sites. The data sets from each algorithm were then cross compared for target gene intersections, and only those genes that appeared on at least two data sets were retained for on the miR-21 predicted target gene list (Table I).
Table 1.
Predicted miR-21 Target Genes.
| M/Ts/Pt | M/Ts | M/Pt | |
|---|---|---|---|
| CCL1 | BRD1 | PCLO | ABCD2 |
| CNTFR | CHD7 | ACBD5 | |
| GLCCI1 | CNOT6 | ACVR2A | |
| JAG1 | ITGB8 | ARHGAP24 | |
| KCNA1 | KCNA3 | ASPN | |
| KRIT1 | LEMD3 | BCL2 | |
| MTAP | MAP3K7IP3 | CART1 | |
| PCBP1 | MRPL9 | CASC4 | |
| PCBP2 | NFIB | CASKIN1 | |
| PDCD4 | PIK3R1 | CBX4 | |
| PELI1 | PLAG1 | CPEB3 | |
| PITX2 | PSRC1 | CREBL2 | |
| RECK | RHOB | DAZL | |
| SOX2 | RP2 | DLX2 | |
| SOX5 | SATB1 | ELF2 | |
| SOX6 | TGFBR2 | EPHA4 | |
| STAG2 | THRB | FAM107B | |
| STAT3 | FASLG | ||
| TIAM1 | FCHO2 | ||
| ZNF704 | GLIS2 | ||
| HIP2 |
Bold indicates experimentally validated miR-21 regulated genes. Underline Indicates additional genes whose mRNAs were regulated by miR-21 in Gabriely et al.( 2008). Italicized genes were not represented in the annotated population of genes examined in figure 4. M-Miranda; Ts-Targetscan; Pt-PicTar.
We examined three putative miR-21 targets (PDCD4, Tiam1, and pellino-1) that were predicted by all three algorithms for changes in protein expression level at 24hr and 3d post-TBI to determine if there was a correlation with altered miR-21 RNA expression. PDCD4 has been previously shown to be a miR-21 target (Frankel et al., 2008; Lu et al., 2008; Zhu et al., 2008; Talotta et al., 2008), while Tiam1 and pellino-1 have just recently been identified as miR-21 targets (Cottonham et al., 2010; Marquez et al., 2010). As figure 3A shows, PDCD4 immunoreactivity was significantly decreased relative to sham levels at 24hr, while its expression level rebounded at 3d post-injury and was significantly elevated above sham levels (1-way ANOVA, 24hr: 71.1±12.7%; 3d: 215.6±12.5%; F(2,8)=182.3; p<0.001). Likewise, Tiam1 immunoreactivity was decreased in ipsilateral hippocampal total protein extracts at 24hr (83.0±7.5%), but remained suppressed at 3d (76.2±12.8%) post-TBI (1-way ANOVA, F(2,9)=7.693; p=0.01; Fig. 3B). Although not statistically different, there was a progressive trend toward decreased pellino-1 expression in the hippocampus after TBI (1-way ANOVA, 24hr: 89.6±18.8%; 3d: 76.1±22.9%; F(2,9)=1.733; p=0.23; Fig. 3C). Equal protein loading was confirmed as described in the methods section.
Figure 3.
