Abstract
Rationale: The biological mechanisms of long-term cognitive impairment and disability after critical illness are unclear.
Objectives: To test the hypothesis that markers of acute inflammation and coagulation are associated with subsequent long-term cognitive impairment and disability.
Methods: We obtained plasma samples from adults with respiratory failure or shock on Study Days 1, 3, and 5 and measured concentrations of CRP (C-reactive protein), IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12, MMP-9 (matrix metalloproteinase-9), TNF-α (tumor necrosis factor-α), soluble TNF receptor 1, and protein C. At 3 and 12 months after discharge, we assessed global cognition, executive function, and activities of daily living. We analyzed associations between markers and outcomes using multivariable regression, adjusting for age, sex, education, comorbidities, baseline cognition, doses of sedatives and opioids, stroke risk (in cognitive models), and baseline disability scores (in disability models).
Measurements and Main Results: We included 548 participants who were a median (interquartile range) of 62 (53–72) years old, 88% of whom were mechanically ventilated, and who had an enrollment Sequential Organ Failure Assessment score of 9 (7–11). After adjusting for covariates, no markers were associated with long-term cognitive function. Two markers, CRP and MMP-9, were associated with greater disability in basic and instrumental activities of daily living at 3 and 12 months. No other markers were consistently associated with disability outcomes.
Conclusions: Markers of systemic inflammation and coagulation measured early during critical illness are not associated with long-term cognitive outcomes and demonstrate inconsistent associations with disability outcomes. Future studies that pair longitudinal measurement of inflammation and related pathways throughout the course of critical illness and during recovery with long-term outcomes are needed.
Keywords: inflammation, coagulation, dementia, disability, critical illness
At a Glance Commentary
Scientific Knowledge on the Subject
Despite the pervasiveness of poor long-term outcomes after critical illness, the underlying biological mechanisms are unclear. Activation of inflammatory and coagulation pathways promotes organ dysfunction during critical illness, but the relationships between these pathways and longer-term outcomes are unclear.
What This Study Adds to the Field
This large, prospective, multicenter cohort study shows that, after adjusting for potential confounders, markers of acute systemic inflammation and coagulation collected early during critical illness are not consistently associated with long-term cognitive or disability outcomes 3 and 12 months after hospital discharge. Future studies that measure inflammation and related pathways throughout the course of critical illness and during recovery in conjunction with cognitive and disability assessments are needed.
Up to one-third of patients who survive critical illness suffer from long-term cognitive impairment and disabilities in activities of daily living (1–7). Although previous studies have begun to describe the clinical risk factors associated with these outcomes (1–5, 8–10), the biological mechanisms that may underlie long-term cognitive impairment and disability in survivors of critical illness remain unknown.
Activation of inflammatory and coagulation pathways promotes acute organ dysfunction during critical illness syndromes such as sepsis, acute respiratory distress syndrome, and surgery (11–13). In those without critical illness, these interrelated mechanisms are associated with greater odds of developing dementia and disability (14–17). Whether these mechanisms are associated with similar adverse long-term outcomes in survivors of critical illness is unknown.
To address these knowledge gaps, we measured circulating plasma markers of acute inflammation and coagulation and assessed long-term cognition and disability outcomes in a multicenter, prospective cohort study of adults with critical illness. We tested the hypothesis that higher concentrations of markers of inflammation and lower concentrations of a marker of coagulation during critical illness are associated with worse cognition and disability 3 and 12 months after critical illness.
Portions of these data were presented in abstract form (20).
Methods
Study Design and Population
During the identical (except for different enrolling sites) BRAIN-ICU (Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors; NCT00392795) and MIND-ICU (Delirium and Dementia in Veterans Surviving ICU Care; NCT00400062) multicenter, prospective cohort studies, we enrolled patients ≥18 years old who were treated in medical and surgical ICUs for acute respiratory failure and/or shock. Detailed inclusion and exclusion criteria have been previously published (3, 9, 18, 19) and are provided in the online supplement. For this long-term outcomes study, we also excluded those who died, withdrew, or were lost to follow-up before 3 months and those who did not have at least 1 day of complete biomarker measurement. Each center’s institutional review board approved the study protocol. Patients or their proxies provided informed consent.
