Abstract
Higher dietary intake of calcium (Ca), magnesium (Mg), and vitamin D has been associated with reduced risk of type 2 diabetes (T2DM), and a higher intracellular ratio of Ca to Mg leads to insulin resistance. Previous epidemiological studies did not examine the combined effects of dietary Ca, Mg, and vitamin D as well as ratio of Ca to Mg with T2DM. Therefore, we assessed the relationship between dietary intakes of Mg, Ca, and vitamin D (using 24‐hr recalls) individually and in composite and T2DM in the National Health and Nutrition Examination Survey 2007–2014, which involved 20,480 adults (9,977 men and 10,503 women) with comprehensive information on related nutrients, and anthropometric, demographic, and biomarker variables using multivariable logistic regression. The results indicated that dietary calcium at Q3 (812 mg/day) was significantly linked with T2DM in women (OR: 1.30; 95% CI: 1.02, 1.65). Dietary vitamin D at Q3 (5.25 μg/day) significantly reduced the odds of T2DM by 21% in men (OR: 0.79; 95% CI: 0.64, 0.98). This is an interesting study that has important implications for dietary recommendations. It is concluded that US adults having dietary Ca below the RDA were associated with increased risk of T2DM in all population and women, while higher ratio of Ca to Mg was associated with increased risk of T2DM in all population and increased vitamin D intake is related to decreased risk of T2DM in men. Moreover, further research is needed to make more definitive nutritional recommendations.
Keywords: cross‐sectional study, dietary calcium, magnesium, NHANES, T2DM, vitamin D
This is an interesting study that has important implications for dietary recommendations. It is concluded that dietary calcium intake level of 812 mg/day (Q3) will increase the risk of developing T2DM in the general population and women. Additionally, the findings conclude that US adults having dietary calcium below the RDA were associated with increased risk of T2DM in all population and women, while higher ratio of Ca to Mg was associated with increased risk of T2DM in all population and increased vitamin D intake was associated with decreased risk of T2DM in men. Moreover, the conclusions should probably call for more studies to confirm these results in other settings.

1. INTRODUCTION
Definition of diabetes according to the American Diabetes Association (ADA) is a group of metabolic diseases characterized by hyperglycemia resulting in either deficiency of insulin secretion, insulin action, or both (Diabetes Care, 2004). Type 2 diabetes is increasing worldwide; hence, it is a chronic disease (). The prevalence of type 2 diabetes in 2005 was 1 in 10 in every 5 adults, while that was predicted to be 1 in 3 adults by the end of 2050 (Boyle et al., 2010). T2DM is associated with poor nutrient intake (McNaughton SA, Mishra GD, Brunner EJ 2008) especially calcium and vitamin D were considered as “nutrient of concerned” (Dietary Guidelines for American (2010) while magnesium was considered as “shortfall nutrient” (United States Department of Agriculture, 2010). Dietary factors have a key role in the incidence and development of chronic diseases, especially T2DM (McNaughton et al., 2008).
Moreover, about 40% of American populations did not meet the daily requirements of calcium from their diet (US Department of Agriculture, 2015). Calcium has a vital role in the prevention of diabetes by improving insulin sensitivity and pancreatic β‐cell functions (Pittas et al., 2007). Insulin secretion depends on calcium, and thus, alterations in calcium flux adversely affect beta‐cell secretion (Pittas et al., 2007). Also, dietary calcium was inversely associated with T2DM in several epidemiological studies (Pittas et al., 2006; Villegas et al., 2009). However, some studies showed inconclusive results (De Boer et al., 2008; Kirii et al., 2009). Meta‐analysis and other research studies conducted on the American populations show a significant inverse association between risk of T2DM and low magnesium intake (Dong et al., 2011; Hruby et al., 2014). Especially, magnesium status is low in populations that eat processed diets; therefore, there is a need to explore the concerns of suboptimal magnesium status because low magnesium was related to the threat of type 2 diabetes (Rosanoff et al., 2016).
Potentially, cellular glucose metabolism is directly regulated through magnesium by affecting rate‐limiting enzymes of glycolysis (Musso, 2009). Magnesium significantly reduces the risk by 36% in Japanese men and women when compared to highest (303 mg/day) quartile versus lowest (158 mg/day) quartile (Kirii et al., 2010). Oral magnesium supplementation of 250 and 365 mg/day for the period of 3 and 6 months decreased insulin resistance in the randomized control trial (RCT; Mooren et al., 2011). A higher intracellular ratio of Ca: Mg leads to insulin resistance and hypertension in the NHANES study (Moore‐Schiltz et al., 2015). According to the hypothesis of Roasanoff (2010), low magnesium and high calcium diet may lead to cellular calcium activation from low magnesium levels, and future studies are obligatory to realize the potential effects of the ratio of Ca to Mg (Nielsen, 2010; Rosanoff, 2010). However, foods fortified with vitamin D were the main food for vitamin D intake in the United States (Holick, 2007). Human studies, and animal and human cell experiment show the protective effects of vitamin D and T2DM (Danescu et al., 2009; Kadowaki & Norman, 1984). NHANES data indicated that serum 25‐OHD was inversely found to be in dose–response patterns in non‐Hispanic whites and Mexican Americans (Scragg et al., 2004). Vitamin D stimulates the expression of insulin receptor and bindings with the beta‐cell receptor, thus affects directly the impaired beta‐cell function and insulin resistance and indirectly changeable calcium flux and extracellular calcium through β‐cell (Pittas et al., 2007).
Using NHANES (2007‐2014), however, no previous epidemiological studies have directly examined the effect of dietary calcium, magnesium, vitamin‐D and ratio of Ca:Mg and risk of T2DM, therefore, the purpose of this study was to examine the individually as well combine effect of dietary calcium, magnesium , ratio of calcium to magnesium (Ca:Mg) and vitamin D using their relevant cutoff points (RDA, Quintiles) and T2DM in US adults, the hypothesis shows that dietary calcium, magnesium, and vitamin‐D individually and in combination would decrease the risk of T2DM.
Hence, the aim to conduct this study was to examine the potential association of dietary intake for magnesium, calcium separately and in combinations consuming their ratio (Mg:Ca), and vitamin D, with type 2 diabetes among US adults, and the previous hypothesis reported that dietary calcium, magnesium, and vitamin D separately and in grouping would decrease the risk of T2DM.
2. MATERIALS AND METHODS
2.1. Study populations
This Cross sectional Study used NHANES data sets 2007–2014 data, NHANES is US national representative survey managed by center for disease control and prevention (CDC). NHANES is a consecutive survey conducted every two years, representing one cycle of the US civilian noninstitutionalized population, and NHANES data set is publically available at http://www.cdc.gov/nchs/nhanes.htm.
2.2. Exclusion and inclusion criteria
We included the adult's participants’ NHANES 2007–2014 data sets from mobile examination center (MEC) (n = 40,617) having age ≥ 18 years (men, nonpregnant women), whereas we excluded those participants having age < 18 years (16,504), pregnant women (n = 180), missing value daily energy intake (n = 4,929), daily energy intake < 500 kcal/day (n = 256) and > 4,500 kcal/day (n = 357), missing value of body mass index (n = 4,687), and the missing value of dietary intakes of calcium, magnesium, and vitamin D (n = 4,929). The detailed descriptions are also provided in Figure 1; moreover, our final data set comprised 20,480 adults (diabetes n = 3,432; nondiabetes n = 17,048).
FIGURE 1.
