Abstract
Intensive care unit (ICU) admissions and mortality in severe COVID-19 patients are driven by “cytokine storms” and acute respiratory distress syndrome (ARDS). Interim clinical trial results suggest that the corticosteroid dexamethasone displays better 28-day survival in severe COVID-19 patients requiring ventilation or oxygen. In this study, 10 out of 16 patients (62.5%) that had an average plasma IL-6 value over 10 pg/mL post administration of corticosteroids also had worse outcomes (i.e., ICU stay >15 days or death), compared to 8 out of 41 patients (19.5%) who did not receive corticosteroids (p-value = 0.0024). Given this potential association between post-corticosteroid IL-6 levels and COVID-19 severity, we hypothesized that the glucocorticoid receptor (GR or NR3C1) may be coupled to IL-6 expression in specific cell types that govern cytokine release syndrome (CRS). Examining single-cell RNA-seq data from BALF of severe COVID-19 patients and nearly 2 million cells from a pan-tissue scan shows that alveolar macrophages, smooth muscle cells, and endothelial cells co-express NR3C1 and IL-6, motivating future studies on the links between the regulation of NR3C1 function and IL-6 levels.
Subject terms: Predictive markers, Drug development, Viral infection
Introduction
Infection with SARS-CoV-2 is largely asymptomatic or presents with mild-to-moderate symptoms in a majority of patients1, but can result in progressive respiratory illness leading to acute respiratory distress syndrome (ARDS) in a subset of severely ill COVID-19 patients2,3. Multiple clinical trials have been underway to investigate therapies ranging from antivirals (e.g., Lopinavir–Remdesivir) to corticosteroids and immunosuppressive agents (e.g., Dexamethasone, Tocilizumab)4. In line with previous reports of IL-6 as a biomarker of severe disease5, some studies examining small patient cohorts have suggested treatment with tocilizumab (anti-IL6 receptor) may improve outcomes in severe COVID-19 patients6,7. Consequently, the use of plasma IL-6 testing has gained traction during the ongoing COVID-19 pandemic.
A recent interim update from the randomized evaluation of COVID-19 therapy (RECOVERY) trial that is examining larger cohorts of severe COVID-19 patient outcomes revealed the maximal reduction in mortality among patients treated with dexamethasone over other therapies8. In this trial, 2104 randomized patients received 6 mg of dexamethasone once per day for ten days, compared with 4321 patients that received standard care alone. Dexamethasone reduced mortality by one-third in ventilated patients (rate ratio 0.65 [95% confidence interval 0.48–0.88]; p = 0.0003) and by one-fifth in other patients receiving oxygen only (0.80 [0.67–0.96]; p = 0.0021)8. Although corticosteroids have long been utilized clinically for their immunosuppressive and anti-inflammatory capacities9–12 the precise mechanisms by which dexamethasone mediates clinical improvement in severe COVID-19 patients are not well understood. Indeed, the success of dexamethasone in treating COVID-19 was somewhat unexpected in light of an early report during this pandemic urging clinicians to not prescribe corticosteroids for lung injury in COVID-19 patients, based on the lack of efficacy as well as an elevated risk of adverse events associated with steroid use in the previous SARS and MERS epidemics13. Such ostensibly contradictory evidence and guidance underline the need for improved mechanistic stratification of corticosteroid efficacy in different subsets of severely ill COVID-19 patients.
Rapid advances in genomic and transcriptomic technologies over the past decade hold great potential to characterize drug targets at unprecedented levels. We recently released the nferX platform single-cell application as a resource to help researchers analyze publicly deposited single-cell RNA-sequencing datasets and readily contextualize these expression-derived insights using quantified literature associations14. We and others have harnessed this wealth of gene expression data at single-cell resolution to profile human tissues and cells based on their expression of ACE2, the putative entry receptor for SARS-CoV-214–17. Notably, although the primary glucocorticoid receptor (GR) (NR3C1) has been previously reported to be ubiquitously expressed18–20, the global expression profile of this important drug target has not been systematically evaluated across the hundreds of thousands of bulk RNA-seq samples and millions of single-cell RNA-sequencing data points which are available.
Here, we perform longitudinal analysis of real-world data of COVID-19 patients and triangulate our findings with publicly available transcriptomic datasets to nominate putative mechanisms by which dexamethasone results in reduced mortality. Our findings provide molecular support for the known immunomodulatory effects of corticosteroids and pinpoint the immune cell types which are likely to be affected by systemic and pulmonary exposure to dexamethasone. Specifically in one study of COVID-19 alveolar immune infiltrates21, we identify multiple populations of macrophages that co-express NR3C1 and IL-6 genes, including one coexpressing population which tends to downregulate NR3C1 in severely ill patients compared to those with mild disease. We also find that non-immune cells including endothelial cells and smooth muscle cells are among the cell types which most frequently coexpress these genes.
Results
The use of high sensitivity plasma IL-6 tests at the Mayo Clinic has increased during the COVID-19 pandemic. From 2010 to 2019, 1463 tests were conducted and 274 of these tests (18%) resulted above 10 pg/mL (normal range between 0.31 and 5 pg/mL). In contrast, during the first half of 2020, 377 out of 537 tests (70%) resulted above 10 pg/mL. The distribution of observed IL-6 levels during the ongoing COVID-19 pandemic (mean = 68 pg/mL) is thus shifted towards higher levels compared to the IL-6 levels from earlier years (mean = 11 pg/mL). For example, pulmonary phenotypes such as ARDS are associated with high levels of IL6 (Supplementary Fig. 1). We also observed that IL-6 levels did not correlate with levels of the inflammatory markers C-reactive protein and erythrocyte sedimentation rate in the COVIDpos patients (Fig. 1 and Supplementary Fig. 2).
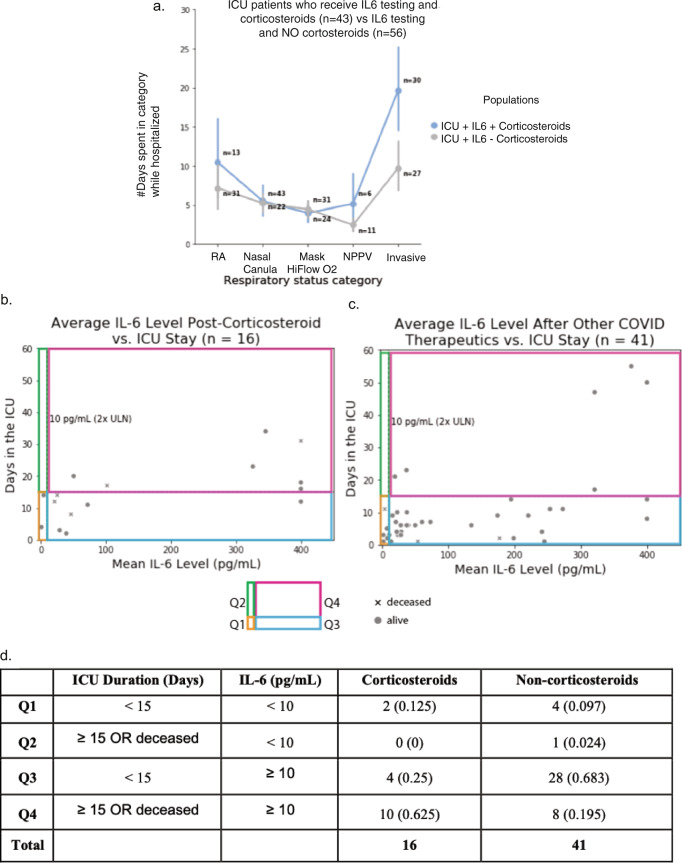
Fig. 1. Relationship between IL-6 levels and ICU duration for COVID-19 ICU patients who had at least one plasma IL-6 measurement after administration of either corticosteroids or other classes of drugs.
