Abstract
Background
The pathology of Parkinson’s disease leads to morphological changes in brain structure. Currently, the progressive changes in gray matter volume that occur with time and are specific to patients with Parkinson’s disease, compared to healthy controls, remain unclear. High-tesla magnetic resonance imaging might be useful in differentiating neurological disorders by brain cortical changes.
Purpose
We aimed to investigate patterns in gray matter changes in patients with Parkinson’s disease by using an automated segmentation method with 7-tesla magnetic resonance imaging.
Material and Methods
High-resolution T1-weighted 7 tesla magnetic resonance imaging volumes of 24 hemispheres were acquired from 12 Parkinson’s disease patients and 12 age- and sex-matched healthy controls with median ages of 64.5 (range, 41–82) years and 60.5 (range, 25–74) years, respectively. Subgroup analysis was performed according to whether axial motor symptoms were present in the Parkinson’s disease patients. Cortical volume, cortical thickness, and subcortical volume were measured using a high-resolution image processing technique based on the Desikan-Killiany-Tourville atlas and an automated segmentation method (FreeSurfer version 6.0).
Results
After cortical reconstruction, in 7 tesla magnetic resonance imaging volume segmental analysis, compared with the healthy controls, the Parkinson’s disease patients showed global cortical atrophy, mostly in the prefrontal area (rostral middle frontal, superior frontal, inferior parietal lobule, medial orbitofrontal, rostral anterior cingulate area), and subcortical volume atrophy in limbic/paralimbic areas (fusiform, hippocampus, amygdala).
Conclusion
We first demonstrated that 7 tesla magnetic resonance imaging detects structural abnormalities in Parkinson’s disease patients compared to healthy controls using an automated segmentation method. Compared with the healthy controls, the Parkinson’s disease patients showed global prefrontal cortical atrophy and hippocampal area atrophy.
Keywords: Parkinson’s disease, 7-tesla magnetic resonance imaging, cortex, subcortex, atrophy
Introduction
Widespread cortical and subcortical atrophic changes in Parkinson’s disease (PD) patients have been observed in several structural magnetic resonance imaging (MRI).1–6 These results have been confirmed by pathological studies, which have shown that the degenerative process is first detectable in the brain stem, subsequently extends into the limbic system and finally progresses to increasingly involve the cerebral cortex.7
In recent years, there has been growing interest in gray matter (GM) volume and white matter (WM) density changes in PD patients, including voxel-based morphometry and surface-based techniques. These methods have been used to assess cortical GM changes in patients with PD. Several cross-sectional studies have been conducted to compare the differences between patients with PD and healthy controls (HCs). However, the results of these studies are inconsistent. These inconsistencies may result from the inclusion of a small number of subjects from single centers; heterogeneity in patient characteristics, including age, disease duration, and disease severity; and differences in the variable covariates used in volumetric analyses, which may confound the results of between-group differences. Therefore, brain volume changes in patients with PD need to be examined further. 7-tesla (T) MRI is useful in detecting structural changes in cortical as well as subcortical regions. A stronger static magnetic field leads to a higher signal-to-noise ratio, which reduces the measurement time and increases the spatial resolution, as well as improves the image contrast of tissues because of increased sensitivity to susceptibility.
Currently, 7-T MRI is used to study patients with PD. However, the automated segmentation method has not yet been reported. Thus, in this study, we investigated the parcellation of brain structures and provided corrected reconstructions in high-resolution images taken using 7-T MRI. We evaluated the volume of several cerebral regions on MRI scans in patients with PD and HCs by using automated segmentation software.8 We hypothesized that PD patients would exhibit characteristic cortical and subcortical atrophy compared to HC which has not been seen in previous studies.
Material and Methods
Participants
The patients were recruited from Chungbuk National University Hospital. All patients were examined by a movement disorder specialist (YS Park). PD was diagnosed clinically on the basis of the United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria for PD.9 All PD patients had a positive response to levodopa. The severity and stage of parkinsonism in each patient was assessed by the modified Hoehn and Yahr (H–Y) scale10 and the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating scale (MDS-UPDRS)11 in the “on” state.
