To the Editor:
The coronavirus disease (COVID-19) pandemic has brought into sharp focus the importance of protecting patients and healthcare workers (HCW) from nosocomial infection (1). Hospital-associated infection can occur through contact with contaminated surfaces, either by droplets generated by coughing or sneezing settling on exposed mucous membranes or through direct inhalation of aerosols. Although the contribution of each route differs according to the setting and type of infection, the ventilation within hospitals is a key determinant of infection risk from airborne pathogens (2, 3), with low ventilation rates associated with increased risks of transmission of, for example, tuberculosis and Middle East respiratory syndrome (4, 5). For patients with cystic fibrosis (CF), acquisition of multidrug-resistant pathogens from other patients represents one of the greatest threats to health (6), with both experimental data (7) and room sampling (8) highlighting the risk of cross-infection through long-lived infectious aerosols.
As a direct response to these challenges, Royal Papworth Hospital installed a bespoke ventilation system during its relocation from a 100-year-old facility to a new state-of-the-art cardiothoracic hospital in Cambridge, United Kingdom. Whereas the old CF center relied on passive, natural ventilation, with airflow created by opening doors and windows, the mechanical ventilation in the new hospital provides 15 air changes per hour (ACH) in the CF center (both patient rooms and corridors), and 6 ACH in other clinical areas.
We examined the impact of high-frequency air changes within patient rooms by monitoring the dispersal, mixing, and ventilation of an injected tracer gas (CO2) using eight CozIR-A sensors (Gas Sensing Solutions) in the new and old hospitals, just before occupation and just after vacation respectively, while the heating and ventilation systems were operational. Although CO2 persisted in passively ventilated rooms for over 58 minutes, rooms actively ventilated at 6 or 15 ACH experienced exponential decay of tracer concentrations to 10% of peak concentration within 26.7 and 11.7 minutes, respectively (Figure 1A).
Figure 1.
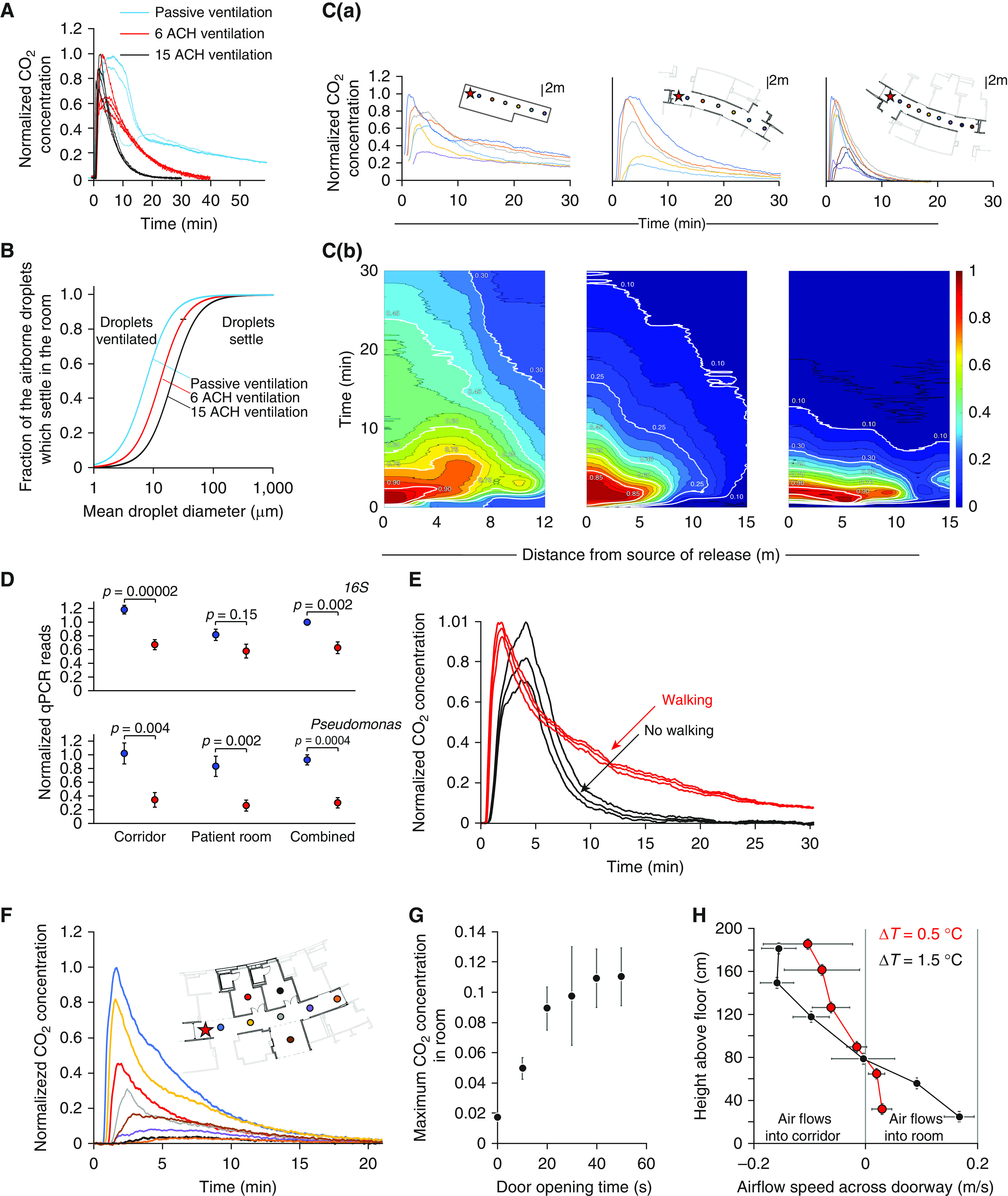
The impact of ventilation on air-handling in hospitals. (A) Concentrations of tracer gas (CO2) monitored over time after a pulse release in three patient rooms with 15 air changes per hour (ACH) (black line) within New Papworth Cystic Fibrosis Centre, 6 ACH (red line) within New Papworth Hospital, or passive ventilation (blue line) from Old Papworth Hospital. The concentrations normalized to the maximum recorded value. (B) Model prediction of the fraction of airborne droplets that sediment to the floor or are ventilated in a patient room as a function of mean droplet diameter, shown for rooms passively ventilated (blue) or with 6 (red) or 15 (black) ACH. (C) Variations in normalized CO2 concentration after tracer release (at the point marked by a star) measured by sensors located along ward corridors (as shown in floor plans) in Old Papworth Hospital under passive ventilation (left) and