a Among the COVID19 patients that were admitted to ICU at some point during their hospital stay and had undergone an IL6 test, the patients that were on corticosteroids (n = 43) experienced longer durations on higher levels of respiratory support compared to the patients that were not on corticosteroids (n = 56). b Relationship between ICU duration and IL-6 levels following the administration of non-topical corticosteroids for n = 21 ICU patients who received corticosteroids. c Relationship between ICU duration and IL-6 levels following the administration of one or more of the following drugs: tocilizumab, azithromycin, hydroxychloroquine, or antivirals (n = 64 patients); these patients did not receive corticosteroids. d Summarizing the number of patients in each of the four quadrants (Q1, Q2, Q3, and Q4) in (b) and (c) above. A Fisher exact test of proportions suggests that a higher proportion of patients who receive corticosteroids and have an IL-6 value over 10 pg/mL have poor outcomes (ICU stay ≥15 days or death) when compared to the equivalent proportion of those treated with non-corticosteroid drugs including antivirals, tocilizumab, azithromycin, and hydroxychloroquine (p = 0.0006).
Stratifying COVID-19 patients by intensive care unit (ICU) duration highlights IL-6 levels post administration of corticosteroids, but not non-corticosteroids, as an indicator of COVID-19 outcomes
To understand whether or not corticosteroids benefited inflammation-induced respiratory distress among the ICU patients, we started by characterizing respiratory status in patients who received corticosteroids and IL-6-testing versus those who received IL-6-testing alone. Among COVID19 patients that were admitted to ICU at some point during their hospital stay and had undergone an IL-6 test, patients that were on corticosteroids experienced longer durations on invasive respiratory support (median = 16 days) compared to those that did not receive corticosteroids (median = 10 days) (Mann–Whitney U = 532.5, p = 0.008) (Fig. 1a).
To understand how corticosteroids either benefited or not the inflammation-induced respiratory distress among the ICU patients, we plotted plasma IL-6 levels versus ICU durations for patients on corticosteroid and non-corticosteroid therapies (antivirals, azithromycin, hydroxychloroquine, tocilizumab). Thresholds were set for IL-6 level (10 pg/mL) and ICU status (15 days) to group patients into four categories, with mortality grouped with an ICU stay of 15 days or more. Ten out of 16 patients (62.5%) that had an average plasma IL-6 value over 10 pg/mL post administration of corticosteroids also had worse outcomes (i.e., ICU stay >15 days or death), compared to 8 out of 41 patients (19.5%) who did not receive corticosteroids (Fig. 1b, c). The Fisher’s exact test p-value for this observation is 0.0024 (Fig. 1d). The split-up of non-corticosteroid drugs into the specific therapeutics is also shown (Fig. 1 and Supplementary Fig. 3), with consistent observations to those summarized above.
Longitudinal comparison of plasma IL-6 levels pre- versus post-administration of corticosteroids
The timing of SARS-CoV-2 PCR testing, non-topical corticosteroid administration, tocilizumab administration, and IL-6 measurements available for 63 hospitalized COVID-19 patients are summarized in Fig. 2 and Supplementary Fig. 1. Of these patients, we considered ten critically ill COVID-19 patients who received IL-6 plasma tests both before and after corticosteroid administration. These patients met the following criteria: (1) hospitalized in the ICU for COVID-19 related illness, (2) received non-topical corticosteroid therapy during COVID-19 related hospitalization, and (3) underwent plasma IL-6 testing at least one time both before and after the first administration of a corticosteroid. Of these patients, 4/10 were male and the median age was 62 (interquartile range: 55–69).
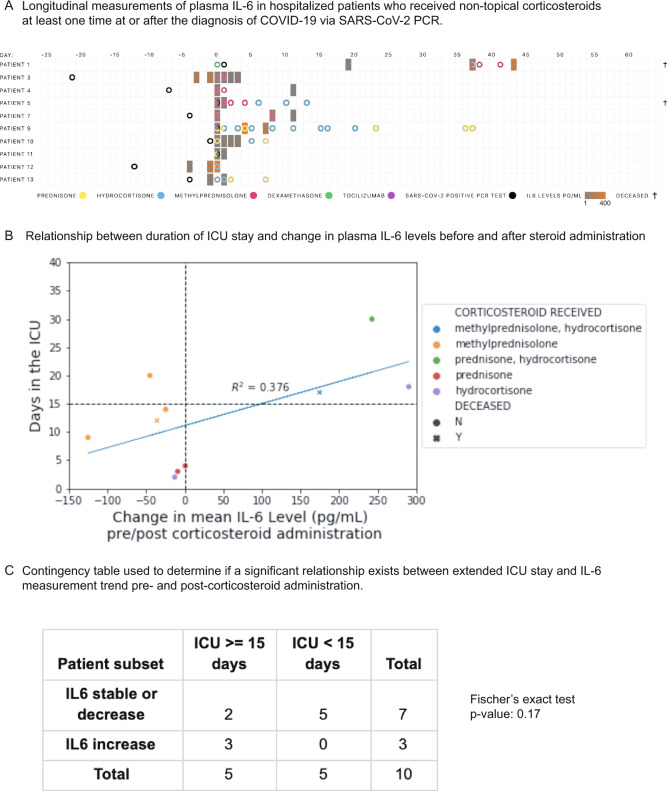
Fig. 2. Constraining cohort of COVID-19 ICU patients to those who had at least one plasma IL-6 measurement before and after corticosteroid administration.
A Longitudinal measurements of plasma IL-6 in hospitalized patients who received non-topical corticosteroids at least one time at or after the diagnosis of COVID-19 via SARS-CoV-2 PCR. B Relationship between duration of ICU stay and change in plasma IL-6 levels before and after steroid administration (patient numbers are mapped onto Fig. 8a). If multiple pre/post-corticosteroid administration levels are available, the mean is used. C Contingency table used to determine if a significant relationship exists between extended ICU stay and IL-6 measurement trend pre- and post-corticosteroid administration.
