To the Editor:
Pulmonary arterial hypertension (PAH) negatively impacts health-related quality of life (HRQoL) and is associated with increased hospitalizations. Therapy is tailored according to the risk of adverse outcomes and several prediction rules for mortality have been proposed. We evaluated whether risk prediction models for mortality were associated with patient-related outcomes. Using the Pulmonary Hypertension Association Registry (PHAR), we hypothesized that higher risk assessment would be associated with worse HRQoL and an increased risk for hospitalization.
The PHAR is a prospective registry of individuals with PAH or chronic thromboembolic pulmonary hypertension, enrolled at participating centers throughout the United States. We included individuals aged 18 years and older with PAH who were enrolled between 2015 and September 2019, with follow-up to March 2020. Two HRQoL questionnaires are administered at each visit: The Medical Outcome Study Short Form-12 (SF-12) with general physical and mental component scores (1) and the emPHasis-10 (e10), a pulmonary hypertension–specific instrument (2). Using the baseline data, we assigned patients into low-, intermediate-, and high-risk categories using Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) (3, 4) and Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) risk calculator (REVEAL 2.0) (5, 6) prediction rules. The outcomes were HRQoL by SF-12 and e10 and the risk of hospitalization. Because hospitalization was an outcome of interest, it was not used in calculating the REVEAL 2.0 score.
We fitted mixed-effects generalized linear regression models with P values for linear trend by risk category. These models were adjusted for potential confounders of PAH risk: age, sex, race and ethnicity, and PAH medications (parenteral therapy and the total number of vasodilator medications). We performed a sensitivity analysis imputing the worst possible HRQoL score for participants who died or were lost to follow-up. Negative binomial regression was used to estimate the incidence rate ratio (IRR) for all-cause hospitalization by risk category with an offset term for follow-up time. We then performed a sensitivity analysis in which death, lung transplantation, or loss to follow-up were counted as hospitalizations to account for potential unrecorded events. To estimate the relative risk of hospitalization with death as a competing risk, we calculated subdistribution hazard ratios (sHRs) and cumulative incidence functions using the Fine-Gray competing-risks regression. These models were then adjusted for the same potential confounders. The statistical analyses were performed using Stata Version 15.1 (StataCorp LLC).
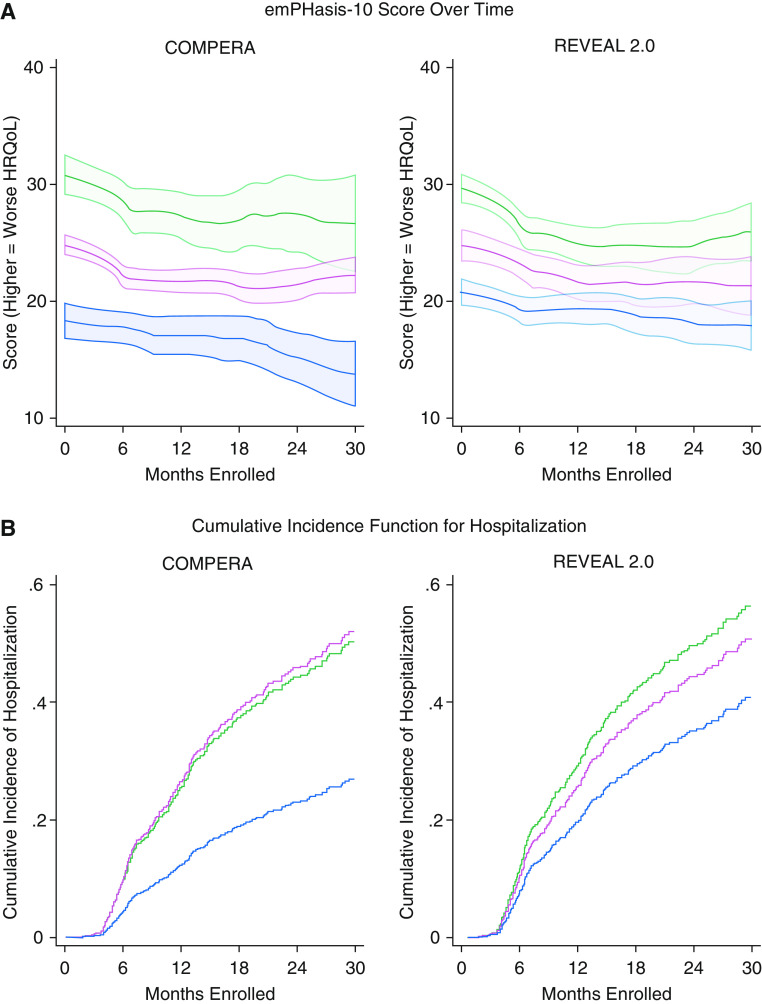
Of the 1,021 participants enrolled in PHAR, 869 were included (Table 1). Using COMPERA, 16% were low, 70% intermediate, and 14% high risk. A total of 796 participants had at least seven variables necessary for REVEAL 2.0, which classified 43% as low-, 24% as intermediate-, and 32% as high-risk patients (6). A higher baseline risk by either method was associated with higher (worse) e10 score at baseline and over time (Figure 1A) by COMPERA and REVEAL 2.0 (β = 5.81; 95% confidence interval [CI], 3.72–7.90 vs. β = 3.78; 95% CI, 1.85–5.71 for intermediate risk and β = 12.12; 95% CI, 9.36–14.89 vs. β = 8.10; 95% CI, 6.28–9.91 for high risk, respectively). Higher predicted risk was also associated with statistically lower (worse) SF-12 physical scores (P < 0.05) but an unclear clinical significance because of a small magnitude of difference. Differences in SF-12 mental scores were only noted for COMPERA (P = 0.04). These associations persisted after multivariate adjustment, in sensitivity analyses using complete data, and when imputing the worst possible HRQoL score for those who died or were lost to follow-up.
