In idiopathic pulmonary fibrosis (IPF), a fibrotic niche is established that leads to persistent collagen deposition. The mechanisms underlying initiation and persistence of this niche and continuing progression of collagen deposition are poorly understood. In this issue of the Journal, Yao and colleagues (pp. 707–717) have developed a new mouse model of pulmonary fibrosis, induced by genetic Sin3a loss of function (Sin3a-LOF) in alveolar type 2 (AT2) cells (1). Unlike the single-dose bleomycin model of fibrosis, in which fibrosis peaks 21 days after exposure and then largely resolves, the fibrosis in Sin3a-LOF mice progresses steadily over 4–8 weeks, eventually causing death.
Yao and colleagues show that loss of Sin3a causes AT2 cells to adopt a complex cellular phenotype. The cells upregulate genes associated with hypoxia, mitochondrial dysfunction, DNA damage, and senescence. Similar patterns of gene expression were seen in single-cell sequencing of epithelial cells from patients with IPF. These cells expressed p21 protein and were hypoproliferative both in vivo and in organoids. Yao and colleagues further showed that the p53 pathway was critically downstream of Sin3a-LOF, as genetic and pharmacological p53 inhibition protected Sin3a-LOF mice from fibrosis. The persistence of dysfunctional epithelial cells was also critical to fibrosis, as a “senolytic” cocktail of dastinib and quercetin decreased the numbers of Sin3a-LOF cells and protected mice from fibrosis.
The transcriptional and functional phenotype of Sin3a-LOF cells has strong similarities to that of a recently described transient population of progenitor cells that emerges after various injuries, including bleomycin exposure, diphtheria toxin ablation, and LPS (2–6). In IPF lungs, KRT17+ cells are probably an analogous population (3, 7). In mice, these cells can be derived from airway or AT2 cells and have the capacity to differentiate into mature AT1 and AT2 cells. The progenitor cells, like Sin3a-LOF cells, are hypoproliferative, and their gene expression phenotype includes upregulation of the p53, TGFβ, hypoxia, and senescence-associated pathways. Sin3a-LOF cells, like these recently described progenitors, upregulate Krt7, Krt8, Krt19, and Lgals3, which selectively mark and perhaps contribute (5) to the transient progenitor cell state.
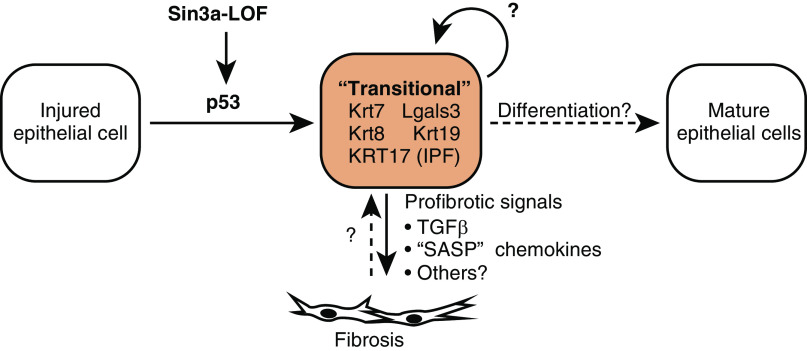
These studies suggest a model in which epithelial cells normally respond to severe injury by entering a transitional state characterized by the activation of pathways often described as pathological. These pathways could actually be homeostatic if injured epithelial cells must transition through this state before then committing to a tissue repair program and differentiating into mature epithelial cells. In this view, pathology would arise when cells get “stuck in a moment”—the transitional state—and cannot get out of it, thereby impairing normal differentiation and repair (Figure 1).
Figure 1.
Model for Sin3a loss of function–induced fibrosis and outstanding questions. IPF = idiopathic pulmonary fibrosis; LOF = loss of function; SASP = senescence-associated secretory phenotype.
If the Sin3a-LOF cellular phenotype is indeed congruent with the transitional progenitor cell state, then the Sin3a model would be a uniquely valuable model to study the intrinsic signals that instruct epithelial progenitor cells to abnormally remain trapped in the transitional state and the consequences of this entrapment. Sin3a-LOF–induced p53 activation has particular relevance to IPF because a substantial proportion of familial IPF probands have mutations that map to telomerase pathway genes, with attendant activation of DNA damage signaling (8, 9). Telomere maintenance defects may be common in sporadic IPF as well (10). It will be interesting to see if other cell-intrinsic pathways that are activated in the transitional state and in IPF, such as the unfolded protein response (3, 11), would likewise contribute to abnormal persistence of the transitional state. Similar efforts to understand the mechanisms by which extrinsic cues contribute to induction and/or persistence of this state will also be important.
The consequences of cells persisting in the transitional state appear to be profound. Some genes upregulated in Sin3a-LOF cells and described as the “senescence-associated secretory phenotype” are actually familiar intercellular signals that are not unique to senescence. One is TGFβ1, a key activator of collagen-producing fibroblasts. Others are inflammatory chemokines that could help recruit monocyte-derived macrophages that infiltrate fibrotic regions and can, in turn, help support the proliferation and activation of fibroblasts (12, 13). Cells stuck in the transitional state are therefore key players in establishing the fibrotic niche.
To translate these findings into new treatments for pulmonary fibrosis, it will be important to characterize the pathways that physiologically induce the transitional state and those that abnormally maintain it. Therapies that prevent entry into the transitional state or eliminate such cells might also deplete the pool of progenitor cells available for lung repair. For example, Yao and colleagues show that loss of p53 function protects Sin3a-LOF mice from fibrosis by decreasing the profibrotic phenotype of Sin3a-LOF cells. Yet, loss of p53 signaling also impairs progenitor cell differentiation into mature epithelium (4). In the Sin3a-LOF model, the net effect of p53 loss of function does seem to be beneficial, as p53 knockout mice had improved mortality. Whether that is true in other models or in human disease might depend on the nature of the primary injury and perhaps the stage of disease.
It will also be important to determine whether the analogous cells in human IPF are strictly senescent in the classical sense of irreversible cell cycle arrest after stress or injury (14). Though the cells are hypoproliferative and express markers associated with senescence, this state may be reversible. If so, disrupting the signals that maintain entrapment of these epithelial cells in the transitional state could potentially ameliorate fibrosis and promote repair and regeneration at the same time.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202010-3898ED on November 23, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med. 2021;203:707–717. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kathiriya JJ, Brumwell AN, Jackson JR, Tang X, Chapman HA. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell. 2020;26:346–358, e4. doi: 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun. 2020;11:3559. doi: 10.1038/s41467-020-17358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol. 2020;22:934–946. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang P, Gil de Rubio R, Hrycaj SM, Gurczynski SJ, Riemondy KA, Moore BB, et al. Ineffectual type 2-to-type 1 alveolar epithelial cell differentiation in idiopathic pulmonary fibrosis: persistence of the KRT8hi transitional state. Am J Respir Crit Care Med. 2020;201:1443–1447. doi: 10.1164/rccm.201909-1726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi J, Park JE, Tsagkogeorga G, Yanagita M, Koo BK, Han N, et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell. 2020;27:366–382, e7. doi: 10.1016/j.stem.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6:a1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armanios MY, Chen JJL, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 9. Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alder JK, Chen JJL, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 14. Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.