We found that IL-6 levels remained stable or decreased after steroid administration in seven of ten patients, while the remaining three patients showed large increases in IL-6 (Fig. 2A). Nine of the ten patients began with pre-corticosteroid IL-6 levels more than twice the upper limit of normal (normal reference range: 0.31–5 pg/mL). Following corticosteroid administration, five of the ten patients returned to mean IL-6 levels less than twice the upper limit of normal (10 pg/mL) and four of the ten patients had IL-6 levels less than 5 pg/mL. The was a weak correlation between change in average plasma IL-6 levels following corticosteroid administration and worse clinical outcomes (Fig. 2B). However, an ICU duration of 15 days or more was not significantly associated with post-steroid increases in IL-6 (p-value = 0.17; Fig. 2C). Admittedly, this analysis is limited by the small number of patients. An analysis in the future on data from a larger number of patients can be used to explore whether longitudinal IL6 testing can be a useful biomarker for COVID-19 severity.
The corticosteroid target NR3C1 is expressed across immune cell types including alveolar macrophages
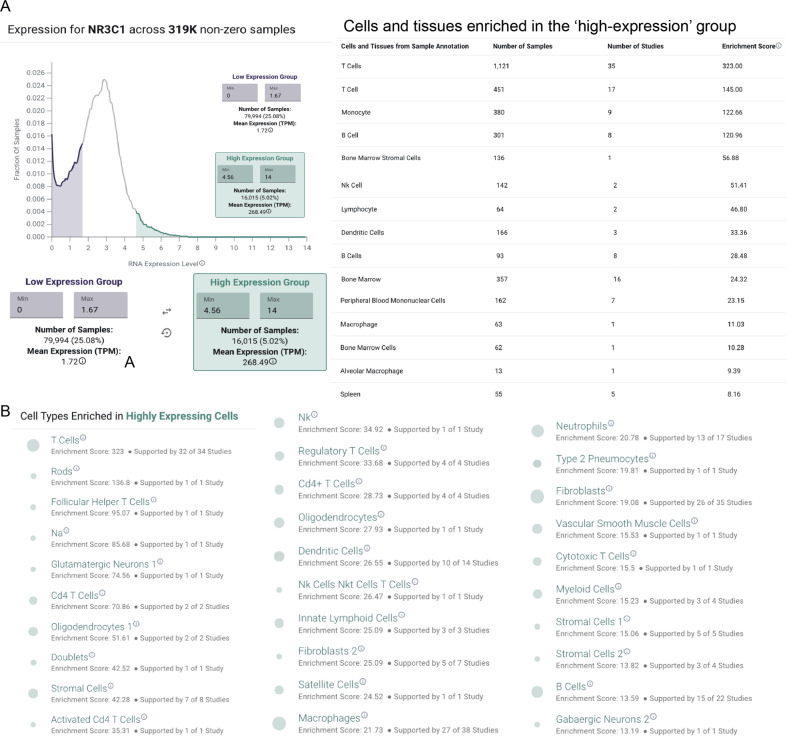
To better understand the mechanisms of potential corticosteroid-induced effects on IL-6 levels, we next systematically profiled the gene expression of NR3C1—the highest affinity target of dexamethasone, methylprednisolone, and prednisone (Fig. 3 and Supplementary Fig. 1) from a resource of over 450,000 bulk RNA-sequencing samples from more than 10,000 studies. Such an analysis is timely and critical to identifying potential mechanisms of steroid-induced immunomodulation. Indeed, the immunomodulatory function of corticosteroids has long been appreciated9,10, yet the expression profile of NR3C1 has not been formally established across all human tissues and cell types. The delineation of this profile in both healthy and diseased patients may help us to better understand the cell types which are most critical to mediate the beneficial effects of steroids in severely ill COVID-19 patients. We found that various immune cells—including T cells, B cells, monocytes, natural killer cells, dendritic cells, and macrophages—are enriched among the samples with the highest (top 5%) of NR3C1 expression (Fig. 3A), consistently with previously reported effects of glucocorticoids on the function of each of these cells types22–26. Interestingly, we also identified one study in which alveolar macrophages specifically showed high NR3C1 expression (Fig. 3 and Supplementary Fig. 2; n = 20 samples from ten individuals), and we found that NR3C1 was consistently expressed at appreciable levels in alveolar macrophages from two other independent studies as well (Fig. 3 and Supplementary Fig. 2; n = 25 samples).
Fig. 3. Analysis of bulk tissue RNA sequencing data from gene expression omnibus reveals increased NR3C1 expression in human immune cells.
A Expression profile of NR3C1 across ~319,000 non-zero expressing bulk RNA-sequencing samples from the gene expression omnibus. Cells of the immune system are enriched among the highest expressing samples, including both lymphocytes and myeloid cells. B Single-cell RNA-sequencing expression profile of NR3C1 across ~1.9 million individual human-derived cells. Cells of the immune system are enriched among the highest expressing samples, including both lymphocytes and myeloid cells.
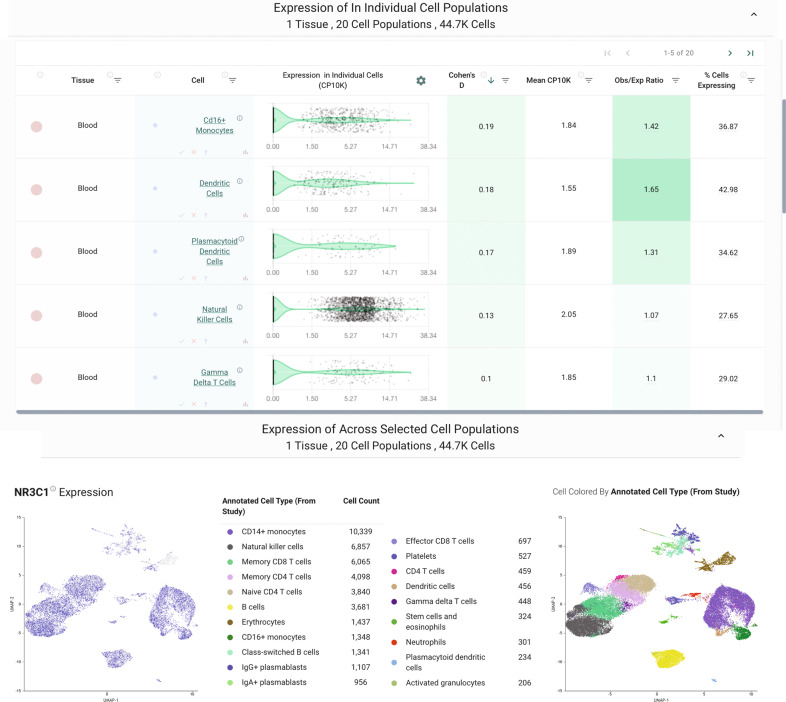
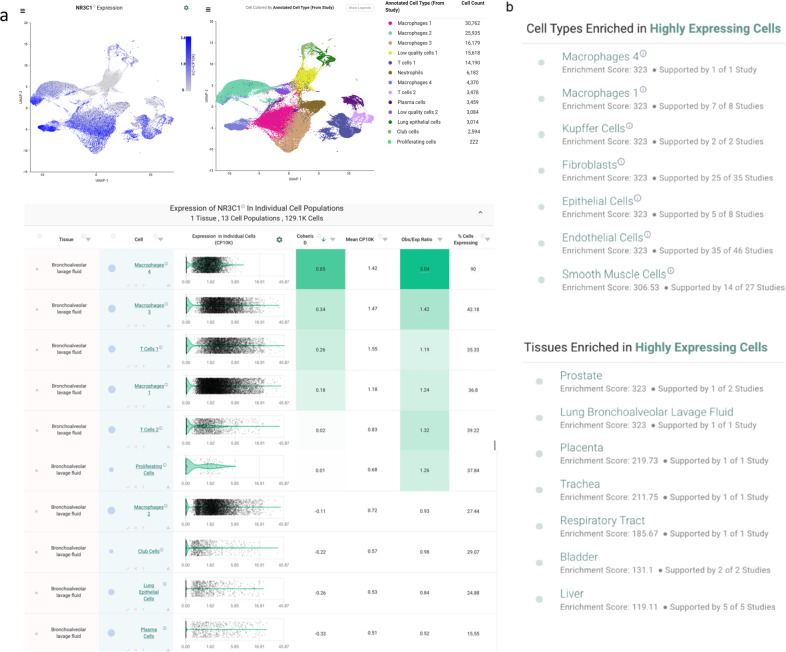