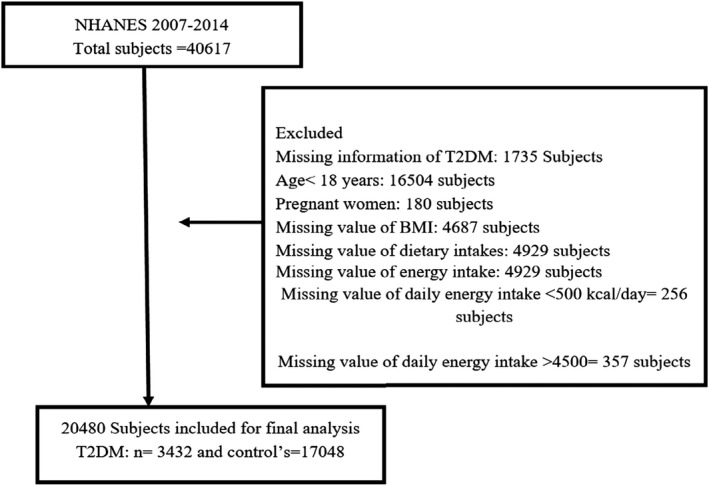
Study flowchart on selection of the participant
2.3. Ascertainment of T2DM
The respondents diagnosed with T2DM or one of the criteria as recommended by the American Diabetes Association (Classification, 2014) will be counted as cases, having hemoglobin A1C ≥ 6.5% and/or fasting glucose ≥ 7 mmol/L and/or having diagnosed T2DM.
2.4. Dietary assessment
The multipass approach method was used to measure dietary intake of magnesium, vitamin D, and calcium during in‐person 24‐hr dietary recall (Moshfegh et al., 2008). This method is a precise and brief list of all foods consumed by an individual during the period of 24 hr. The second 24‐hr recall was collected by a phone call at least 3–10 days later (NHANES, 2013). Moreover, NHANES dietary interviewers' procedure manuals provided detailed descriptions about dietary interview methods (National Health and Nutrition Examination Survey, 2017).
2.5. Study covariates
Data about sex, age, annual household income, ethnicity, educational level, smoking, and drinking position were in use from NHANES in‐person household interviews (Zipf et al., 2013). The covariates are as follows: smoking status (used tobacco/nicotine for the last 5 days), drinking status (had at least 12 alcohol drinks/year), physical activity (yes/no), overweight, and obesity classification (Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, 1998). Also, total energy intakes were divided into 10 groups, while fasting glucose and glycohemoglobin (HbA1c) were used as indicators to define our outcome.
2.6. Statistical analysis
Data from NHANES 2007–2008, 2009–2010, 2011–2012, and 2013–2014 household interviews, dietary data, anthropometry, and laboratory data for each member were combined using unique participant identifier and took into complex sampling design. The complex and four‐stage probability sampling design was used to select participants of each NHANES cycle (NHANES, 2010). Dietary calcium, magnesium, a ratio of Ca:Mg, and vitamin D intakes were adjusted for energy intake (as a continuous variable) by the residual method explained by Willett and Stampfer) (Willett, 1998). Categorical and constant variables were presented using percentages and mean ± SD, respectively. Logistic regression models were used to examine the association between T2DM and calcium, magnesium, vitamin‐D and Ca:Mg intake separately and each nutrient categorized into quintiles with the lowest quintile as reference (models 1–3) and Quintile 4 as reference (model 4–6) by using logistic regression models, p‐value .05. Furthermore, we also stratified our models by sex and evaluated the intake of calcium and magnesium based on RDA (further down RDA or meeting the RDA based on sex‐ and age‐specific recommendations) for individual nutrient (Institute of Medicine, 2012; Institute of Medicine, 1997).
Model 1 was accustomed to sex, race, and age. Model 2 was moreover adjusted for body mass index groups (kg/m2), educational level, exercise regularly (yes/no), current smoker (yes/no), current drinker (yes/no), annual household income, energy intake in groups, total vitamin D (mg/day) (for the model including calcium, magnesium quintile, Ca:Mg ratio quintile), or total Ca:Mg ratio intake (mg/day) (for the model including vitamin D quintile). Model 3 was further additionally adjusted for total calcium (mg/day) (model including magnesium quintile), total calcium (mg/day) (model including magnesium quintile).
3. RESULTS
The study included 20,480 adults, using data from NHANES 2007–2014, which included cases (T2DM, n = 3,432) and noncases (nondiabetes, n = 17,048) (Table 1). The occurrence of type 2 diabetes in the total population was 16.8%, and women account for 48.4% of patients with type 2 diabetes. The average age was 48.4 years. The study populations with T2DM were Mexican American (9.4%), non‐Hispanic white (60.3%), non‐Hispanic black (16.4%), and other ethnic races (13.9%). The percentage of current smokers, current drinkers, exercise regularly, and family history of diabetes were 19.6%, 67.4%, 13.9%, and 3.7%, respectively. The daily mean intake of magnesium, calcium, and vitamin D, and the ratio of calcium to magnesium for individuals with diabetes were 278.9 mg/day, 890.3 mg/day, 4.7 μg/day, and 3.3 (0.04) mg/day, while daily mean intake of magnesium, calcium, vitamin D, and the ratio of calcium to magnesium for individuals with diabetes for individuals without T2DM were 300 mg, 960 mg, 4.7 μg, and 3.3 (0.02) mg/day, respectively. Only 26.9%, 14.5%, and 2.5% of individuals with T2DM met RDA of calcium, magnesium, and vitamin D.
TABLE 1.
Selected characteristics of participants according to type 2 diabetes status in the National Health and Nutrition Examination Survey (NHANES, 2007–2014) a
| Characteristic b | Diabetes (n = 3,432) | Nondiabetes (n = 17,048) | p‐value c |
|---|---|---|---|
| Age (year) | 59.4 (0.30) | 45.7 (0.30) | <0.001 |
| Female | 185 (48.4) | 994 (52.6) | 0.001 |
| BMI (kg/m2) | 33.1 (0.2) | 28.2 (0.0) | <0.001 |
| Race | |||
| NHW | 1,369 (60.3) | 8,668 (68.6) | 0.013 |
| NHB | 1,055 (16.4) | 3,786 (10.6) | |
| MA | 649 (9.4) | 2,746 (8.3) | |
| Others | 752 (13.9) | 3,901 (12.5) | |
| Education | |||
| Less than 9th grade | 710 (11.4) | 1834 (5.1) | <0.001 |
| 9th−11th grades | 715 (15.3) | 2,785 (11.3) | |
| High school grad./GED or equivalent | 888 (24.9) | 4,345 (22.2) | |
| Some college or AA degree | 960 (30.2) | 5,545 (31.0) | |
| College graduate or above | 543 (18.0) | 4,569 (30.2) | |
| Current smokers | 652 (19.6) | 4,252 (25.4) | <0.001 |
| Current drinkers | 2,161 (67.4) | 12,125 (78.6) | <0.001 |
| Exercised regularly | 432 (13.9) | 3,300 (20.7) | <0.001 |
| Income | |||
| Under $20,000 | 1,043 (27.3) | 3,935 (14.1) | 0.075 |
| $20,000 to $45,000 | 1,386 (36.2) | 6,098 (27.1) | |
| $45,000 to $75,000 | 627 (16.4) | 3,358 (20.2) | |
| $75,000 to $100,000 | 242 (6.3) | 1691 (11.5) | |
| Over $100,000 | 334 (8.7) | 3,100 (23.6) | |
| Family history of type 2 diabetes | 128 (3.7) | 1714 (10.1) | <0.001 |
| Energy intake (kcal/day) | 1895.3 (21.4) | 2092.28 (8.6) | <0.001 |
| Calcium(mg/day) | 890.3 (13.4) | 960.36 (6.9) | <0.001 |
| Magnesium (mg/day) | 278.9 (3.5) | 300.56 (2.1) | <0.001 |
| Ratio of calcium to magnesium(mg/day) | 3.3 (0.0) | 3.32 (0.0) | 0.335 |
| Vitamin D (μg/day) | 4.7 (0.1) | 4.67 (0.0) | 0.742 |
| Above RDA calcium | 923 (26.9) | 5,966 (35.0) | <0.001 |
| Above RDA magnesium | 497 (14.5) | 3,696 (21.7) | <0.001 |
| Above RDA vitamin D | 87 (2.5) | 528 (3.1) | 0.937 |
| Fasting insulin (pmol/L) | 129.2 (5.4) | 71.9 (1.0) | <0.001 |
| Fasting glucose (mmol/L) | 8.65 (0.1) | 5.4 (0.0) | <0.001 |
| 2‐hr glucose (mmol/L) | 12.41 (0.3) | 6.1 (0.0) | <0.001 |
| HDL (mmol/L) | 1.2 (0.0) | 1.3 (0.0) | <0.001 |
| TC (mmol/L) | 4.8 (0.0) | 5.0 (0.0) | 0.015 |
| TG (mmol/L) | 1.9 (0.0) | 1.3 (0.0) | <0.001 |
| SBP (mmHg) | 129.9 (0.4) | 120.5 (0.2) | <0.001 |
| DBP (mmHg) | 69.0 (0.3) | 70.6 (0.2) | 0.001 |
| HOMA‐IR | 9.3 (0.3) | 2.5 (0.0) | <0.001 |
| HOMA‐beta | 90.1 (3.5) | 100.7 (7.0) | <0.001 |
All data analyses conducted in the present study were based on weighted estimates with sample weight provided by NHANES.