Exposures
We enrolled participants within 72 hours of acute organ failure and collected blood samples on Study Days 1, 3, and 5. Using commercially available immunoassays and validated laboratory protocols (21), we measured, in duplicate, plasma concentrations of CRP (C-reactive protein), IFN-γ, IL-1β, IL-6, IL-8, IL-10, IL-12, MMP-9 (matrix metalloproteinase-9), TNF-α (tumor necrosis factor-α), sTNFR1 (soluble tumor necrosis factor receptor 1), and protein C. Descriptions of sample collection and processing and the rationale for inclusion of each marker are provided in the online supplement.
Outcomes
At 3 and 12 months after hospital discharge, study personnel blinded to events of the hospitalization measured participants’ global cognition using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (22), executive function using the Trail Making Test Part B (Trails B) (23), disability in basic activities of daily living (BADLs) using the Katz ADL (24), and disability in instrumental ADLs (IADLs) using the Functional Activities Questionnaire (FAQ) (25).
Covariates
Based on prior research and biological plausibility, we chose covariates a priori that we hypothesized may confound the association of interest between marker levels and outcome. In all models, we adjusted for age, sex, years of education, Charlson comorbidity index score (26), Short Informant Questionnaire on Cognitive Decline in the Elderly score (27), and mean daily doses of sedatives and opioids. In addition, in cognitive outcome models, we adjusted for underlying stroke risk using the Framingham Stroke Risk Score (28), and in disability models, we adjusted for baseline disability using the Katz ADL (24) and FAQ scores (25). Details of each covariate are provided in the online supplement.
Statistical Analysis
We calculated the mean value of each marker and then performed log10-transformation, using the transformed value as the exposure variable for all models. In addition to these primary analyses, we conducted a sensitivity analysis using Study Day 1 biomarker levels as the exposure variable and another using the percent change in biomarker level as the exposure variable. To measure associations with long-term cognitive outcomes, we performed multiple linear regression, adjusted for covariates. To measure the associations with disability outcomes, we performed zero-inflated negative binomial regression, adjusted for covariates.
To reduce the effect of bias due to differences between patients who were included in these analyses and those who were not because of death, study dropout, or loss to follow-up (i.e., attrition-related selection bias), we used inverse probability of attrition weighting (29, 30). In brief, we estimated the probability that each patient would be alive, not withdrawn, and included in each of the four potential follow-up cohorts (e.g., cognitive or disability at 3 or 12 mo). We included the inverse of these weighted variables in the respective models. A description of models used to calculate the weighted variables is included in the online supplement.
In cognitive outcome models, we allowed associations with continuous covariates to be nonlinear using restricted cubic splines. In disability outcome models, all covariates were forced linear except biomarkers, which were allowed to be nonlinear using restricted cubic splines. In all models, we excluded nonlinear terms if the global test for nonlinearity was P > 0.20. We used multiple imputation to account for missing follow-up outcomes data in those who participated in some follow-up testing using predictive mean matching, but we did not impute outcomes for those who died, withdrew, or were lost to follow-up. All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna Austria). P values < 0.05 were considered significant.