We then further characterized NR3C1 expression by single-cell RNA-sequencing across 1.9 million human cells using the nferX single-cell platform14. Consistent with our findings from bulk RNA-seq, we found that various hematopoietic lineages were enriched among the cells with the highest expression of NR3C1 (Fig. 3B). Several T cell populations showed particularly strong enrichment among NR3C1-high samples, but we also found evidence for high expression in macrophages, dendritic cells, B cells, and innate lymphoid cells (Fig. 3B). Similarly, in a study of peripheral blood samples from healthy donors (n = 6) and COVID-19 patients between days 2 and 16 of symptom onset (n = 7), NR3C1 was appreciably expressed in diverse immune cell types including T cells, B cells, dendritic cells, monocytes, and plasmacytoid dendritic cells (Fig. 4). In bronchoalveolar lavage fluid (BALF) of COVID-19 patients, both macrophages and T cells strongly expressed NR3C1, while plasma cells, neutrophils, and the recovered epithelial populations showed lower levels of NR3C1 expression (Fig. 5; nferX platform single cell app).
Fig. 4. nferX single-cell platform analysis of NR3C1 expression.
In a study of peripheral blood samples from healthy donors (n = 6) and COVID-19 patients between days 2 and 16 of symptom onset (n = 7), NR3C1 was appreciably expressed in diverse immune cell types including T cells, B cells, dendritic cells, monocytes, and plasmacytoid dendritic cells.
Fig. 5. Analysis of available single-cell RNA sequencing data from cells recovered from bronchoalveolar lavage fluid of COVID-19 patients.
A Expression of NR3C1 in cells from bronchoalveolar lavage fluid (BALF) of COVID-19 patients (n = 9; 3 mild, 6 severe) by single-cell RNA-sequencing. B Summary of IL-6/NR3C1 co-expression across 1.9 million human cells from single-cell RNA-sequencing datasets. Shown are cell and tissue types most highly enriched for detection of both NR3C1 and IL-6 in the same individual cells.
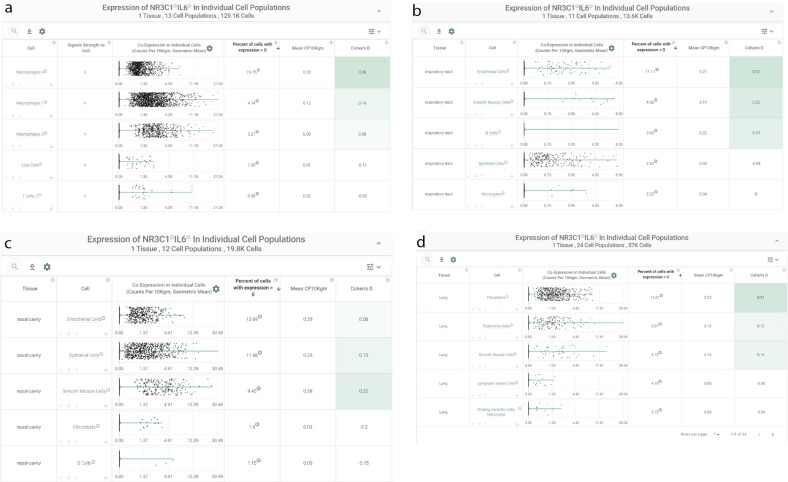
NR3C1 and IL-6 are co-expressed in alveolar macrophages of COVID-19 patients and systemically in endothelial cells and smooth muscle cells
To connect this expression profile to our clinical observation of reduced plasma IL-6 in a subset of patients following corticosteroid administration, we specifically evaluated co-expression of NR3C1 and IL-6 across these same 1.9 million human single cells. We found that two populations of alveolar macrophages from a study of healthy controls (n = 3) and COVID-19 patients (n = 9) were among the most strongly enriched cell types for this co-expression, with both genes detected in 20 and 5% of these two populations (“Macrophages 4” and “Macrophages 1”, respectively; Fig. 6A). Interestingly, in contrast to the immune-centric expression profile of NR3C1 alone, we found that several other non-immune cell types showed notable co-expression with IL6 in both respiratory (Fig. 6B) and non-respiratory (Fig. 6C, D) tissues including fibroblasts, epithelial cells, endothelial cells, and smooth muscle cells. This observation of co-expression in both endothelial cells and smooth muscle cells is particularly interesting given the reports of systemic vascular inflammation in the context of COVID-1927–29. Given the well-established mechanisms of glucocorticoid-mediated suppression of IL-6 transcription30, we propose that agonism of NR3C1 in these various co-expressing cell types may serve to dampen IL-6 production both in the lungs and systemically.
Fig. 6. IL-6/NR3C1 co-expression by single-cell RNA-sequencing.
A Bronchoalveolar lavage fluid of COVID-19 patients; and (B)–(D) nasal cavity, respiratory tract, lungs.
