Abstract
Background.
In Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF), high-dose spironolactone (100 mg daily) did not improve efficacy endpoints over usual care (placebo or continued low-dose spironolactone [25 mg daily] in patients already receiving spironolactone) in the treatment of acute heart failure (HF). We hypothesized that low concentrations of the long-acting active metabolites of spironolactone (canrenone and 7-α-thiomethylspironolactone [7α-TMS]) in the high-dose group could have contributed to these neutral results.
Methods and Results.
In patients randomized to high-dose spironolactone not previously treated with spironolactone (high-dose-naïve, n=112), concentrations of canrenone and 7α-TMS increased at 48 and 96 hours compared to baseline, and between 48h and 96h (all p<0.005), indicating that steady-state concentrations had not been reached by 48h. In patients previously on low-dose, high-dose spironolactone (high-dose-previous, n=37), concentrations of canrenone increased at 48h and 96h compared to baseline (both p<0.0005), with a marginal increase between 48h and 96h (p=0.0507). At 48h, both high-dose groups had higher concentrations of both metabolites than the low-dose spironolactone group (p<0.0001). Moreover, concentrations of both metabolites were higher in high-dose-previous vs. high-dose-naïve patients (p<0.01), indicating that previous spironolactone use was significant, and that steady-state has not been reached in high-dose-naïve patients at 48h. We found limited and inconsistent evidence of correlation between metabolite concentrations and endpoints.
Conclusions:
Lower-than-anticipated concentrations of spironolactone active metabolites were observed for at least 48h in the high-dose spironolactone group and may have contributed to the absence of pharmacological effects of spironolactone in the ATHENA-HF trial.
Introduction
Acute heart failure (HF) is associated with an increased risk of mortality and morbidity.1, 2 The incremental activation of the renin-angiotensin-aldosterone system during decompensation may contribute to these adverse outcomes,3 in addition to diuretic resistance. In particular, increased aldosterone concentrations while receiving an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker, commonly referred to as “aldosterone escape”, is associated with adverse prognosis in patients with acute HF.3 Mineralocorticoid receptor antagonists (MRA) in the context of HF decompensation, particularly at high doses, may be useful to relieve congestion, overcome diuretic resistance, and mitigate neurohormonal activation.4-6 However, in the Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, high-dose (100 mg daily) spironolactone in patients with acute HF previously receiving no or low-dose (12.5 or 25 mg daily) spironolactone, did not improve N-terminal-proB-type natriuretic peptide (NT-proBNP) concentrations from baseline to 96h (primary endpoint), or any other secondary endpoints, more than usual care (continuing low-dose spironolactone or placebo).7 Safety endpoints (changes in serum potassium and renal function) did not differ between study groups also.
One explanation behind these results could be that concentrations of canrenone (available as a drug in Europe) and 7-α-thiomethylspironolactone (7α-TMS),8-11 the two main active metabolites of spironolactone,11-13 may not have reached effective concentrations within the timeframe (96h) of the trial and in the context of acute HF.14 Both metabolites exhibit MRA effects and have longer half-lives than spironolactone (16.5 and 15.0 hours, for canrenone and 7α-TMS respectively in healthy individuals) and thus mediate a significant part of the effects of spironolactone.11-13 Therefore, we examined concentrations of spironolactone and its metabolites as part of ATHENA-HF and whether these concentrations correlated with ATHENA-HF endpoints. We hypothesized that canrenone and 7α-TMS did not reach their anticipated concentrations early during the study in the high-dose group, thus contributing to the neutral results of the trial.
METHODS
Overview of the study design
The methods and results of ATHENA-HF (clinicaltrials.gov Identifier: NCT02235077) which was conducted by the Heart Failure Clinical Research Network and sponsored by the National Heart, Lung, and Blood Institute, have been reported previously.7, 15 Briefly, ATHENA-HF was a multicenter, randomized, placebo-controlled trial investigating the impact of high-dose spironolactone (100 mg once daily) compared to usual care in patients aged 21 years or older with at least one sign and one symptom of acute HF and NT-proBNP ≥1000 pg/ml or BNP ≥250 pg/ml measured within 24 hours of randomization. Patients were included irrespective of left ventricular ejection fraction (LVEF). Patients receiving low-dose spironolactone (12.5 or 25 mg daily) were allowed, but those receiving >25 mg daily were excluded, as were patients receiving eplerenone.
Patients not previously taking spironolactone were randomized to 100 mg of spironolactone or placebo, while individuals taking low-dose spironolactone prior to admission were randomized to 100 mg or 25 mg per day. Randomization was double-blinded for both strata. The study drug was given once daily, at baseline, 24, 48 and 72h after randomization or until discharge if the patient was discharged before 72h. The daily dose was adjusted based on daily serum potassium, renal function and congestion status, as detailed previously.15
The primary endpoint was change in NT-proBNP from randomization to 96h or discharge (if earlier than 96h). Several secondary endpoints were evaluated from randomization to 96h or discharge. Amongst those, we evaluated clinical congestion score, dyspnea relief, daily cumulative net urine output, and net weight loss in the current analysis. Safety endpoints included changes in serum potassium and creatinine from baseline to 96h or discharge.
Substudy design
We analyzed samples only from patients who received spironolactone, i.e. patients randomized to high-dose (100 mg) spironolactone from both the naïve and previous low-dose strata and those randomized to continue low-dose (25 mg) spironolactone among the previous low-dose stratum. In ATHENA-HF, blood samples for biomarkers were collected at ±2 hours from baseline, 48h, and 96h, unless the patient was discharged prior to these time points, in which case blood samples were collected at discharge (i.e. 24h and 72h). Samples were stored locally at −70°C and shipped to the central repository (University of Vermont) on dry ice. We included patients who provided informed consent to participate in both the genomic/pharmacogenomic and biomarker substudies and who had available serum samples. Three patients who had consented to be included in the biomarker substudy with available serum had not provided consent for the genomic substudy and two who consented to participate in the genomic substudy but not the biomarker substudy were not included. ATHENA-HF was approved by all local Institutional Review Boards (IRB).7 All participants provided written informed consent to participate. The genomics and pharmacogenomics and biorepository substudies were separately approved in participating centers and all participants willing to participate provided separate written informed consents.
Study objectives and endpoints
The primary aim of this substudy was to describe concentrations of canrenone and 7α-TMS at 48h and 96h in patients randomized to high-dose (100 mg) spironolactone or continued low-dose (25 mg) spironolactone and compare concentrations according to study arm and stratum. We grouped patients into three groups: (1) previous low-dose stratum randomized to continue low dose (“low-dose”); (2) previous low-dose stratum randomized to high-dose (“high-dose-previous”); and (3) naïve stratum randomized to high-dose (“high-dose-naïve”). We investigated the association between metabolite concentrations and changes in selected efficacy and safety endpoints. Finally, we also measured concentrations of spironolactone and the metabolite 6β-Hydroxy-7α-thiomethylspironolactone (6β-OH-7α-TMS).