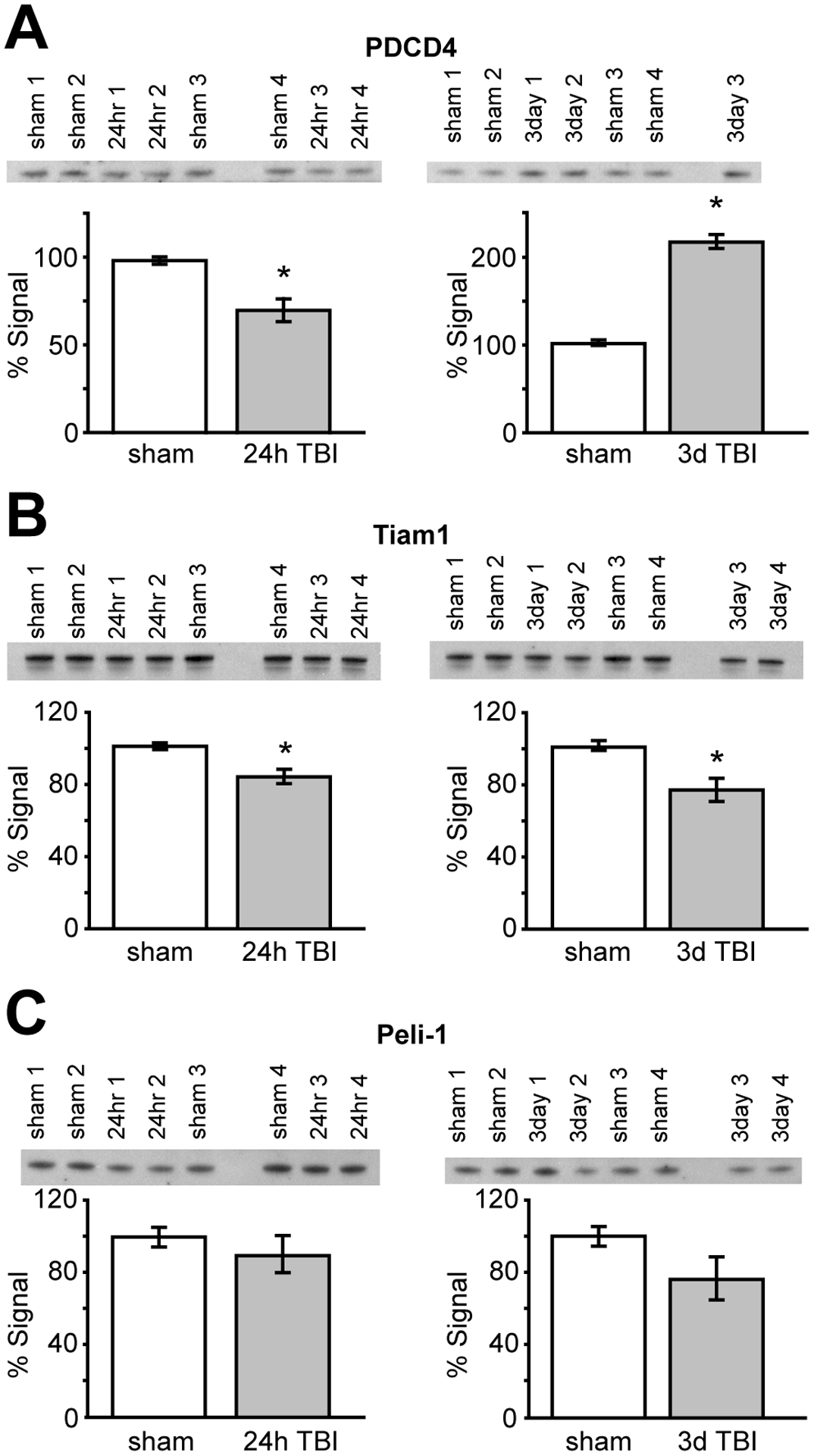
Western blots for the miR-21 predicted targets Tiam1, peli-1, and PDCD4. Total hippocampal extract (8 μg) was separated by SDS-PAGE and the resulting blot probed with antibodies directed against (A) PDCD4, (B) Tiam1, and (C) pellino-1, Images of the immunoreactive bands are shown above their respective bars graphs. Data are presented as mean±SEM of percent signal intensity, with sham signal intensity set to 100%. N=4/group; Asterisks (*) indicate p≤0.01 by 1-way ANOVA followed by post-hoc comparisons.
