Abstract
Infections are increasingly recognized as a common trigger of autoimmune disease, including autoimmune encephalitis. A significant association is particularly shown between HSV-1 encephalitis (HSVE) and a post-infectious autoimmune encephalitis mediated by neuronal autoantibodies, most notably anti-N-methyl-D-aspartate receptor (NMDAR) antibodies. The clinical significance of these and other novel post-infectious autoantibodies has led to new diagnostic and treatment challenges for clinicians. Here we present a case of a 19-year-old female with premorbid psychiatric disease and neuropsychiatric sequelae from HSVE who presented over a year after her initial HSVE with behavioral changes and positive anti-NMDAR antibodies. The clinical challenges encountered during this case are explored in detail based on a review of the literature. Research is needed to help guide management in these complex clinical situations.
Keywords: autoimmune diseases of the nervous system, central nervous system viral diseases, central nervous system infections, encephalitis, central nervous system infections, neuroimmunology, clinical specialty
Introduction
A significant association is identified between Herpes Simplex Virus-1 encephalitis (HSVE) and a post-infectious autoimmune encephalitis mediated by autoantibodies, most significantly N-methyl-D-aspartate receptor (NMDAR) antibodies.1-3 It is shown that 10-30% of patients with HSVE will later develop anti-NMDA receptor antibodies in the serum and CSF.2-4 In a mouse model, 4 of 6 mice inoculated with HSV-1 later develop serum antibodies to NMDAR and decreased expression of NMDAR in the hippocampus.5 The exact pathophysiology underlying this phenomenon remains ill-defined, but evidence suggests that HSV-mediated neuroinflammation exposes host NMDARs to the immune system, triggering CNS autoimmunity.2,3,6,7
Auto-antibodies detected in the post-infectious period must be interpreted within the context of clinical presentation. Importantly, a small proportion of patients without suspected autoimmune encephalitis will also develop auto-antibodies following central nervous system infection.1,3,8 In 1 prospective cohort trial, neuronal surface auto-antibodies were detected in either the serum or CSF of 30% (11/34) of patients following HSVE without clinical signs of encephalitis.1 Studies have also shown that a percentage of patients will test positive for more than 1 auto-antibody following infection,1,3,8,9 posing the question of which, if any, of these auto-antibodies are truly pathogenic. It remains unknown if neuronal auto-antibodies in patients with atypical clinical presentations represent a wide phenotypic range or may be incidental in a subset of patients.
Here we present a challenging case of post-HSV NMDARE in a patient with comorbid psychiatric disease, extensive structural damage from her initial infection, and a nebulous timeline of symptom onset.
Case Presentation
A 19-year-old female with significant psychiatric comorbidities presented to a local hospital with altered mental status and seizures. Her past medical history was notable for psychiatric disease; including generalized anxiety disorder, panic attacks, agoraphobia, claustrophobia, self-mutilation behaviors, and depression. Her parents described behavioral and verbal disinhibition since a young age. She was withdrawn from school due to debilitating anxiety, but was not known to have cognitive deficits. There was no history of hallucinations, psychosis, or manic episodes.
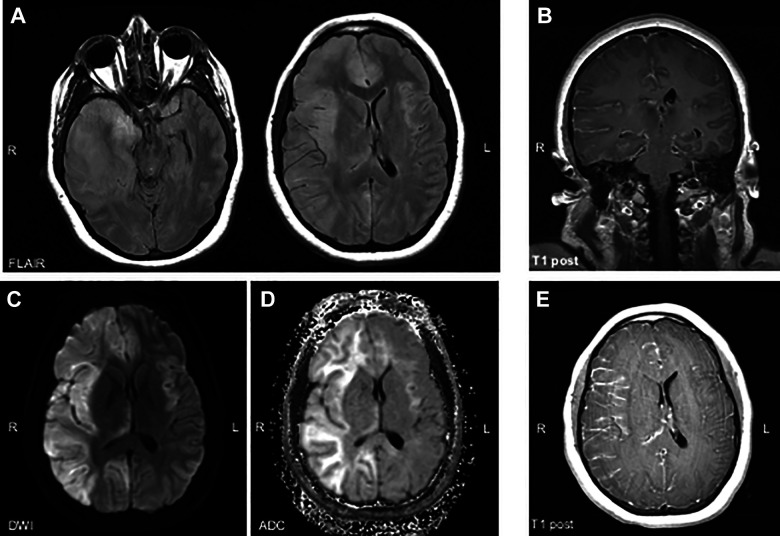
The patient’s presenting examination was notable for agitation, but otherwise non-focal and without meningismus. Her hospital course was complicated by fevers, headache, seizures, and progressive altered mental status. MRI of the brain showed partially enhancing FLAIR/T2 hyperintensities in the frontal and temporal lobes with right to left midline shift and diffuse leptomeningeal enhancement [ Figure 1 ]. She was intubated and empirically prescribed broad spectrum antibiotics, acyclovir, and intravenous antiepileptic medications for potential non-convulsive status epilepticus. She was then transferred to our hospital for further care.