In severe COVID-19 patients, NR3C1 is downregulated in macrophages that co-express IL-6 and NR3C1
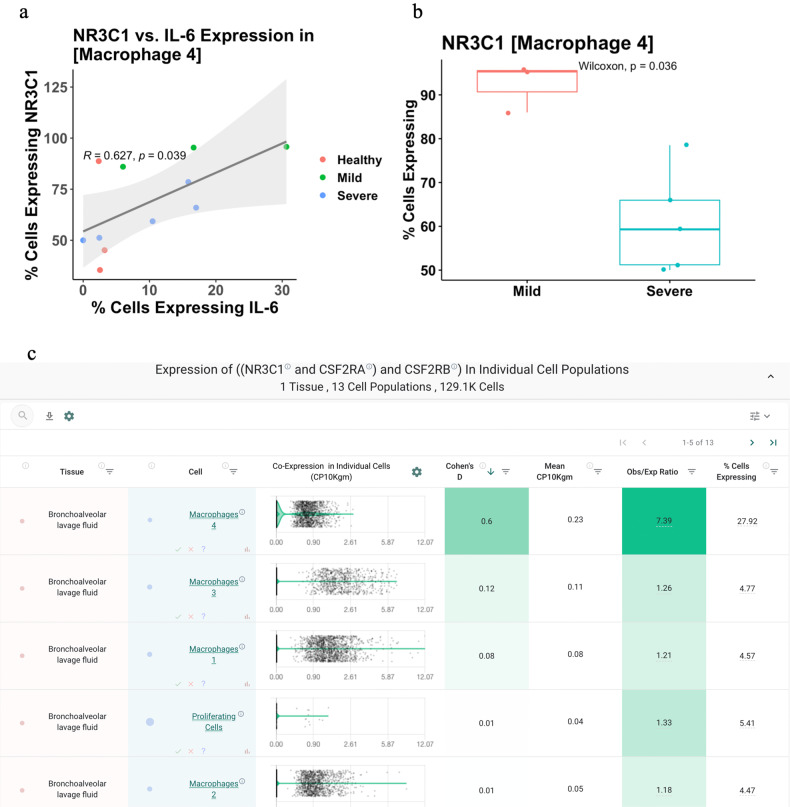
Given that dexamethasone appears to reduce mortality among only severe cases of COVID-19, we finally asked how NR3C1 and IL-6 co-expression varies between mild and severe disease. To answer this, we assessed NR3C1 and IL-6 expression levels in each recovered BALF cell population between cases of mild COVID-19 (n = 3) and severe COVID-19 (n = 6), along with healthy controls (n = 3). Interestingly we found that expression of IL-6 and NR3C1 is positively correlated in the strongest co-expressing population (“Macrophages 4” identified above), and further that NR3C1 expression in this cell population was significantly lower in patients with severe compared to mild disease (Fig. 7A, B). We hypothesize that this overall direct correlation may reflect a cell-intrinsic mechanism wherein activated inflammatory macrophages are simultaneously primed for homeostatic steroid-mediated immunosuppression. Indeed, such programmed feedback loops are well-established to dampen immune responses, including most notably the immune checkpoint pathways (e.g., PD-1/PD-L1) which restrain excessive T-cell activation upon antigen recognition31. Accordingly, our observation that NR3C1 expression is decreased in severe COVID-19 patients compared to mild COVID-19 patients may reflect a pathologic downregulation of this endogenous immunomodulatory system which can be restored pharmacologically via corticosteroid-mediated agonism of NR3C1. Together with potential broader effects exerted through the various other previously identified NR3C1/IL-6 co-expressing cell types (see Fig. 7), corticosteroid therapy may thereby dampen both local pulmonary and systemic inflammation to reduce the likelihood of patient progression to outright cytokine storm.
Fig. 7. Relationship of NR3C1 and IL-6 expression to COVID-19 status and clinical severity.
A Correlation between IL-6 and NR3C1 expression in the macrophage population found to most robustly co-express these two genes (“Macrophages 4”). Each dot represents the percentage of cells from this cluster (i.e., cell population) in which IL-6 and NR3C1 were detected, and dots are colored by COVID-19 status and clinical severity. Values shown are the Pearson correlation coefficient (“R”) and corresponding p-value (“p”). B Comparison of NR3C1 expression in the “Macrophage 4” cell population from patients with mild versus severe COVID-19. C Coexpression of NR3C1 with the two subunits of the GM-CSF receptor (CSF2RA and CSF2RB) in the bronchoalveolar lavage fluid of COVID-19 patients (n = 9; 3 mild, 6 severe). Top coexpressing populations, all of which are macrophages, are shown.
Discussion
The COVID-19 pandemic is unquestionably one of the most urgent global health crises of the modern era. Its rapid spread around the world has sparked clinical and basic research in efforts to discover interventions that can effectively eliminate infection or at least mitigate disease severity. While antiviral drugs, antibiotics, and antimalarial have shown little to no clinical benefit in COVID-19 patients32–34, modulation of host inflammatory responses with drugs that inhibit IL-6 signaling (tocilizumab) or stimulate GR activity (dexamethasone) have been suggested to improve clinical outcomes in critically ill patients6,35 negative results have also been reported36. There are also suggestions for using IL-6 as a biomarker for COVID-19 severity5.
The present work has several limitations. Firstly, the analysis of the relationship between IL-6 levels and duration of ICU stay is based on a small number of patients. Due to the small size of the groups being compared, propensity matching for factors such as age, gender, ethnicity, etc. was not performed. Secondly, the clinical rationale underlying the selection and dosage of the corticosteroids administered was not taken into account. Thirdly, the causality of the association between the plasma IL6 levels and ICU length of stay cannot be established in a retrospective observational study. The limitations of a retrospective study aside, this study motivates prospective pre-clinical and clinical research to compare the efficacy of corticosteroids and other drugs on disease severity.
In this study, we performed an integrated analysis of publicly available molecular data and curated EHR data as well as associated lab test results to study mechanisms of corticosteroid mitigation of COVID-19 cytokine storms and ARDS. Strikingly, despite the long history of corticosteroid use in the clinic, expression of the glucocorticoid receptor (NR3C1) has never been systematically profiled at single-cell resolution in the modern genomic era. Such profiling will help understand the tissues and cell types that are most likely to be directly targeted by systemic steroid therapies. Consistent with the well-established and clinically utilized anti-inflammatory effects of corticosteroids, our analysis of gene expression by bulk and single-cell RNA-sequencing strongly suggests that hematopoietic cells could be impacted by the corticosteroid dexamethasone via its NR3C1 agonism. Specifically, we find that T cells are the most strongly enriched human cell type for high NR3C1 expression, but other adaptive and innate immune cells are also notably strong expression.
After NR3C1, the second-highest affinity target known for the corticosteroid dexamethasone is NR3C2, which was not explored in this study. Primarily, the intersection between IL6 and NR3C2 appears to be limited to the ovaries (Fig. 3 and Supplementary Fig. 3). The NR3C2 activity of corticosteroids, and indeed the other targets including progesterone receptors, may well be beneficial for other aspects of COVID-19 severity mitigation beyond the IL-6 cytokine nexus that was explored in this study via clinical-omics triangulation. The polypharmacology of corticosteroids as they pertain to COVID-19 will be the topic of a follow-up study.
