Abstract
Enteroendocrine cells such as duodenal cholecystokinin (CCK cells) are generally thought to be confined to certain segments of the gastrointestinal (GI) tract and to store and release peptides derived from only a single peptide precursor. In the current study, however, transgenic mice expressing enhanced green fluorescent protein (eGFP) under the control of the CCK promoter demonstrated a distribution pattern of CCK-eGFP positive cells that extended throughout the intestine. Quantitative PCR and liquid chromatography-mass spectrometry proteomic analyses of isolated, FACS-purified CCK-eGFP-positive cells demonstrated expression of not only CCK but also glucagon-like peptide 1 (GLP-1), gastric inhibitory peptide (GIP), peptide YY (PYY), neurotensin, and secretin, but not somatostatin. Immunohistochemistry confirmed this expression pattern. The broad coexpression phenomenon was observed both in crypts and villi as demonstrated by immunohistochemistry and FACS analysis of separated cell populations. Single-cell quantitative PCR indicated that approximately half of the duodenal CCK-eGFP cells express one peptide precursor in addition to CCK, whereas an additional smaller fraction expresses two peptide precursors in addition to CCK. The coexpression pattern was further confirmed through a cell ablation study based on expression of the human diphtheria toxin receptor under the control of the proglucagon promoter, in which activation of the receptor resulted in a marked reduction not only in GLP-1 cells, but also PYY, neurotensin, GIP, CCK, and secretin cells, whereas somatostatin cells were spared. Key elements of the coexpression pattern were confirmed by immunohistochemical double staining in human small intestine. It is concluded that a lineage of mature enteroendocrine cells have the ability to coexpress members of a group of functionally related peptides: CCK, secretin, GIP, GLP-1, PYY, and neurotensin, suggesting a potential therapeutic target for the treatment and prevention of diabetes and obesity.
Hormone-producing enteroendocrine cells are scattered throughout the gastrointestinal (GI) tract from the esophagus to the rectum, collectively constituting the largest and most diverse endocrine organ of the body (1–4). Enteroendocrine cells are generally flask shaped with an apical, microvillus-decorated sensory extension reaching the gut lumen and with dense core secretory granules at the base from which the gut hormones are released (5–7). About 15 different types of enteroendocrine cell types have been identified, and a similar, large number of peptide hormones have been assigned to each distinct cell type. Enteroendocrine cells were originally defined on the basis of distinct electron microscopic characteristics of their secretory granules (8). However, immunohistochemistry soon revealed that different enteroendocrine cell types could be distinguished by expression of distinct peptide hormones. For example, the so-called I-cells and S-cells of the duodenum were found to store cholecystokinin (CCK) and secretin, respectively (6, 9, 10), whereas enteroglucagon and later glucagon-like peptide 1 (GLP-1) were found in the L-cells of the ileum (11). At present, it is still generally assumed that each enteroendocrine cell type stores and releases peptide hormones derived from only a single peptide hormone precursor. Well-established exceptions to this concept are few and include L-cells, which costore and corelease GLP-1 and GLP-2 (both derived from posttranslational processing of proglucagon) and peptide YY (PYY) (12, 13).
Enteroendocrine cells differ from other peptide hormone- and neuropeptide-producing endocrine and neuronal cells in the body in that they continuously are renewed, with a generation time of only 5–7 d (14, 15). Importantly, all the enteroendocrine cell types, as well as all other cell types of the GI tract epithelium, are derived from single pluripotent stem cells residing in the mucosal crypts. These stem cells divide to give rise to a pool of rapidly dividing transit-amplifying cells, which enter either an absorptive or a secretory lineage as they reach the upper crypt region (16–18). The enteroendocrine cells are derived from a secretory lineage that is dependent upon the expression of ngn3 (19). During maturation, most enteroendocrine cells, as well as goblet cells and absorptive enterocytes, migrate upward in the crypt, whereas Paneth cells migrate to the bottom of the crypt and have a longer generation time (1). Before leaving the crypts, enteroendocrine cells are believed to become terminally differentiated, such that each mature subtype produces only peptides derived from a single peptide hormone precursor. It was noted early on, however, that a subpopulation of enteroendocrine cells migrate downward in the crypts to reside close to the LGR5-positive progenitor and Paneth cells (20–22). These enteroendocrine cells located deep in the crypts are known to coexpress a broad selection of gut hormones: CCK, secretin, gastric inhibitor peptide (GIP), and neurotensin as well as stem cell markers (20). In contrast, the enteroendocrine cells, which migrate up into the villi, comply with the current one cell-one hormone dogma for enteroendocrine cells (1, 14, 15, 20).
The present study was undertaken to better characterize CCK cells, which previously had been thought to be limited to the duodenum. Using primarily material from transgenic reporter mice expressing enhanced green fluorescent protein (eGFP) under the control of the CCK promoter, we demonstrate both a more widely distributed expression pattern of CCK cells throughout the GI tract and coexpression of functionally related gut hormones (i.e. GLP-1, PYY, GIP, secretin, and neurotensin) with CCK. The coexpression was not limited to enteroendocrine cells in the crypts but was also found in presumably mature enteroendocrine cells of the villi. We did not detect coexpression of CCK with the functionally distinct peptide somatostatin. Expression of peptides derived from more than one preprohormone in the same enteroendocrine cells was further verified through a cell ablation study using transgenic mice in which the human diphtheria toxin receptor is expressed under the control of the glucagon promoter. Key elements of the coexpression were demonstrated also in the human small intestine by double staining immunohistochemistry.
Materials and Methods
Transgenic mice
Cck-eGFP transgenic mice were developed by the Gensat project (www.gensat.org) and obtained from the Mutant Mouse Regional Resource Center.
Humanized Renilla green fluorescent protein (Ghrelin-hrGFP) (line hrGFP10) mice were generated as previously described (23).
Gcg-hDTR mice were generated using a bacterial artificial chromosome (BAC) clone (RP23-343C17) in which the sequence coding for the human diphtheria toxin receptor was inserted at the start codon for Gcg. The mice were generated at DAGMAR (Danish Genetically Modified Animal Resource).
All animal studies were carried out in accordance with the Institutional Animal Care and Use Committees at the Universities of Copenhagen and Texas Southwestern Medical Center.
Cell isolation and FACS purification
Intestinal CCK cell isolation and purification
Cck-eGFP transgenic mice were euthanized and duodenal, jejunal, and ileal segments were inverted, inflated, and digested for 20 min with 0.13 Wünsch units of Liberase (Roche, Indianapolis, IN) in DMEM 1885 while being slowly shaken at 37 C. Every fifth minute the tissue was shaken vigorously for 5 sec. This procedure was repeated three times. Before sorting, the cells were slowly shaken for a second period of 20 min at 37 C, passed through a 70-μm pore diameter cell strainer, pelleted at 1500 rpm for 5 min, and resuspended in DMEM 1885 with 10% fetal bovine serum.
Fractionation of crypt vs. villus cells
Similar to the method developed by Stegmann et al. (24), we fractionated cells using the procedure described above; importantly, during the first 20-min period the vigorous shaking every fifth minute was replaced by a gentle inverting of the tube 10 times. Cells liberated during this first enzyme digestion were used as the villus fraction. Over the next 35 min the tissue was shaken vigorously every fifth minute and cells liberated were discarded. A final incubation for 20 min with fresh enzyme solution yielded the crypt fraction. The crypt and villus fractions were verified by QPCR for markers for the villus (Alpi and Apoa4) and crypt (Lyz1 and Defa5). Primers are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://end.endo.journals.org.
Cells were sorted as eGFP positive or eGFP negative using either a BD FACSAria II (BD Biosciences, Palo Alto, CA) or a MoFlo XDP (Beckman Coulter, Brea, CA). Cells were sorted on dry ice or into lysis buffer for RNA extraction or into 6m acetic acid for peptide analysis.