Twelve patients diagnosed with PD (median age = 64.5 years; range = 41–82 years) and 12 age- and sex-matched HCs (median age = 60.5 years; range = 25–74 years) were enrolled in this study.
Subgroup analysis was performed according to whether axial motor symptoms were present. We defined the presence of axial symptoms when they have two or more points in the composite score of six items in the MDS-UPDRS III suggestive axial motor features: speech, rigidity of the neck, arising from a chair, posture, gait, and postural stability.
Individuals with MRI signals of abnormal intensity, a history of other neuropsychiatric disorders, alcohol or drug abuse, and clinically significant systemic diseases were excluded. The informed consent was obtained from all participants. All study protocols were approved by the Institutional Review Board of Chungbuk National University Hospital (No. 2016-12-009-005), and informed consent was obtained from all participants.
MRI acquisition
Magnetic resonance images from all subjects were acquired using a 7-T MRI system (Philips Healthcare, Cleveland, OH, USA) with a 32-channel head coil (Nova Medical, Wilmington, MA, USA). Three-dimensional anatomical brain scans were acquired using a magnetization-prepared rapid-acquisition gradient-echo sequence-induced T1-weighted imaging system with the following settings: repetition time (TR) = 4.4 ms, echo time (TE) = 2.2 ms, slice thickness = 0.5 mm, in-plane resolution = 0.5 × 0.5 mm2, matrix size = 432 × 432, number of axial slices = 432, and acquisition time (TA) = 5 min 57 s.
Image analysis
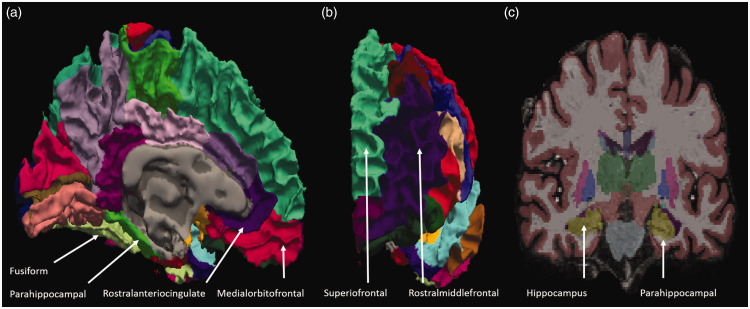
To acquire surface-based high-resolution images, modifications to the default reconstruction settings were required, and the nonparametric, nonuniform intensity, normalization (N3) algorithm12 for inhomogeneity correction for 7-T MRI was used. Cortical reconstruction and volumetric segmentation were carried out using FreeSurfer software version 6.0. After cortical reconstruction, the GM cortex was parcellated with allocations for labeling with the Desikan-Killiany-Tourville (DKT) neuroanatomical atlas, which divided the cortex into 31 cortical regions per hemisphere, as shown in Fig. 1.
Fig. 1.
Representative MRI images taken using 7-T MRI. (a) Brain structures from the medial aspect view, (b) anterior aspect view, and (c) coronal view of patients with PD in images taken with 7-T MRI and processed with N3 algorithms. The gray matter (GM) thickness of patients with PD was calculated using these algorithms.
Statistical analysis
Statistical evaluations were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). The data are shown as the means ± SDs. Normality tests were done using Kolmogorov–Smirnov method. The independent t test was applied when samples had a standard normal distribution, while two- or multiple-independent samples nonparametric test (Mann–Whitney U test) was applied when samples did not have a standard normal distribution. We used the chi-squared test to examine differences between categorical variables. Effect sizes are reported in terms Cohen’s d where appropriate. The threshold for significant differences was a P-value of < 0.05.
Results
Clinical characterization and demographics
There were no significant differences in age, sex, or disease duration between the PD and HC groups. In the subgroup analysis, there was no significant difference in age, sex, disease duration, the MDS-UPDRS III score, or the H–Y stage between the “PD with axial symptoms” group and the “PD without axial symptoms” group. Detailed characteristics are shown in Tables 1 and 2.
Table 1.