Results
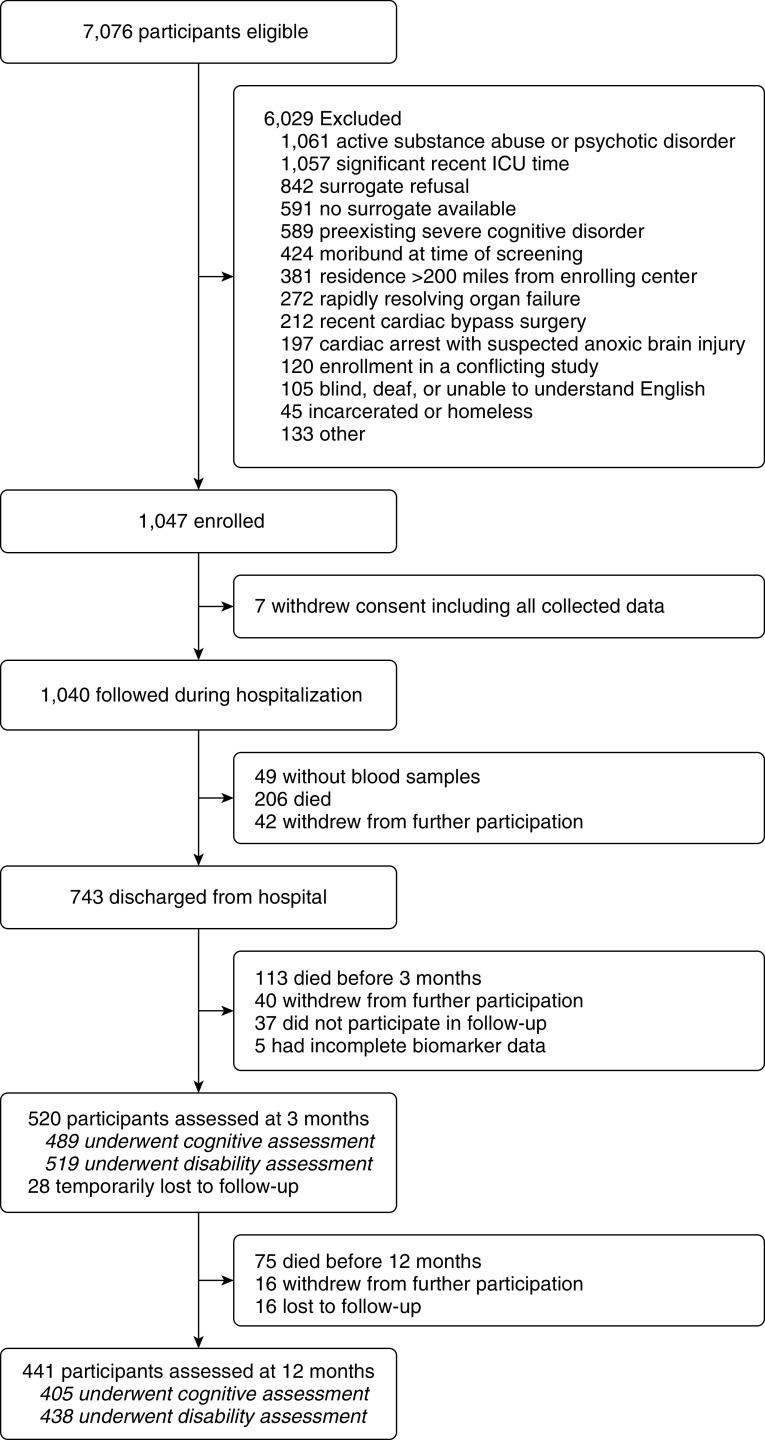
Between March 2007 and December 2010, we recruited 1,047 participants, 7 of whom withdrew permission to use their data. We followed 1,040 participants during their hospitalization (Figure 1). Blood samples were not obtained from 49 of these participants. Thus, 991 participants were eligible for this cohort study (Table E1 in the online supplement). Before hospital discharge, 206 participants died and 42 withdrew consent. Before 3-month follow-up, an additional 113 participants died, 40 withdrew, and 37 did not participate in follow-up. Among those who participated in follow-up, there were five who did not have at least 1 day of complete biomarker data. Therefore, 548 unique participants were analyzed for the current study, the majority of whom were mechanically ventilated and who had a high severity of illness (Table 1). Among survivors (i.e., including participants who withdrew or were lost to follow-up), we assessed cognition in 489/672 (73%) and disability in 519/672 (77%) at 3 months and cognition in 405/597 (68%) and disability in 438/597 (73%) at 12 months.
Figure 1.
Flow of participant enrollment and follow-up.
Table 1.
Demographic and Clinical Characteristics of Participants
| Characteristic | Participants (N = 548)* |
|---|---|
| Age, yr | 61 (52–70) |
| Sex, M, n (%) | 328 (60) |
| White race, n (%) | 488 (89) |
| Years of education | 12 (12–14) |
| IQCODE score at enrollment | 3.0 (3.0–3.1) |
| Charlson comorbidity index score | 2 (1–4) |
| APACHE II score at admission | 23 (17–29) |
| Mean SOFA score at admission | 8 (7–11) |
| Diagnosis at admission, n (%) | |
| Sepsis | 166 (30) |
| Acute respiratory failure | 90 (16) |
| Cardiogenic shock, myocardial infarction, or arrhythmia | 96 (17) |
| Airway protection/upper airway obstruction | 55 (10) |
| Surgical procedure† | 99 (18) |
| Neurologic disease or seizure | 7 (1) |
| Other diagnosis | 35 (6) |
| Enrolling center, n (%) | |
| Academic | 295 (54) |
| Community | 135 (25) |
| Veterans Affairs | 118 (22) |
| Mechanical ventilation | |
| Patients, n (%) | 483 (88) |
| Days of mechanical ventilation‡ | 2 (1–6) |
| Delirium | |
| Patients, n (%) | 389 (71) |
| Days of delirium‡ | 3 (2–7) |
| Coma | |
| Patients, n (%) | 288 (53) |
| Days of coma‡ | 2 (1–5) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; IQCODE = Short Informant Questionnaire on Cognitive Decline in the Elderly; SOFA = Sequential Organ Failure Assessment.