Table 1.
Baseline Characteristics of the Study Cohort
| Baseline Characteristics (n = 869) | Value |
|---|---|
| Age, yr | 55.4 ± 16.0 |
| Sex, n (%) | |
| M | 217 (25.0) |
| F | 642 (73.9) |
| Other/unknown | 10 (1.1) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 582 (67.0) |
| Black, non-Hispanic | 107 (12.3) |
| Hispanic | 97 (11.2) |
| Asian | 40 (4.6) |
| Other | 43 (4.9) |
| Body mass index, kg/m2 (n = 845) | 29.4 ± 7.2 |
| WHO group I diagnosis, n (%) | |
| Idiopathic PAH | 344 (39.6) |
| Heritable PAH | 23 (2.6) |
| Drug/toxin-induced PAH | 103 (11.9) |
| CTD PAH | 283 (32.6) |
| HIV-related PAH | 15 (1.7) |
| Portopulmonary hypertension | 57 (6.6) |
| CHD PAH | 44 (5.1) |
| Baseline WHO functional class, n (%) (n = 815) | |
| I | 58 (7.1) |
| II | 289 (35.5) |
| III | 411 (50.4) |
| IV | 57 (7.0) |
| Six-minute-walk distance, m (n = 744) | 340 (253–425) |
| EmPHasis-10 score (n = 853) | 26 (16–34) |
| SF-12 physical score (n = 854) | 34.3 (30.0–38.5) |
| SF-12 mental score (n = 854) | 48.7 (42.2–54.6) |
| Baseline right heart catheterization | |
| Right atrial pressure, mm Hg (n = 826) | 9.0 (5.0–13.0) |
| Mean pulmonary artery pressure, mm Hg (n = 843) | 49 (40–58) |
| Pulmonary artery wedge pressure, mm Hg (n = 809) | 11.0 (7.0–14.0) |
| Q̇, L/min (n = 791) | 4.0 (3.2–5.1) |
| Cardiac index, L/min/m2 (n = 830) | 2.2 (1.8–2.7) |
| Pulmonary vascular resistance, Wood units (n = 803) | 9.0 (6.0–13.0) |
| PAH therapy use by drug class, n (%) | |
| Phosphodiesterase-5 inhibitor | 531 (61.1) |
| Endothelin receptor antagonist | 374 (43.0) |
| Prostacyclin analog (inhaled) | 25 (2.9) |
| Prostacyclin analog (oral) | 36 (4.1) |
| Prostacyclin analog (parenteral) | 129 (14.8) |
| Soluble guanylate cyclase stimulator | 20 (2.3) |
Definition of abbreviations: CHD = congenital heart disease; CTD = connective tissue disease; PAH = pulmonary arterial hypertension; SF-12 = The Medical Outcome Study Short Form-12; WHO = World Heath Organization.
Summary statistics are presented as mean ± SD if normally distributed or as median (interquartile range) if skewed.
Figure 1.
Disease-specific quality-of-life scores and cumulative incidence of hospitalization over time. (A) Local polynomial smoothed plot of emPHasis-10 scores over follow-up time for COMPERA and REVEAL 2.0, with shaded areas representing 95% confidence intervals. Higher scores indicate a worse HRQoL. (B) Cumulative incidence functions for hospitalization over follow-up time for COMPERA and REVEAL 2.0. The groups were stratified by low (blue), intermediate (red), and high (green) risk. COMPERA = Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; HRQoL = health-related quality of life; REVEAL = Registry to Evaluate Early and Long-Term PAH Disease Management.