Demographic and clinical characteristics of Parkinson’s disease (PD) patients and healthy controls (HCs).
| Parkinson’s disease (N = 12) | Healthy controls (N = 12) | P-values | |
|---|---|---|---|
| Age (years)a | 64.50 ± 10.53 | 57.92 ± 13.10 | 0.19 |
| Sex (males/females)b | 3/9 | 2/10 | 1.00 |
| Disease duration (year) | 3.42 ± 1.68 | ||
| MDS-UPDRSIIIc | 28.33 ± 14.56 | ||
| H–Y | 2.38 ± 0.48 |
MDS-UPDRS: Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating scale; H–Y: Hoehn and Yahr scale.
Age was compared using an independent t test.
Sex was assessed using Fisher’s exact test.
P < 0.05.
Note: UPDRS was measured during the “on” state regarding PD medication.
Table 2.
Demographic and clinical characteristics of Parkinson’s disease patients with and without axial symptoms.
| PD with axial Sx. (N = 5) | PD without Axial Sx. (N = 7) | P-values | |
|---|---|---|---|
| Age (years)a | 66.00 ± 10.42 | 63.43 ± 11.30 | 1.00 |
| Sex (males/females)b | 4/1 | 5/2 | 1.00 |
| Disease duration (year) | 4.40 ± 1.14 | 2.71 ± 1.70 | 0.11 |
| MDS-UPDRSIIIc | 35.20 ± 19.11 | 23.43 ± 8.85 | 0.27 |
| H–Y | 2.60 ± 0.22 | 2.21 ± 0.57 | 0.27 |
MDS-UPDRS: Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating scale; H–Y: Hoehn and Yahr scale.
Age, duration, and the MDS-UPDRS III and H–Y scales were compared using the Mann-Whitney U test.
Sex was assessed using Fisher’s exact test.
P < 0.05.
Note: UPDRS was measured during the “on” state regarding PD medication.
Comparison of cortical volume between the patients with PD and HCs
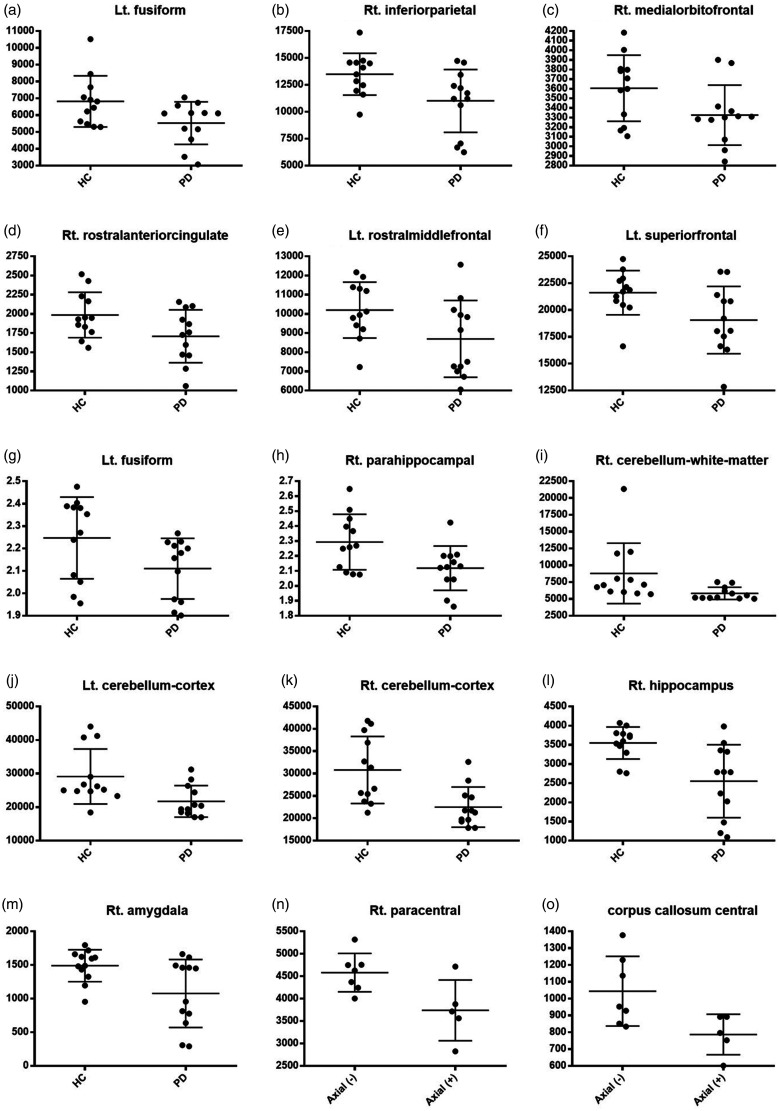
The patients with PD showed significant global cortical volume loss not only in prefrontal areas, including the left (Lt.) rostral middle frontal (P = 0.047, Cohen’s d = 0.86), Lt. superior frontal (P = 0.028, Cohen’s d = 0.96), right (Rt.) rostral anterior cingulate (P = 0.046, Cohen’s d = 0.86), and Rt. medial orbitofrontal areas (P = 0.049, Cohen’s d = 0.85), but also in other areas, including the Lt. fusiform (P = 0.034, Cohen’s d = 0.92) and Rt. inferior parietal areas (P = 0.023, Cohen’s d = 1.00). There were no significant differences in any of the other gyral-based regions of interest (ROIs) in either hemisphere according to the DKT atlas (Table 3, Fig. 2(a) to (f)).