Data are shown as median (interquartile range) unless noted otherwise.
N represents the number of participants who were assessed at either 3- or 12-month follow-up. Overall, we assessed 520 patients at 3 months and an additional 28 patients at 12 months who were temporarily lost to follow-up at 3 months.
Includes gastric, colonic, vascular, urologic, orthopedic, obstetric/gynecologic, hepatobiliary/pancreatic, otolaryngologic, or transplant.
Among participants who had the clinical condition.
Marker Concentrations
Marker concentrations were obtained on more than 98% of eligible participant-days. The median time between ICU admission and study enrollment was 1 (0.7–2.0) day. Median concentrations of each marker from Study Days 1, 3, and 5 are presented in Table E2.
Cognitive Function and Disability at Follow-up
Complete cognitive outcomes assessments were performed in 423/489 (87%) participants at 3 months and in 365/405 (90%) participants at 12 months. Likewise, complete disability outcomes assessments were performed in 462/519 (89%) participants at 3 months and in 391/439 (89%) participants at 12 months. At both 3- and 12-month follow-up, RBANS and Trails B scores were approximately 1 SD below age-adjusted population means, where lower scores indicate worse cognitive function (Table 2). Katz ADL and FAQ scores at 3 and 12 months indicated that disabilities in BADLs and IADLs were present in one-quarter of survivors (Table 2).
Table 2.
Cognitive and Disability Outcomes at Follow-up
| Outcome | 3 mo | 12 mo |
|---|---|---|
| RBANS global cognition composite score*† | 80 (71–88) | 82 (72–90) |
| Trail Making Test Part B score*‡ | 41 (33–49) | 43 (35–51) |
| Katz ADL score§‖ | 0 (0–2) | 0 (0–1) |
| Functional Activities Questionnaire score§¶ | 3 (0–9) | 2 (0–8) |
Definition of abbreviations: Katz ADL = Katz Index of Independence in Activities of Daily Living; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
Data are shown as median (interquartile range).
Among 489 participants at 3 months and 405 participants at 12 months.
RBANS scores have an age-adjusted mean of 100 with an SD of 15. Lower scores indicate worse cognition.
Trail Making Test Part B scores have an age-, sex-, and education-adjusted mean of 50, with an SD of 10. Lower scores indicate worse cognition.
Among 519 participants at 3 months and 438 participants at 12 months.
Katz ADL scores range from 0 to 12. Scores of ≥1 indicate disability.
Functional Activities Questionnaire scores range from 0 to 30. Scores ≥1 indicate disability.
Association of Markers of Inflammation and Coagulation with Long-Term Cognitive Outcomes
At 3 and 12 months, none of the markers studied were associated with RBANS global cognition scores (Figure 2A). Likewise, none of the markers were associated with Trails B scores at either 3- or 12-month follow-up (Figure 2B). Sensitivity analyses that used Study Day 1 biomarker level or percent change in biomarker level as the main exposure did not alter these findings (Tables E3 and E4).
Figure 2.