Gastric ghrelin cell isolation and FACS purification
hrGFP-positive and negative cells were isolated and FACS purified from ghrelin-hrGFP mice as previously described (23).
RNA extraction and quantitative RT-PCR analysis
Total RNA was extracted from 5000–30,000 cells using NucleoSpin RNA XS kit (Macherey-Nagel, Duren, Germany), and RT-PCR was performed using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Custom-designed StellARray qPCR arrays (Lonza Group, Basel, Switzerland) were assayed according to the manufacturer's instructions with SYBR Premix Ex Taq (TaKaRa, Otsu, Shiga, Japan) using a Mx3000P (Stratagene, La Jolla, CA) or a LightCycler480 (Roche). Target regions are listed in Supplemental Table 1.
To enable direct comparison of RNA levels from different genes, a genomic DNA sample containing all assayed transcripts was used as calibrator, and the data were further normalized using Ywhaz as reference gene as previously described (25).
Single-cell quantitative RT-PCR analysis
Single cells from duodenum were sorted directly into 10 μl 2 × RT buffer (100 mm Tris, 150 mm KCl, 6 mm MgCl2, 10 mm dithiothreitol) in 96-well plates using a BD FACS Aria II and stored at −80 C. cDNA was transcribed as follows: 5 μl H2O containing random primers (0.008 A260 U, Roche) were added to each well, heated to 70 C for 3 min, and cooled to 4 C before adding 10 μl H2O containing 0.05 mm deoxynucleotide triphosphates, 0.6 U RNaseOUT, 1 U SuperScript III (Invitrogen). Subsequently the samples were heated to 25 C for 5 min, 50 C for 60 min, and 70 C for 15 min. The cDNA was diluted five times in a final reaction volume of 12.5 μl and assayed on a LightCycler480 (Roche) using SYBR Premix Ex Taq (TaKaRa). Four genes were assayed from each cell. Ct values were calculated using the second derivative method. Ct values less than 40 were counted as positive, and non-RT samples were included to validate the specificity of the assay. To correlate expression of Cck with Sct, the Ct values were transformed to relative expression levels using the standard delta ct method (26) setting the average expression for each gene to 1.
A nonparametric Spearman correlation of the relative expression of Cck vs. Sct at single cell level was performed (GraphPad Software, San Diego, CA). Primers are listed in Supplemental Table 1.
Liquid chromatography-mass spectrometry (LC-MS)
Seventy-thousand cells obtained from FACS sorting of the small intestine were analyzed for natural occurring small peptide fragments without enzymatic treatment using LC-MS as previously described (27).
Immunohistochemistry
Intestinal tissue from Cck-eGFP transgenic mice
Tissue was fixed in freshly prepared 4% paraformaldehyde (24 h at 4 C), followed by 2 d cryoprotection (20% sucrose at 4 C). The tissues were embedded in Tissue-Tek (Sakura Finetek, Torrance, CA), snap frozen in isopentane and stored at −20 C. (8 μm) Sections were cut with a cryostat (cm3050 S, LEICA) and placed on glass slides to air dry for 1 h at room temperature, washed three times for 5 min. in PBS. Sections were preblocked using 2% BSA in PBS for 1 h and incubated overnight at 4 C with the primary antibodies (Supplemental Table 2). The primary antibodies were washed off (three times for 5 min, PBS) and replaced by secondary antibodies (Supplemental Table 2) and incubated 1 h at room temperature. Secondary antibodies were washed off, and PBS containing Hoechst stain (Thermo Scientific, Rockford, IL) was added for 5 min. The sections were washed (three times for 5 min) in PBS and coverslipped in mounting medium (S3023; DAKO Corp., Carpenteria, CA). Using a fluorescence microscope (Olympus IX70, Olympus Corp., Lake Success, NY), pictures were captured with a CMOS camera (ProgRes CT3 USB, Jenoptik).
Ablation study and immunohistochemistry on Gcg-hDTR transgenic mice
Transgenic Gcg-hDTR and WT mice (20 wk of age) received ip injections of diphtheria toxin (100 ng/g bodyweight) (Sigma Aldrich, St. Louis, MO). Mice were killed at time zero or 24 h after diphtheria toxin administration. Twenty cross-sections of distal ileum tissue were collected for each peptide and mice analyzed (five cross-sections from four interspaced positions). All cells staining positive for a given peptide were counted and finally normalized as counts per cross-section. The tissue was excised and submerged into freshly prepared 4% paraformaldehyde for 48 h at 4 C, stored in 70% alcohol, and paraffin embedded using a Shandon Excelsior (Thermo Fisher). Sections (4–6 μm) were cut and dried (60 C, 1 h) and stored at 4 C. Sections were deparaffinized, rehydrated, submerged, and boiled for 15 min in 0.01 mol/liter citrate buffer (pH 6.0). Tissue sections were permeabilized with PBST [PBS-Ca-Mg (pH 7.4) + 0.1% triton] three times for 2 min. Sections were preblocked using 2% BSA (15 min room temperature) and incubated overnight at 4 C with the primary antibodies (Supplemental Table 2), washed in PBS (three times 2 min), and incubated for 1 h with biotinylated secondary antibodies (Supplemental Table 2). Endogenous tissue peroxidase was blocked using 3% H2O2 in PBS for 8 min followed by 30-min incubation in vectastain reagents (Vector Laboritories, Burlingame, CA) and 15-min incubation with freshly made 3,3 diaminobenzidine solution (Kementec Diagnostics, Copenhagen, Denmark). Aqueous solution of CuSO4 in TNT buffer (0.1 m Tris-HCl, 0.15 m NaCl, and 0.05% Tween 20) was applied for 1 min to enhance coloration. Slides were counterstained with Mayer's hematoxylin (Ampliqon, Odense, Denmark), dehydrated in alcohol, and coverslipped with Pertex (catalog no. 00801; Histolab, Gothenburg, Sweden).
Immunohistochemistry of human small intestinal tissue
Untraceable historical archival samples of normal human intestinal tissues that were originally collected during surgery of the GI tract were used. Immunohistochemistry in these paraffin-embedded tissues were performed in a manner similar to that described above for mice tissues.
Results
Immunohistochemical analysis of eGFP expression in CCK-eGFP mice
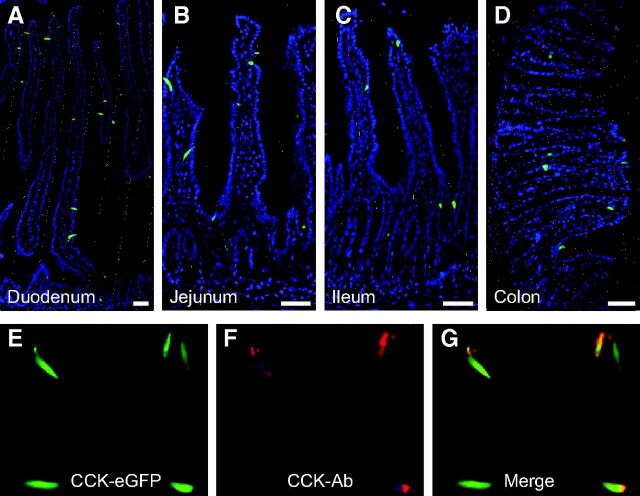
CCK has previously been identified as mainly a duodenal hormone. Accordingly, eGFP expression was found in classical enteroendocrine-like cells scattered in the duodenal mucosa from CCK-eGFP transgenic mice. These CCK-eGFP-positive cells were located both in the crypts and villi (Fig. 1A). As in previous reports, the majority of these duodenal eGFP-positive cells contained CCK immunoreactive material (28–30). A relatively large population of CCK-eGFP-positive enteroendocrine cells was observed in the jejunum, and surprisingly, also in the ileum and colon, where classical CCK cells were not expected to be found in great numbers (Fig. 1, B–D). A small fraction of the CCK-eGFP-positive enteroendocrine cells did not contain CCK immunoreactivity (Fig. 1, E–G); these were characterized further by single-cell QPCR analysis (see below).