Measurement of spironolactone and metabolites.
All analyses were performed at the Platform of Biopharmacy, Université de Montréal, blinded to arm assignment and dosage. Serum samples (40 μL) were extracted by protein precipitation with acetonitrile containing the internal standard (Testosterone-D3, 75 ng/mL, 80 μL). After centrifugation, the supernatant (75 μL) was diluted with an aqueous ammonium formate solution (5 mM, pH 5, 50 μL). A 11-point calibration curve including the four compounds and ranging from 0.2 to 500 ng/mL was prepared in blank human serum and was extracted the same way. The extracted samples for spironolactone and its metabolites were quantified with high pressure liquid chromatography coupled to electrospray ionization tandem mass spectrometry in positive ion mode. Calibration curves for spironolactone and 6β-OH-7α-TMS were plotted using peak area ratios and for 7α-TMS and canrenone using peak height ratios (analyte/internal standard) vs. nominal analyte concentration, using a weighted 1/x quadratic regression. The lower limit of quantification (LLOQ) were 0.5 ng/mL for spironolactone, 0.2 ng/mL for canrenone (adapted from 16), 1 ng/mL for 7α-TMS and for 6β-OH-7α-TMS. Additional details are provided in the supplementary methods.
Statistical analysis
All patients meeting the selection criteria of the substudy were included in the analyses if they had at least one available measurement of spironolactone and its metabolites measurement, whether it was at baseline or follow-up. For continuous variables, descriptive statistics are presented as median and interquartile range (IQR) or as mean ± standard deviation, while categorical variables are presented as counts and percentages of each category.
Comparisons between groups and correlations between drug concentrations and study endpoints between drug concentrations and study endpoints are provided when a sufficient number of patients were available for analysis, that is, only at baseline, 48h and 96h. Comparison between groups were conducted using the X2 test or Fisher’s exact test for categorical variables. For continuous variables, the Kruskal-Wallis test was used. To compare serial concentrations of spironolactone and metabolites, we conducted Wilcoxon signed-rank tests between measurement times. We calculated correlation coefficients using Spearman’s rank test, because the concentrations of spironolactone and its metabolites were not normally distributed. Per-protocol sensitivity analyses were also conducted at 48 and 96 hours that were limited to individuals who received all study drug doses from baseline to each time point. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, NC, USA).
RESULTS
Study population
A total of 188 of patients were included; 39 patients in the low-dose group, 37 in the high-dose-previous group, and 112 in the high-dose-naive group (Table 1). Patients from the two prior-use groups (high-dose-previous and low-dose) presented no significant differences in baseline characteristics and pre-hospital spironolactone doses were similar (21.0±5.9 mg vs 23.0±4.6 mg, respectively; median: 25 mg in both). Finally, a similar proportion of patients were still receiving spironolactone at randomization in the high-dose-previous (62.2%) and the low-dose (53.9%) group (p=0.49). Compared with these two groups of prior spironolactone users, the high-dose-naive group were older, had a higher LVEF, systolic blood pressure, and serum sodium, and a history of hypertension. No significant difference between the groups was observed in regard to baseline serum creatinine, eGFR, weight, body mass index, or potassium.
Table 1.
Baseline characteristics
| Baseline characteristics |
All N=188 |
High-dose-naïve N=112 |
High-dose- previous N=37 |
Low-dose N=39 |
p* | p† High-dose- naïve Vs High- dose- previous |
p† High-dose- -naïve Vs Low- dose |
p† High-dose- previous vs Low- dose |
|
|---|---|---|---|---|---|---|---|---|---|
| Men, n (%) | 127 (67.6) | 72 (64.3) | 28 (75.7) | 27 (69.2) | 0.43 | 0.23 | 0.70 | 0.61 | |
| Patient age, years | 64.0 (55.0;74.0) | 66.0 (57.5;76.5) | 60.0 (82.0;70.0) | 64.0 (53.0;70.0) | 0.047 | 0.03 | 0.08 | 0.70 | |
| Ethnicity | White | 104 (55.3) | 64 (57.1) | 20 (54.1) | 20 (51.3) | 0.43 | 0.48 | 0.58 | 0.60 |
| Black | 77 (41.0) | 43 (38.4) | 15 (40.5) | 19 (48.7) | |||||
| Asian | 3 (1.6) | 3 (2.7) | 0 (0.0) | 0 (0.0) | |||||
| Others | 4 (2.1) | 2 (1.8) | 2 (5.4) | 0 (0.0) | |||||
| HF etiology | Ischemic | 125 (66.5) | 67 (59.8) | 27 (73.0) | 31 (79.5) | 0.053 | 0.17 | 0.03 | 0.59 |
| Non-ischemic | 63 (33.5) | 45 (40.2) | 10 (27.0) | 8 (20.5) | |||||
| NYHA HF class | III | 119 (63.3) | 66 (58.9) | 27 (73.0) | 26 (66.7) | 0.77 | 0.53 | 0.90 | 0.75 |
| IV | 39 (20.7) | 24 (21.4) | 6 (16.2) | 9 (23.1) | |||||
| LVEF | 30.0 (20.0;45.0) | 35.0 (22.0;51.0) | 25.0 (15.0;35.0) | 25.0 (15.0-35.0) | 0.0004 | 0.004 | 0.0007 | 0.64 | |
| Baseline LVEF | ≤40% | 131 (69.7) | 65 (58.0) | 31 (83.8) | 35 (89.7) | 0.0008 | 0.006 | 0.001 | 0.87 |