Univariate logistic regression models were used for continuous and categorical variables.
Continuous variables are presented as mean (standard error). Categorical variables are presented as n (%).
Table 2 shows a significant inverse association between highest quintile (median: 1502 mg) as compared to lowest quintile (median: 397 mg/day) of calcium and T2DM after adjustment of age, sex, and race (model 1:0.79; 95% CI: 0.64, 0.98, p trend: 0.042). However, this inverse association did not remain statistically significant between highest versus. lowest quintiles after further adjustment for total vitamin D, BMI groups (kg/m2), education level, exercise, smoking, drinking, annual household income, energy groups, and positive association observed at quintile 3 intake (Q3 812 mg/day) (model 2: OR: 1.22; 95% CI: 1.01, 1.44). A positive association was remained at quintile 3, after further adjustment for total magnesium intake (model 3: OR: 1.20; 95% CI: 1.01, 1.43). A significant inverse association remained between highest (median: 445 mg/day) versus. lowest quintiles (median: 152 mg/day) of magnesium and T2DM after alteration for race, sex, and age (model 1: OR: 0.55; 95% CI: 0.45, 0.68), and such inverse association did not remain statistically significant for model 2: OR: 1.03 95% CI (0.78, 1.36) and model 3: OR: 0.99; 95% CI (0.74, 1.33). We found a counter‐relationship among the highest quintile (445 mg/day) and magnesium versus the lowest quintile and T2DM (data not shown) when energy intake was excluded from our statistical models. The results were as follows: model 2: OR: 0.76; 95% CI: 0.60, 0.96, and model 3: OR: 0.76; 95% CI: 0.59, 0.99. We found a positive association of vitamin D at quintile 2 and T2DM when compared to Q4 reference in model 4; OR: 1.43; 95% CI: 1.14, 1.74, and such association remained at quintile 2 for model 5: OR: 1.29; 95% CI: 1.04, 1.59, and model 6: OR: 1.52; 95% CI: 1.14, 2.04. We found a significant positive association between highest (median: 4.98 mg/day) versus lowest quintiles (median: 1.76 mg/day) of calcium‐to‐magnesium ratio and T2DM after adjustment for model 1: OR: 1.23; 95% CI: 1.03, 1.48, P trend: 0.016, and such positive association remained after further adjustment for model 2: OR: 1.29; 95% CI: 1.05, 1.59, p trend: .010). Table 3 shows, stratification by sex revealed that association between highest (median 1457 mg/d) vs lowest (369 mg/d) quintile of dietary calcium and T2DM which shows statistically significant association only remained in female (model 1: OR: 0.71; 95% CI: 0.51, 0.99). When the model was further additionally adjusted for total vitamin‐D intake, BMI groups (kg/m2), education level, exercise, smoking, drinking, annual household income, Energy‐Groups which reveals the positive association at quintile 3 (812mg/d) the value of model 2: OR 1.29; 95% CI: 1.01, 1.63 that shows 1.29 times more odds of T2DM, when the model was additionally adjusted for magnesium intake such association at quintile 3 remained/ retained (model 3: OR: 1.30; 95% CI: 1.02, 1.65, p trend: .22).
TABLE 2.
Prevalence of type 2 diabetes by quintiles of dietary intake in the National Health and Nutrition Examination Survey (2007–2014)
| Variable | OR (95% CI) per quintile category of dietary intake a | p linear b | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Calcium (mg/day) | |||||||
| 397 | 617 | 812 | 1,051 | 1502 | |||
| Case/n | 812/4,100 | 761/4,096 | 742/4,098 | 578/4,091 | 539/4,095 | ||
| Model 1 | 1.00 | 0.9 (0.7,1.1) | 0.9 (0.8,1.0) | 0.8 (0.6, 0.9) | 0.7 (0.6, 0.9) | .042 | |
| Model 2 | 1.00 | 1.0 (0.8,1.2) | 1.2 (103,1.4) | 1.1 (0.8,1.4) | 1.1 (0.8 ,1.5) | .169 | |
| Model 3 | 1.00 | 1.0 (0.8,1.2) | 1.2 (1.0 , 1.4) | 1.1 (0.8, 1.1) | 1.1 (0.8, 1.5) | .226 | |
| Model 4 | 1.2 (1.0, 1.5) | 1.1 (0.9,1.4) | 1.1 (0.9,1.3) | Reference | 0.9 (0.8, 1.1) | ||
| Model 5 | 0.8 (0.70, 1.1) | 0.9 (0.7,1.1) | 1.0 (0.8,1.3) | Reference | 1.0 (0.8, 1.3) | ||
| Model 6 | 0.8 (0.7,1.14) | 0.9 (0.7,1.1) | 1.0 (0.8,1.3) | Reference | 1.0 (0.8, 1.2) | ||
| Magnesium (mg/day) | |||||||
| 152 | 215 | 265 | 328 | 445 | |||
| Case/n | 855/4,137 | 739/4,089 | 712/4,096 | 616/4,068 | 510/4,090 | ||
| Model 1 | 1.000 | 0.8 (0.7, 0.9) | 0.8 (0.6, 0.9) | 0.7 (0.5, 0.8) | 0.5 (0.4, 0.6) | .000 | |
| Model 2 | 1.000 | 0.9 (0.7, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.0 (0.7, 1.3) | .699 | |
| Model 3 | 1.000 | 0.9 (0.7, 1.1) | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.3) | 0.9 (0.7,1.33) | .906 | |
| Model 4 | 1.4 (1.1, 1.7) | 1.1 (1.0,1.41) | 1.1 (1.0, 1.3) | Reference | 0.7 (0.6 ,0.9) | ||
| Model 5 | 0.8 (0.7, 1.0) | 0.8 (0.71, 1.0) | 1.0 (0.8 ,1.2) | Reference | 0.9 (0.7, 1.1) | ||
| Model 6 | 0.9 (0.7, 1.1) | 0.8 (0.7, 1.0) | 1.0 (0.8 ,1.2) | Reference | 0.9 (0.7 ,1.1) | ||
| Ratio of calcium to magnesium | |||||||