Table 3.
Comparison of cortical volume, cortical thickness, and subcortical volume between the PD and HC groups.
|
Parkinson’s disease (N = 12) |
Healthy controls (N = 12) |
P-values |
||||
|---|---|---|---|---|---|---|
| Left hemi | Right hemi | Left hemi | Right hemi | Left hemi | Right hemi | |
| Comparison of cortical volume between the PD and HC groups | ||||||
| Fusiform | 5529.17 | 5820.75 | 6817.08 | 6114.92 | *0.034 | NS |
| Inferior parietal | 10,132.25 | 11,011 | 11,417.58 | 13,497.42 | NS | *0.023 |
| Medial orbitofrontal | 3591.58 | 3325.67 | 3788 | 3605.58 | NS | *0.049 |
| Rostral anterior cingulate | 2744.25 | 1707.25 | 2927 | 1985.08 | NS | *0.046 |
| Rostral middle frontal | 8694.33 | 8892.33 | 10,198.83 | 9685.67 | *0.047 | NS |
| Superior frontal | 19,067.33 | 22,063.58 | 21,613.92 | 23,243.42 | *0.028 | NS |
| Comparison of cortical thickness between the PD and HC groups | ||||||
| Fusiform | 2.11025 | 2.22325 | 2.24717 | 2.24958 | *0.049 | NS |
| Parahippocampal | 2.24175 | 2.11825 | 2.24608 | 2.29317 | NS | *0.018 |
| Comparison of subcortical volume between the PD and HC groups | ||||||
| Cerebellum-white-matter | 7082.28 | 5829.66 | 9892.57 | 8338.94 | NS | *0.045 |
| Cerebellum-cortex | 21,729.63 | 22,506.33 | 27,758.12 | 29,117.14 | *0.013 | **0.004 |
| Hippocampus | 3210.77 | 2549.78 | 3327.99 | 3513.46 | NS | **0.005 |
| Amygdala | 1152.23 | 1076.71 | 1274.57 | 1462.43 | NS | *0.021 |
NS: not significant; PD: Parkinson’s disease; HC: healthy control.
Notes: The PD and HC groups were compared using the independent t test, and nonparametric data analysis was performed using the Mann–Whitney U test. The thresholds of significance were as follows: *P < 0.05, *P < 0.01, and **P < 0.001.
Fig. 2.
Comparison of GM volume between PD patients and controls, (a) to (f); comparison of GM thickness between PD patients and controls, (g) and (h); comparison of subcortical volume between PD patients and controls, (i) to (m); comparison of GM volume between PD patients with and without axial symptoms, (n); and comparison of subcortical volume between PD patients with and without axial symptoms, (o).
Lt.: left; HC: healthy control; PD: Parkinson’s disease; Rt.: right.