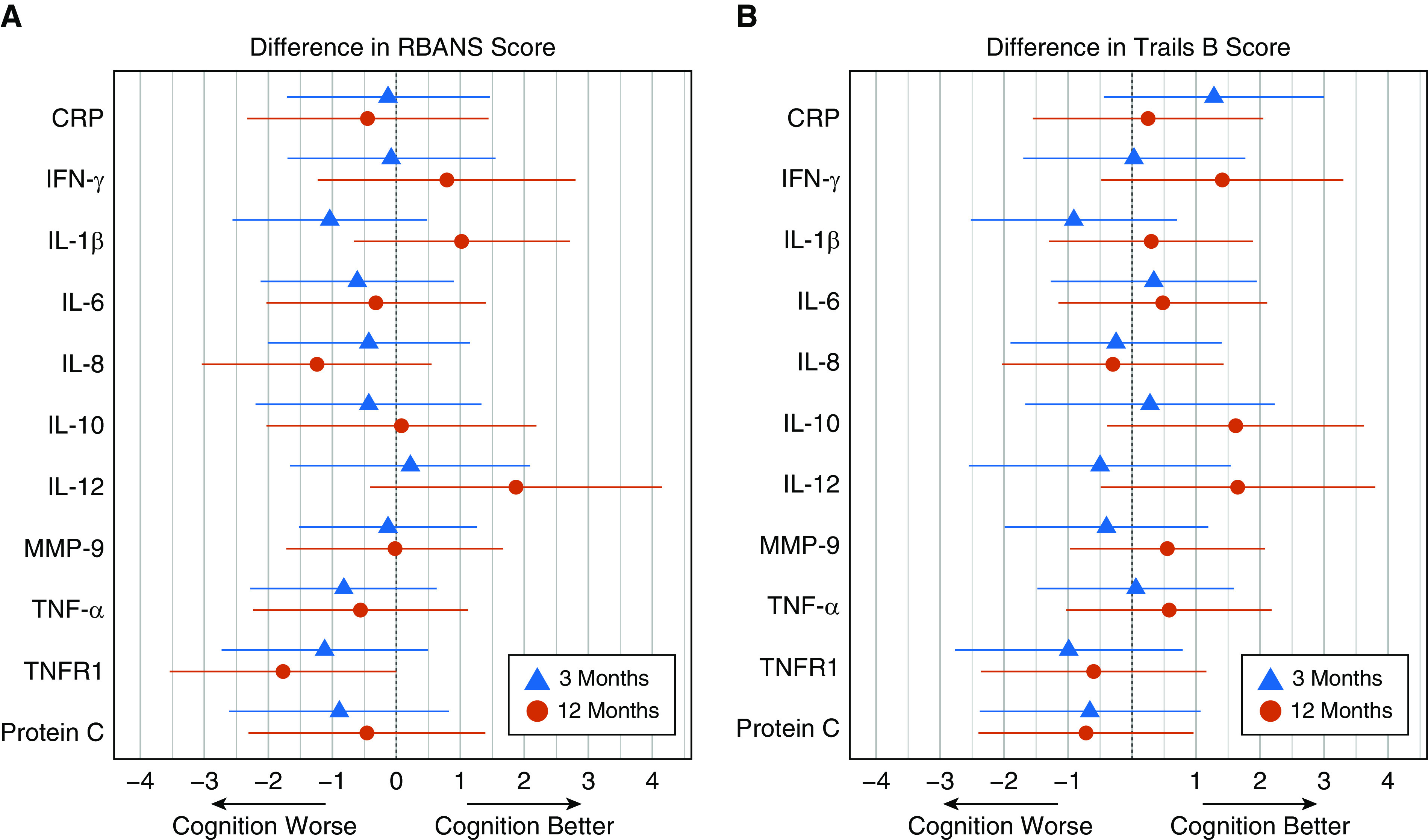
Associations of markers of inflammation and coagulation with long-term cognition. These figures display the difference in score on the (A) RBANS or (B) Trails B tests for each of the 11 biomarkers of interest. Blue triangles represent the point estimate at 3 months and red circles represent the point estimate at 12 months. Horizontal lines represent the 95% confidence interval. Each comparison is of participants with biomarker concentration at the 75th percentile to participants with a biomarker concentration at the 25th percentile, with all covariates adjusted to their mean or mode value. Negative changes in scores (i.e., point estimates to the left of 0) represent worse cognitive function. CRP = C-reactive protein; MMP = matrix metalloproteinase; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor; Trails B = Trail Making Test Part B.