| 1.76 | 2.45 | 3.06 | 3.75 | 4.98 | |||
| Case/n | 725/4,096 | 681/4,096 | 698/4,096 | 660/4,096 | 668/4,096 | ||
| Model 1 | 1.000 | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) | 1.0 (0.9, 1.2) | 1.2 (1.0 ,1.4) | .016 | |
| Model 2 | 1.000 | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.3) | 1.2 (1.0, 1.5) | .010 | |
| Vitamin D (μg/day) | |||||||
| 1.40 | 3.45 | 5.20 | 7.45 | 12.70 | |||
| Case/n | 1,320/7,892 | 848/4,639 | 541/3,176 | 430/2,808 | 293/1,965 | ||
| Model 1 | 1.000 | 1.0 (0.9, 1.2) | 0.9 (0.7, 1.0) | 0.7 (0.6 ,0.9) | 0.9 (0.7 ,1.0) | .046 | |
| Model 2 | 1.000 | 1.1 (0.9, 1.2) | 0.8 (0.7, 1.0) | 0.8 (0.6, 1.0) | 1.0 (0.7, 1.3) | .605 | |
| Model 3 | 1.000 | 1.1 (0.9 ,1.2) | 0.8 (0.7 ,1.0) | 0.8 (0.6 ,1.0) | 1.0 (0.7, 1.3) | .567 | |
| Model 4 | 1.1 (1.1, 1.6) | 1.4 (1.1, 1.7) | 1.2 (1.0, 1.4) | Reference | 1.20 (0.9, 1.5) | ||
| Model 5 | 1.1 (0.9, 1.4) | 1.2 (1.0, 1.5) | 1.0 (0.8, 1.2) | Reference | 1.2 (0.9, 1.5) | ||
| Model 6 | 1.1 (0.9 ,1.4) | 1.2 (1.0, 1.5) | 1.0 (0.8, 1.2) | Reference | 1.1 (0.9, 1.5) | ||
Model 1 was adjusted for age, gender and race; model 2 was additionally adjusted for BMI groups (kg/m2), education level, exercise regularly (yes/no), current smoker (yes/no), current drinker (yes/no), annual household income, Energy‐Groups, total vitamin‐D (for the model including calcium, magnesium quintile, Ca:Mg ratio quintile) or total Ca:Mg ratio intake (for the model including Vitamin‐D quintile) Model 3 was additionally adjusted for total calcium (model including magnesium quintile), total calcium for the (model including magnesium quintile).
Model included the median value for each quintile of consumption as a continuous variable.
TABLE 3.
Prevalence of type 2 diabetes by quintiles of dietary intake by gender in the National Health and Nutrition Examination Survey (2007–2014)
| OR (95% CI) per quintile category of dietary intake a | p linear b | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Male | ||||||
| Calcium (mg/day) | ||||||
| mg/day | 397 | 618 | 816 | 1,058 | 1538 | |
| Case/n | 365/1683 | 372/1722 | 347/1865 | 333/2146 | 358/2561 | |
| Model 1 | 1.000 | 1.0 (0.8, 1.3) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.0) | 0.8 (0.6, 1.0) | .093 |
| Model 2 | 1.000 | 1.2 (0.9, 1.6) | 1.1 (0.9, 1.5) | 1.1 (0.8 ,1.6) | 1.3 (0.9, 1.9) | .295 |
| Model 3 | 1.000 | 1.2 (0.90,1.64) | 1.1 (0.8, 1.5) | 1.1 (0.8, 1.5) | 1.2 (0.8, 1.8) | .391 |
| Model 4 | 1.1 (0.9,1.5) | 1.2 (0.9 ,1.7) | 1.0 (0.8, 1.3) | Reference | 1.0 (0.8, 1.3) | |
| Model 5 | 0.8 (0.6,1.1) | 1.0 (0.7, 1.4) | 0.9 (0.7, 1.2) | Reference | 1.12 (0.8, 1.4) | |
| Model 6 | 0.8 (0.6,1.1) | 1.0 (0.7, 1.1) | 0.9 (0.7, 1.2) | Reference | 1.0 (0.8, 1.4) | |
| Magnesium (mg/day) | ||||||
| mg/day | 153 | 216 | 268 | 329 | 451 | |
| Case/n | 338/1416 | 322/1615 | 362/1900 | 372/2244 | 381/2802 | |
| Model 1 | 1.000 | 0.9 (0.6, 1.2) | 0.8 (0.6, 1.1) | 0.7 (0.5 ,0.9) | 0.6 (0.4 ,0.8) | .01 |
| Model 2 | 1.000 | 1.1 (0.8, 1.5) | 1.2 (0.9, 1.7) | 1.2 (0.9, 1.7) | 1.2 (0.8 ,1.8) | .566 |
| Model 3 | 1.000 | 1.1 (0.7, 1.5) | 1.2 (0.9, 1.7) | 1.2 (0.8 ,1.7) | 1.1 (0.7 ,1.7) | .787 |
| Model 4 | 1.3 (1.0,1.7) | 1.2 (0.9, 1.5) | 1.1 (0.9, 1.4) | Reference | 0.8 (0.6, 1.0) | |
| Model 5 | 0.7 (0.5 ,1.0) | 0.8 (0.6 ,1.1) | 1.0 (0.8, 1.2) | Reference | 09 (0.7 ,1.2) | |
| Model 6 | 0.8 (0.5,1.1) | 0.8 (0.6, 1.1) | 1.0 (0.8, 1.2) | Reference | 0.9 (0.7 ,1.1) | |
| Ratio of calcium to magnesium | ||||||
| mg/day | 1.73 | 2.44 | 3.06 | 3.75 | 5.01 | |
| Case/n | 427/2243 | 346/1972 | 341/1919 | 324/1873 | 337/1970 | |
| Model 1 | 1.000 | 1.01 (0.8 ,1.2) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 1.2 (0.9, 1.5) | .173 |
| Model 2 | 1.000 | 0.9 (0.7, 1.2) | 0.8 (0.6, 1.1) | 0.9 (0.7, 1.2) | 1.0 (0.7, 1.3) | .848 |
| Vitamin D | ||||||
| μg/day | 1.4 | 3.5 | 5.25 | 7.50 | 12.70 | |
| Case/n | 627/3504 | 428/2174 | 278/1558 | 240/1521 | 202/1220 | |
| Model 1 | 1.000 | 1.1 (0.9 ,1.3) | 0.8 (0.6, 1.0) | 0.6 (0.5, 0.9) | 0.9 (0.7, 1.2) | .177 |
| Model 2 | 1.000 | 1.1 (0.9, 1.4) | 0.7 (0.6, 0.9) | 0.7 (0.5, 1.0) | 1.00 (0.7, 1.4) | .333 |
| Model 3 | 1.000 | 1.1 (0.9 ,1.4) | 0.7 (0.6, 0.9) | 0.7 (0.5, 1.0) | 0.9 (0.7, 1.3) | .299 |
| Model 4 | 1.4 (1.0,1.8) | 1.6 (1.2, 2.0) | 1.2 (0.9, 1.5) | Reference | 1.4 (1.0, 1.8) | |
| Model 5 | 1.2 (0.9,1.7) | 1.5 (1.1, 2.0) | 1.0 (0.7, 1.3) | Reference | 1.2 (0.9, 1.8) | |
| Model 6 | 1.2 (0.9,1.7) | 1.5 (1.1, 2.0) | 1.0 (0.7, 1.3) | Reference | 1.2 (0.9, 1.7) | |
| Female | ||||||
| Calcium (mg/day) | ||||||
| mg/day | 369 | 615 | 809 | 1,042 | 1,457 | |
| Case/n | 447/2,417 | 389/2,374 | 395/2,233 | 245/1,945 | 181/1,534 | |
| Model 1 | 1.000 | 0.8 (0.6 ,1.0) | 0.9 (0.8, 1.2) | 0.7 (0.5, 1.0) | 0.7 (0.5, 0.9) | .179 |
| Model 2 | 1.000 | 0.8 (0.7, 1.1) | 1.2 (1.0, 1.6) | 1.1 (0.7, 1.5) | 1.0 (0.7, 1.5) | .227 |