in New Papworth Hospital in areas with ventilation with 6 ACH (middle) or ventilation with 15 ACH (right). (Ca) Normalized CO2 concentrations detected over time by each sensor. (Cb) Normalized CO2 concentrations as a distance–time plot. (D) Comparison of the relative amounts of total bacteria (16S) (quantified by qPCR) detected in air samples from ward corridors and unoccupied rooms in Old Papworth Hospital (blue) under passive ventilation, compared with equivalent areas in New Papworth Hospital receiving ventilation with 15 ACH (red) during periods of similar ward-bed occupancy levels. (E) CO2 concentrations over time after tracer release in a corridor with ventilation with 15 ACH, in the presence (red) or absence (black) of people walking along it. (F) CO2 concentrations over time after tracer released in the corridor (red star) measured by sensors deployed along the corridor and inpatient rooms. (G) Peak CO2 concentrations detected in rooms (expressed as a fraction of maximum corridor concentration) as a function of the time the doorway is kept open. (H) Variation of the airflow speed across a doorway as a function of height above the floor (quantified from a video of smoke released from an Artic Hayes pen) when the room is 0.5°C (red) or 1.5°C (black) hotter than the corridor. ΔT = change in temperature; qPCR = quantitative PCR.
The type of ventilation determines the fraction of airborne droplets that settle in the patient’s room before being removed. Because the fall speeds of droplets vary with particle size, the impact of changes in ventilation between old and new hospitals is particularly marked for particles in the respirable size range produced by coughing (9) (1–10 μm in diameter) (Figure 1B), indicating that high-frequency air changes may reduce transmission risks from aerosols.
We next explored air mixing and dispersal along hospital-ward corridors, again using a CO2 tracer with sensors deployed 1.7–14.7 m from the CO2 source (Figure 1C). With passive ventilation at the Old Papworth site, CO2 spread rapidly along the corridor and persisted for 30 minutes. At the new site, active ventilation (at 6 ACH) limited dispersal distances and improved clearance rates (although detectible amounts of CO2 remained after 30 min), whereas high ventilation with 15 ACH initially caused faster dispersal than conventional ventilation but led to clearance of CO2 to below 10% of peak concentration by 13.3 minutes.
To examine the impact of ventilation on patient exposure to airborne pathogens, we deployed static NIOSH BC 251 two-stage cyclone aerosol samplers (9), positioned at 90 cm and 180 cm from the floor, to sample the air in unoccupied patient rooms and ward corridors in both the old and new CF centers during periods of similar ward–bed occupancy (92% ± 3.5% new ward vs. 94% ± 7.9% old ward; mean ± SD, P = 0.43). The amounts of total bacteria (quantified by 16S quantitative PCR) were reduced in rooms and corridors with high-frequency air changes compared with those with passive ventilation (Figure 1D), suggesting reduced patient and HCW exposure to nosocomial airborne infections.