We next compared the Gene Ontology annotations of the predicted miR-21 gene target data set (Table 1, study group) against all annotated human genes (population group) to determine if any molecular function or biological process terms were overrepresented (Ashburner et al., 2000; Harris et al., 2004). This analysis provides a high-level view of functions or processes that might be impacted after TBI by miR-21-mediated regulation of target gene expression. We utilized the web-based program Ontologizer to compare the study group against the genome population set using the conservative parent-child method (Grossmann et al., 2007). The parent-child analysis method takes into account the structural relationships of GO terms, resulting in the preferential identification of core GO terms and a reduced false-positive identification rate as compared to a term-for-term analysis. A total of 28721 annotated genes contained in the population group were compared against the 94 study group genes that were represented in the annotated database. Three molecular function GO terms (binding, protein binding, and transcription regulator activity) and four biological process terms (biological regulation, multicellular organismal process, developmental process, and enzyme linked receptor protein signaling pathway) were identified as being statistically overrepresented in the study group relative to the population group (Fig. 4, filled ovals; supplemental Table 1).
Figure 4.
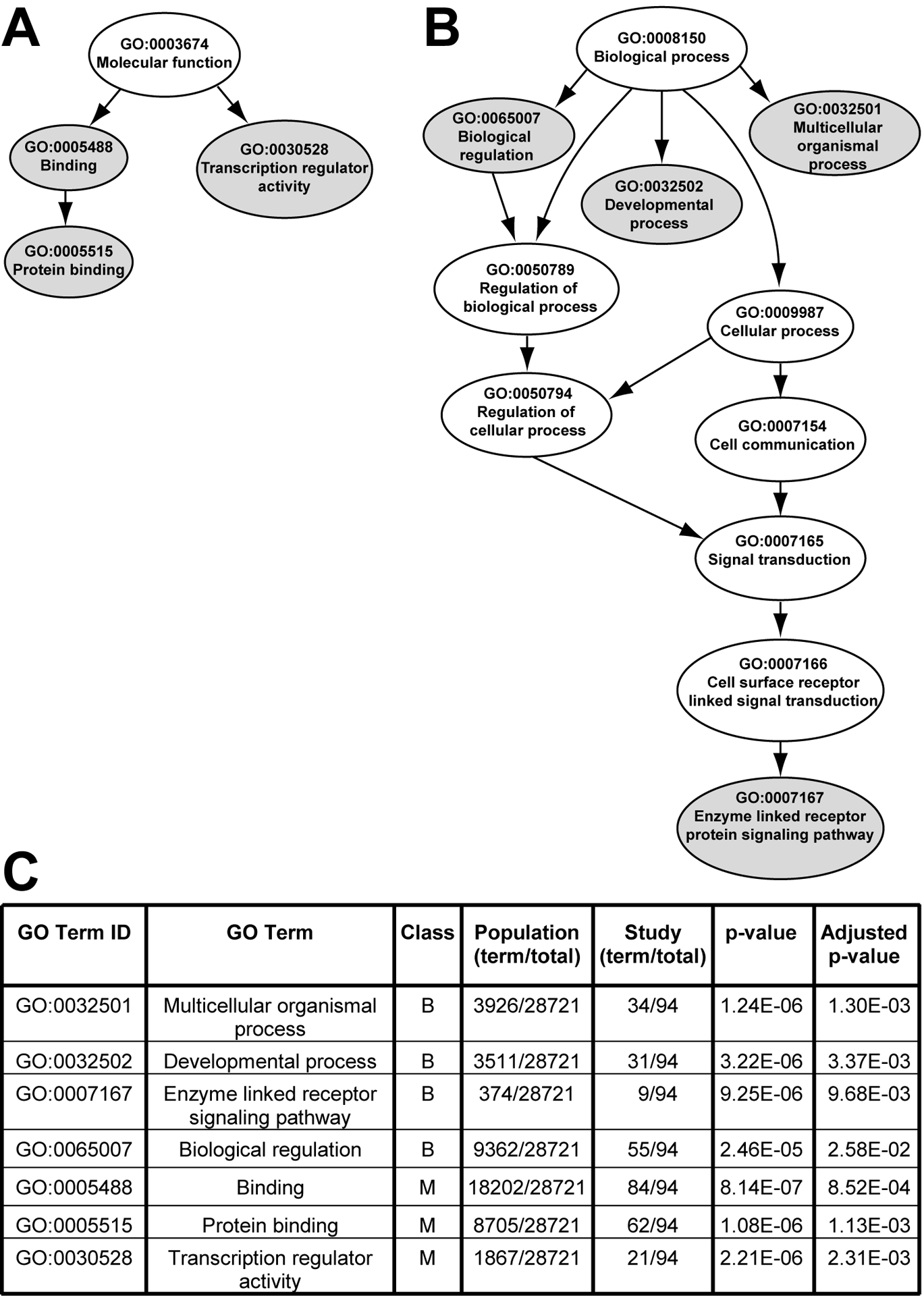
Overrepresented gene ontologies within the predicted miR-21 target genes. (A) Graphical representation of the relationships of the overrepresented molecular function terms, (B) Graphical representation of the relationships of the overrepresented biological process terms, (C) Relative representation of each GO term within the population group versus the study group, and the associated calculated p-values. Data was compared using Bonferroni’s method with a conservative correction for multiple comparisons. An adjusted p-value <0.05 was considered significant. B-biological process; M-molecular function.
Discussion
MicroRNAs have been reported to play an important role in the initiation and progression of human cancers, and have been shown to modulate several important biological processes that are relevant to TBI-induced pathophysiology, including apoptosis and neuronal plasticity. The three key findings from our studies are: 1) the level of miR-21 is elevated in the rat ipsilateral hippocampus 1–3 days following TBI, 2) increased expression was observed in a number of areas including the pyramidal and granule cell layers of the hippocampus, cells associated with the microvasculature, the cerebral cortex, and the thalamus, and 3) protein levels of 3 miR-21 targets identified by bioinformatic analysis (PDCD4, Tiam1, and pellino-1) were altered in injured hippocampus.