Fig. 1.
Distribution of eGFP-positive enteroendocrine cells in the GI tract of CCK-eGFP transgenic mice. Panels A–D demonstrate localization of CCK promoter-driven eGFP fluorescence (green) within duodenal (A), jejunal (B), ileal (C), and colonic (D) mucosa along its full glandular extent from the crypts (bottom) to the villus tips. General architecture of the mucosa is visualized by counterstaining with Hoechst nuclei staining (blue). Panels E–G demonstrate localization of CCK promoter-driven eGFP fluorescence (E) with CCK immunoreactivity (F) and merged (G) in jejunum. Bar, 50 μm. Ab, Antibody.
QPCR analysis of isolated FACS-purified CCK-eGFP positive cells
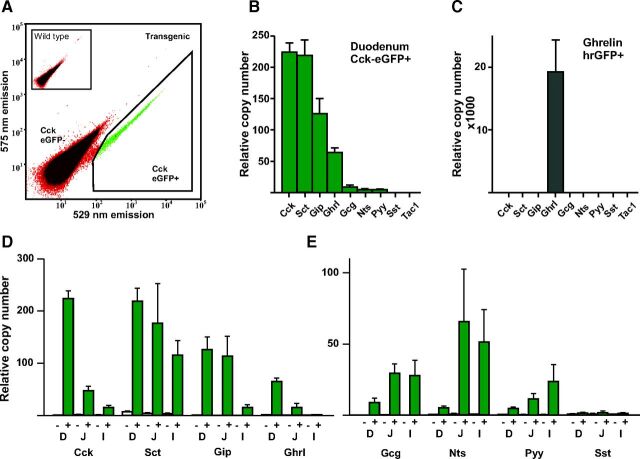
To characterize the CCK-eGFP-positive cells in more detail, single cell preparations were generated through enzymatic digestion of mucosa from different segments of the small intestine, followed by FACS (Fig. 2A). The CCK-eGFP-positive cells constituted on average 0.2% of cells released from the duodenal mucosa by enzymatic digest. After FACS sorting, the CCK-eGFP-positive cells were more than 90% pure as judged by microscopy. QPCR analysis revealed that the CCK-eGFP-positive cells purified from duodenum expressed RNA coding for the CCK precursor (Cck), as expected (Fig. 2B). The expression of Cck was enriched 250-fold in the CCK-eGFP-positive cells compared with the eGFP-negative cells (Fig. 2B). Unexpectedly, the CCK-eGFP-positive cells expressed equal amounts of RNA encoding the secretin precursor (Sct) as that encoding Cck. They also expressed relatively large amounts of Gip and ghrelin (Ghrl) RNA, i.e. about half and one fourth of Cck RNA, respectively (Fig. 2B). RNA encoding the proglucagon/GLP-1 precursor (Gcg), the neurotensin (Nts) precursor, and the Pyy precursor were also detected in the CCK-eGFP-positive cells from duodenum albeit at relatively low copy numbers compared with Cck (Fig. 2B). In contrast, RNA encoding somatostatin and substance P precursors were barely detectable (Tac1 in Fig. 2B).
Fig. 2.
QPCR analysis of RNAs encoding peptide precursors in isolated, FACS-purified CCK-eGFP positive cells. Panel A, A representative FACS diagram showing the gate (black line surrounding green cells) used for sorting of the CCK-eGFP-positive cells from duodenum based on the green (529 nm) vs. yellow (575 nm) emission after excitation at 488 nm. Panels B and C show relative expression of Cck, Sct, Gip, Ghrl, Gcg, Nts, Pyy, Sst, and Tac. In CCK-eGFP-positive cells from duodenum (B) and ghrelin-hrGFP-positive cells isolated from stomach (C). Panel D shows the expression of the abundant peptide precursor RNAs Cck, Sct, Gip, and Ghrl in CCK-eGFP-positive cells from the duodenum (D) jejunum (J), and ileum (I), respectively. Panel E shows the expression of the scarce peptide precursor RNAs Gcg, Nts, Pyy and Sst in CCK-eGFP-positive cells from the duodenum (D), jejunum (J), and ileum (I), respectively.
A similar QPCR analysis was performed on FACS-purified CCK-eGFP positive cells from jejunum and ileum. As shown in Fig. 2D, the relative copy number of Cck was much lower in jejunum and ileum compared with duodenum, in accordance with the expected overall expression pattern for the CCK hormone. Similarly, the expression of Ghrl was much lower in cells from the jejunum compared with the duodenum and basically was not present in cells from the ileum (Fig. 2D). Gip RNA expression in the eGFP-positive cells was similar in jejunum as is duodenum, but much lower in the ileum. In contrast, secretin was expressed at almost the same level in the CCK-eGFP-positive cells from both jejunum, ileum, and duodenum. The expression of Gcg, Nts, and Pyy increased in jejunum and ileum compared with duodenum (Fig. 2E) (3). It should be noted that the expression profiles shown in Fig. 2, D and E, correspond to the expression of the RNA coding for these peptide precursors in the CCK-eGFP-positive cells, not their general expression profile in the mucosa as such.
Thus, although CCK is the most highly expressed peptide hormone at the RNA level in the CCK-eGFP-positive cells in the duodenum, a number of other peptides including secretin are expressed to a similar level within these cells. Unexpectedly, purified CCK-eGFP-positive cells from the jejunum and ileum express nearly the same amount or more of Sct, Nts, Gcg, and Pyy than Cck itself.
QPCR analysis of ghrelin-hrGFP positive cells from the stomach
To probe whether the relatively broad expression pattern of gut hormones is a phenomenon related to the BAC GFP reporter approach as such, we applied the QPCR array for peptide hormones to FACS-purified ghrelin cells isolated from the gastric mucosa of transgenic ghrelin-hrGFP mice prepared as previously reported (23). Similar to the CCK-eGFP line on which the current manuscript is focused, the ghrelin-hrGFP line also was generated by placing a GFP reporter construct under the control of ghrelin transcriptional control elements present in a BAC. As shown in Fig. 2C, a very different, clean expression profile was obtained in gastric ghrelin-hrGFP cells. More specifically, the expression of Ghrl was several orders of magnitude higher than the expression of any of the other genes encoding gut hormone precursors. The second most highly expressed gene was Sst with a copy number of 5.4, which is 0.1% of that of Ghrl (4200 copies).
It is concluded that the BAC GFP reporter gene approach, when applied to the rapidly renewed enteroendocrine cells of the GI tract, does not inherently lead to promiscuous labeling of multiple types of enteroendocrine cells.