Comparison of cortical thickness between the patients with PD and HCs
The patients with PD showed significant cortical thinning in the limbic/paralimbic areas: Lt. fusiform (P = 0.049, Cohen’s d = 0.85) and Rt. parahippocampal areas (P = 0.018, Cohen’s d = 1.04). There were no significant differences in any of the other gyral-based ROIs in either hemisphere according to the DKT atlas (Table 3, Fig. 2(g) and (h)).
Comparison of subcortical volume between the patients with PD and HCs
Subcortical atrophy around limbic/paralimbic areas was observed. The volumes of the Rt. hippocampus (P = 0.005, Cohen’s d = 1.36) and Rt. amygdala (P = 0.021, Cohen’s d = 1.05) were smaller in the patients with PD than in the HCs. The patients with PD showed significant volume loss in the Lt. cerebellar cortex (P = 0.013, Cohen’s d = 1.11), Rt. cerebellar WM (P = 0.045, Cohen’s d = 0.92), and Rt. cerebellar cortex (P = 0.004, Cohen’s d = 1.34). Other regions showed no significant differences in any of the other gyral-based ROIs in either hemisphere according to the DKT atlas (Table 3, Fig. 2(i) to (m)).
Comparison between the groups of PD patients with and without axial motor symptoms
The PD patients with axial symptoms showed significant cortical and subcortical atrophy in the Rt. paracentral area (P = 0.03, Cohen’s d = –1.49) and corpus callosum central area (P = 0.03, Cohen’s d = –1.52). There were no significant differences in any of the other gyral-based ROIs in either hemisphere according to the DKT atlas (Fig. 2(n) and (o)).
Discussion
We have found that 7-T MRI detects structural abnormalities in PD patients compared to HCs using an automated segmentation method. Previous studies on cortical atrophy in PD patients have reported that various parts of the brain are affected. However, the studies have yielded inconclusive findings,13,14 and earlier longitudinal studies have assessed only global measures such as whole brain atrophy rates.15 To date, the only two longitudinal studies on cortical thickness have been conducted, and they are limited by their relatively small sample sizes16 and heterogeneous PD cohorts.17
Nevertheless, there are some patterns in atrophy: limbic/paralimbic areas were affected in the early stages of PD, while neocortical areas, such as the temporal and frontal cortices, became more affected as disease severity increased.
To date, the MRI detection rate of purely intracortical lesions has been disappointingly low.18,19 However, 7-T MRI is feasible and can detect cortical abnormalities fairly well.20–23 In patients with PD, which is long-standing chronic progressive disease, subtle volumetric changes can be detected better by 7-T MRI than by conventional MRI in accordance with the DKT protocol. Most of the current research is related to hardware improvements and sequence optimization for diagnostic examinations to overcome limitations associated with inhomogeneous transmit fields, a large amount of artifacts, and poor absorption rate.24,25
Regarding inhomogeneity, 7-T MRI negatively impacts structure analysis. The N3 method may be a useful tool for bias field correction to overcome limitations in 7-T MRI analysis. Intensity inhomogeneity correction is a necessary preprocessing step to obtain better image segmentation.26
Our methods lie in developing methods for normalizing images to ensure that patients with PD could be compared with HCs and subgroups of patients with PD could be compared. This method consisted of the N3 algorithm, which was quantified by a comparison with the standardized area through human brain mapping, and reduced bias.
The DKT27 adult cortical parcellated atlas is the successor to the Desikan-Killiany atlas, from which some regions with unclear or arbitrary boundaries were removed and many existing boundaries were revised to conform to sulcal fundi. This modified version provides greater anatomical consistency across individuals because the landmarks are clearer and more reproducible. The use of sulcal-based landmarks also optimizes the applicability of surface-based labeling, such as that performed in FreeSurfer.8
Any automated labeling method can be used to initialize the labels for further editing by a human.27 In this study, FreeSurfer performed well for registration-based labeling, as its classifier uses geometric properties, such as local curvatures, that are similar to those of our DKT protocol, and it offers a good interface for editing labels.28
Neurodegeneration is expected to involve the prefrontal cortex in Braak’s stage 4/5.29 Structural changes in the frontal lobe have been hypothesized to contribute to dysexecutive syndrome,4,30,31 which is the hallmark symptom of cognitive impairment in early PD patients and has been attributed to functional derangement in specific prefrontal cortex-striatal circuits.32 In our study, significant volume loss of the hippocampus and surrounding regions was observed in the PD patients.