| Model 3 | 1.000 | 0.8 (0.7, 1.1) | 1.3 (1.0, 1.6) | 1.1 (0.7, 1.5) | 1.0 (0.7, 1.5) | .225 |
| Model 4 | 1.2 (0.9,1.6) | 1.0 (0.8, 1.3) | 1.2 (1.0, 1.5) | Reference | 0.9 (0.6 ,1.2) | |
| Model 5 | 0.9 (0.6,1.2) | 0.8 (0.5, 1.0) | 1.1 (0.8, 1.5) | Reference | 0.9 (0.6, 1.3) | |
| Model 6 | 0.8 (0.6,1.2) | 0.7 (0.5 ,1.0) | 1.1 (0.8, 1.5) | Reference | 0.9 (0.6, 1.3) | |
| Magnesium (mg/day) | ||||||
| mg/day | 151 | 214 | 265 | 326 | 431 | |
| Case/n | 517/2721 | 417/2474 | 350/2196 | 244/1824 | 129/1288 | |
| Model 1 | 1.000 | 0.7 (0.6 ,0.9) | 0.7 (0.6 ,0.9) | 0.6 (0.5, 0.8) | 0.4 (0.3, 0.6) | .00 |
| Model 2 | 1.000 | 0.8 (0.6, 1.1) | 1.0 (0.8, 1.3) | 1.0 (0.7, 1.5) | 0.9 (0.6, 1.4) | .936 |
| Model 3 | 1.000 | 0.8 (0.6, 1.1) | 1.0 (0.8, 1.3) | 1.0 (0.7 ,1.5) | 0.9 (0.5 ,1.4) | .972 |
| Model 4 | 1.4 (1.1,1.9) | 1.1 (0.8, 1.5) | 1.1 (0.9, 1.4) | Reference | 0.7 (0.5, 0.9) | |
| Model 5 | 0.9 (0.6,1.3) | 0.8 (0.5, 1.1) | 1.0 (0.7, 1.3) | Reference | 0.8 (0.6, 1.2) | |
| Model 6 | 0.9 (0.6,1.3) | 0.8 (0.5, 1.1) | 1.0 (0.7, 1.3) | Reference | 0.8 (0.6 ,1.2) | |
| Ratio of calcium to magnesium | ||||||
| mg/day | 1.79 | 2.47 | 3.05 | 3.74 | 4.96 | |
| Case/n | 298/1853 | 335/2142 | 335/2124 | 357/2177 | 336/2223 | 331/21,26 |
| Model 1 | 1.000 | 0.9 (0.7, 1.2) | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.4) | 1.2 (0.9, 1.6) | .015 |
| Model 2 | 1.000 | 0.9 (0.70 1.3) | 0.9 (0.7, 1.3) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.4) | .597 |
| Vitamin D | ||||||
| μg/day | 1.45 | 3.45 | 5.20 | 7.40 | 12.555 | |
| Case/n | 693/4388 | 420/2465 | 263/1618 | 190/1287 | 91/745 | |
| Model 1 | 1.000 | 1.0 (0.8, 1.2) | 0.9 (0.7 ,1.2) | 0.8 (0.6,0.9) | 0.7 (0.5, 1.1) | .096 |
| Model 2 | 1.000 | 1.0 (0.8, 1.2) | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.2) | 1.0 (0.6, 1.7) | .812 |
| Model 3 | 1.000 | 1.0 (0.8, 1.2) | 1.0 (0.7 ,1.3) | 1.0 (0.7, 1.2) | 1.0 (0.6, 1.7) | .244 |
| Model 4 | 1.2 (1.0,1.4) | 1.2 (0.9, 1.5) | 1.1 (0.9, 1.5) | Reference | 0.9 (0.6, 1.4) | |
| Model 5 | 0.9 (0.7,1.2) | 1.0 (0.8, 1.3) | 1.0 (0.7, 1.4) | Reference | 1.0 (0.6 ,1.6) | |
| Model 6 | 0.9 (0.7,1.2) | 1.0 (0.8, 1.2) | 1.0 (0.7, 1.4) | Reference | 1.0 (0.6, 1.6) | |
Model 1 was adjusted for age, race, BMI groups (kg/m2), education level, exercise regularly (yes/no), current smoker (yes/no), current drinker (yes/no), and annual household income; model 2 was adjusted for total vitamin D (for the model containing calcium, magnesium quintile, Ca:Mg ratio quintile). Model 3 was adjusted for total calcium (model together with magnesium quintile), total calcium for the (model including magnesium quintile)
Model included the median value for each quintile consumption as a continuous variable.
A significant inverse association was found for women across dietary magnesium quintile and T2DM (model 1: OR: 0.47; 95% CI: 0.33, 0.67, and p trend: .00). Results showed a significant inverse association in women and men between highest (median: 431 mg/day) versus. lowest quintiles (median: 151 mg/day) of magnesium and T2DM after adjustment of age, sex, and race (model 1: OR: 0.47; 95% CI: 0.33, 0.67 p trend: .00), while in men (model 1: OR: 0.63; 95% CI: 0.47, 0.85 p trend: .01), respectively, such inverse association did not exist further in models 2 and 3. Dietary vitamin D in male has significantly reduced the odds of T2DM at quintile 4 (7.50 μg/day) (model 1: OR: 0.69; 95% CI: 0.53, 0.91), and we found that vitamin D intake at quintile 4 (7.50 μg/day) reduced the odds of T2DM in men (model 1: OR: 0.69; 95% CI: 0.53, 0.91), after further adjustment at quintile 3 (5.25 μg/d) (model 2: OR: 0.79; 95% CI: 0.64, 0.98), which shows inverse association to reduce the odds of T2DM. Furthermore, vitamin D intake at quintile (Q2) as compared to Q4 (reference) contains the recommended amount for model 4: OR: 1.61; 95% CI: 1.24, 2.09, model 5: OR: 1.53; 95% CI: 1.14, 2.04, and model 6: OR: 1.52; 95% CI: 1.14, 2.04, which revealed that T2DM participants at quintile 2 (3.5 μg/day) had more odds of T2DM when compared with reference Q4 (7.50 μg/day); however, no association was found for Ca: Mg ratio in men and women.
Several arrangements of intake for vitamin D and Ca and Mg used RDA (cutoff points, Table 4). Results showed no significant association was found in all populations and those who met the RDA for calcium depend on age (1,000–1,200 mg/day) (OR: 0.97; 95% CI: 0.76, 1.24), magnesium (310–320) (OR: 1.17; 95% CI: 0.97, 1.42), and vitamin D ≥ 15 μg/(600 IU/day) (OR: 1.10; 95% CI: 0.63, 1.92). By sex‐specific analysis for the evaluation of OR 95% CI as well multiplicative interactions between sex and those subjects meeting the RDA for Ca and Mg and vitamin‐D (shown in Table 4) that reveals non‐significant interaction between sex and meeting the RDA for Ca, Mg and vitamin‐D.
TABLE 4.