Association of Markers of Inflammation and Coagulation with Long-Term Disability Outcomes
Higher levels of four markers—CRP, IL-1β, IL-6, and MMP-9—were associated with higher 3-month Katz ADL scores, indicating worse disability in BADLs (Figure 3A). In contrast, higher levels of the antiinflammatory marker IL-10 were associated with lower Katz ADL scores at 3 months. At 12 months, higher levels of two markers, CRP and MMP-9, were associated with higher Katz ADL scores (Figure 3A). Higher levels of TNF-α, however, were associated with lower Katz ADL scores at 12 months. No other markers were associated with Katz ADL scores at 3 or 12 months.
Figure 3.

Associations of markers of inflammation and coagulation with disability in activities of daily living. These figures display the incident rate ratio for (A) Katz ADL or (B) FAQ scores for each of the 11 biomarkers of interest. Blue triangles represent the point estimate at 3 months and red circles represent the point estimate at 12 months. Horizontal lines represent the 95% confidence interval. Each comparison is of participants with biomarker concentration at the 75th percentile to participants with a biomarker concentration at the 25th percentile, with all covariates adjusted to their mean or mode value. Incident rate ratios (IRRs) of 1 represent no change in disability, IRRs greater than 1 (i.e., to the right of 1.0) represent greater disability, and IRRs less than 1 (i.e., to the left of 1.0) represent less disability. CRP = C-reactive protein; FAQ = Functional Activities Questionnaire; Katz ADL = Katz Index of Independence in Activities of Daily Living; MMP = matrix metalloproteinase; TNF = tumor necrosis factor; TNFR = tumor necrosis factor receptor.
Higher levels of two markers—CRP and MMP-9—were associated with higher 3- and 12-month FAQ scores (Figure 3B) indicating worse disability in IADLs. Higher levels of IL-8 were associated with lower FAQ scores at 3 months. Higher levels of three markers—IFN-γ, IL-8, and TNF-α—were associated with lower 12-month FAQ scores (Figure 3B). No other markers were associated with FAQ scores at either 3 or 12 months. In models using Study Day 1 biomarker level or percent change in biomarker level as the main exposure, CRP and MMP-9 were no longer associated with Katz ADL or FAQ scores (Tables E5 and E6).
Discussion
In this large, multicenter, prospective cohort study, we found that markers of acute systemic inflammation and coagulation measured early in the course of a critical illness are not associated with long-term cognitive function in survivors. Likewise, these markers demonstrated inconsistent associations with disabilities in basic and instrumental activities of daily living. These results do not support the hypothesis that the interrelated pathways of systemic inflammation and coagulation during the acute phase of critical illness are risk factors for the development of adverse long-term outcomes in survivors.
The present study, the largest to date to measure the association of markers of inflammation and coagulation with long-term cognitive function in survivors of critical illness, extends the findings from two prior Dutch studies. Van den Boogaard and colleagues found no association between markers of inflammation (e.g., CRP, IL-1β, IL-1ra [IL-1 receptor antagonist], IL-6, IL-8, IL-10, IL-17, IL-18, MIF [macrophage inhibitory factor], and TNF-α) collected once during critical illness and self-reported cognitive function in 52 survivors (31). A second study measured daily CRP levels during critical illness and self-reported cognitive function in 363 survivors at 1 year and found no association between serial CRP measurements and 1-year cognition (32). In contrast, we performed serial measurement of a number of a priori defined biomarkers and used objective, robust, and well-validated direct assessments of global cognition and executive function to measure cognitive function in more than 500 survivors at follow-up, with high follow-up rates. Moreover, we used advanced statistical techniques to reduce bias related to death and study dropout. In total, this emerging body of evidence suggests that markers of acute systemic inflammation and coagulation, measured early in the course of a critical illness, are not associated with long-term cognitive impairment in survivors.
The findings that markers of acute inflammation are not associated with long-term cognitive impairment seem to stand in contrast to those from preclinical studies that implicate such a relationship exists and is mediated through microglial activation and ongoing neuroinflammation (33–38). Several possible explanations for this divergence exist. It may be the case that animal models subject to inflammatory insults (e.g., via exposure to LPS, cecal ligation and puncture, or bacterial injection) do not adequately model the complexity and heterogeneity of critical illness, effects related to coexisting illnesses and frailty, or effects of multiple organ failures (39). Many of these preclinical studies, for example, include younger animals, whereas in human studies most patients are in their 50s or 60s or older (40). Thus, differences related to biological processes of aging (e.g., cellular senescence and epigenetic modifications) could account for differences between preclinical and human studies. On the other hand, most clinical studies, including the current investigation, have been limited by the use of markers of peripheral inflammation rather than direct measures of neuroinflammation. In addition, the neuroanatomical and neuropathologic changes seen in animal models of cognitive impairment after critical illness have only been studied in small case–control studies (34–36, 38, 41). Future studies should seek to address these limitations and to evaluate the effect of critical illness–related chronic inflammation and related pathways on blood–brain barrier integrity, endothelial function, neuronal injury, and neurotransmitter pathways hypothesized to underlie other forms of acquired cognitive impairment and dementia.