Figure 1.
MRI at initial presentation demonstrated FLAIR hyperintensities in the right frontal, temporal, and parietal lobes with subsequent right to left midline shift and mild uncal herniation (A). There was high signal on DWI in these areas, which (B) most likely represented T2 shine-through, based on lack of ADC drop out (C). There was diffuse right greater than left leptomeningeal enhancement, demonstrated here on coronal (D) and axial (E) post-contrast images. FLAIR= fluid-attenuated inversion recovery; DWI= diffusion weighted imaging; ADC = Apparent diffusion coefficient; T1 post= T1 weighted image after contrast administration.
Upon transfer, lumbar puncture (LP) revealed an opening pressure (OP) of >55 cm H2O, 629 WBC /uL (70% lymphocytes, 17% Monocytes), 18 RBC /uL, glucose 54 (serum glucose 101) mg/dL, and protein 109 mg/dL; BioFire Diagnostics Inc. FilmArray Meningitis/Encephalitis PCR panel (M/E panel) was positive for HSV-1. All other infectious and inflammatory CSF studies returned negative; NMDAR antibodies were not tested at that time. After weaning of sedation, she had persistently poor mental status and intermittent loss of brainstem reflexes despite ICP management and acyclovir. Repeat imaging revealed herniation and she underwent emergent hemicraniectomy.
Following the procedure, she was intermittently able to follow simple commands, had intact cranial nerves/brainstem reflexes, but had developed left hemiplegia. This improved with time, but her mental status continually fluctuated. EEG showed generalized slowing and left rhythmic delta activity without seizures or epileptiform discharges while on levetiracetam monotherapy. Imaging several days into her course showed interval development of bilateral frontal lobe infarcts. CT angiogram demonstrated right middle cerebral artery and bilateral anterior cerebral artery stenosis. The differential included vascular compression from parenchymal inflammation and subfalcine herniation, and HSV-vasculitis.
The patient completed 21 days of IV acyclovir. She underwent cranioplasty 1 month after hemicraniectomy, which was complicated by subgaleal fluid collection requiring subgaleal to peritoneal shunt placement. The remainder of her hospital course was notable for persistent fevers, dysautonomia, and minor infections. With treatment, she became more alert and interactive, though developed fluctuating levels of agitation, restlessness, and confusion that required management with multiple antipsychotic medications and psychiatric liaison support. These symptoms were attributed to delirium, though her lack of participation with interview limited more thorough characterization. She was ultimately discharged to an acute traumatic brain injury (TBI) rehabilitation center.
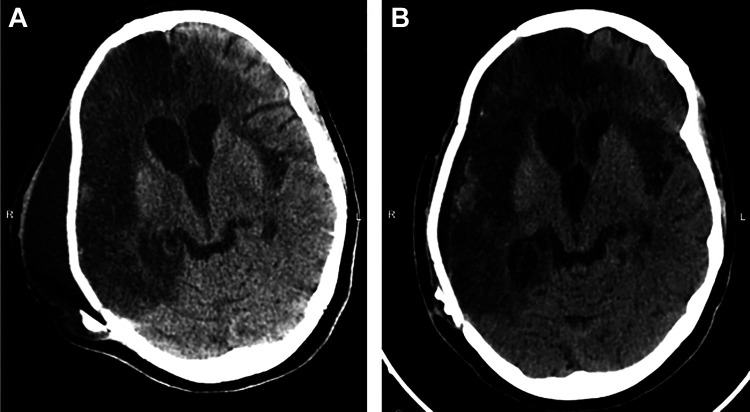
Approximately 13 months after her initial hospitalization, the patient was readmitted for progressive behavioral disturbances. While the patient had been intermittently agitated and impulsive after her initial hospitalization, both her family and the staff at the TBI facility noted a distinct change a month prior to presentation. In addition to worsening inattention, several new behaviors were described; including disobedience, recurrent nudity, delusions, and vandalism. Exam at the time of admission was notable for inattentiveness, pressured speech, verbal and behavioral disinhibition, hypersexuality, grandiose delusions, frontal release signs, and subtle left pyramidal signs. MRI of the brain and CT scan of the head performed after symptom onset was stable from a recent hospital discharge 6 months earlier [ Figure 2 ].