Single-cell QPCR analysis of FACS-sorted CCK-eGFP cells
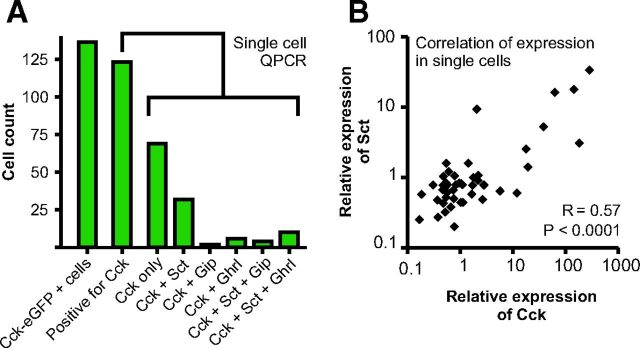
Single CCK-eGFP-positive cells isolated from the duodenum of transgenic mice were sorted individually into 96-well plates and subjected to QPCR analysis for Cck, Sct, Ghrl, and Gip gene expression without preamplification. We examined 135 FACS-separated cells in the eGFP-enriched pools (Fig. 3). Of these, 123 cells (or rather, 91%) were identified as expressing Cck RNA. Of those cells, 44% confirmed as being positive for Cck were shown to express RNA encoding one of the three other peptide hormones: i.e. 37% also expressed Sct (46 of 123), 13% also expressed Ghrl (16 of 123), and 5% also expressed Gip (6 of 123). Of the Cck-positive cells, 11% expressed RNA for three hormones: 8% coexpressed Cck, Sct, and Ghrl, and 3% coexpressed Cck, Sct, and Gip (Fig. 3A). None of the tested CCK-positive cells coexpressed all three of the other hormones. A positive correlation was found between the expression level for Cck and Sct (Fig. 3B).
Fig. 3.
Single-cell QPCR of the four most abundant peptide precursor RNAs (see Fig. 2) Cck, Sct, Gip, and Ghrl in FACS-sorted single CCK-eGFP-positive cells from duodenum. Panel A, In the unamplified QPCR analysis a Ct value less than 40 is considered to be positive for expression of the peptide precursor RNA (see Materials and Methods). The expression pattern for the 123 of the 135 CCK-eGFP-positive cells (far left column) which express Cck itself are shown in the green columns. Of the remaining 12 cells in which Cck expression could not be detected, five expressed Sct, four Ghrl, two Gip, and one both Sct and Ghrl. Panel B, Correlation of the relative expression of Cck and Sct in individual single cells, R = 0.57 (P < 0.0001), using nonparametric Spearman correlation analysis.
Thus, confirming the QPCR analysis of pooled populations of FACS-separated CCK-eGFP cells, single-cell QPCR of individual cells from those CCK cell-enriched pools demonstrates that a relatively large fraction of duodenal CCK-eGFP-positive cells express more than one hormone gene, of which Sct is dominant. However, only a relatively small fraction of the cells express more than two hormone genes.
Villus vs. crypt analysis of CCK-eGFP-positive cells
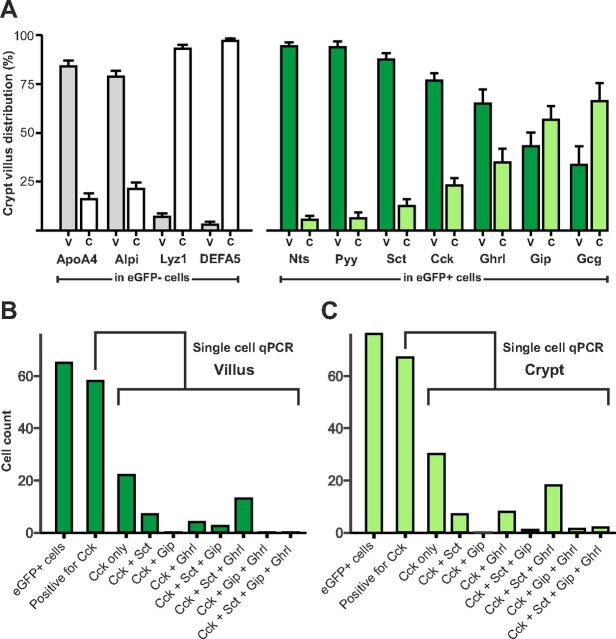
One explanation for the relatively broad repertoire of peptide hormones being expressed in the CCK-eGFP cells could be that this was mainly a phenomenon occurring in immature cells of the crypts. To assess this, populations of cells derived from duodenal villi or crypts were generated based on the fact that cells from the villi are detached before cells from the crypts during the enzymatic treatment of the mucosa (24). The separation of crypt vs. villus cells was validated by QPCR analysis for control marker enzymes of which lysozyme and Defa5 (both Paneth cell markers), as expected, were highly enriched in the CCK-eGFP-negative cells from the crypts, whereas alkaline phosphatase and ApoA4 (both enterocyte markers), also as expected, were enriched in the CCK-eGFP-negative cells from the villi (Fig. 4A).
Fig. 4.
Relative expression of RNAs encoding peptide precursors in FACS-sorted populations of CCK-eGFP-positive cells isolated from villus (V) vs. crypt (C) fractions from duodenum of CCK-eGFP transgenic mice. Populations of cells derived from villus vs. crypts were obtained by collecting cells early vs. late, respectively, during the enzymatic release of cells from the duodenal mucosa (see Material and Methods for details) Panel A (to the left), The relative expression of markers for intestinal villus (Alpi and Apoa4 from enterocytes) and crypt (Lyz1 and Defa5 from Paneth cells) in the CCK-eGFP-negative cells derived from villus (V, gray columns) vs. crypt (C, white columns). Panel A (to the right), The relative expression for the gut hormones: Nts, Pyy, Sct, Cck, Ghrl, Gip, and glucagon/GLP-1 (Gcg) in CCK-eGFP-positive cells derived from villus (V, dark green) vs. crypt (C, light green). The gene expression is indicated as percentage of total, i.e. crypt plus villus. It should be noted that although the actual total expression in the CCK-eGFP-positive cells is relatively high for Cck, Sct, Gip, and Ghrl it is relatively low for Gcg, Pyy, and Nts (see Fig. 2B) the total is here indicated as 100%. Panel B and C, Single-cell QPCR (see legend to Fig. 3) of cells derived from the villus and crypt, respectively. The numbers of cells positive for Cck, Sct, Gip, and ghrelin are given, in this unamplified QPCR analysis a Ct value <40 is considered to be positive for expression of the peptide precursor RNA (see Materials and Methods).
Among the peptide hormones, not only Cck but also Sct, Pyy, and Nts were all enriched in the villus compared with the crypt fraction of CCK-eGFP-positive cells, in contrast to Gip, Ghrl, and Gcg, which were relatively evenly expressed in the villus and crypt fractions of CCK-eGFP-positive cells from duodenum (Fig. 4A).
Single-cell QPCR performed on cells isolated either from the villus (Fig. 4B) or the crypt (Fig. 4C) of duodenum supported the notion that the coexpression of multiple peptide hormones in the CCK-eGFP-positive cells is a general phenomenon, which is not limited to cells residing in the crypts.
Immunohistochemical localization of peptides in CCK-eGFP cells
The hormonal expression within CCK-eGFP cells at the peptide level was further illuminated by means of performing immunohistochemistry with antibodies selective for each of the discussed peptide hormones.
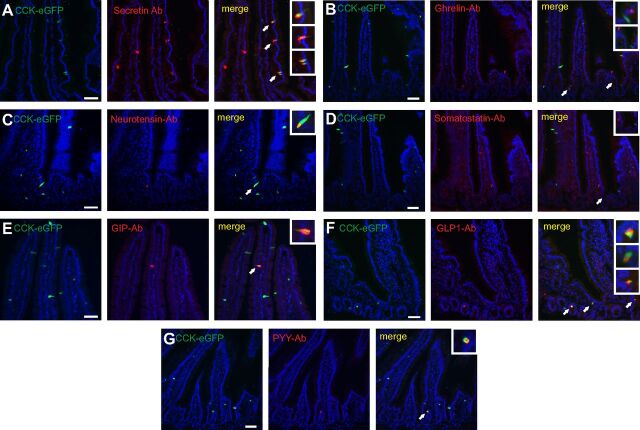
As shown in Fig. 5, CCK-eGFP-positive cells in the duodenum costained with antibodies against secretin, GIP, GLP-1, neurotensin, and PYY. Approximately 60% of the secretin-positive cells were CCK-eGFP positive and, interestingly, these cells occasionally were found clustered within the same crypt-villus segment (Fig. 5 and see Fig. 7). A relatively large fraction of the rather scarce PYY- and GLP-1-positive cells were also positive for CCK-eGFP. CCK-eGFP-positive cells costaining with antibodies for the various non-CCK peptide hormones were not only found in the crypts but frequently also along the length of the villi (Fig. 5). We did not detect any costaining of CCK-eGFP with antibodies directed against somatostatin, which is in agreement with the QPCR data. Also, no costaining of CCK-eGFP with ghrelin was observed, which, however, was surprising because the QPCR analysis indicated expression of Ghrl mRNA in FACS-separated CCK-eGFP-positive cells (Fig. 2, B and 2D).