| 41-49% | 15 ( 8.0) | 12 (10.7) | 2 ( 5.4) | 1 ( 2.6) | |||||
| ≥50% | 40 (21.3) | 34 (30.4) | 3 ( 8.1) | 3 ( 7.7) | |||||
| Baseline core lab NT-proBNP (pg/mL) | 4092.0 (2559.9;8656.7) | 4061.9 (2584.4;9166.6) | 5260.3 (3182.0;7904.2) | 3792.8 (2123.4;8545.1) | 0.61 | 0.48 | 0.60 | 0.34 | |
| Prior MI, n (%) | 58 (30.9) | 34 (30.4) | 11 (29.7) | 13 (33.3) | 0.94 | 1.00 | 0.84 | 0.81 | |
| Atrial fibrillation, n (%) | 89 (47.3) | 57 (50.9) | 13 (35.1) | 19 (48.7) | 0.29 | 0.13 | 0.85 | 0.35 | |
| Hypertension, n (%) | 157 (83.5) | 102 (91.1) | 28 (75.7) | 27 (69.2) | 0.002 | 0.02 | 0.003 | 0.61 | |
| Diabetes, n (%) | 71 (37.8) | 50 (44.6) | 12 (32.4) | 9 (23.1) | 0.04 | 0.25 | 0.02 | 0.44 | |
| COPD, n (%) | 39 (20.7) | 24 (21.4) | 5 (13.5) | 10 (25.6) | 0.38 | 0.35 | 0.51 | 0.25 | |
| Systolic BP, mmHg | 117.0 (104.0;134.0) | 122.0 (105.5;142.5) | 108.5 (101.0;119.5) | 112.0 (101.0;126.0) | 0.002 | 0.002 | 0.02 | 0.44 | |
| Diastolic BP, mmHg | 69.0 (61.0;82.0) | 72.0 (61.5;85.0) | 67.0 (62.0;77.5) | 67.0 (59.0;76.0) | 0.20 | 0.28 | 0.10 | 0.56 | |
| Heart rate (bpm) | 78 (69;90) | 78 (69;91) | 78 (70;89) | 77 (66;89) | 0.68 | 0.85 | 0.43 | 0.44 | |
| Baseline weight (lbs) | 197.6 (164.5;235.1) | 198.7 (165.2;248.0) | 183.9 (156.5;229.1) | 200.00 (170.0;233.3) | 0.61 | 0.38 | 0.51 | 0.80 | |
| Body mass index | 30.4 (25.5;35.4) | 31.0 (26.5;37.6) | 28.8 (24.9;34.3) | 31.7 (23.9;34.9) | 0.35 | 0.15 | 0.55 | 0.47 | |
| Baseline eGFR (mL/min/1.73 m2) | 60.5 (48.1;78.2) | 63.2 (47.5;78.2) | 59.9 (48.8;74.8) | 58.7 (48.9;78.2) | 0.88 | 0.78 | 0.64 | 0.84 | |
| Baseline sodium (mEq/L) | 139 (138;142) | 140 (138;143) | 139 (137;141) | 138 (137;141) | 0.002 | 0.01 | 0.002 | 0.56 | |
| Baseline potassium (mEq/L) | 3.9 (3.6;4.2) | 3.9 (3.6;4.2) | 3.9 (3.6;4.2) | 3.9 (3.7;4.2) | 0,72 | 0,4302 | 0,6775 | 0,7982 | |
| Pre-hospital loop diuretics, n (%) | 149 (79.3) | 79 (70.5) | 34 (91.9) | 36 (92.3) | 0.002 | 0.01 | 0.008 | 1.00 | |
| Baseline medication use, n (%) | |||||||||
| ACE inhibitor or ARB use, n (%) | 112 (59.6) | 62 (55.4) | 24 (64.9) | 26 (66. 7) | 0.35 | 0.34 | 0.26 | 1.00 | |
| Beta-blockers | 139 (73.9) | 79 (70.5) | 30 (81.1) | 30 (76.9) | 0.40 | 0.29 | 0.54 | 0.78 | |
| Digoxin | 17 ( 9.0) | 5 ( 4.5) | 8 (21.6) | 4 (10.3) | 0.007 | 0.004 | 0.24 | 0.22 | |
| Spironolactone | 46 (24.5) | 2 ( 1.8)** | 23 (62.2) | 21 (53.9) | <0.0001 | <0.0001 | <0.0001 | 0.49 | |
| Loop diuretics | 188 (100.0) | 112 (100.0) | 37 (100.0) | 39 (100.0) | |||||
| Clinical congestion score | 10.0 (9;12) | 10.0 (8.0;12.0) | 10.0 (9.0;12.0) | 10.5 (9.0;12.0) | 0,87 | 0.83 | 0.59 | 0.82 | |
| Visual analog scale | 60 (45;75) | 60 (45;75) | 70 (50;75) | 60 (40;75) | 0,39 | 0,37 | 0,44 | 0.17 | |
| MRA strata | On low dose MRA on admission | 76 (40.4) | 0 ( 0.0) | 37 (100.0) | 39 (100.0) | <0.0001 | <0.0001 | <0.0001 | NA |
BP: Blood pressure; COPD: Chronic obstructive pulmonary disease; HF: heart failure; LVEF: Left ventricular ejection fraction; NT-proBNP: N-terminal Pro B-type natriuretic peptide
p-value for continuous variables: Kruskal-Wallis test, categorical variables: Chi-Square test
p-value for continuous variables: Kruskal-Wallis test, categorical variables: Fisher exact test
Two patients in whom spironolactone was initiated between hospital admission and randomization.
Study drug and open-label use
The administration of study drug was similar throughout the study between groups, with only modest differences at baseline, with between 89 and 100% of patients receiving the study drug at baseline, and 92 to 100% receiving the prescribed dose at baseline (Supplemental Table 1). Thereafter, study drug administration and adherence to prescribed dose was excellent at 24h and 48h but slightly lower at 72h. The use of open-label spironolactone was generally low throughout the study (less than 5% overall at 24, 48 and 72 hours), but it tended to be slightly higher in those receiving spironolactone prior to the hospitalisation (Supplemental Table 2).
Concentration of canrenone and 7α-TMS
Concentrations of canrenone and 7α-TMS throughout the study are presented in Figures 1 and 2, respectively. At baseline, canrenone levels were similar in the two groups with prior spironolactone use (p=0.33). As expected, canrenone levels were higher in these groups vs. spironolactone-naïve patients (p<0.0001). Similar observations were made for 7α-TMS.
Figure 1. Canrenone concentrations in the ATHENA-HF trial.
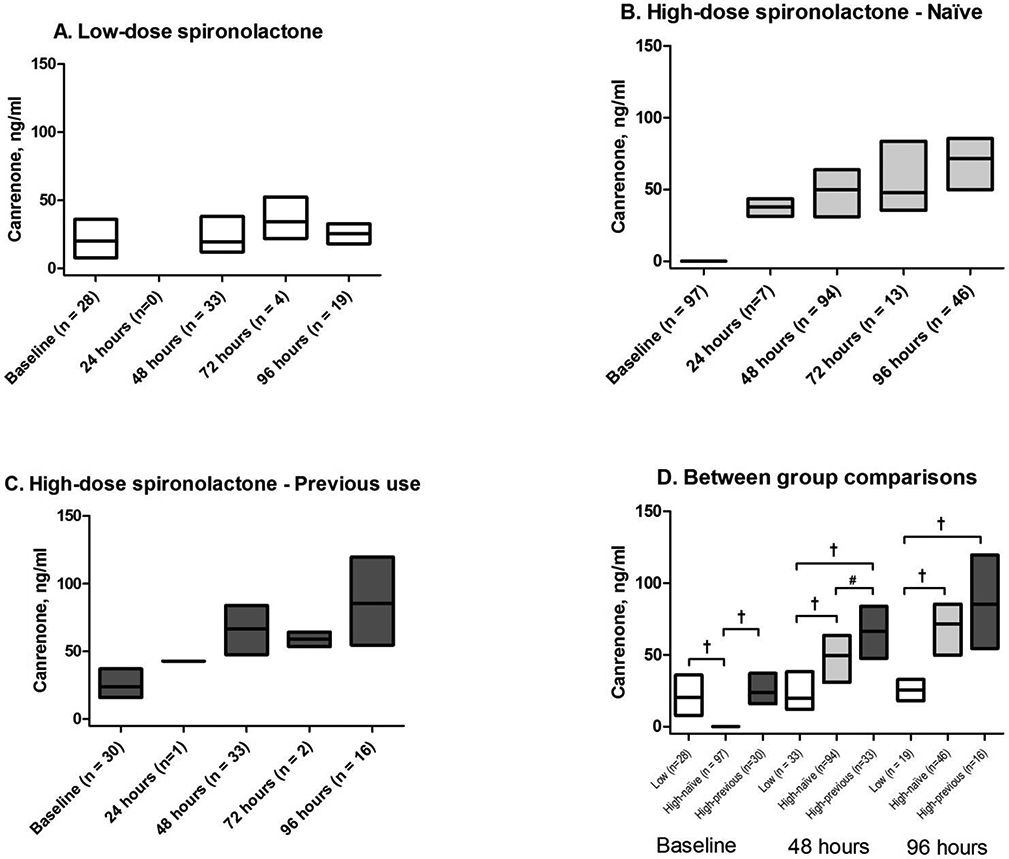
A) Previous low-dose stratum randomized to low-dose spironolactone (Low-dose group); B) Naïve stratum randomized to high-dose spironolactone group (High-dose-naïve group); C) Previous low-dose stratum randomized to high-dose spironolactone group (High-dose-previous group); D) Between group comparison at baseline, 48 and 96 hours. Data presented as median and interquartile range. All between group comparisons performed using Kruskal-Wallis test. *p < 0.05; # p < 0.005; †p < 0.0001
Figure 2. 7-α-thiomethyl-spironolactone concentrations in the ATHENA-HF trial.