OR for being below the RDA or meeting or being above the RDA for calcium and magnesium combinations for all individuals (odds ratios and 95% confidence intervals)
| All | Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR a | 95% CI | p | OR a | 95% CI | p | OR a | 95% CI | P | |
| Below Mg RDA b | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| Met Mg RDA | 0.97 | 0.7–1.2 | .83 | 0.88 | 0.2–2.7 | .82 | 0.97 | 0.7–1.2 | 0.92 |
| Pinteraction term (sex × Mg_RDA) e | <.001 | ||||||||
| Below Ca RDA c | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| Met Ca RDA | 1.17 | 0.9–1.4 | .08 | 1.08 | 0.8–1.4 | .55 | 1.25 | 0.9–1.6 | 0.07 |
| Pinteraction term (sex × Ca_RDA) | .53 | ||||||||
| Below VD RDA d | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | |||
| Met VD RDA | 1.10 | 0.6–1.9 | .71 | 1.25 | 0.4–3.8 | .68 | 1.08 | 0.6–1.9 | 0.78 |
| Pinteraction term (sex × VD_RDA) | .84 | ||||||||
| Met Ca RDA and met Mg RDA | 1.18 | 0.9–1.4 | 1.11 | 0.88 | 0.5–1.4 | .61 | 1.28 | 0.9–1.6 | 0.051 |
| Pinteraction term (sex × Mg _Ca _RDA) | .89 | ||||||||
Ref., reference values.
Model was adjusted for gender, age, race, BMI groups (kg/m2), education level, exercise regularly (yes/no), current smoker (yes/no), current drinker (yes/no), annual household income, BMI groups (kg/m2) total energy intake groups (kcal/day), total vitamin D (for the model including RDA of calcium and magnesium), total calcium (for the model including RDA of vitamin D and magnesium), and adjusted for total magnesium (for the model including RDA of vitamin D and calcium).
RDA for Mg is 400 mg/day for males aged 18–30 years and 420 mg/day for males > 30 years of age; RDA for Mg is 310 mg/day for females aged 18–30 years and 320 mg/day for females > 50 years of age.
RDA for calcium is 1,000 mg/day for males aged 20–70 years and 1,200 mg/day for males > 70 years of age; RDA for Ca is 1,000 mg/day for females aged 20–50 years and 1,200 mg/day for females > 50 years of age.
RDA for vitamin D is 15 μg/day (600 IU) for both males and females aged ≤ 70 years and RDA > 15 μg/day (>600 IU/day) for aged ≥ 71 for both males and females.
p‐value for the multiplicative interaction term.
4. DISCUSSION
To our information, this is the first study aimed to assess the combined effect of dietary calcium, magnesium, Ca: Mg intake ratio, and vitamin D on T2DM using 8 years of NHANES data (2007–2014). It is concluded that dietary calcium intake of 812 mg/day (below RDA) increased the odds by 1.20 times, while the calcium‐to‐magnesium ratio between highest versus lowest quintiles increased the odds by 1.29 times in all populations. Calcium intake (809 mg/day, below RDA) increased the odds by 1.30 times in women, while vitamin D intake (5.25 μg/day) reduced the odds by 21% in men. Moreover, the importance of RDA indicated vitamin D intake when compared with the reference (Q4 recommended intake), and the odds of T2DM increased by 1.29 times in Q2 in both genders while 1.52 times in men.
There was no relationship between the lowest versus highest quintiles of dietary intake of calcium, magnesium, and vitamin D and T2DM. Mostly, studies reported that one‐third of Americans did not meet adequate intake (AI) for calcium (Bailey et al., 2010; USDA, 2005), while some studies showed that about half of the US populations did not meet the RDA of magnesium (Moshfegh et al., 2008; Nicklas et al., October, 2014; USDA, 2005). Similar findings reported low dietary intake of magnesium, calcium, and vitamin D below RDA level. Moreover, the results for dietary calcium intake in our conclusions are stable with the results of dietary calcium intake between highest (629 mg/day) versus lowest quartiles (254 mg/day) with multivariable odds ratio of 0.93; 95% CI: 0.71–1.22, for men and women, 810 versus. 365, 0.76; 95% CI: 0.56–1.03 (Kirii et al., 2009). Similar study were conducted in Australia the relative risk RR; 0.94; 95%; CI: 0.61 ‐1.46 for highest (1,060–2,317) versus. lowest (171–740) (Gagnon et al., 2011). However, no association between calcium and diabetes were found and such association disappeared (RR: 1.04, 95% CI: 0.88–1.24). However, inverse association with magnesium remained, RR: 0.65, 95% CI: 0.54–0.78 (Van Dam et al., 2006), and similarly, studies reported the same results (Gagnon et al., 2011; Pittas et al., 2006; Van Dam et al., 2006).
The culprit mechanism in our study for such observed association) as vitamin‐D facilities intestinal calcium absorption and insufficient calcium (due to vitamin‐D insufficiency, low calcium intake) because calcium is required in regulating glucose intolerance due to vitamin‐D deficiency in vivo (Beaulieu et al., 1993).
The results of the meta‐analysis show 600 mg/day calcium intake as desirable, while 1,200 mg daily intake was considered to be preferred (Pittas et al., 2006). Some studies showed a nonsignificant association between dietary magnesium and diabetes (Hopping et al., 2010; Wang et al., 2005). Likewise, the intake of magnesium in our study was low as the intake in the national sample of African American women (median: 183 mg/day) that is below the RDA (320 mg/day) (Ford & Mokdad, 2003). Also, in Mg and glucose metabolism the results are inconsistent (De Valk, 1999). Our study have similar finding with study conducted for 5 years which showed dietary magnesium highest (348 mg/day) versus. lowest quintile (213mg/day) men, RR; 0.86; 95 % CI; 0.63‐1.16 while the results for women RR; 0.92; 95% CI: 0.66–1.28 (Nanri et al., 2010). The ARIC study conducted in the United States for 6 years found a nonsignificant association between dietary magnesium highest (418 mg/day) versus. lowest quintiles (308 mg/day) in the black subjects, RR: 1.05; (0.58, 1.93) but not in white (Kao et al., 1999). Our results had a significant association among race (p trend = .013), while one of the studies reported ethnic differences to exist among NHANES participants (Scragg et al., 2004). Also, a nonsignificant association between dietary magnesium and diabetes, OR: 0.73; 95% CI: 0.51, 1.04, conclusions from the Melbourne collaborative cohort study (Hodge et al., 2004; Li et al., 2009), supports our findings. The results of our study are generally consistent with previous cross‐sectional studies conducted on non‐Hispanic whites and Mexican Americans aged ≥ 20 years and found an inverse relationship between vitamin D and diabetes (Scragg et al., 2004). Our study reported dietary vitamin D at quintile 3 reduced the odds of T2DM by 21% only among men. Nurses’ health study shows vitamin D intake > 800 IU and calcium > 1,200 reduced T2DM risk by 33% as compared to calcium intake < 600 mg/day and vitamin D < 400 IU/day, RR: 0.67; 95% CI: 0.49–0.90 (Pittas et al., 2006). The result of our study matched with finding RR; 0.87;95% CI; 0.75‐1.00 when the model were adjusted for several factors including dietary magnesium, retinol, and calcium, a non‐significant association was observed between vitamin‐D3 intake and T2DM (Pittas et al., 2006), while similar findings from another nested case–control study (Robinson et al., 2011). The findings of our study showed that 60% of participants were non‐Hispanic white and few participants fall in our highest quintile (Q5). Conversely, overweight is still a well‐known risk factor for T2DM in many studies (Colditz et al., 1995). The results of other studies indicated that genetic variants of dietary magnesium channels such as TRPM6 and TRPM7 increased the incidence risk of T2DM when the dietary intake for magnesium is less than 250 mg/day (Song et al., 2009). Higher calcium‐to‐magnesium ratio leads to inappropriate cellular calcium activation, which leads to T2DM, CVD, and other diseases if the magnesium deficiency is not corrected (Rosanoff, 2010). In this study, first time we evaluated the individual and combined effects of magnesium, calcium, vitamin D, and Ca: Mg ratio using 8 years of NHANES data, which are the US nationally large representative sample, which demonstrates the strength of this study; secondly, adjustment for several confounding factors; thirdly, the trained staff with standardized protocols of NHANES and efficacy and precision of the data; and fourthly, the criteria been used for case ascertainment ensured all cases were included in the study. However, processed foods, soda/ soft drinks, beverages, poor nutrient choices, and unhealthy diet will not able the American to meet the daily recommended intake of these micronutrients and health professionals need strategies and dietary interventions, which are an important aspect of epidemiology and public health (O’Neil et al., 2012).