Thus, through these neuronal loops, dopaminergic deficits can indirectly impair frontal functions. Although the caudate nucleus did not show a significant difference in our results, the results of our study are thought to indicate dopaminergic deficits in the fronto-striatal network of patients with PD.33,34
The cerebellum is increasingly being recognized to play an important role in the pathology of PD.35 Both functional and structural abnormalities of the cerebellum in PD patients have received an increasing amount of attention. Our findings are consistent with those in previous studies supporting cerebellar involvement in the pathology of PD.
The analysis of morphometric associations of GM with clinical subdomains in PD patients is a logical and powerful method of identifying functionally meaningful brain structural patterns that may inform PD biotypes. However, relatively little attention has been paid to studying the relationship between MRI GM intensity changes and clinical subdomains in PD patients. Furthermore, previous studies have shown inconsistent findings.36,37
In our study, the Rt. paracentral area and corpus callosum in the “PD with axial symptoms” group showed significant atrophy. Considering the function of these areas, a loss of WM integrity in the corpus callosum and paracentral atrophy may be associated with axial symptoms of PD.
We hypothesized that 7-T MRI could produce PD patient’s characteristic cortical and subcortical atrophy by using with DKT atlas and N3 algorithm. Although the sample size of this study is small, the results deserve consideration because no differences in these areas between the PD and HC groups were detected.
The present study has some limitations. First, the number of subjects in both the PD and HC groups was relatively small, which was the reason we used nonparametric, individual analyses instead of general linear model group analysis. We need to use larger sample sizes in future studies. Second, even though various normalization and correction methods were used, measurement imprecision due to changes in the position of the object with respect to the scanner can be pronounced if the structure being measured is relatively small and the imaging slices are fairly thick.38 Third, advanced PD patients were not included due to the high risk of severe motion artifacts and difficulty in performing long-term examinations; therefore, this study analyzed relatively mild symptoms of PD. Compared with individuals with mild stages of PD, those with advanced stages of PD can show more severe atrophy and atrophy in other areas. In future research, we should conduct longitudinal observations of changes in PD patients.
In conclusion, we observed that GM volume in global cortical and subcortical lesions is smaller in PD patients than in HCs according to 7-T MRI and high-resolution images, which were processed with the N3 algorithm for MRI inhomogeneity correction. This study is the first clinical study in which high-resolution MRI and a surface-based automated segmentation method was used to assess PD, a neurodegenerative disease. Compared with the HCs, the PD patients showed global prefrontal cortical atrophy, limbic/paralimbic area atrophy. Compared with conventional MRI, 7-T MRI with the DKT protocol can be more useful to detect long-standing subtle volumetric changes in earlier stages of PD.
Supplementary Material
Acknowledgments
We thank Hyun-Jeong Kim (PA) and KBSI members for allowing and setting up the 7-T MRI study. All images were obtained using an ultrahigh field 7-T MRI in KBSI.
Author roles: Conception and design: BHO, HCM, and YSP. Acquisition of data, analysis, and interpretation: BHO, HCM, CJC, and YSP. Writing of the article: BHO, HCM, YSP, and AK. Critical review of the article: BHO, HCM, and YSP. Final approval of the article for publication: YSP. Agreed to be accountable for all aspects of the work: BHO, HCM, and YSP.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the National Research Foundation of Korea (NRF-2016H1D5A1908909). This work was supported in part by Korea Basic Science Institute (KBSI) grant by T36800,T38401.