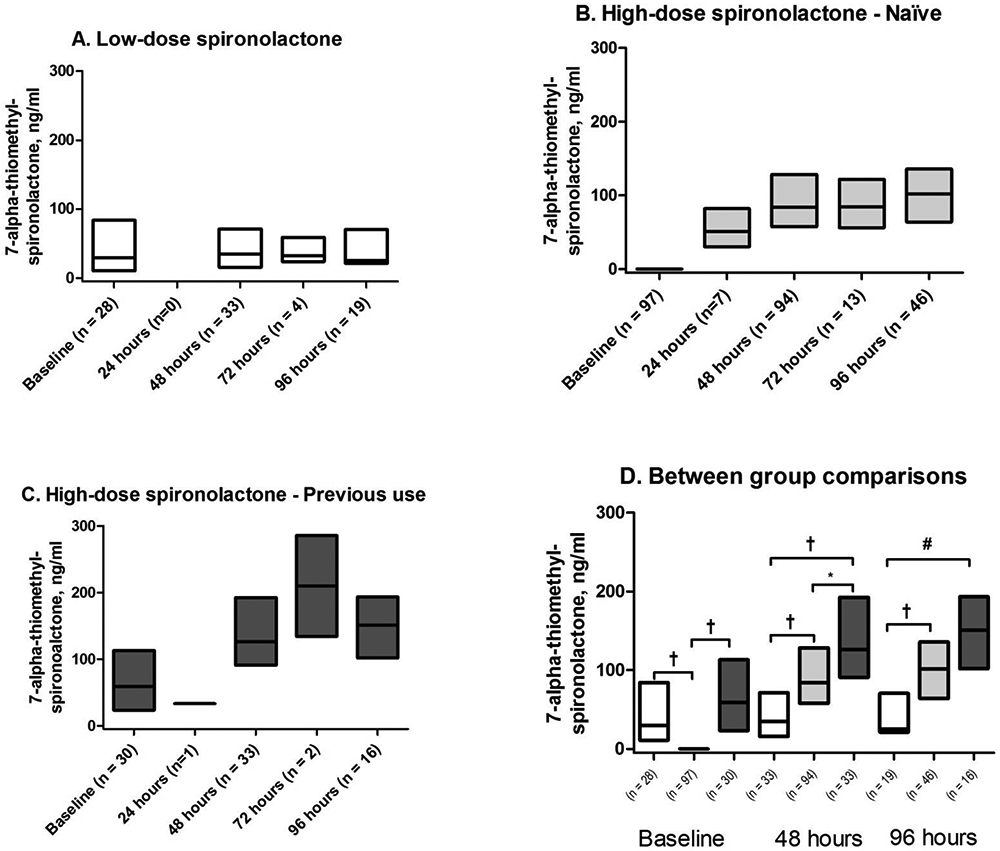
A) Previous low-dose stratum randomized to low-dose spironolactone (Low-dose group); B) Naïve stratum randomized to high-dose spironolactone group (High-dose-naïve group); C) Previous low-dose stratum randomized to high-dose spironolactone group (High-dose-previous group); D) Between group comparison at baseline, 48 and 96 hours. Data presented as median and interquartile range. All between group comparisons performed using Kruskal-Wallis test. *p < 0.05; # p < 0.005; †p < 0.0001
Concentrations of canrenone and 7α-TMS progressively increased over time in both high-dose groups (Figures 1 and 2). Concentrations of both metabolites remained more constant in the low-dose group. At 48h, both high-dose groups had higher levels than the low-dose group for these metabolites (all p<0.0001). Moreover, at 48h, concentrations of both canrenone and 7α-TMS in the high-dose-previous group remained higher than in the high-dose-naïve (both p<0.01), indicating that the impact of the pre-hospitalisation low-dose spironolactone use on canrenone concentrations was still significant after 48 hours.
In patients remaining at the 96h time point, both high-dose groups had higher concentrations of canrenone and 7α-TMS vs. the low-dose group (both p≤0.0001). However, differences between the high-dose groups were no longer significant for canrenone (p=0.26) or 7α-TMS (p=0.11).
In patients with >1 time point available, we evaluated changes between baseline and 48h (n=130), baseline and 96h (n=73), and 48h and 96h (n=80). In the high-dose-naive group, levels were significantly increased at 48h and 96h compared to baseline (n=79 and n=44 respectively), as well as between 48h and 96h (n=46) for canrenone and 7α-TMS (all p<0.005). In the high-dose-previous group, canrenone levels increased at 48h and 96h compared to baseline (n=27 and 15, respectively; both p<0.0005), but the increase between 48h and 96h was not significant (n=16; p=0.0507). For 7α-TMS, the increase between baseline and 48h (p<0.0001), but not between baseline and 96h (p=0.08) or between 48h and 96h (p=0.71) was significant. Finally, in the low-dose group, a slight increase was observed between baseline and 48h (n=24; p=0.03) for canrenone, but not between baseline and 96 hours (n=14; p=0.63) or between 48 and 96 hours (n=18; p=0.90). No increase in 7α-TMS occurred in the low-dose group.
Concentrations of spironolactone and 6β-OH-7α-TMS
Concentrations of spironolactone and 6β-OH-7α-TMS are shown in Supplementary Figures 1 and 2, respectively. Concentrations of 6β-OH-7α-TMS were consistent with those of canrenone, both in terms of progression across time points, as for differences between groups at each time point. Results are provided in the supplementary material.