Fig. 5.
Immuohistochemical colocalization of selected hormones with CCK-eGFP in duodenum of transgenic CCK-eGFP mice. Vertical panel I, Cck-eGFP-expressing cells (green). Vertical panel II, Immunohistochemical localization of hormones (red) using primary antibodies against secretin (horizontal lane A), ghrelin (lane B), neurotensin (lane C), somatostatin (lane D), gastric inhibitory polypeptide (GIP) (lane E), GLP-1 (lane F), and peptide YY (PYY) (lane G). Vertical panel III, Merged pictures showing colocalization (yellow). Arrows point to colocalization (or, in the case of ghrelin and somatostatin, lack of colocalization) shown in higher magnification in the insets at the top right corner of each panel. Cells storing GLP-1, PYY, and neurotensin show a high degree of colocalization with CCK-eGFP. Secretin- and GIP-producing cells display an approximately 50–60% colocalization with CCK-eGFP. In contrast, somatostatin and ghrelin do not colocalize with CCK-eGFP. Nuclei are visualized with Hoechst counter staining (blue). Bar, 50 μm. Ab, Antibody.
Fig. 7.
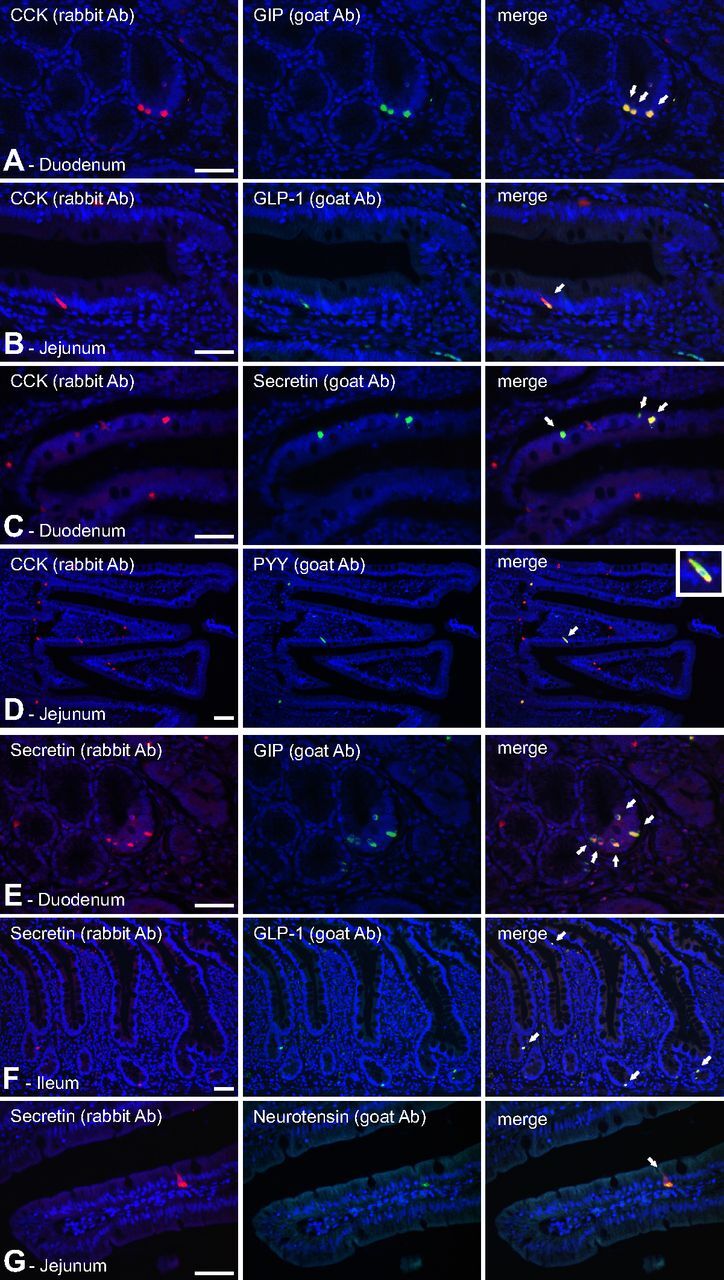
Immunohistochemical colocalization of selected gut hormones in human small intestine. Panel A, Colocalization of CCK (red, rabbit Ab) and GIP (green, goat Ab) in human duodenum. Panel B, Colocalization of CCK (red, rabbit Ab) and GLP-1 (green, goat Ab) in human jenunum. Panel C, Colocalization of CCK (red, rabbit Ab) and secretin (green, goat Ab) in human duodenum. Panel D, Colocalization of CCK (red, rabbit Ab) and PYY (green, goat Ab) in human jejunum. Panel E, Colocalization of secretin (red, rabbit Ab) and GIP (green, goat Ab) in human duodenum. Panel F, Colocalization of secretin (red, rabbit Ab) and GLP-1 (green, goat Ab) in human ileum. Panel G, Colocalization of secretin (red, rabbit Ab) and neurotensin (green, goat Ab) in human jejunum. For identification of antibodies see Supplemental Table 2. Hoechst nuclei counterstaining (blue) and bar, 50 μm. Ab, Antibody.
It is concluded that subpopulations of the CCK-eGFP-positive cells costore secretin, GLP-1, PYY, GIP, and neurotensin peptides, but apparently not somatostatin- and ghrelin-immunoreactive material.
LC-MS analysis of peptides from FACS-purified CCK-eGFP cells
To study the occurrence of the peptides in the CCK-eGFP-positive cells more directly, acetic acid extracts of FACS-purified CCK-eGFP cells from the whole small intestine were subjected to LC-MS analysis using an LC protocol for small to medium peptides. The purity of the preparation was substantiated by the fact that the top nine scoring proteins, based on the sum of the single peptides recorded in the LC-MS analysis, all were derived from either gut hormone precursors or progranins. Among the granins, peptides from secretogranin-1 (chromogranin-B), secretogranin-2, and chromogranin-A were detected in the CCK-eGFP-positive cells in numbers and with a total intensity corresponding to the peptide precursors (Table 1).
Table 1.
The 11 most abundant proteins identified by LC-MS analysis of peptide fragments occurring in acetic acid extract of FACS-sorted CCK-eGFP-positive cells from the entire small intestine
| Peptide precursor | Total peptide intensity | No. of different peptides |
|---|---|---|
| Procholecystokinin | 4.1 × 108 | 68 |
| Proglucagon | 2.4 × 108 | 34 |
| Prosecretogranin-1 | 2.3 × 108 | 49 |
| Proneurotensin | 1.6 × 108 | 18 |
| Pro-PYY | 1.2 × 108 | 26 |
| Pro-GIP | 0.9 × 108 | 34 |
| Prosecretogranin-2 | 0.7 × 108 | 42 |
| Prochromogranin-A | 0.7 × 108 | 22 |
| Prosecretin | 0.2 × 108 | 18 |
| Keratin, type II cytoskeletal 8 | 1.6 × 107 | 38 |
| Protachykinin β | 0.1 × 107 | 8 |
The number of peptide fragments from each of the peptide precursors and the total intensity of these peptides are indicated.