We also considered how people walking might influence air mixing and residence times in corridors. Given the high Reynolds number (Re) associated with a person walking (Re = ρuL/μ ≈ 0.5 × 105; based on a speed [u] of 1 m/s, size [L] of 0.5 m, air viscosity [μ] of 10−5 Pa·s, and density [ρ] of 1 kg/m3), we expect the resultant wake to drive highly turbulent mixing. When measured in hospital corridors with high ventilation, a person walking along the corridor every 30 seconds considerably increased the residence time of CO2 after a pulse release (Figure 1E), lengthening the half-life from 1.3 minutes to 6.7 minutes (Figure 1E).
Finally, we explored the potential for movement of infectious aerosols between corridors and rooms. We found that CO2 released in the corridor rapidly penetrated into patient rooms with open doors (Figure 1F), despite the fact that both corridor and rooms had their own balanced system of inflow and outflow ducts and were at the same pressure. We saw a steep relationship between the duration of door opening and the resulting CO2 concentrations in the room (Figure 1G), suggesting that limiting door opening could greatly reduce corridor-to-room pathogen spread.
We also noticed that movement of CO2 into rooms increases with temperature contrasts. Airflow speeds across a doorway based on video recordings of tracer smoke show air flowed out of the room near the top (with mean speeds of 15–16 cm/s); whereas at lower heights, air flowed into the room at 16–18 cm/s (P = 0.002) (Figure 1H). The associated exchange flow driven by a 3°C temperature difference between the room and corridor (0.16 m3/s) is comparable in magnitude to the high-frequency ventilation (about 0.20 m3/s) and may contribute to pathogen transmission between room and corridor.
In summary, our results indicate that high-frequency ventilation can reduce residence times of infectious aerosols, despite residual impacts of people walking in corridors and temperature-dependent exchange flow across doorways. We believe that this strategy has the potential to improve safety for CF patients and other vulnerable individuals in the hospital and impede cross-infection between HCW and patients. Airborne transmission has been implicated in the spread of several nosocomial infections (3, 6), most recently severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1), which remains viable in aerosols for over 3 hours (10) and is likely to be transmitted by both large and small airborne droplets (3). Enhanced hospital ventilation, coupled with limiting door opening times, equilibration of temperature, minimizing corridor traffic, and wearing facemasks could play a critical role in the response to this current pandemic.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank William G. Lindsley from the U.S. CDC for providing the NIOSH air samplers and Colin Glen for providing information about hospital floor plans and ventilation systems.
Footnotes
Supported by the Wellcome Trust, Cambridge National Institute of Health Research Biomedical Research Centre, and Royal Papworth Hospital.
Author Contributions: All authors contributed to the manuscript according to the four International Committee of Medical Journal Editors criteria for authorship.
Originally Published in Press as DOI: 10.1164/rccm.202009-3634LE on November 19, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson J, Chartier Y, Pessoa-Silva CL, Jensen P, Yuguo L, Wing-Hong S World Health Organization. Geneva, Switzerland: World Health Organization; 2009. Natural ventilation for infection control in health-care settings: WHO Guidelines 2009. [PubMed] [Google Scholar]
- 3.Morawska L, Tang JW, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fennelly KP, Nardell EA. The relative efficacy of respirators and room ventilation in preventing occupational tuberculosis. Infect Control Hosp Epidemiol. 1998;19:754–759. doi: 10.1086/647719. [DOI] [PubMed] [Google Scholar]
- 5.Jo S, Hong J, Lee S-E, Ki M, Choi BY, Sung M. Airflow analysis of Pyeongtaek St Mary’s Hospital during hospitalization of the first Middle East respiratory syndrome patient in Korea. R Soc Open Sci. 2019;6:181164. doi: 10.1098/rsos.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saiman L, Siegel JD, LiPuma JJ, Brown RF, Bryson EA, Chambers MJ, et al. Cystic Fibrous Foundation; Society for Healthcare Epidemiology of America. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol. 2014;35:S1–S67. doi: 10.1086/676882. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright CE, France MW, O’Rourke P, Anuj S, Kidd TJ, Nissen MD, et al. Cough-generated aerosols of Pseudomonas aeruginosa and other Gram-negative bacteria from patients with cystic fibrosis. Thorax. 2009;64:926–931. doi: 10.1136/thx.2008.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood ME, Stockwell RE, Johnson GR, Ramsay KA, Sherrard LJ, Kidd TJ, et al. Cystic fibrosis pathogens survive for extended periods within cough-generated droplet nuclei. Thorax. 2019;74:87–90. doi: 10.1136/thoraxjnl-2018-211567. [DOI] [PubMed] [Google Scholar]
- 9.Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 10.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