Correlation with study endpoints
We found limited and inconsistent evidence for correlation between concentrations of canrenone and study endpoints. Correlations with the primary endpoint (change in NT-proBNP) are provided in Table 2. No significant correlation was observed at 48h. At 96h, at which time the number of patients was lower, canrenone levels were inversely correlated with changes in NT-proBNP when considering all three groups together (p=0.03), and in the high-dose-naïve group alone (p=0.02). For 7α-TMS, no correlation with the primary endpoint was observed. Globally, few correlations were observed between any of the end points and spironolactone or its metabolites. These should be interpreted with caution in the light of the multiple testing (Table 2 and Supplementary Table 3).
Table 2.
Spearman correlation between concentrations of spironolactone and metabolites at 48 and 96 hours with changes in NT-proBNP and biochemistry endpointxs
| All | High-dose-naïve | High-dose-previous | Low-dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSpearman | P-value | N | RSpearman | P-value | N | RSpearman | P-value | N | RSpearman | P-value | N | |
| NT-proBNP change at 48h | ||||||||||||
| Spironolactone | 0.0446 | 0.58 | 157 | 0.0741 | 0.48 | 94 | 0.0477 | 0.80 | 32 | 0.2079 | 0.26 | 31 |
| Canrenone | −0.0897 | 0.26 | 157 | −0.1159 | 0.27 | 94 | −0.1598 | 0.38 | 32 | 0.0093 | 0.96 | 31 |
| 7α-TMS | −0.0135 | 0.87 | 157 | 0.0270 | 0.80 | 94 | −0.2166 | 0.23 | 32 | 0.0565 | 0.76 | 31 |
| 6β-OH-7α-TMS | −0.0656 | 0.41 | 157 | −0.0389 | 0.71 | 94 | −0.1059 | 0.56 | 32 | −0.0230 | 0.90 | 31 |
| NT-proBNP change at 96h (Primary endpoint) | ||||||||||||
| Spironolactone | −0.2270 | 0.04 | 80 | −0.1478 | 0.33 | 46 | −0.0619 | 0.82 | 16 | −0.4698 | 0.049 | 18 |
| Canrenone | −0.2388 | 0.03 | 80 | −0.3452 | 0.02 | 46 | −0.2647 | 0.32 | 16 | −0.3375 | 0.17 | 18 |
| 7α-TMS | −0.1353 | 0.23 | 80 | −0.0236 | 0.88 | 46 | −0.0500 | 0.85 | 16 | −0.4159 | 0.09 | 18 |
| 6β-OH-7α-TMS | −0.1784 | 0.11 | 80 | −0.0720 | 0.63 | 46 | −0.0912 | 0.74 | 16 | −0.4925 | 0.04 | 18 |
| Creatinine change at 48h | ||||||||||||
| Spironolactone | 0.0931 | 0.24 | 159 | 0.0474 | 0.65 | 93 | 0.0970 | 0.59 | 33 | 0.0028 | 0.99 | 33 |
| Canrenone | 0.1371 | 0.08 | 159 | 0.1011 | 0.34 | 93 | 0.1350 | 0.45 | 33 | 0.0333 | 0.85 | 33 |
| 7α-TMS | 0.1194 | 0.13 | 159 | 0.1081 | 0.30 | 93 | 0.0290 | 0.87 | 33 | 0.1085 | 0.55 | 33 |
| 6β-OH-7α-TMS | 0.1346 | 0.09 | 159 | 0.1445 | 0.17 | 93 | 0.1345 | 0.46 | 33 | 0.0523 | 0.77 | 33 |
| Creatinine change at 96h | ||||||||||||
| Spironolactone | −0.0794 | 0.48 | 81 | 0.1259 | 0.40 | 46 | −0.6175 | 0.01 | 16 | 0.1729 | 0.48 | 19 |
| Canrenone | −0.0168 | 0.88 | 81 | 0.3414 | 0.02 | 46 | −0.4606 | 0.07 | 16 | 0.4091 | 0.08 | 19 |
| 7α-TMS | −0.0020 | 0.99 | 81 | 0.2789 | 0.06 | 46 | −0.5048 | 0.046 | 16 | 0.3213 | 0.18 | 19 |
| 6β-OH-7α-TMS | 0.0279 | 0.80 | 81 | 0.3581 | 0.01 | 46 | −0.5504 | 0.03 | 16 | 0.5138 | 0.02 | 19 |
| Potassium change at 48h | ||||||||||||
| Spironolactone | 0.1216 | 0.13 | 159 | 0.1186 | 0.26 | 93 | −0.0780 | 0.67 | 33 | 0.0849 | 0.64 | 33 |
| Canrenone | 0.1707 | 0.03 | 159 | 0.2063 | 0.047 | 93 | −0.0287 | 0.87 | 33 | 0.0978 | 0.59 | 33 |
| 7α-TMS | 0.1628 | 0.04 | 159 | 0.1413 | 0.17 | 93 | 0.0920 | 0.61 | 33 | 0.2178 | 0.22 | 33 |
| 6β-OH-7α-TMS | 0.1313 | 0.10 | 159 | 0.0783 | 0.46 | 93 | 0.1065 | 0.56 | 33 | 0.2068 | 0.25 | 33 |
| Potassium change at 96h | ||||||||||||
| Spironolactone | 0.0470 | 0.68 | 81 | 0.0314 | 0.84 | 46 | 0.1617 | 0.55 | 16 | −0.2218 | 0.36 | 19 |
| Canrenone | 0.1674 | 0.14 | 81 | 0.1573 | 0.30 | 46 | 0.5111 | 0.04 | 16 | −0.1127 | 0.64 | 19 |
| 7α-TMS | 0.0844 | 0.454 | 81 | 0.0415 | 0.78 | 46 | 0.5985 | 0.01 | 16 | −0.3248 | 0.17 | 19 |
| 6β-OH-7α-TMS | 0.0969 | 0.39 | 81 | −0.0123 | 0.94 | 46 | 0.6267 | 0.009 | 16 | −0.2757 | 0.25 | 19 |
Per-protocol sensitivity analyses
In the per-protocol analysis, 141 and 59 patients were included at 48h and 96h, respectively, compared to 160 and 81 of the total patients. The per-protocol analyses provided consistent results for the concentrations of spironolactone and its metabolites. For example, median concentrations of canrenone at 48h were 20.9 ng/ml in the low-dose; 51.8 ng/ml in the high-dose-naive (p<0.0001 vs low-dose) and 66.5 ng/ml in the high-dose-previous group (p<0.0001 vs low-dose and <0.05 vs high-dose-naïve). At 96h, median concentrations were 26.2 ng/ml in the low-dose; 74.6 ng/ml in the high-dose-naïve (p<0.0001 vs low-dose) and 92.0 ng/ml in the high-dose-previous group (p<0.001 vs low-dose and =0.65 vs high-dose naïve). The per-protocol analyses provided observations consistent with those in the total population for spironolactone and other metabolites (data not shown) and did not reveal substantially different correlations between metabolites and study endpoints (data not shown).