To understand the cellular context around the intersection of glucocorticoids and IL-6, in this study, we have performed the first directed co-expression analysis of NR3C1 and IL-6 across human single-cell RNA-sequencing datasets totaling almost 2 million cells. Interestingly, this analysis highlighted that while NR3C1 alone is highly expressed in T cells throughout the human body, co-expression with IL-6 is prominently observed in alveolar macrophages of the lung along with various non-immune cells across multiple tissues including endothelial, smooth muscle, epithelial, and stromal cells. We hypothesize that the engagement of the glucocorticoid receptor by dexamethasone in these co-expressing cell types reduces local and systemic IL-6 production, which in turn restores immune homeostasis and mitigates the progression of the COVID-19 associated acute respiratory distress syndrome. It is important to note that other inflammatory pathways may also be activated in COVID-19 patients and that blockade of these pathways may provide a clinical benefit similar to that seen with tocilizumab. For example, early results indicate that blockade of the GM-CSF pathway with the monoclonal antibody mavrilimumab may improve outcomes in several ill patients37. GM-CSF is known to orchestrate the activity of various innate immune cells including dendritic cells38 and macrophages38, and single-cell RNA-seq data confirms that NR3C1 is co-expressed with both the alpha and beta subunits of the GM-CSF receptor (CSF2RA and CSF2Rb) in several macrophage populations from the BALF of COVID-19 patients (Fig. 7C). Whether agonism of the glucocorticoid signaling pathway in these cells impacts their response to GM-CSF is certainly relevant to investigate as this could have direct clinical implications regarding the utility of coadministration of corticosteroids with both tocilizumab and mavrilimumab.
The results reported in this study emphasize that follow-up studies with larger cohorts of longitudinal data are warranted to investigate the clinical efficacy of dexamethasone and other corticosteroids in COVID-19 patients with ARDS and cytokine release syndrome (CRS). Such larger follow-up studies will help shed light on the mechanisms underlying heterogeneity in patient responsiveness to corticosteroids. If suppression of IL-6 production by corticosteroids like dexamethasone is indeed confirmed to be responsible for mitigating the respiratory and vascular onslaught of SARS-CoV-2 in severely ill COVID-19 patients, then longitudinal monitoring of plasma IL-6 levels before and after initiation of steroids may be warranted in clinical practice. We speculate that such practice will help determine whether a patient is likely to respond to corticosteroid therapy alone or if they should be considered as candidates for alternative intervention, including combination therapies with IL-6 receptor antagonists like tocilizumab (actemra) and sarilumab (kevzara), or direct IL-6 antibodies like siltuximab (sylvant).
Methods
Cohort definition for patients receiving IL-6 measurements and corticosteroids
All patients who tested positive for SARS-CoV-2 (COVIDpos), as determined by at least one positive PCR test, within the Mayo Clinic Health system and were hospitalized were selected as candidates for further analysis. These COVIDpos patients were then filtered by requiring an administration of a systemic corticosteroid at some point during their hospital stay; these corticosteroids included dexamethasone, prednisone, triamcinolone, methylprednisolone, prednisolone, betamethasone, and hydrocortisone. Tocilizumab was also included as a control given it is known effect on IL-6 signaling. In patients who received a systemic corticosteroid, we required that they underwent plasma IL-6 testing at least once both before and after the first administration of any of the corticosteroid agents listed above. Only 13 patients met these criteria, with 7 patients receiving IL-6 testing before and after methylprednisolone administration, four patients receiving IL-6 testing before and after prednisone administration, and four patients receiving IL-6 testing before and after hydrocortisone administration. Two patients overlapped drug categories, with one receiving first administrations of both methylprednisolone and hydrocortisone between IL-6 tests and the other receiving first administrations of both prednisone and hydrocortisone between IL-6 tests. In total, six patients received IL-6 testing both before and after any first corticosteroid administration within the timeframe considered (−25 days to +64 days). In addition to IL-6 levels and corticosteroid/tocilizumab administration data, we extracted outcomes including death, admission to an ICU, and length of time in ICU, as well as demographic information such as age, sex, and race.
For patients with IL-6 measured post-corticosteroid administration, over half of the patients (11 out of 21) were in the ICU at least 15 days or did not survive and had IL-6 levels above 10 pg/mL. As a corollary, fewer patients given corticosteroids (30%, n = 21) had high IL-6 levels and shorter ICU stays compared to tocilizumab (80%, n = 5), azithromycin (61%, n = 49), and hydroxychloroquine (60%, n = 10). While not statistically significant due to small cohort sizes, together these results may indicate that patients with high IL-6 levels post-corticosteroids are more likely to have longer ICU durations compared to the other drugs considered here. This points to the necessity for IL-6 measurement post-corticosteroid administration to monitor if further intervention is needed.
Characterizing the survival curves of hospitalized and ICU COVID-19 patients
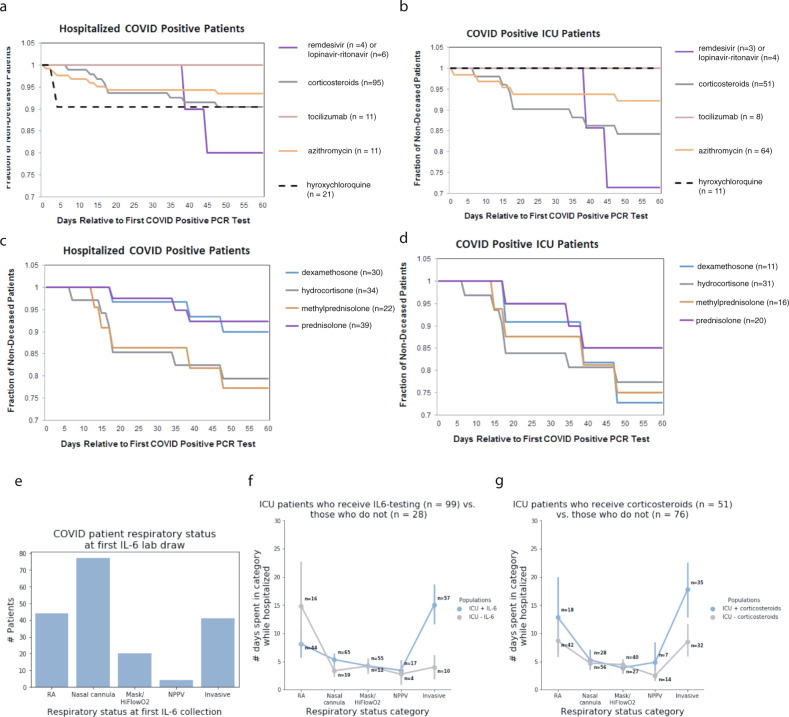
Kaplan–Meier curves were generated for 185 COVID-19 positive patients that were hospitalized at the Mayo Clinic (Fig. 8). Patient cohorts were defined by each drug or drug class that was administered. Patients receiving multiple drugs of different classes were counted as part of both cohorts. The drugs analyzed include corticosteroids, antivirals (remdesivir, lopinavir–ritonavir), antibacterial azithromycin, hydroxychloroquine, and the IL-6 inhibitor tocilizumab. As shown, all 11 hospitalized patients receiving tocilizumab survived 60 days post-diagnosis. Hospitalized cohorts receiving corticosteroids (n = 95) and azithromycin (n = 123) have similar survival rates between 90 and 95% when considering all hospitalized patients. Hydroxychloroquine (n = 21) also has a 60-day post-diagnosis survival rate of 90% but compared to corticosteroids and azithromycin, mortality occurred much earlier in the hydroxychloroquine cohort, i.e., 2 out of 21 patients died within 5 days of COVID-19 diagnosis. However, when considering more severe COVID patients requiring ICU admission, this subset of hydroxychloroquine patients (n = 11) have a 100% survival rate, the azithromycin cohort (n = 64) remains relatively unchanged (92% versus 93% for all hospitalized patients), and the corticosteroid cohort survival rate decreased from 91 to 84% at 60 days post-diagnosis. In both hospitalized and ICU patient cohorts, antivirals performed most poorly, with the caveat that these cohorts also have the smallest patient counts. Similar trends were also observed in the subset of patients with IL-6 measurements in these cohorts.