Aberrant miR-21 expression has been reported for many different types of human cancers, and increased miR-21 expression has been linked to increased cell survival, growth, proliferation, and decreased apoptosis (Meng et al., 2006; Si et al., 2007; Ji et al., 2007; Gabriely et al., 2008; Asangani et al., 2008; Zhu et al., 2008). Mir-21 expression has been shown to modulate these processes by directly or indirectly regulating the expression of target genes involved in these pathways. We used a combination of three different search algorithms to identify at least 99 potential gene targets containing miR-21 binding sites in their 3′UTRs (Table 1). Among these predicted targets were several gene products that have been previously experimentally verified as being regulated by miR-21, including PDCD4, TGFβ1, BCL-2, NFIB, TPM, RECK, and TIMP-3 (Meng et al., 2006; Si et al., 2007; Zhu et al., 2007; Ji et al., 2007; Fujita et al., 2008; Gabriely et al., 2008; Lu et al., 2008; Zhu et al., 2008; Talotta et al., 2008). In addition, the mRNA expression levels of at least 7 additional predicted target genes (CPEB3, GLCCL1, PELI1, PLEKHA1, SOX2, STAT3, and YOD1) were inversely regulated by the over expression or inhibition of miR-21 in A172 glioma cells (Gabriely et al., 2008). When we examined the molecular function and biological processes associated with the miR-21 predicted targets, we found an over representation of 7 gene ontology terms, including transcription regulator activity, developmental process, and enzyme linked receptor signaling (Fig. 4). The over representation of these terms is consistent with the established role of miR-21 in regulating cell growth and proliferation. In addition, we noted an approximately 3-fold enrichment in apoptosis-related genes (population group, 901 genes out of 28721 examined; study group 10 out of 94). Although this was not a significant enrichment by our conservative assessment, it is consistent with miR-21’s recognized role in regulating apoptosis, and identifies additional apoptosis-related genes (BCL-2, CBX4, FASLG, IL12A, MSX1, NTF3, PDCD4, PIK3R1, RhoB, and TRAF4) that may prove to be interesting targets for further investigation.