DISCUSSION
The results of this substudy bring important insights into the interpretation of ATHENA-HF and, more broadly, for the use of spironolactone in patients with HF. First, our results indicate that the concentrations of the two primary active metabolites of spironolactone, canrenone and 7α-TMS, steadily increased during the study in patients randomized to high-dose spironolactone. Second, the concentrations of these metabolites were lower than anticipated in the high-dose spironolactone groups for at least 48h, particularly in patients not previously on spironolactone. This suggests that steady-state concentrations of these metabolites had not yet been reached during a significant portion of the study, which could explain why the diuretic and the potassium-sparing effects of spironolactone were not observed in ATHENA-HF. Third, previous low-dose spironolactone use had a significant impact on concentrations of active metabolites for at least 48h. Therefore, inclusion of this stratum of patients may have contributed in minimizing the differences between the high-dose and control (placebo or low-dose) arms. Finally, we did not find consistent evidence of correlation between non-steady state metabolite concentrations and the clinical effects of spironolactone, as measured by the study endpoints, which is aligned with an absence of a clinically significant effect in the ATHENA-HF trial.
Spironolactone dosing and metabolite concentrations
In a previous report from the TOPCAT study, spironolactone daily dose and canrenone levels were closely correlated, and spironolactone daily dose in mg and median canrenone levels in ng/ml after 12 months were proportionate in a ~1:1 ratio.16 Consistent with this observation, in the current study, baseline concentrations of canrenone in both groups of prior spironolactone users were of 23.7 ng/ml and 20.2 ng/ml, with previous mean dose of 21.0±5.9 mg and 23.0±4.6 mg, respectively. Moreover, in patients in the Usual-care group randomized to continue low dose spironolactone (25 mg daily), median concentrations of 19.6 ng/ml and 25.5 ng/ml at 48 and 96 hours, respectively. This consistency is attributable to the long half-life of canrenone which translates into stable concentrations over the daily dosing interval of spironolactone.
One could thus expect that steady-state concentrations of canrenone following administration of 100 mg of spironolactone daily to be ~100 ng/ml. However, median canrenone concentrations were 49.6 ng/ml at 48h in the high-dose-naive group, the majority of the “High-dose spironolactone” group in ATHENA-HF. Therefore, one could surmise that only ~50% of the steady-state concentration of canrenone was reached in these patients by mid-study.
Several hypotheses can be proposed to explain these observations. First, one could argue that the congestion of the gut and liver, and the decrease in cardiac output patients with HF decompensation could significantly reduce spironolactone absorption or hepatic metabolism into its active metabolites.14 Yet, canrenone concentrations at baseline in previous low-dose users and throughout the study in those randomized to continue low-dose spironolactone were consistent with concentrations measured in stable, chronic spironolactone users, therefore arguing against this hypothesis.16
We believe it is more likely that these observations are the result of a much longer than anticipated half-life of canrenone, which resulted in a longer time to reach steady-state concentrations for canrenone, and potentially, of spironolactone’s other metabolites. Although prescribing information for spironolactone and most references state that spironolactone, canrenone and 7α-TMS have half-lives of 1.4, 16.5 and 13.8 hours, respectively, these values were obtained from 13 healthy men, aged a median of 22 years,17 a group obviously not representative of ATHENA-HF patients or the wider HF patient population.18, 19 An extreme illustration of the importance of liver impairment in prolonging the half-lives of spironolactone metabolites is a study of nine individuals with biopsy-proven cirrhosis and ascites, in whom the half-lives of canrenone and 7α-TMS were 57.8 and 23.9 hours, respectively.20
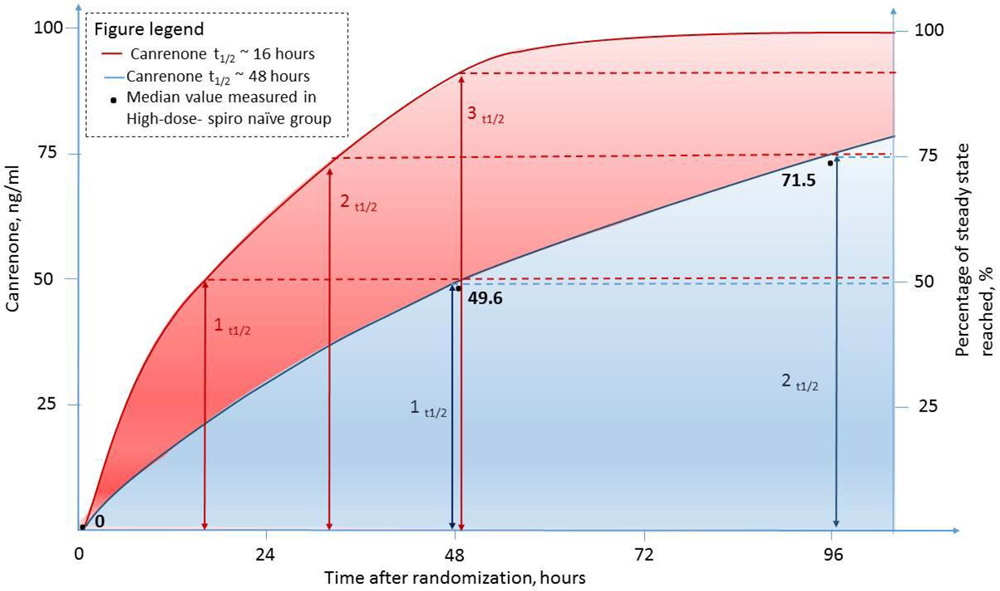
If canrenone half-life was indeed 16h in patients with acute HF, then one would expect that canrenone would have reached ~87.5% of its steady-state concentrations by 48h (which corresponds to ~3 half-lives, Figure 3)21 in the high-dose-naïve group in ATHENA. Again, despite the caveats mentioned, a ~50 ng/mL canrenone concentration at 48h in this group suggests that approximately 50% of steady-state was reached, which corresponds to 1 half-life (Figure 3).21 This would suggest a canrenone half-life of ~48 hours in these patients.21
Figure 3. Observed and theoretical canrenone concentrations in patients randomized to high-dose spironolactone who were not treated with spironolactone prior to hospitalisation.
The figure illustrates anticipated canrenone concentrations in the scenario where canrenone would have an elimination half-life of 16 hours (red line) and 48 hours (blue line). The black dots represent the median canrenone concentrations measured at baseline, 48 and 96 hours in patients randomized to high-dose spironolactone who did not receive spironolactone group before hospital admission.