Fig. 8. Survival curves and respiratory status for the study cohort.
Survival curves for (a) hospitalized and (b) ICU-COVID-19 patients who received corticosteroids, tocilizumab, azithromycin, hydroxychloroquine, or antivirals (remdesivir or lopinavir–ritonavir) following their SARS-CoV-2-positive PCR test. c Survival curves for each corticosteroid administered patient who is hospitalized, split up by the exact corticosteroid drug. d Survival curves for each corticosteroid administered patient who is in the ICU, split up by the exact corticosteroid drug, i.e., dexamethasone, hydrocortisone, methylprednisolone, or prednisone. e Bar plot of COVID-19 patient respiratory status at first IL-6 lab draw, showing that ICU patients who receive IL-6 testing are typically initially tested early in the course of respiratory failure (panel A), (f) yet spend a long time in critical respiratory failure, indicating that physicians may be using IL-6 as part of their assessment of patients who are rapidly progressing towards critical respiratory failure. Here, RA indicates that the patient is breathing room air. The nasal cannula includes all nasal cannula with oxygen flow rates up to 15 L/min. Mask/HiFlowO2 includes devices designed to increase the percentage of oxygen inspired with each breath, such as a non-rebreather, Oxymizer, OxyMask, high flow nasal cannula, etc. NPPV includes all forms of non-invasive positive pressure ventilation, such as CPAP (continuous positive airway pressure), BiPAP (bilevel positive airway pressure), and oxygen-supplemented CPAP/BiPAP. Invasive ventilation includes intubation and mechanical ventilation. g Across every respiratory status, COVID-19 patients who received corticosteroids generally are worse from a respiratory standpoint than patients who did not receive corticosteroids.
To further understand the specific effects of the different corticosteroids given to COVID positive patients, survival rates of hospitalized and ICU patients are given dexamethasone, hydrocortisone, prednisone, and methylprednisolone were also generated. For hospitalized patients, dexamethasone (n = 30) and prednisone (n = 39) perform similarly (90 and 92% 60-day survival, respectively) and have higher 60-day survival post-diagnosis compared to hydrocortisone (n = 34) and methylprednisolone (n = 22) (79 and 77% 60-day survival, respectively). While a decrease in survival is observed in the more severe subset of each of these cohorts who are admitted to the ICU, dexamethasone (n = 11) shows the most significant decrease in 60-day survival, dropping from 90 to 72%.
Comparison of patient IL6/ICU pattern between drug classes
We defined four categories of patient IL-6 level/ICU stay length (i.e., the four quadrants mentioned in Fig. 1b). We split patient IL-6 level into two categories (high and low) and ICU stay length into two categories (poor outcome—i.e., 15+ days or ending in death, and not poor outcome—i.e., <15 days in ICU). We then compared the proportion of high IL-6 patients who had poor outcomes for (i) cohort of patients who took corticosteroids versus (ii) the cohort of patients who did not take corticosteroids (but did take one of our other drugs of interest); as well as the proportion of low IL-6 patients who had poor outcomes in each of those cohorts. We performed a Fisher exact test to compute p-values on these proportion comparisons.
This research was conducted under IRB 20-003278, “Study of COVID-19 patient characteristics with augmented curation of electronic health records (EHR) to inform strategic and operational decisions”. All analysis of EHRs was performed in the privacy-preserving environment secured and controlled by the Mayo Clinic. nference and the Mayo Clinic subscribe to the basic ethical principles underlying the conduct of research involving human subjects as set forth in the Belmont Report and strictly ensures compliance with the common rule in the code of federal regulations (45 CFR 46) on the protection of human subjects.
Bulk RNA-sequencing analysis
Data accession and processing
Datasets were downloaded in raw fastq format and uniformly processed using salmon as previously described14.
Global expression analysis
To identify highly expressing cells and tissues for a given gene, the following steps are followed:
Plot the distribution of gene expression (in units of transcripts per million, or TPM) across all samples from all studies.
Divide distribution into “high expression group” (e.g., cells in top 5% of expressing samples for query gene) and “low expression group” (e.g., cells in the bottom 25% of expressing samples for query gene).
Count the number of individual samples from each annotated cell or tissue type falling in the high and low expression groups. Note that cells and tissues are extracted from sample-level metadata available through the gene expression omnibus and other databases including the genotype-tissue expression project, the cancer genome atlas, and the cancer cell line encyclopedia.
Compute Fisher’s exact test p-value to measure the enrichment of cell type C (or tissue T) among the high expression group. Enrichment scores displayed correspond to −log10(adjusted p-value), where p-values are adjusted using the Benjamini–Hochberg (BH) correction.
Single-cell RNA-sequencing analysis
Data accession and processing
Datasets were downloaded and processed as previously described14, and processed datasets have been made available for investigation upon registration in the nferX single-cell platform (https://academia.nferx.com/).
Global expression analysis
To identify highly expressing cells for a given gene, the following steps are followed:
Plot the distribution of gene expression (in units of counts per 10,000, or CP10K) across all single cells from all studies.
Divide distribution into “high expression group” (e.g., cells in top 10% of expressing samples for query gene) and “low expression group” (e.g., cells in the bottom 90% of expressing samples for query gene).
Count the number of individual cells from each annotated cell population (or tissue) falling in the High and low expression groups.
Compute Fisher’s exact test p-value to measure the enrichment of cell population C (or tissue T) among the high expression group. Enrichment scores displayed correspond to −log10(adjusted p-value), where p-values are adjusted using the Benjamini–Hochberg (BH) correction.
Coexpression analysis
For a given set of genes, a single coexpression vector is computed as the geometric mean of CP10K values of all genes in each cell. The geometric mean is used as a coexpression metric as it will only yield a positive value in cells that express all genes in the defined set (i.e., one or more zero values in an individual cell will result in a coexpression value of zero for that cell). As such, all cells with a coexpression value (CP10Kgm) greater than zero can be considered as “coexpressing cells”, whereas all cells with a CP10Kgm value equal to zero can be considered as “non-coexpressing cells.” After this coexpression vector has been computed, it is treated identically to a gene expression vector for a single gene in the context of the global expression or single study-level analyses described above.