ORCID iDs: Hyeong C Moon https://orcid.org/0000-0002-2087-5352
Young S Park https://orcid.org/0000-0001-7685-6292
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Beyer MK, Janvin CC, Larsen JP, et al. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 2007; 78:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004; 127:791–800. [DOI] [PubMed] [Google Scholar]
- 3.Melzer TR, Watts R, MacAskill MR, et al. Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 2012; 83:188–194. [DOI] [PubMed] [Google Scholar]
- 4.Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 2005; 64:224–229. [DOI] [PubMed] [Google Scholar]
- 5.Summerfield C, Junque C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 2005; 62:281–285. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 2011; 68:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004; 318:121–134. [DOI] [PubMed] [Google Scholar]
- 8.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355. [DOI] [PubMed] [Google Scholar]
- 9.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988; 51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology 2001; 57:S11–S26. [PubMed] [Google Scholar]
- 11.Movement Disorder Society Task Force on Rating Scales for Parkinson’s D. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003; 18:738–750. [DOI] [PubMed] [Google Scholar]
- 12.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998; 17:87–97. [DOI] [PubMed] [Google Scholar]
- 13.Zarei M, Ibarretxe-Bilbao N, Compta Y, et al. Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2013; 84:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak E, Bergsland N, Dwyer MG, et al. Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: a combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am J Neuroradiol 2014; 35:2257–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton EJ, McKeith IG, Burn DJ, et al. Brain atrophy rates in Parkinson’s disease with and without dementia using serial magnetic resonance imaging. Mov Disord 2005; 20:1571–1576. [DOI] [PubMed] [Google Scholar]
- 16.Hanganu A, Bedetti C, Degroot C, et al. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 2014; 137:1120–1129. [DOI] [PubMed] [Google Scholar]
- 17.Ibarretxe-Bilbao N, Junque C, Segura B, et al. Progression of cortical thinning in early Parkinson’s disease. Mov Disord 2012; 27:1746–1753. [DOI] [PubMed] [Google Scholar]
- 18.Geurts JJ, Bo L, Pouwels PJ, et al. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 2005; 26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 19.Kilsdonk ID, Jonkman LE, Klaver R, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain 2016; 139:1472–1481. [DOI] [PubMed] [Google Scholar]
- 20.Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol 2010; 67:812–818. [DOI] [PubMed] [Google Scholar]
- 21.Tallantyre EC, Morgan PS, Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J Magn Reson Imaging 2010; 32:971–977. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen AS, Kinkel RP, Tinelli E, et al. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging 2012; 35:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluestein KT, Pitt D, Sammet S, et al. Detecting cortical lesions in multiple sclerosis at 7 T using white matter signal attenuation. Magn Reson Imaging 2012; 30:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Kolk AG, Hendrikse J, Zwanenburg JJ, et al. Clinical applications of 7 T MRI in the brain. Eur J Radiol 2013; 82:708–718. [DOI] [PubMed] [Google Scholar]
- 25.Theysohn JM, Maderwald S, Kraff O, et al. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA 2008; 21:63–72. [DOI] [PubMed] [Google Scholar]
- 26.Vovk U, Pernus F, Likar B. A review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans Med Imaging 2007; 26:405–421. [DOI] [PubMed] [Google Scholar]
- 27.Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 2012; 6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein A, Ghosh SS, Avants B, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage 2010; 51:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessa C, Lucetti C, Giannelli M, et al. Progression of brain atrophy in the early stages of Parkinson’s disease: a longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Hum Brain Mapp 2014; 35:3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruck A, Kurki T, Kaasinen V, et al. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry 2004; 75:1467–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rae CL, Correia MM, Altena E, et al. White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage 2012; 62:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams-Gray CH, Foltynie T, Brayne CE, et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 2007; 130:1787–1798. [DOI] [PubMed] [Google Scholar]
- 33.Foltynie T, Brayne CE, Robbins TW, et al. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 2004; 127:550–560. [DOI] [PubMed] [Google Scholar]
- 34.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol 2005; 18:149–154. [DOI] [PubMed] [Google Scholar]
- 35.Mirdamadi JL. Cerebellar role in Parkinson’s disease. J Neurophysiol 2016; 116:917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acharya HJ, Bouchard TP, Emery DJ, et al. Axial signs and magnetic resonance imaging correlates in Parkinson’s disease. Can J Neurol Sci 2007; 34:56–61. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Xing Y, Martin-Bastida A, et al. Patterns of grey matter loss associated with motor subscores in early Parkinson’s disease. Neuroimage Clin 2018; 17:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunter JL, Shiung MM, Manduca A, et al. Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging 2003; 18:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.