Among patients remaining in the study at 96h, canrenone concentrations in patients randomized to high-dose spironolactone were still increasing, indicating that steady-state had not been reached. Furthermore, the naïve group at 96h provides additional evidence for a 48-h half-life for canrenone. Indeed, the median concentration of 71.5 ng/ml is extremely close to the 75% of steady state concentration expected to be reached after 2 elimination half-lives.21 This would be highly concordant with the time since drug initiation in these patients; 96h (or two half-lives of 48h). Thus, ultimately, in this patient population, one would expect to reach 87.5% (3 half-lives) and 97% of steady state (5 half-lives) only after 6 and 10 days of treatment, respectively.21 The concentrations of 7α-TMS and 6β-OH-7α-TMS also point towards a potential lack of early achievement of steady-state concentrations in ATHENA-HF spironolactone-naïve patients.
Prior spironolactone use
Another important observation is related to the inclusion of prior spironolactone users. For both ethical and safety reasons, spironolactone could not have been halted during the study period in previous users. However, canrenone, 7α-TMS, and 6β-OH-7α-TMS concentrations all remained higher after 48h in previous users randomized to receive high-dose spironolactone compared to spironolactone-naïve patients. This indicates that prior use may have had a residual effect for at least 48h in ATHENA-HF.
Correlation between spironolactone metabolites and study end points
The lower-than-anticipated concentrations of active metabolites observed at 48h may also partially explain why no significant reduction in NT-proBNP or diuresis was observed by that time point, or during the trial. Indeed, chronic doses associated with such low canrenone concentrations16 have not been associated with a major diuretic effect in most patients.22 Moreover, as existing data suggest average delays of two to four days are needed to achieve 20 to 30% reductions in natriuretic peptides in HF patients undergoing decongestion,23-25 the complete effect of high-dose spironolactone on efficacy endpoints may not have been observed during the timeframe of the study. Thus, measurement of study endpoints beyond 96 hours may have demonstrated improvements in these parameters. This hypothesis requires prospective validation. Finally, considering that ATHENA-HF was neutral with respect to efficacy and safety endpoints and that steady-state had not been reached, it is not surprising that we saw no clear correlation between the metabolites and the study endpoints.
Broader perspective
A variety of reasons have been suggested to explain the difficulties in developing therapeutic approaches for acute HF, including an incomplete understanding of drug pharmacokinetics.26 This substudy underscores this possibility. Indeed, despite having been used for more than six decades, little pharmacokinetic evidence is available for spironolactone for patients with HF. Relying on data from healthy individuals may be suboptimal to guide therapy in HF patients.14, 27, 28 In the case of spironolactone, only limited pharmacokinetic data from patients with stable HF are available29, 30 and these studies were conducted decades ago, using fluorometric assays. These assays have since been found to be not specific to canrenone, as other metabolites, such as 7α-TMS, were measured concurrently.31 Yet, consistent with our findings, two small studies using fluorometric measurements in patients with HF showed mean half-lives of 18 and 36.7 hours, respectively, for canrenone.29, 30 An assessment of spironolactone pharmacokinetics in HF patients using contemporary measurement methods appears necessary.14
Our results could be of interest in the design of future trials. Given the linear pharmacokinetics of spironolactone and canrenone, one could estimate that in spironolactone-naïve patients, an initial oral bolus of 300 to 400 mg could lead to concentrations similar to the steady-state concentrations of 100 mg daily. In fact, such high-doses doses have been used in patients with cirrhotic ascites.20 This hypothesis requires prospective validation. Alternatively, the use of IV MRAs e.g. canrenoate, which is rapidly converted to canrenone,32 followed by oral MRA, should also be investigated.
Our results have other potential implications. First, when initiating or titrating spironolactone, clinicians should be aware that, in some patients, reaching steady-state concentrations might be delayed, particularly when a patient presents with advanced or decompensated HF. Thus, this delay should be taken into consideration when individualizing the interval between increases in spironolactone dosing. Second, our results support existing evidence suggesting that HF patients demonstrate significant differences in the pharmacokinetics of both cardiovascular and non-cardiovascular drugs.33 Given that HF patients have a heavy burden of prescription medications, and thus are at increased risk of drug interactions,34 the impact of HF on pharmacokinetics and dosing requirements warrants further investigation.
Limitations
The measurement of spironolactone and its metabolites in ATHENA included a limited number of samples per patient preventing the performance of a true pharmacokinetic study. Thus, any pharmacokinetic estimates, such as half-life of canrenone, should be interpreted with caution until this evidence is validated by a prospective pharmacokinetic study in HF. The small number of patients in the high-dose-previous and low-dose groups, and the small number of patients who had drug and metabolite concentrations at 96 hours, also limited our ability to perform more complex regression models. Also, not all patients randomized to high-dose spironolactone could be included in the substudy. Nevertheless, as the 149 patients included from the high-dose arm represent 81.9% of patients initially randomized to the high-dose arm in ATHENA-HF, we believe that our results are reasonably generalizable to the rest of this treatment arm.
Conclusion
In conclusion, the results of the current study suggest that lower than anticipated concentrations of spironolactone metabolites in the high-dose spironolactone group may have contributed to the absence of pharmacological effects of spironolactone in ATHENA-HF. More broadly, these results highlight the necessity to conduct comprehensive pharmacokinetic studies in HF patients to optimize dosing strategies and guide decisions both in daily clinical practice and the design of clinical trials.
Supplementary Material
Acknowledgments
Funding: Research reported in this article was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award U10 HL084904 (for the Coordinating Center) and awards U10 HL110297, U10 HL110342, U10 HL110309, U10 HL110262, U10 HL110338, U10 HL110312, U10 HL110302, U10 HL110336, and U10 HL110337 (for Regional Clinical Centers). This project was also supported by the Université de Montréal Beaulieu-Saucier Chair in Pharmacogenomics and the Montreal Heart Institute Foundation.