Numerous studies have shown that PDCD4, a gene upregulated during apoptosis (Shibahara et al., 1995; Zhang et al., 2006), is a direct target of miR-21 (Asangani et al., 2008; Frankel et al., 2008; Lu et al., 2008; Zhu et al., 2008; Talotta et al., 2008). Our western blot data showed that PDCD4 immunoreactivity in hippocampal extracts is decreased by 24hr post-TBI (Fig. 3A), a time point at which miR-21 expression levels are increased approximately 2-fold (Fig.1A, B). As determined by in situ localization, miR-21 expression was increased in the hippocampal neuronal cell layers, especially in regions known to be apoptosis-sensitive such as the dentate gyrus and CA3 cell layer (Fig. 2). It is possible that increased miR-21 expression may serve as a pro-survival mechanism for hippocampal neurons after injury, perhaps by blunting PDCD4 signaling. Further experiments will be necessary to specifically address the influence of enhanced miR-21 expression on apoptotic cell death after TBI.
Tiam1 (T-lymphoma invasion and metastasis) is a ubiquitously expressed guanine exchange factor (GEF) that activates Rac, a member of the Rho-like GTPases involved in regulating cytoskeletal dynamics, by catalyzing the exchange of GDP for GTP (Habets et al., 1994; Michiels et al., 1995; Heasman and Ridley, 2008). In addition to activating Rac in vivo, there is in vitro evidence that Tiam1 can also act as a GEF for Rho and CDC42 (Minard et al., 2004). In cultured rodent neurons, Tiam1 is localized to growth cones where it is involved in regulating neurite extension and axon formation/guidance (Kunda et al., 2001; Matsuo et al., 2003). More recently, a role for Tiam1 in regulating dendritic spine morphogenesis has also been described (Tolias et al., 2005; Zhang and Macara, 2006; Tolias et al., 2007). Since all three computer algorithms predicted Tiam1 was a miR-21 target gene, we investigated whether Tiam1 expression level changed after TBI. Western blot analysis of hippocampal protein extracts (Fig. 3B) found that Tiam1 protein levels had an inverse relationship to miR-21 expression levels in the hippocampus (Figure 1B). This is consistent with a recent report showing miR-21 directly regulates TIAM1 expression level in human colon carcinoma cells (Cottonham et al., 2010). This suggests the possibility that increased miR-21 expression may contribute to altered hippocampal dendritic plasticity after TBI. In addition to its function in neurons, many studies have shown that Tiam1 also plays a key role in the regulation of adherens and tight junctions in epithelial and endothelial cells (Malliri et al., 2004; Mertens et al., 2005; Chen and Macara, 2005; Birukova et al., 2007a; Birukova et al., 2007b; Guillemot et al., 2008; Knezevic et al., 2008). We observed increased miR-21 expression in vessel-like structures in the hippocampal fissure, thalamus and cortex, suggesting the possibility that miR-21 may also impact blood-brain barrier function after TBI. Since the blood-brain barrier exhibits chronic dysfunction after TBI (Shlosberg et al., 2010), future experiments examining the role of enhanced miR-21 expression on TBI-induced enhanced BBB permeability are warranted.
In summary, the findings presented herein demonstrate that the expression of miR-21 is increased in the hippocampus as a result of TBI, and that this increase is associated with changes in the protein levels of known and predicted miR-21 targets. While these studies do not provide a direct causal relationship between the observed changes in miRNA expression and protein levels, these findings identify potential signaling pathways that may influence the progression of TBI pathology. At present, the impact of miRNA-mediated regulation on cellular biology and function is only just beginning to be explored. The development of new techniques and tools for efficiently manipulating miRNAs both in vitro and in vivo will enable a more complete characterization of the consequences of altered miRNA expression following TBI, and possibly identify new targets for therapeutic intervention.