Figure 2.
CT head at most recent hospital discharge several months prior (A) in comparison to CT head upon readmission for behavioral changes (B). Findings were unchanged other than interval improvement of previous extra-axial fluid collection.
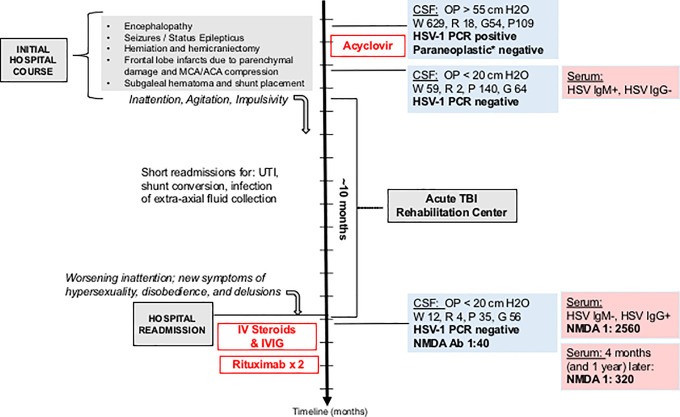
Upon readmission, LP showed an opening pressure <20 cm H2O, 4 RBC /uL, 12 WBC/uL, glucose 56 mg/dL (serum 100 mg/dL), protein 35 mg/dL, and M/E panel negative (including HSV-1). CSF anti-NMDA antibodies returned positive at a titer of 1:40 and serum anti-NMDA antibody titer of 1:2560 [ Figure 3 ]. CT scans and an abdominal ultrasound were negative for ovarian teratoma and other neoplasms. She was treated with a 5-day course of IVIg and intravenous methylprednisolone without improvement in symptoms, and received 2 doses of IV rituximab. Her levetiracetam was converted to valproate and she was prescribed additional antipsychotic, anxiolytic, and mood stabilization medications for symptomatic management.
Figure 3.
Timeline of clinical course with relevant diagnostic data and therapeutic interventions. Y axis scale (along timeline arrow)- 1 month per increment; IVIG= intravenous immunoglobulin; UTI= urinary tract infection; OP= opening pressure; W= white blood cells (/uL); R= red blood cells (/uL); G= glucose (mg/dL); P= protein(mg/dL); *Mayo Clinic Paraneoplastic (PAC1) panel.
Over the course of several months to a year after treatment, serum NMDA antibody titers decreased from 1:2560 to 1:320. CSF titers were not trended. No clinical change was observed despite treatment. Rituximab was discontinued and she remained clinically stable over a year off of immunosuppressive therapy at her last follow up.
Discussion
Only a small body of literature exists for patients with post-infectious causes of NMDARE. Clinical decisions in these patients are therefore often extrapolated from data in primary (e.g. paraneoplastic or idiopathic autoimmune) NMDARE. With that in mind, several features of this case fit the classic description of anti-NMDARE. Anti-NMDARE occurs most commonly in females (80%), young adults (median age 21), and immunocompetent individuals.3,9,10 Symptoms vary by age, but most commonly include: behavioral changes, psychosis, cognitive impairment, memory deficits, seizures, movement disorders, dysautonomia, altered awareness, and focal neurologic deficits.1,3,11,12 In young adults, the most common presenting symptom is abnormal behavior and cognition.12 Accordingly, this patient’s exam was notable for symptoms of disinhibition, delusions, pressured speech, and hypersexuality. Her CSF also demonstrated the typical findings of a low grade lymphocytic pleocytosis and CSF-specific oligoclonal bands. Most importantly, she was found to have IgG antibodies against the NR1 subunit of NMDAR in both the serum and CSF., which is shown to be both sensitive and specific.13 Overall, this favored true disease, with the degree of positive serum titers suggesting poor prognosis.14
Two features of this case are important to point out. While most cases of NMDARE following HSV infection occur 1-3 months after the initial infection,1,3 this patient presented over a year later [ Figure 3 ]. In addition, despite over a month without treatment prior to hospitalization, she did not progress to develop additional symptoms. Previous multi-institutional observational studies found that 90% of patients with primary NMDARE left untreated will meet 4 or more typical symptom categories, while only 1% will remain mono-symptomatic.12 However, this has not been studied in populations of post-infectious NMDARE where isolated psychiatric and cognitive symptoms may be more common.