5. LIMITATIONS
The cross‐sectional study does not allow the formation of a temporal relationship. United States have 14% gestational diabetes ratio due to that we excluded gestational diabetes cases from our study (BA, I, JC, & WPT, 2004). Moreover, dietary supplement data inevitably have missing values which is why we do not include supplementation data. However, intake was done by the recall. Also, the results for the study may not be universal because the study was done in one geographic area; thus, further research is needed to make more definitive nutritional recommendations. Therefore, further prospective studies are warranted due to public health importance. Thus, essential micronutrients (vitamin D, calcium, magnesium) related to chronic ailments of global concern include T2D as their public health importance.
6. CONCLUSIONS
This is an interesting study that has important implications for dietary recommendations. It is concluded that dietary calcium intake level of 812 mg/day (Q3) will raise the threat of developing T2DM in the general population and women. Additionally, the findings conclude that US adults having dietary calcium below the RDA were related to an enhanced risk of T2DM in all population, while higher ratio of Ca to Mg was associated with increased risk of T2DM in all population and increased vitamin D intake was associated with decreased risk of T2DM in men. Therefore, the conclusions should probably call for further studies to confirm these results in other settings.
CONFLICT OF INTEREST
The authors declare no conflict of interest. “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.”
ACKNOWLEDGMENTS
This research was funded by the National Key R&D Program of China to Changhao Sun (CHS), Grant Number 2017YFC1307401. The authors appreciate all participants of NHANES contributing to the study.
Shah IU, Sameen A, Manzoor MF, et al. Association of dietary calcium, magnesium, and vitamin D with type 2 diabetes among US adults: National health and nutrition examination survey 2007–2014—A cross‐sectional study. Food Sci Nutr. 2021;9:1480–1490. 10.1002/fsn3.2118
Contributor Information
Changhao Sun, Email: changhaosun2002@163.com.
Azhari Siddeeg, Email: azhari_siddeeg@uofg.edu.sd.
REFERENCES
- Bailey, R. L. , Dodd, K. W. , Goldman, J. A. , Gahche, J. J. , Dwyer, J. T. , Moshfegh, A. J. , Sempos, C. T. , & Picciano, M. F. (2010). Estimation of total usual calcium and vitamin D intakes in the United States. Journal of Nutrition, 140(4), 817–822. 10.3945/jn.109.118539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu, C. , Kestekian, R. , Havrankova, J. , & Gascon‐Barre, M. (1993). Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes, 42(1), 35–43. 10.2337/diab.42.1.35 [DOI] [PubMed] [Google Scholar]
- Boyle, J. P. , Thompson, T. J. , Gregg, E. W. , Barker, L. E. , & Williamson, D. F. (2010). Projection of the year 2050 burden of diabetes in the US adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics, 8, 1–12. 10.1186/1478-7954-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (1998). NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). National Heart, Lung, and Blood Institute. [Google Scholar]
- Colditz, G. A. , Willett, W. C. , Rotnitzky, A. , & Manson, J. E. (1995). Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine, 122(7), 481–486. 10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- Diabetes Care . (2004). American Diabetes Association, Diabetes Care Volume 37, Supplement 1, 27(5), 1047–1053. [Google Scholar]
- Danescu, L. G. , Levy, S. , & Levy, J. (2009). Vitamin D and diabetes mellitus. Endocrine, 35(1), 11–17. 10.1007/s12020-008-9115-5 [DOI] [PubMed] [Google Scholar]
- de Boer, I. H. , Tinker, L. F. , Connelly, S. , Curb, J. D. , Howard, B. V. , Kestenbaum, B. , Larson, J. C. , Manson, J. E. , Margolis, K. L. , Siscovick, D. S. , & Weiss, N. S. (2008). Calcium plus vitamin D supplementation and the risk of incident diabetes in the women’s health initiative. Diabetes Care, 31(4), 701–707. 10.2337/dc07-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valk, H. W. (1999). Magnesium in diabetes mellitus. Netherlands Journal of Medicine, 54(4), 139–146. 10.1016/S0300-2977(99)00005-4 [DOI] [PubMed] [Google Scholar]
- Dong, J. Y. , Xun, P. , He, K. , & Qin, L. Q. (2011). Magnesium intake and risk of type 2 diabetes meta‐analysis of prospective cohort studies. Diabetes Care, 34(9), 2116–2122. 10.2337/dc11-0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, E. S. , & Mokdad, A. H. (2003). Nutritional Epidemiology — Research communication dietary magnesium intake in a. Journal of Nutrition, 133(9), 2879–2882. [DOI] [PubMed] [Google Scholar]
- Gagnon, C. , Lu, Z. X. , Magliano, D. J. , Dunstan, D. W. , Shaw, J. E. , Zimmet, P. Z. , Sikaris, K. , Grantham, N. , Ebeling, P. R. , & Daly, R. M. (2011). Serum 25‐hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: Results from a national, population‐based prospective study (the Australian diabetes, obesity and lifestyle study). Diabetes Care, 34(5), 1133–1138. 10.2337/dc10-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge, A. M. , English, D. R. , O’Dea, K. , & Giles, G. G. (2004). Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care, 27(11), 2701–2706. 10.2337/diacare.27.11.2701 [DOI] [PubMed] [Google Scholar]
- Holick, M. F. (2007). Vitamin D deficiency. New England Journal of Medicine, 357, 266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Hopping, B. N. , Erber, E. , Grandinetti, A. , Verheus, M. , Kolonel, L. N. , & Maskarinec, G. (2010). Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. Journal of Nutrition, 140(1), 68–74. 10.3945/jn.109.112441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby, A. , Meigs, J. B. , O’Donnell, C. J. , Jacques, P. F. , & McKeown, N. M. (2014). Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle‐aged americans. Diabetes Care, 37(2), 419–427. 10.2337/dc13-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (1997). Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press. [PubMed] [Google Scholar]
- Institute of Medicine (2012). Dietary Reference Intakes for Calcium and Vitamin D. National Academy Press. [Google Scholar]
- Kadowaki, S. , & Norman, A. W. (1984). Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest., 73(3), 759–766. 10.1172/JCI111269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, W. H. L. , Folsom, A. R. , Nieto, F. J. , Mo, J. P. , Watson, R. L. , & Brancati, F. L. (1999). Serum and dietary magnesium and the risk for type 2 diabetes mellitus: The Atherosclerosis Risk in Communities Study. Archives of Internal Medicine, 159(18), 2151–2159. 10.1001/archinte.159.18.2151 [DOI] [PubMed] [Google Scholar]
- Kirii, K. , Iso, H. , Date, C. , Fukui, M. , & Tamakoshi, A. (2010). Magnesium Intake and Risk of Self‐Reported Type 2 Diabetes among Japanese. Journal of the American College of Nutrition, 29(2), 99–106. 10.1080/07315724.2010.10719822 [DOI] [PubMed] [Google Scholar]