There were 1,255 person-years of follow-up; 281 (34%) participants with follow-up reported a hospitalization, 12 (1%) underwent lung transplantation, 102 (12%) died, and 119 (14%) transferred care or were lost to follow-up. Intermediate and high predicted risk by both COMPERA and REVEAL 2.0 were associated with an increased rate of hospitalization (COMPERA: IRR 1.88; 95% CI, 1.27–2.77 and IRR 2.34; 95% CI, 1.42–3.82; and REVEAL 2.0: IRR 1.45; 95% CI, 1.03–2.03 and IRR 1.88; 95% CI, 1.38–2.59, respectively). This persisted after multivariable adjustment and in the sensitivity analysis in which death, lung transplantation, and transfer and/or loss to follow-up were counted as hospitalizations. Higher risk was associated with an increased sHR for hospitalization for COMPERA and REVEAL 2.0 (intermediate: sHR 2.34; 95% CI, 1.53–3.60 vs. sHR 1.35; 95% CI, 0.99–1.85; and high: sHR 2.23; 95% CI, 1.34–3.72 vs. sHR 1.58; 95% CI, 1.20–2.09, respectively). Cumulative incidence functions for hospitalization are shown in Figure 1B.
In a large multicenter national cohort of patients with PAH, a higher predicted risk of mortality by two methods was associated with a worse HRQoL and increased hospitalizations. We observed a larger relative difference in the e10 scores than in the SF-12 scores, suggesting the disease-specific tool may be more sensitive with an approximate 10-point difference between low- and high-risk groups, similar to the published difference between those with World Health Organization functional class II and III symptoms (2). In contrast, differences in SF-12 scores were smaller and of unclear clinical significance (7).
Our findings support prior reports of the importance of hospitalizations as a prognostic indicator, similar to findings reported in REVEAL (5). Hospitalizations represent a period of potential high morbidity and mortality and are often driven by PAH-related complications (8, 9). Although the cause of hospitalization was not available and to account for potential undercounting of hospitalizations, we conducted sensitivity analysis imputing death, transplant, or loss to follow-up as hospitalization events, which confirmed our findings. Our study was not powered to detect differences between the intermediate and high-risk groups, but the sHRs for these groups were similar, suggesting a lack of discrimination in the higher risk strata—an area for potential improvement.
The multicentered PHAR cohort included a diverse population from centers throughout the country, making generalizability a particular strength. We calculated risk scores with available data in a “real-world” setting and conducted sensitivity analyses assuming the “worst-case” outcome. We only calculated the predicted risk from data at baseline; the strength of the relationships support the importance of the baseline “risk profile” irrespective of subsequent treatment. Unmeasured or residual confounding could explain the results; however, these prediction rules are designed to be used in a “stand-alone” fashion, so even if present, the clinical importance of the findings remains.
In conclusion, we found that a higher predicted risk for death by COMPERA and REVEAL 2.0 was associated with worse disease-specific HRQoL, a higher rate of hospitalizations, and increased risk for nonfatal hospitalizations. Improved risk stratification will allow for targeted strategies to improve HRQoL and reduce hospitalizations in these vulnerable patients with PAH.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and particularly the participants of the Pulmonary Hypertension Association Registry for their valuable contributions.
Footnotes
Supported by NIH grants T32 HL007891 (J.M.) and K23 HL141584 (N.A.-N.) and by the Aldrighetti Research Award for Young Investigators (N.A.-N.).
Originally Published in Press as DOI: 10.1164/rccm.202010-3967LE on November 19, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the PHAR Investigators, Abhijit Raval, Amresh Raina, Anna Hemnes, Charles Burger, D. Dunbar Ivy, Delphine Yung, Dianne Zwicke, Erika Berman-Rosenzweig, Gautam Ramani, Granthem Farr, H. James Ford, James Runo, Jeffrey Fineman, Jeremy Feldman, John Swisher, John Ryan, John Wesley McConnell, Kenneth Presberg, Kishan Parikh, Linda Cadaret, Mark Avdalovic, Michael Duncan, Michael Eggert, Nidhy Varghese, Paul Boyce, Peter Leary, Raymond Foley, R. James White, Robert Frantz, Roham Zamanian, Russel Hirsch, Sahil Bakshi, Sonja Bartolome, Steven Kawut, Stephen Mathai, Teresa De Marco, Timothy Williamson, and Todd Bull
References
- 1.Ware J, Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yorke J, Corris P, Gaine S, Gibbs JSR, Kiely DG, Harries C, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J. 2014;43:1106–1113. doi: 10.1183/09031936.00127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50:1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 4.Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39:4175–4181. doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 7.Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J Clin Epidemiol. 2017;89:188–198. doi: 10.1016/j.jclinepi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Hoeper MM, Channick RN, Chin KM, Delcroix M, Gaine S, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71:752–763. doi: 10.1016/j.jacc.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38:359–367. doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.