While these features may represent an atypical presentation of disease, they could also be explained by an alternative timeline. It is possible that several of the symptoms attributed to complications of HSVE during the patient’s initial hospitalization (e.g. dysautonomia, fluctuating mental status, behavioral changes) were actually manifestations of undiagnosed NMDARE. Her CSF was evaluated for persistent HSV at that time, but NMDAR antibodies were not sent. This highlights the importance of considering this diagnosis in a patient with lack of recovery or new symptoms weeks to months out from an HSV infection.
The patient discussed here had no improvement of symptoms despite treatment with steroids, IVIg, and rituximab. At this time, it remains unknown if patients with post-infectious NMDARE will respond to immunotherapy in the same way that patients with ovarian teratomas are shown to respond after tumor removal. Young patients and those without ovarian teratomas are found to have worse clinical outcomes and higher rates of relapse.3,14 While no formal treatment guidelines exist, first-line therapy for all forms of NMDARE usually consists of combination regimens of intravenous steroids, IVIg, and plasmapheresis. Second-line therapy involves rituximab, with potential escalation to cyclophosphamide.3,15 As patients without an identified teratoma are at high risk of relapse,6,12 some are prescribed oral immunosuppressive therapies for an additional year after achieving clinical improvement.6
After discontinuation of immunotherapy, our patient’s serum antibody titers decreased significantly. Prospective data regarding the clinical significance of antibody titers is currently lacking. While higher antibody titers at the time of diagnosis predict worse clinical outcomes, studies suggest that majority of cases will demonstrate a decrease in both CSF and serum titers regardless of clinical course.14 Recommendations are to use clinical progression and response to immunotherapy to guide treatment rather than antibody trends.3,12 In our case, it was not clear if the patient’s lack of improvement was due to refractory disease, confounding pre-morbid psychiatric disease, or extensive structural damage [ Figure 2 ]. Despite discontinuation of treatment, she had no clinical progression nor change in antibody titers at 1 year.
Research and long-term follow up data are needed to better guide the diagnosis and appropriate management of patients with post-infectious NMDARE in the context of complex clinical situations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial Disclosures: Samantha Epstein, MD: reports no disclosures.
Jyoti Ankam, MBBS, MPH: reports no disclosures.
Wendy Vargas, MD has served as a consultant for Biogen Idec Inc., Octapharma, and Alexion Pharmaceuticals.
Kiran T Thakur, MD has received NIH funding support: NIH/NINDS 1K23NS105935-01.
NIH/NICHD 1R01HD074944-01A1.
Informed consent was obtained from the patient’s health care proxy. This can be provided upon request.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Samantha Epstein, MD  https://orcid.org/0000-0003-1158-5430
https://orcid.org/0000-0003-1158-5430
Kiran T. Thakur, MD  https://orcid.org/0000-0003-0050-0323
https://orcid.org/0000-0003-0050-0323
References
- 1. Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armangue T, Leypoldt F, Malaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75(2):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045–1057. [DOI] [PubMed] [Google Scholar]
- 4. Pruss H, Finke C, Holte M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72(6):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linnoila JJ, Pulli B, Armangué T, et al. Mouse model of anti-NMDA receptor post–herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm. 2019;6(2):e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavaliere E, Nosadini M, Pelizza MF, Ventura G, Toldo I, Satori S. Anti-NMDAR encephalitis preceded by non-herpetic central nervous system infection: systematic literature review and first case of tick-borne encephalitis triggering anti-NMDAR encephalitis. J Neuroimmunol. 2019;332:1–7. doi:10.1016/j.jneuroim.2019.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e245. doi:10.1212/NXI.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexopoulos H, Akrivou S, Mastroyanni S, et al. Postherpes simplex encephalitis: a case series of viral-triggered autoimmunity, synaptic autoantibodies and response to therapy. Ther Adv Neurol Disord. 2018;11:1756286418768778. doi:10.1177/1756286418768778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mann AP, Grebenciucova E, Lukas RV. Anti-N-methyl-D-aspartate-receptor encephalitis: diagnosis, optimal management, and challenges. Ther Clin Risk Manag. 2014;10:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. doi:10.1002/ana.21050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Coevorden-Hameete MH, Titulaer MJ, Schreurs MW, de Graaff E, Sillevis Smitt PA, Hoogenraad CC. Detection and characterization of autoantibodies to neuronal cell-surface antigens in the central nervous system. Front Mol Neurosci. 2016;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2013;13(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin YW, Lee ST, Park KI, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2017;11:1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]