With respect to peptides derived from hormone precursors, the pro-CCK peptides were dominating both in terms of number and total peptide MS intensity; 68 pro-CCK-derived peptides with a total peptide intensity signal of 4.1 × 108 were observed (Table 1). Additionally, 34 peptides from proglucagon/GLP-1 were detected in the extracts of the CCK-eGFP-positive cells giving a total intensity of 2.4 × 108. Similarly, a large number of peptides derived from proneurotensin, pro-PYY, and pro-GIP gave total intensities in the same order of magnitude as pro-CCK and proglucagon/GLP-1 (Table 1). However, in view of the dominating position of Sct RNA in the QPCR analysis, only relatively small amounts of peptides derived from prosecretin were detected, i.e. 0.2 × 108, which is just above the intensity of the first contaminating proteins, keratin (Table 1). Proghrelin (two peptides with an intensity of 1.1 × 106) and prosomatostatin peptides were only found in what was considered to be noise level, in accordance with the immunohistochemical data (Fig. 5).
At the proteomics level, it is concluded that the CCK-eGFP-positive cells contain not only pro-CCK-derived peptides, but also relatively large amounts of peptides derived from especially proglucagon, pro-GIP, pro-PYY, proneurotensin, and to a lesser degree prosecretin.
Cell ablation based on proglucagon promoter-driven diphtheria toxin receptor expression
In mice expressing the human diphtheria toxin receptor under the control of the proglucagon promoter, treatment with diphtheria toxin eliminated nearly all GLP-1 cells in the mucosa of the ileum within 24 h (Fig. 6A). As expected based on the previously well-characterized coexpression of GLP-1 with PYY, PYY-containing cells were ablated to an almost similar extent. Based on the above immunohistochemistry, QPCR, and LC-MS studies, it was not surprising that treatment with diphtheria toxin also resulted in a marked reduction in CCK cells (Fig. 6B). It was not expected that reductions in neurotensin cells, GIP cells, and secretin cells would also be observed (Fig. 6, A–C). Numbers of somatostatin cells and substance P cells were unaffected (Fig. 6 and Supplemental Fig. 1). These changes seemingly were not due to a generalized diphtheria toxin-induced gut mucosal injury because no obvious histological changes to the mucosal appearance were observed (Supplemental Fig. 1).
Fig. 6.
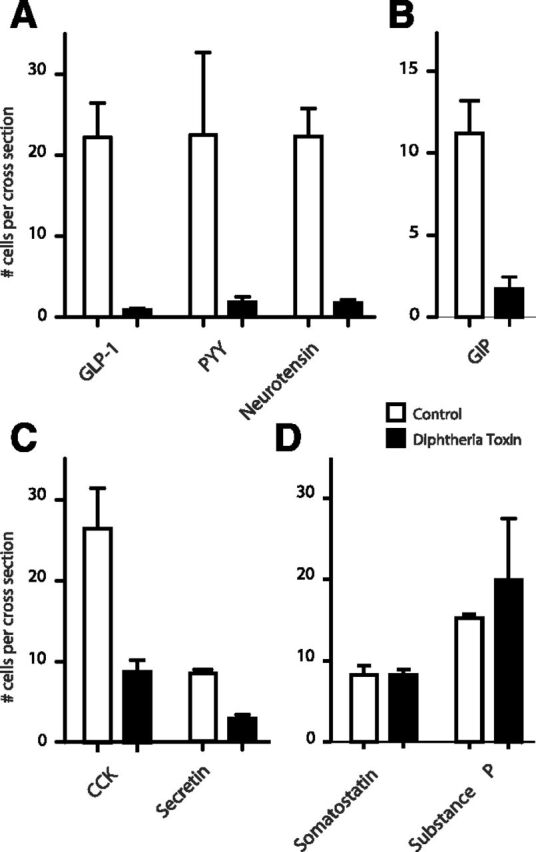
Diphtheria toxin-mediated ablation of enteroendocrine cells in the ileum of transgenic mice expressing the human diphtheria toxin receptor under the control of the proglucagon promoter. Enteroendocrine cells were stained with antibodies selective for GPL-1, PYY, neurotensin, GIP, CCK, secretin, substance P, and somatostatin and counted as described in Materials and Methods in control animals (n = 2, open bars) and 24 h after treatment with low doses of diphtheria toxin (n = 3, closed bars).
These experiments indicate that the proglucagon promoter is active not only in GLP-1-producing cells, but also in the majority of PYY, neurotensin, and GIP cells and a major fraction of the CCK and secretin cells, but not in the somatostatin and substance P cells.
Costaining of peptide hormones in human enteroendocrine cells
The above data relate to murine tissues and are based on cells genetically tagged by GFP or diphtheria toxin receptor. To probe for similar coexpression patterns within human tissues, we used classical double-staining immunohistochemical analysis employing combinations of rabbit and goat antisera raised against the gut peptide hormones. Inclusion criteria for commercial goat-derived antibodies were that they should show identical staining patterns of enteroendocrine cells as our prevalidated rabbit antibodies (Supplemental Fig. 2).
Here we focused on costaining experiments based on CCK and secretin, respectively. In the human duodenum, CCK immunoreactivity was found in almost all GIP-positive cells, which mainly were located in the crypts (Fig. 7). Nevertheless, most CCK-positive cells did not store GIP. In contrast, CCK immunoreactivity was found in at least 50% of the human duodenal secretin cells, mainly in the villi, and in nearly all duodenal PYY cells, which were few in number. In the jejunum, CCK also was found in many neurotensin-positive cells and in the majority of the GLP-1-positive cells.
The other major duodenal hormone, secretin, was costored with GIP in the majority of human duodenal GIP-positive cells, again mainly in the crypts. In the human jejunum, secretin immunoreactivity was found to be costored with neurotensin, mainly in the villi. In the ileum, secretin was found in the majority of GLP-1-positive cells, mainly in the crypts but also in the villi (Fig. 7).
In contrast, somatostatin cells were observed throughout the human gut, and we did not observe costaining of somatostatin with any of the other hormones tested. This lack of costaining of somatostatin with other gut hormones is shown for CCK in the duodenum in Supplemental Fig. 1. With respect to ghrelin, we observed an almost obligatory costaining with motilin in the small intestine (31), as shown for duodenum in Supplemental Fig. 3; however, costaining of ghrelin with other tested gut hormones was not observed. The motilin gene is not found in the mouse and rat, where ghrelin alone is thought to control, for example, gut motility, which in many other animals conceivably is taken care of by the two peptides jointly.
Although not comprehensive, this immunohistochemical analysis demonstrates coexpression and costorage of CCK, secretin, GIP, GLP-1, PYY, and neurotensin in subsets of enteroendocrine cells of the human small intestine, not only in the crypts but also on the villi. In contrast, but in agreement with the mouse data, no indication of coexpression of somatostatin or ghrelin with this larger group of gut hormones was observed.
Discussion
According to textbooks, CCK is a peptide hormone expressed and released from I-cells of the duodenum and, to some extent, the jejunum (4, 6, 32–35). CCK cells are not generally believed to be found throughout the GI tract, nor are they expected to store and release other biologically active peptides other than CCK, such as GLP-1. It was therefore surprising to find substantial numbers of CCK-eGFP-positive cells in regions of the small and large intestines other than the duodenum and jejunum, as we report here. Furthermore, it was surprising to find that CCK cells express not only relatively high levels of RNA encoding peptide precursors in addition to CCK, but also that they store peptides derived from these precursors. Similarly, GLP-1 is generally believed to be expressed mainly in a limited population of enteroendocrine cells of the lower small intestine and the colon (35). However, as observed in the CCK-eGFP reporter mice, we found through diphtheria toxin-mediated cell ablation that the proglucagon promoter is active not only in GLP-1 and PYY cells, but also in neurotensin and GIP cells as well as CCK and secretin cells of the ileum. Importantly, a similar broad peptide coexpression phenomenon could be demonstrated by classical immunohistochemical costaining in the human small intestine, totally independent of any transgenic methodology.