- Kirii, K. , Mizoue, T. , Iso, H. , Takahashi, Y. , Kato, M. , Inoue, M. , Noda, M. , & Tsugane, S. (2009). Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia, 52(12), 2542–2550. 10.1007/s00125-009-1554-x [DOI] [PubMed] [Google Scholar]
- Li, Y. , Ma, A. , Sun, Y. , Liang, H. , Wang, Q. , Yi, X. , & Han, X. (2009). Magnesium status and dietary intake of mid‐old people in a rural area of China. Magnesium Research, 22, 66–71. [PubMed] [Google Scholar]
- McNaughton, S. A. , Mishra, G. D. , & Brunner, E. J. (2008). Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the whitehall II study. Diabetes Care, 31(7), 1343–1348. 10.2337/dc07-1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren, F. C. , Krüger, K. , Völker, K. , Golf, S. W. , Wadepuhl, M. , & Kraus, A. (2011). Oral magnesium supplementation reduces insulin resistance in non‐diabetic subjects ‐ a double‐blind, placebo‐controlled, randomized trial. Diabetes, Obesity & Metabolism, 13(3), 281–284. 10.1111/j.1463-1326.2010.01332.x [DOI] [PubMed] [Google Scholar]
- Moore‐Schiltz, L. , Albert, J. M. , Singer, M. E. , Swain, J. , & Nock, N. L. (2015). Dietary intake of calcium and magnesium and the metabolic syndrome in the National Health and Nutrition Examination (NHANES) 2001–2010 data. British Journal of Nutrition, 114(6), 924–935. 10.1017/S0007114515002482 [DOI] [PubMed] [Google Scholar]
- Moshfegh, A. J. , Rhodes, D. G. , Baer, D. J. , Murayi, T. , Clemens, J. C. , Rumpler, W. V. , Paul, D. R. , Sebastian, R. S. , Kuczynski, K. J. , Ingwersen, L. A. , Staples, R. C. , & Cleveland, L. E. (2008). The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. American Journal of Clinical Nutrition, 88(2), 324–332. 10.1093/ajcn/88.2.324 [DOI] [PubMed] [Google Scholar]
- Musso, C. G. (2009). Magnesium metabolism in health and disease. International Urology and Nephrology, 41(2), 357–362. 10.1007/s11255-009-9548-7 [DOI] [PubMed] [Google Scholar]
- Nanri, A. , Mizoue, T. , Noda, M. , Takahashi, Y. , Kirii, K. , Inoue, M. , & Tsugane, S. (2010). Magnesium intake and type II diabetes in Japanese men and women: The Japan Public Health Center‐based Prospective Study. European Journal of Clinical Nutrition, 64(10), 1244–1247. 10.1038/ejcn.2010.138 [DOI] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey (NHANES) . MEC In‐Person Dietary Interviewers Procedures Manual. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/dietary_mec.pdf(accessed on 30 August 2017).
- NHANES (2010). Sampling design. http://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/SampleDesign/intro.htm (accessed December 2012). [Google Scholar]
- NHANES (2013). NHANES dietary web tutorial. Dietary data overview: NHANES dietary data collection. www.cdc/gov/nchs/tutorials/Dietary/SurveyOrientation/DietaryDataOverview/info2.htm (accessed December 2013).
- Nicklas, T. A. , O’Neil, C. E. , & Fulgoni, V. L. (2014). The role of dairy in meeting the recommendations for shortfall nutrients in the american diet. Journal of the American College of Nutrition, 2009(28), 73S–81S. 10.1080/07315724.2009.10719807 [DOI] [PubMed] [Google Scholar]
- Nielsen, F. H. (2010). Magnesium, inflammation, and obesity in chronic disease. Nutrition Reviews, 68(6), 333–340. 10.1111/j.1753-4887.2010.00293.x [DOI] [PubMed] [Google Scholar]
- O’Neil, C. E. , Keast, D. R. , Fulgoni, V. L. , & Nicklas, T. A. (2012). Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients., 4(12), 2097–2120. 10.3390/nu4122097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas, A. G. , Dawson‐Hughes, B. , Li, T. , Van Dam, R. M. , Willett, W. C. , Manson, J. E. , & Hu, F. B. (2006). Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care, 29(3), 650–656. 10.2337/diacare.29.03.06.dc05-1961 [DOI] [PubMed] [Google Scholar]
- Pittas, A. G. , Lau, J. , Hu, F. B. , & Review, D.‐H. (2007). The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism, 92(6), 2017–2029. 10.1210/jc.2007-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J. G. , Manson, J. E. , Larson, J. , Liu, S. , Song, Y. , Howard, B. V. , Phillips, L. , Shikany, J. M. , Allison, M. , Curb, J. D. , Johnson, K. C. , & Watts, N. (2011). Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care, 34(3), 628–634. 10.2337/dc10-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanoff, A. (2010). Rising Ca: Mg intake ratio from food in USA adults: A concern? Magnesium Research, 23(4), 181–193. [DOI] [PubMed] [Google Scholar]
- Rosanoff, A. , Dai, Q. , & Shapses, S. A. (2016). Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv Nutr., 7(1), 25–43. 10.3945/an.115.008631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scragg, R. , Sowers, M. , & Bell, C. (2004). Serum 25‐hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care, 27, 2813–2818. 10.2337/diacare.27.12.2813 [DOI] [PubMed] [Google Scholar]
- Song, Y. , Hsu, Y. H. , Niu, T. , Manson, J. A. E. , Buring, J. E. , & Liu, S. (2009). Common genetic variants of the ion channel transient receptor potential membrane melastatin 6 and 7 (TRPM6 and TRPM7), magnesium intake, and risk of type 2 diabetes in women. BMC Medical Genetics, 10, 1–12. 10.1186/1471-2350-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture (2010). Dietary Guidelines for Americans. Available online: http://www.cnpp.usda.gov/DGAs2010‐PolicyDocument.htm (accessed on 13 December 2011). [Google Scholar]
- US Department of Agriculture, US Department of Health & Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; 2015. Available at: https://health.gov/dietaryguidelines/2015‐scientific‐report/ Accessed 4/23/18.
- USDA (2005). What We Eat in America, NHANES 2001‐2002: Usual Nutrient Intakes from Food Compared to Dietary Reference Intakes, 1–56.
- Van Dam, R. M. , Hu, F. B. , Rosenberg, L. , Krishnan, S. , & Palmer, J. R. (2006). Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care, 29(10), 2238–2243. 10.2337/dc06-1014 [DOI] [PubMed] [Google Scholar]
- Villegas, R. , Shu, X. O. , Li, H. , Cai, H. , Gong, Y. , Gao, Y. T. , & Zheng, W. (2009). Dietary Patterns and the Incidence of Type 2 Diabetes: The Shanghai Women Health Study. In Diabetes [Internet]. (Vol. 58, pp. A253–A253). Alexandria, USA: American Diabetes Association. [Google Scholar]
- Wang, J. L. , Shaw, N. S. , Yeh, H. Y. , & Kao, M. D. (2005). Magnesium status and association with diabetes in the Taiwanese elderly. Asia Pac J Clin Nutr., 14(3), 263–269. [PubMed] [Google Scholar]
- Willett, W. , & Stampfer, M. (1998). Implications of total energy intake for epidemiological analysis. In Willett W. (Ed.), Nutritional epidemiology (pp. 273–301). Oxford University Press. [Google Scholar]
- Zipf, G. , Chiappa, M. , Porter, K. S. , Lewis, B. G. , Ostchega, Y. , & Dostal, J. National Health And Nutrition Examination Survey: Plan and operations, 1999–2010. Vital Heal Stat Ser 1 Programs Collect Proced 2013;(56):1‐37. [PubMed] [Google Scholar]