We found that two markers, CRP and MMP-9, were associated with worse long-term disability in activities of daily living in survivors of critical illness. Nevertheless, because these markers were evaluated in the context of nine other markers at two time points, and our analyses found only inconsistent associations with disability outcomes, the small number of significant associations should be interpreted as exploratory. A body of literature has reported an association between greater inflammation and worse disability among older adults without critical illness (42–46). To our knowledge, however, the present study is among the first to explore this association in patients who survive critical illness. Prior studies enrolled older adults without acute or critical illness and therefore reported associations between chronic inflammation and disability. In contrast, we studied the association between acute inflammation during critical illness with subsequent disability. Indeed, a limitation of our study is that we did not measure inflammatory markers through the full course of recovery from critical illness (e.g., at or after hospital discharge). Moreover, in prior studies, the association between inflammation and disability was shown over years of follow-up, in contrast to our 12 months of follow-up. Finally, the biological mechanisms of progressive disability in community-dwelling older adults may differ from those that cause the accelerated disability in survivors of critical illness. Future longitudinal studies are needed to explore the time course of inflammation after critical illness with longer follow-up of disability than was available in the present study.
In addition to the strengths of the current investigation described above, we included, of patients from a diverse group of academic, community, and Veterans Affairs hospitals with heterogeneous reasons for medical and surgical critical illness, an approach that enhances the generalizability to our findings. We assessed cognition and disability at two time points, allowing a determination of the association between acute inflammation and these outcomes at both the intermediate and long-term phase of recovery. Finally, all follow-up assessments and biomarker measurements were performed by different personnel masked to each other’s findings.
Nevertheless, our findings should be considered in the context of several limitations. We measured biomarkers during the first week of critical illness. Therefore, our data cannot comment on potential associations between persistent inflammation and coagulation and long-term outcomes after critical illness (47–49). Future longitudinal studies, which pair the measurement of biomarkers of inflammation throughout the course of critical illness and recovery with cognitive and disability assessments, are needed.
Second, the 11 markers we studied may not represent all of the direct and indirect pathways by which systemic inflammation can affect the central nervous system to result in long-term cognitive impairment. Thus, future studies using biomarkers obtained from both peripheral blood and cerebrospinal fluid should evaluate inflammation-associated pathways—such as endothelial dysfunction, microvascular thrombosis, blood–brain barrier disruption, neuroinflammation, and neuronal injury—implicated in other forms of cognitive impairment, dementia, and acquired brain injury. Finally, we did not capture data on events following the hospitalization (e.g., rehospitalizations, rehabilitation interventions, nutritional status, and perceived stress) that may affect the relationship between inflammation and cognitive status or disability. Nevertheless, in our statistical models, we accounted for a number of potential confounders related to a patient’s baseline health status and function as well as those related to index critical illness.
Conclusions
In conclusion, we found no consistent associations between markers of acute systemic inflammation and coagulation during critical illness and long-term cognitive function or disability in survivors. Future longitudinal studies are needed to determine whether the subset of critical illness survivors who develop chronic inflammation are at increased risk for long-term cognitive impairment and disability.
Supplementary Material
Footnotes
Supported by the NIH (K76AG054864, K23AG034257, R01AG035117, R01AG027472, R01HL111111, R01HL135144, and K24HL103836); the Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center; and a VA Merit award.