A series of reports on transgenic reporter mice expressing variants of GFP under the control of promoters for different gut peptide precursors have been published over the last couple of years without mention of coexpression of nonexpected peptide hormones (23, 28, 36, 37). However, very recently, Habib et al. (38) reported that FACS-purified GLU-Venus (proglucagon-Venus) and GIP-Venus cells also express both RNA and immunoreactive peptides corresponding to the precursors for CCK, GIP, and secretin. This broad peptide hormone expression pattern corresponds rather closely to what we find in the present study for CCK-eGFP cells and proglucagon-hDTR cells.
Is the apparent coexpression a phenomenon related to the transgenic BAC-based labeling technology?
Such a genetic tagging artifact possibly could explain the observed broad peptide coexpression pattern described in the present study and that of Habib et al. (38). The expression of CCK-eGFP in cells apparently not expressing CCK itself could indicate such an artifact, which according to the single-cell QPCR analysis is observed in 9–12% of the enteroendocrine cells (Figs. 3 and 4). In principle, this could be a result of ectopic expression of the CCK-eGFP in non-CCK cells. However, it could also reflect that the QPCR reaction for CCK has failed in 9–12% of the wells, although CCK mRNA was present (the QPCR is performed in an unamplified manner on very little RNA material). Or, another likely possibility could be that the highly stable eGFP molecule is still present in mature enteroendocrine cells that no longer express CCK but did express CCK, and CCK-eGFP, earlier in their life cycle, i.e. in the crypts (39). Moreover, gastric ghrelin cells derived from a line of ghrelin-hrGFP transgenic mice, which was made by a similar transgenic approach, showed no sign of expression of enteroendocrine peptide hormones other than ghrelin (Fig. 2). Importantly however, ectopic expression of the eGFP reporter in cells other than CCK cells or the occurrence of stable eGFP in mature cells that no longer express Cck would not explain the single-cell QPCR data in which 44% of the individual cells containing Cck RNA, which happen to have been isolated from CCK-eGFP mice based on their eGFP signature, were found also to contain Sct RNA, Gip RNA, ghrelin RNA, or combinations of those (Fig. 3). Furthermore, key components of the coexpression phenomenon were confirmed in human tissue samples using classical immunohistochemical double-labeling technique, which is totally independent of the GFP labeling technology (Fig. 7).
Does the QPCR coexpression reflect an RNA phenomenon not carried over to the protein level?
This is clearly not the case for the majority of the peptides studied as demonstrated by the immunohistochemical and LC-MS proteomic studies. However, in the case of ghrelin and secretin, the coexpression phenomenon is exclusively or to a large extent observed at the RNA level compared with the protein level. Thus, although coexpression of ghrelin RNA with Cck mRNA was observed in the CCK-eGFP-enriched intestinal enteroendocrine cell pools as well as in individual CCK-eGFP cells (Figs. 2 and 3), we observed only minimal, if any, immunohistochemical coexpression of ghrelin with the other peptides. These immunohistochemical observations agree well with the finding that proghrelin peptides were not detected above the noise level in the LC-MS analysis of FACS-separated CCK-eGFP cells (Table 1). Similarly, very few and only small amounts of prosecretin peptides were detected in the LC-MS analysis, which contrasted with the high levels of secretin RNA observed in the CCK-eGFP-positive cells even in the jejunum and ileum, where the secretin peptide is not normally believed to be expressed (Fig. 2). Nevertheless, and in contrast to ghrelin, secretin could by immunohistochemistry be detected in CCK-eGFP-positive cells, and colocalization of CCK and secretin was detected by costaining experiments in the human tissue samples (Fig. 6). The LC-MS data, however, do indicate that the secretin expression must be rather low at the protein level, e.g. in the distal small intestine.
Is the coexpression only a crypt phenomenon?
It has long been known that a subpopulation of enteroendocrine cells in the crypts can express multiple peptide hormones (40). This phenomenon was recently described in relation to CCK-eGFP cells (20), as was the observation of expression of only a single hormone within enteroendocrine cells localized within the villi, presumably after their migration out of the crypts during the maturation process. In the present study, however, using both immunohistochemistry and QPCR analysis of FACS-separated enriched pools of CCK-eGFP cells or of individual FACS-separated CCK-eGFP cells, isolated from either villi or crypts, demonstrated that the coexpression phenomenon clearly is not restricted to the crypts, but is found in the presumed mature cells that populate the villi, as well. Concerning the FACS analysis, we did not exclude the possibility that expression of CCK-eGFP in the enteroendocrine cells potentially could have affected the expression of the marker enzymes in the enterocytes and Paneth cells.
Cell ablation studies also demonstrate extensive coexpression of more than one peptide hormone within enteroendocrine cells
Our cell ablation study based on the proglucagon promoter-driven expression of human diphtheria toxin receptor supports the phenomenom of a broader existence of enteroendocrine cells with coexpression of gene products from more than one peptide hormone precursor. Not only did this system support the above studies demonstrating coexpression of CCK with GLP-1, but it also demonstrated the existence of cells in which proglucagon-derived peptide hormones were coexpressed with several other peptide hormones.
Previously, Rindi et al. (41) performed a similar cell ablation study aimed at secretin-producing enteroendocrine cells using transgenic expression of the thymidine kinase from herpes simplex virus under the control of the secretin promoter. Similar to our study, they observed markedly reduced numbers of not only secretin cells, but also CCK, GLP-1, and PYY cells and also, to some degree, GIP cells during ganciclovir treatment. However, because Rindi et al. treated with the toxic prodrug for 5 d, their study could only indicate that the secretin gene is expressed in a progenitor cell, which is common to a number of enteroendocrine cell types (41).
Broad coexpression of gut hormones has been reported previously
Although the one cell-one hormone concept has been the prevailing dogma in enteroendocrinology, there are, in fact, strong reports in the literature describing a broad coexpression of peptide hormones similar to what we find in the present study (22, 40, 43). As early as 20 years ago, pioneering transgenic work of Roth et al. (40), combined with classical immunohistochemical techniques, demonstrated that colonic enteroendocrine cells not only share a cellular origin, but also coexpress several gut hormones, even as mature cells. Based on pair-wise comparison of coexpression, they suggested that the colonic enteroendocrine lineage has two branches: one branch produces substance P cells and another branch produces CCK, GLP-1, PYY, and neurotensin cells (40). The latter enteroendocrine lineage or branch very likely corresponds to the lineage identified in the present study in the small intestine, which we find expresses also GIP and secretin, but not somatostatin, and, at the protein level, not ghrelin. Antibodies against the latter four hormones were not employed in the cited study (40).