Supplementary Material
Acknowledgements
The authors would like to thank Anthony N. Moore for his assistance with western blots and critical reading of the manuscript, and Dr. Yin Liu for helpful discussions on the bioinformatic analysis of microRNA target genes. We thank Dr. Christina Thaller and Agnes Liang of the Gene Expression Core, Department of Biochemistry and Molecular Biology, Baylor College of Medicine, for performing the in situ hybridizations.
This research was supported by grants from the National Institutes of Health (NS049160, MH072933, NS05359), American Heart Association (09BGIA2260018), and the Mission Connect/TIRR and Gillson Longenbaugh Foundations.
References
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. 2008. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27:2128–2136. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet 25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. 2008. The impact of microRNAs on protein output. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP, Chen CZ. 2004. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet 5:396–400. [DOI] [PubMed] [Google Scholar]
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res. 36:D149–D153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. 2007a. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 21:2776–2786. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. 2007b. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp. Cell Res 313:2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M, Murphy G, Bennett MR, Newby AC, Baker AH. 2002. Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol. Chem 277:13787–13795. [DOI] [PubMed] [Google Scholar]
- Carson JP, Eichele G, Chiu W. 2005. A method for automated detection of gene expression required for the establishment of a digital transcriptome-wide gene expression atlas. J. Microsc 217:275–281. [DOI] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. 2005. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol 7:262–269. [DOI] [PubMed] [Google Scholar]
- Colicos MA, Dixon CE, Dash PK. 1996. Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 739:111–119. [DOI] [PubMed] [Google Scholar]
- Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R, Shah K. 2007. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res 67:8994–9000. [DOI] [PubMed] [Google Scholar]
- Cottonham CL, Kaneko S, Xu L. 2010. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J. Biol. Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. 1991. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39:253–262. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 19;391:806–811. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Lyeth BG. 2007. Astroglia: important mediators of traumatic brain injury. Prog. Brain Res 161:61–79.:61–79. [DOI] [PubMed] [Google Scholar]
- Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. 2008. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol. Chem 283:1026–1033. [DOI] [PubMed] [Google Scholar]
- Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. 2008. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol 378:492–504. [DOI] [PubMed] [Google Scholar]
- Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. 2008. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell Biol 28:5369–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van DS, Enright AJ. 2008. miRBase: tools for microRNA genomics. Nucleic Acids Res 36:D154–D158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann S, Bauer S, Robinson PN, Vingron M. 2007. Improved detection of overrepresentation of Gene-Ontology annotations with parent child analysis. Bioinformatics. 23:3024–3031. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. 2008. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol. Biol. Cell 19:4442–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets GG, Scholtes EH, Zuydgeest D, van der Kammen RA, Stam JC, Berns A, Collard JG. 1994. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77:537–549. [DOI] [PubMed] [Google Scholar]
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, Rubin GM, Blake JA, Bult C, Dolan M, Drabkin H, Eppig JT, Hill DP, Ni L, Ringwald M, Balakrishnan R, Cherry JM, Christie KR, Costanzo MC, Dwight SS, Engel S, Fisk DG, Hirschman JE, Hong EL, Nash RS, Sethuraman A, Theesfeld CL, Botstein D, Dolinski K, Feierbach B, Berardini T, Mundodi S, Rhee SY, Apweiler R, Barrell D, Camon E, Dimmer E, Lee V, Chisholm R, Gaudet P, Kibbe W, Kishore R, Schwarz EM, Sternberg P, Gwinn M, Hannick L, Wortman J, Berriman M, Wood V, de la CN, Tonellato P, Jaiswal P, Seigfried T, White R. 2004. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res 32:D258–D261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. 2008. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol 9:690–701. [DOI] [PubMed] [Google Scholar]
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. 2007. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res 100:1579–1588. [DOI] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. 2004. Human MicroRNA targets. PLoS. Biol 2:e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. 2006. The diverse functions of microRNAs in animal development and disease. Dev. Cell 11:441–450. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Wienholds E, de BE, Kauppinen S, Plasterk RH. 2006. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods 3:27–29. [DOI] [PubMed] [Google Scholar]
- Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. 2008. Tiam1 and Rac1 are required For PAF-induced endothelial junctional disassembly and increase In vascular permeability. J Biol. Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. 2005. Combinatorial microRNA target predictions. Nat. Genet 37:495–500. [DOI] [PubMed] [Google Scholar]
- Kunda P, Paglini G, Quiroga S, Kosik K, Caceres A. 2001. Evidence for the involvement of Tiam1 in axon formation. J Neurosci 21:2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–5. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 120:15–20. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. 2003. Prediction of mammalian microRNA targets. Cell. 115:787–798. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. 2005. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433:769–773. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. 2008. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27:4373–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliri A, van ES, Huveneers S, Collard JG. 2004. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol. Chem 279:30092–30098. [DOI] [PubMed] [Google Scholar]
- Marquez RT, Wendlandt E, Galle CS, Keck K, McCaffrey AP. 2010. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am. J. Physiol Gastrointest. Liver Physiol 298:G535–G541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Terao M, Nabeshima Y, Hoshino M. 2003. Roles of STEF/Tiam1, guanine nucleotide exchange factors for Rac1, in regulation of growth cone morphology. Mol. Cell Neurosci 24:69–81. [DOI] [PubMed] [Google Scholar]
- Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. 2006. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130:2113–2129. [DOI] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der KR, Collard JG. 2005. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 170:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. 1995. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375:338–340. [DOI] [PubMed] [Google Scholar]
- Minard ME, Kim LS, Price JE, Gallick GE. 2004. The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res Treat. 84:21–32. [DOI] [PubMed] [Google Scholar]
- Raghupathi R 2004. Cell death mechanisms following traumatic brain injury. Brain Pathol. 14:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Liu Y, Dash PK. 2008a. Traumatic Brain Injury Alters Expression of Hippocampal MicroRNAs: Potential Regulators of Multiple Pathophysiological Processes. In. p 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Liu Y, Dash PK. 2008b. Traumatic Brain Injury Alters Expression of Hippocampal MicroRNAs: Potential Regulators of Multiple Pathophysiological Processes. In. p 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Liu Y, Dash PK. 2008c. Traumatic Brain Injury Alters Expression of Hippocampal MicroRNAs: Potential Regulators of Multiple Pathophysiological Processes. In. p 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. 2007. Competing interactions between microRNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci 27:8546–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. 2008. Widespread changes in protein synthesis induced by microRNAs. Nature 455:58–63. [DOI] [PubMed] [Google Scholar]
- Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. 1995. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 166:297–301. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. 2010. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol 6:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. 2007. miR-21-mediated tumor growth. Oncogene 26:2799–2803. [DOI] [PubMed] [Google Scholar]
- Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM. 2005. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3’UTR evolution. Cell 123:1133–1146. [DOI] [PubMed] [Google Scholar]
- Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ. 2009. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol 216:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talotta F, Cimmino A, Matarazzo MR, Casalino L, De VG, D’Esposito M, Di LR, Verde P. 2008. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. 2005. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45:525–538. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. 2007. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc. Natl. Acad. Sci. U. S. A 104:7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. 2006. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20:515–524. [DOI] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. 2005. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature 434:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. 2001. Caspase-dependent apoptotic pathways in CNS injury. Mol. Neurobiol 24:131–144. [DOI] [PubMed] [Google Scholar]
- Yaylaoglu MB, Titmus A, Visel A, varez-Bolado G, Thaller C, Eichele G. 2005. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev. Dyn 234:371–386. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. 2007. Transforming growth factor-beta and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues. Organs 185:157–161. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. 2006. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat. Cell Biol 8:227–237. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S. 2006. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25:6101–6112. [DOI] [PubMed] [Google Scholar]
- Zhu S, Si ML, Wu H, Mo YY. 2007. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol. Chem 282:14328–14336. [DOI] [PubMed] [Google Scholar]
- Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. 2008. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18:350–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