Conflict of Interest:
SdeD was supported through grants from Pfizer, AstraZeneca, Roche Molecular Science, DalCor and Novartis. JLR served as a consultant for Novartis and AstraZeneca. JB is consultant to: Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Mayers Squib, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, Stealth Peptide, SC Pharma, Vifor. MPD is a co-author on a patent pertaining to pharmacogenomics-guided CETP inhibition and has minor equity interest in DalCor. MPD has received honoraria from Dalcor and research support (access to samples and data) from AstraZeneca, Pfizer, Servier, Sanofi and GlaxoSmithKline. RJM receives research support from the National Institutes of Health (U01HL125511-01A1 and R01AG045551-01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and Sanofi; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Sinkeler SJ, Damman K, van Veldhuisen DJ, Hillege H and Navis G. A re-appraisal of volume status and renal function impairment in chronic heart failure: combined effects of pre-renal failure and venous congestion on renal function. Heart Fail Rev. 2012;17:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JGF, Anker SD, Gheorghiade M, Fiuzat M, Rossignol P, Zannad F, Pitt B, O'Connor C and Felker GM. Decongestion in acute heart failure. Eur J Heart Fail. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girerd N, Pang PS, Swedberg K, Fought A, Kwasny MJ, Subacius H, Konstam MA, Maggioni A, Gheorghiade M, Zannad F and investigators E. Serum aldosterone is associated with mortality and re-hospitalization in patients with reduced ejection fraction hospitalized for acute heart failure: analysis from the EVEREST trial. Eur J Heart Fail. 2013;15:1228–35. [DOI] [PubMed] [Google Scholar]
- 4.Hensen J, Abraham WT, Dürr JA and Schrier RW. Aldosterone in congestive heart failure: analysis of determinants and role in sodium retention. Am J Nephrol. 1991;11:441–6. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet AA, Donker AJ, Nauta JJ and Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993;71:21A–28A. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P and Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2013. [DOI] [PubMed] [Google Scholar]
- 7.Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne-Nickens P, Hernandez AF, Braunwald E, National Heart L and Blood Institute Heart Failure Clinical Research N. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017;2:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim A Spironolactone: disposition, metabolism, pharmacodynamics, and bioavailability. Drug Metab Rev. 1978;8:151–88. [DOI] [PubMed] [Google Scholar]
- 9.Los LE, Pitzenberger SM, Ramjit HG, Coddington AB and Colby HD. Hepatic metabolism of spironolactone. Production of 3-hydroxy-thiomethyl metabolites. Drug Metab Dispos. 1994;22:903–8. [PubMed] [Google Scholar]
- 10.Guyonnet J, Elliott J and Kaltsatos V. A preclinical pharmacokinetic and pharmacodynamic approach to determine a dose of spironolactone for treatment of congestive heart failure in dog. J Vet Pharmacol Ther. 2010;33:260–7. [DOI] [PubMed] [Google Scholar]
- 11.McInnes GT, Shelton JR, Ramsay LE, Harrison IR, Asbury MJ, Clarke JM, Perkins RM and Venning GR. Relative potency and structure activity relationships of aldosterone antagonists in healthy man: correlation with animal experience. Br J Clin Pharmacol. 1982;13:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McInnes GT. Relative potency of amiloride and spironolactone in healthy man. Clin Pharmacol Ther. 1982;31:472–7. [DOI] [PubMed] [Google Scholar]
- 13.Adams KF Jr., Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, Committee ASA and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–16. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa R, Stachnik JM and Echizen H. Clinical pharmacokinetics of drugs in patients with heart failure: an update (part 2, drugs administered orally). Clin Pharmacokinet. 2014;53:1083–114. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, Tang WH, Felker GM, Shah MR and Braunwald E. Rationale and Design of the ATHENA-HF Trial: Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure. JACC Heart Fail. 2016;4:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA and Rouleau JL. Spironolactone Metabolites in TOPCAT - New Insights into Regional Variation. N Engl J Med. 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, Stubbs K, Smith M and Karim A. Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Pharmacol. 1989;29:342–7. [DOI] [PubMed] [Google Scholar]
- 18.Korol S, White M, O'Meara E, Rouleau JL, White-Guay B, Dorais M, Ahmed A, de Denus S and Perreault S. Is there a potential association between spironolactone and the risk of new-onset diabetes in a cohort of older patients with heart failure? Eur J Clin Pharmacol. 2019. [DOI] [PubMed] [Google Scholar]
- 19.Allen LA, Shetterly SM, Peterson PN, Gurwitz JH, Smith DH, Brand DW, Fairclough DL, Rumsfeld JS, Masoudi FA and Magid DJ. Guideline concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circ Heart Fail. 2014;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sungaila I, Bartle WR, Walker SE, DeAngelis C, Uetrecht J, Pappas C and Vidins E. Spironolactone pharmacokinetics and pharmacodynamics in patients with cirrhotic ascites. Gastroenterology. 1992;102:1680–5. [DOI] [PubMed] [Google Scholar]
- 21.Bauer LA. Clinical Pharmacokinetic and Pharmacodynamic Concepts Applied Clinical Pharmacokinetics, 3e New York, NY: McGraw-Hill Medical; 2015. [Google Scholar]
- 22.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78:902–7. [DOI] [PubMed] [Google Scholar]
- 23.Stienen S, Salah K, Dickhoff C, Carubelli V, Metra M, Magrini L, Di Somma S, Tijssen JP, Pinto YM and Kok WE. N-Terminal Pro-B-Type Natriuretic Peptide (NT-proBNP) Measurements Until a 30% Reduction Is Attained During Acute Decompensated Heart Failure Admissions and Comparison With Discharge NT-proBNP Levels: Implications for In-Hospital Guidance of Treatment. J Card Fail. 2015;21:930–4. [DOI] [PubMed] [Google Scholar]
- 24.Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E and Solal AC. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43:635–41. [DOI] [PubMed] [Google Scholar]
- 25.Cioffi G, Tarantini L, Stefenelli C, Azzetti G, Marco R, Carlucci S and Furlanello F. Changes in plasma N-terminal proBNP levels and ventricular filling pressures during intensive unloading therapy in elderly with decompensated congestive heart failure and preserved left ventricular systolic function. J Card Fail. 2006;12:608–15. [DOI] [PubMed] [Google Scholar]
- 26.Hamo CE, Butler J, Gheorghiade M and Chioncel O. The bumpy road to drug development for acute heart failure. European Heart Journal Supplements. 2016;18:G19–G32. [Google Scholar]
- 27.Shammas FV and Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15:94–113. [DOI] [PubMed] [Google Scholar]
- 28.Benowitz NL and Meister W. Pharmacokinetics in patients with cardiac failure. Clin Pharmacokinet. 1976;1:389–405. [DOI] [PubMed] [Google Scholar]
- 29.Jackson L, Branch R, Levine D and Ramsay L. Elimination of canrenone in congestive heart failure and chronic liver disease. Eur J Clin Pharmacol. 1977;11:177–9. [DOI] [PubMed] [Google Scholar]
- 30.Sadee W, Schroder R, von Leitner E and Dagcioglu M. Multiple dose kinetics of spironolactone and canrenoate-potassium in cardiac and hepatic failure. Eur J Clin Pharmacol. 1974;7:195–200. [DOI] [PubMed] [Google Scholar]
- 31.Overdiek HW, Hermens WA and Merkus FW. New insights into the pharmacokinetics of spironolactone. Clin Pharmacol Ther. 1985;38:469–74. [DOI] [PubMed] [Google Scholar]
- 32.Krause W, Karras J and Seifert W. Pharmacokinetics of canrenone after oral administration of spironolactone and intravenous injection of canrenoate-K in healthy man. Eur J Clin Pharmacol. 1983;25:449–53. [DOI] [PubMed] [Google Scholar]
- 33.Mangoni AA and Jarmuzewska EA. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: a critical appraisal of the evidence. Br J Clin Pharmacol. 2019;85:20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page RL and Lindenfeld J. The Comorbidity Conundrum: A Focus on the Role of Noncardiovascular Chronic Conditions in the Heart Failure Patient. Current Cardiology Reports. 2012;14:276–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.