Institutional review board (IRB) approval for this research
This research was conducted under IRB 20-003278, “Study of COVID-19 patient characteristics with augmented curation of EHR to inform strategic and operational decisions”. All analysis of EHRs was performed in the privacy-preserving environment secured and controlled by the Mayo Clinic. nference and the Mayo Clinic subscribe to the basic ethical principles underlying the conduct of research involving human subjects as set forth in the Belmont Report and strictly ensures compliance with the common rule in the code of federal regulations (45 CFR 46) on the protection of human subjects. For more information, please visit https://www.mayo.edu/research/institutional-review-board/overview.
Supplementary information
Acknowledgements
The authors thank Patrick Lenehan, Murali Aravamudan, Travis Hughes, Andrew Danielsen, Vineet Agarwal, Sankar Ardhanari, Jason Ross, Najat Khan, Anuli Anyanwu Ofili, Kristopher Standish, and James Merson for their helpful feedback on this manuscript.
Author contributions
S.A.: conceptualization, methodology, data curation, validation, formal analysis, writing, visualization. T.W.: conceptualization, methodology, data curation, validation, formal analysis, writing, visualization. A.J.V.: conceptualization, methodology, writing, visualization. A.P.: methodology, formal analysis, writing. M.H.: project administration. V.A.: data curation, software. I.C.: data curation, software. C.K.: data curation, software. R.A.: data curation, software. J.O’H.: supervision, writing (review and editing). W.K.: writing (review and editing). R.K.: writing (review and editing). W.M.II: writing (review and editing). J.H.: writing (review and editing). A.W.W.: writing (review and editing). W.A.F.Jr.: writing (review and editing). A.D.B.: writing (review and editing). G.J.G.: writing (review and editing). V.S.: conceptualization, methodology, supervision, resources, and writing.
Funding
No external funding was received for this work.
Data availability
All data analyzed in this study were accessed under an IRB approved by the Mayo Clinic and were analyzed in consideration of HPAA regulations to protect patient privacy. Per the HIPAA act and Mayo Clinic’s policies for IRB review, all data availability requests should be directed to the corresponding author and will be directed to the Mayo Clinic colleagues for processing on a case-by-case basis.
Ethics statement
IRB approval was obtained for this work as described above.
Conflict of interest
ADB is supported by Grants AI110173 and AI120698 from NIAID, 109593-62-RGRL from Amfar, and the HH Sheikh Khalifa Bin Zayed Al-Nahyan named professorship from Mayo Clinic. ADB is a consultant for Abbvie, is on scientific advisory boards for Nference and Zentalis, and is founder and President of Splissen therapeutics. WK reports grants from AstraZeneca, grants from Biogen, grants from Roche, outside the submitted work. One or more of the investigators from the Mayo Clinic associated with this project and Mayo Clinic have a Financial Conflict of Interest in the technology used in the research and that the investigator(s) and Mayo Clinic may stand to gain financially from the successful outcome of the research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies. The authors on this manuscript from nference have equity in nference and have a financial interest in nference.
Footnotes
Edited by C. Agrati
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Samir Awasthi, Tyler Wagner
Supplementary information
The online version contains supplementary material available at 10.1038/s41420-021-00429-9.
References
- 1.Wagner, T. et al. Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID-19 diagnosis. 10.1101/2020.04.19.20067660. [DOI] [PMC free article] [PubMed]
- 2.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P, et al. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Studies from the World Health Organization Database—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/who_table.
- 5.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kewan T, et al. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. University of Oxford. http://www.ox.ac.uk/news/2020-06-16-low-cost-dexamethasone-reduces-death-one-third-hospitalised-patients-severe.
- 9.Barnes PJ. Glucocorticosteroids: current and future directions. Br. J. Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematol. Am. Soc. Hematol. Educ. Program. 2016;2016:366–378. doi: 10.1182/asheducation-2016.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Staa TP, et al. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 13.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatakrishnan, A. J. et al. Knowledge synthesis of 100 million biomedical documents augments the deep expression profiling of coronavirus receptors. Elife9 (2020). [DOI] [PMC free article] [PubMed]
- 15.Ziegler CGK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leventhal SM, et al. Uncovering a multitude of human glucocorticoid receptor variants: an expansive survey of a single gene. BMC Genet. 2019;20:16. doi: 10.1186/s12863-019-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aranda CJ, et al. Intestinal epithelial deletion of the glucocorticoid receptor NR3C1 alters expression of inflammatory mediators and barrier function. FASEB J. 2019;33:14067–14082. doi: 10.1096/fj.201900404RR. [DOI] [PubMed] [Google Scholar]
- 20.Sefton C, et al. Elevated hypothalamic glucocorticoid levels are associated with obesity and hyperphagia in male mice. Endocrinology. 2016;157:4257–4265. doi: 10.1210/en.2016-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao M, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 22.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olnes MJ, et al. Effects of systemically administered hydrocortisone on the human immunome. Sci. Rep. 2016;6:23002. doi: 10.1038/srep23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinehart JJ, Balcerzak SP, Sagone AL, LoBuglio AF. Effects of corticosteroids on human monocyte function. J. Clin. Investig. 1974;54:1337–1343. doi: 10.1172/JCI107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshimi K, Gonda N, Sumiya M, Kano S. Effects of corticosteroids on natural killer cell activity in systemic lupus erythematosus. Clin. Exp. Immunol. 1980;40:83–88. [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrchen JM, Roth J, Barczyk-Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front. Immunol. 2019;10:2028. doi: 10.3389/fimmu.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teuwen, L.-A., Geldhof, V., Pasut, A. & Carmeliet, P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 10.1038/s41577-020-0343-0 (2020). [DOI] [PMC free article] [PubMed]
- 29.Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 10.1056/NEJMoa2015432 (2020). [DOI] [PMC free article] [PubMed]
- 30.Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur. J. Immunol. 1990;20:2439–2443. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe AH. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol. Rev. 2017;276:5–8. doi: 10.1111/imr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—preliminary report. N. Engl. J. Med. 10.1056/NEJMoa2007764 (2020). [DOI] [PubMed]
- 33.Wang Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulware DR, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horby, P. et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. Infect Dis (except HIV/AIDS) 10.1101/2020.06.22.20137273 (2020).
- 36.Stone, J. H. et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 10.1056/NEJMoa2028836 (2020). [DOI] [PMC free article] [PubMed]
- 37.De Luca G, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119:3383–3393. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study were accessed under an IRB approved by the Mayo Clinic and were analyzed in consideration of HPAA regulations to protect patient privacy. Per the HIPAA act and Mayo Clinic’s policies for IRB review, all data availability requests should be directed to the corresponding author and will be directed to the Mayo Clinic colleagues for processing on a case-by-case basis.