Author Contributions: N.E.B. and T.D.G. had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: N.E.B., C.G.H., P.P., L.B.W., E.W.E., and T.D.G. Data acquisition, analysis, and interpretation: All authors. Statistical analysis: J.L.T., O.M.O, and R.R. Drafting of the manuscript: N.E.B. Critical revision of the article for important intellectual content: All authors. Final approval of the article: All authors. Obtaining funding: N.E.B., E.W.E., and T.D.G.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2449OC on October 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 2.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196:64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrante LE, Murphy TE, Leo-Summers LS, Gahbauer EA, Pisani MA, Gill TM. The combined effects of frailty and cognitive impairment on post-ICU disability among older ICU survivors. Am J Respir Crit Care Med. 2019;200:107–110. doi: 10.1164/rccm.201806-1144LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29(7) Suppl:S99–S106. doi: 10.1097/00003246-200107001-00032. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 15.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 16.Wichmann MA, Cruickshanks KJ, Carlsson CM, Chappell R, Fischer ME, Klein BE, et al. Long-term systemic inflammation and cognitive impairment in a population-based cohort. J Am Geriatr Soc. 2014;62:1683–1691. doi: 10.1111/jgs.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama A, O’Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2013;68:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel MB, Jackson JC, Morandi A, Girard TD, Hughes CG, Thompson JL, et al. Incidence and risk factors for intensive care unit-related post-traumatic stress disorder in veterans and civilians. Am J Respir Crit Care Med. 2016;193:1373–1381. doi: 10.1164/rccm.201506-1158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes CG, Patel MB, Jackson JC, Girard TD, Geevarghese SK, Norman BC, et al. MIND-ICU, BRAIN-ICU investigators. Surgery and anesthesia exposure is not a risk factor for cognitive impairment after major noncardiac surgery and critical illness. Ann Surg. 2017;265:1126–1133. doi: 10.1097/SLA.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brummel NE, Pandharipand PP, Hughes CG, Jackson JC, Thompson JL, Chandrasekhar R, et al. The association between biomarkers of inflammation and long-term cognitive impairment and disability in survivors of critical illness [abstract] Am J Respir Crit Care Med. 2016;193:A2610. [Google Scholar]
- 21.Bastarache JA, Koyama T, Wickersham NE, Ware LB. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J Immunol Methods. 2014;408:13–23. doi: 10.1016/j.jim.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 23.Reitan RM, Wolfson D. The halstead reitan neuropsychological test battery. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 24.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL. A standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 28.Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham Stroke Risk Profile and poor cognitive function: a population-based study. BMC Neurol. 2008;8:12. doi: 10.1186/1471-2377-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189. [DOI] [PubMed] [Google Scholar]
- 30.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15:R297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolters AE, Peelen LM, Veldhuijzen DS, Zaal IJ, de Lange DW, Pasma W, et al. Long-term self-reported cognitive problems after delirium in the intensive care unit and the effect of systemic inflammation. J Am Geriatr Soc. 2017;65:786–791. doi: 10.1111/jgs.14660. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011;39:945–953. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemstra AW, Groen in’t Woud JC, Hoozemans JJ, van Haastert ES, Rozemuller AJ, Eikelenboom P, et al. Microglia activation in sepsis: a case-control study. J Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 38.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 40.Starr ME, Saito H. Sepsis in old age: review of human and animal studies. Aging Dis. 2014;5:126–136. doi: 10.14336/AD.2014.0500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janz DR, Abel TW, Jackson JC, Gunther ML, Heckers S, Ely EW. Brain autopsy findings in intensive care unit patients previously suffering from delirium: a pilot study. J Crit Care. 2010;25:538.e7–538.e12. doi: 10.1016/j.jcrc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 43.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 44.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 45.Coelho FM, Narciso FM, Oliveira DM, Pereira DS, Teixeira AL, Teixeira MM, et al. sTNFR-1 is an early inflammatory marker in community versus institutionalized elderly women. Inflamm Res. 2010;59:129–134. doi: 10.1007/s00011-009-0079-6. [DOI] [PubMed] [Google Scholar]
- 46.Haren MT, Malmstrom TK, Miller DK, Patrick P, Perry HM, III, Herning MM, et al. Higher C-reactive protein and soluble tumor necrosis factor receptor levels are associated with poor physical function and disability: a cross-sectional analysis of a cohort of late middle-aged African Americans. J Gerontol A Biol Sci Med Sci. 2010;65:274–281. doi: 10.1093/gerona/glp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, et al. GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yende S, D’Angelo G, Mayr F, Kellum JA, Weissfeld L, Kaynar AM, et al. GenIMS Investigators. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One. 2011;6:e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2:e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.