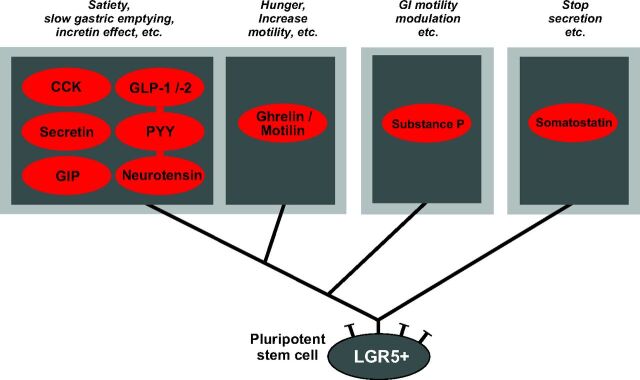
Proposed model of small intestinal enteroendocrine cell lineage
In the context of the results of the present study, which takes a broad spectrum of peptides into account, it would appear that there are two main lineages of enteroendocrine cells in the small intestine: one lineage yielding cells expressing the universally inhibitory peptide hormone somatostatin, and a second lineage that includes cells that have the potential of coexpressing CCK, GLP-1, GIP, PYY, neurotensin, and secretin (Fig. 8). In a broad sense, the peptides produced by the second lineage could all be considered to have similar physiological function: to varying degrees, they act as suppressors of appetite, inhibitors of gastric emptying, and stimulators of insulin secretion (incretins) (3). As shown in Fig. 8, it is suggested that two additional branches split off from this lineage: one leads to cells producing ghrelin and motilin and another leads to the enterochromaffin cells producing substance P, as originally suggested by Roth et al. (40) in the colon. In respect to function, ghrelin, and to some extent motilin, both have orexigenic effects, and both peptides have very similar, stimulatory effects on gut motility (44). These functions can be considered opposite to those of CCK, GLP-1, and PYY from the other branch, and thus it is reasonable that the expression of these two different groups of peptide hormones would occur in cells from different branches. Recently, Beucher et al. (45) demonstrated through an elegant study based on the homeodomain-containing transcription factors Arx and Pax4 that somatostatin cells clearly constitute a separate lineage of enteroendocrine cells, as we also conclude from the present study using entirely different methodology.
Fig. 8.
Schematic overview of proposed main enteroendocrine cell lineages of the small intestine. Based on the results of the present study as well as studies by Roth et al. (40), it is suggested that cells expressing the universally inhibitory peptide somatostatin constitute a separate cell lineage distinct from the cell lineage generating cells expressing the rest of the GI-tract peptide hormones. From the latter lineage are apparently generated enterochromafin cells that express substance P (and 5HT) (40), and cells coexpressing ghrelin and motilin (31) plus a major lineage of enteroendocrine cells that have the potential of expressing the functionally related peptides: CCK, secretin, GIP, GLP-1/-2, PYY, and neurotensin. Some of the major functions of the peptide products are indicated above each cell lineage.
Major parts of the old gut hormone physiology remain valid
The results of the present study, as well as, for example, the studies by Roth et al. (40) and Habib et al. (38), do not change the basic physiological concept that CCK is mainly a duodenal hormone, whereas GLP-1, neurotensin, and PYY are mainly hormones of the jejunum and ileum. Such is clearly observed, for example, at the RNA level in CCK-eGFP cells isolated from the different parts of the small intestine (Fig. 2). It is nevertheless apparent from these studies that enteroendocrine cells have a much broader potential for expression of multiple peptide precursors than is generally believed. Our single-cell QPCR data of CCK-eGFP-labeled cells indicates that in a given cell this potential is, however, only exploited to a limited degree because coexpression of more than one hormone was observed in only half of the cells and expression of more than two hormone genes only rarely was observed (Fig. 3). More studies using other types of approaches and techniques are needed to address the issue of whether a given enteroendocrine cell population in a given location stores and releases peptides from more than one peptide precursor at physiologically meaningful levels.
The enteroendocrine system may be rather adaptive
The apparent, quite broad potential for coexpression of multiple peptide precursors indicates that the enteroendocrine system could be more adaptive than previously believed. The fact that peptide hormones such as CCK, secretin, GIP, GLP-1 and -2, PYY, and neurotensin, to various degrees, are coexpressed in enteroendocrine cells throughout the intestine suggests that the expression pattern potentially could be modulated by environmental factors. This might occur, for example, after bariatric surgery. In principle, such a potential plasticity of the enteroendocrine system could be exploited therapeutically by developing methods to control not only the secretion of the peptide hormones, but also methods to control the number and types of enteroendocrine cells or the ability of a certain enteroendocrine cell type to express a different, given repertoire of peptides, which they already are equipped to express. This will require insight into the factors and receptors that may control the peptide precursor expression pattern and the precursor processing pattern of the enteroendocrine cells.
Supplementary Material
Acknowledgments
We thank Professor Bente Pakkenberg and Ph.D. student Anna Schou Karlsen for essential insights into stereology; and Professors Jens F. Rehfeld, Jan Fahrenkrug, and Ove Schaffalitzky de Muckadell for the generous gifts of antibodies. We also thank laboratory technicians Lisbeth E. Stolpe, Heidi M.Paulsen, Lise S.Strange, Lotte Laustsen, Susanne Hummelgaard, Jovin Suku, and Mette Simons and personnel at the University of Texas Southwestern Medical Center Flow Cytometry Multi-user Core Facility for expert technical assistance.
This work was supported by The Novo Nordisk Foundation Center for Basic Metabolic Research (www.metabol.ku.dk) including an International Research Alliance with J.M.Z. is supported by an unconditional grant from the Novo Nordisk Foundation. The project was also supported by the UNIK project for Food, Fitness & Pharma (http://www.foodfitnesspharma.ku.dk) from the Danish Ministry of Science, Technology and Innovation. The laboratories were further supported by grants from the Novo Nordisk Foundation (to T.W.S. and N.W.) the Lundbeck Foundation (to K.L.E., and T.W.S.), from the Danish Medical Research Council (to K.L.E., and T.W.S.), the Swedish Medical Research Council (projects nos. 522-2008-4216 and K2009-55 × 21111-01-4 to N.W.) as well as the FP7 Full4Health consortium (for J.J.H.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- BAC
Bacterial artificial chromosome
- CCK
cholecystokinin
- eGFP
enhanced green fluorescent protein
- GI
gastrointestinal
- GFP
green fluorescent protein
- GIP
gastric inhibitory peptide
- GLP-1
glucagon-like peptide 1
- hrGFP
humanized Renilla green fluorescent protein
- LC-MS
liquid chromatography-mass spectrometry
- Nts
neurotensin
- PYY
peptide YY
- Sct
secretin
Footnotes
K.L.E. and M.S.E. contributed equally to this work.
References
- 1. Schonhoff SE, Giel-Moloney M, Leiter AB 2004. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 145:2639–2644 [DOI] [PubMed] [Google Scholar]
- 2. Rehfeld JF 1998. The new biology of gastrointestinal hormones. Physiol Rev 78:1087–1108 [DOI] [PubMed] [Google Scholar]
- 3. Miller LJ 2003. Gastrointestinal hormones and receptors. In: , Yamada T, ed. Textbook of gastroenterology. Baltimore: Lippincott Williams & Wilkins; 48–76 [Google Scholar]
- 4. Field BC, Chaudhri OB, Bloom SR 2010. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 6:444–453 [DOI] [PubMed] [Google Scholar]
- 5. Sundler F 2004. GI tract, general anatomy (cells). In: , Martini L, ed. Encyclopedia of endocrine diseases. New York: Elsevier; 208. –215 [Google Scholar]
- 6. Solcia E, Capella C, Buffa R, Usellini L, Fiocca R, Frigerio B, Tenti P, Sessa F 1981. The diffuse endocrine-paracrine system of the gut in health and disease: ultrastructural features. Scand J Gastroenterol Suppl 70:25–36 [PubMed] [Google Scholar]
- 7. Engelstoft MS, Egerod KL, Holst B, Schwartz TW 2008. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab 8:447–449 [DOI] [PubMed] [Google Scholar]
- 8. Bussolati G, Capella C, Solcia E, Vassallo G, Vezzadini P 1971. Ultrastructural and immunofluorescent investigations on the secretin cell in the dog intestinal mucosa. Histochemie 26:218–227 [DOI] [PubMed] [Google Scholar]
- 9. Larsson LI, Rehfeld JF 1978. Distribution of gastrin and CCK cells in the rat gastrointestinal tract. Evidence for the occurrence of three distinct cell types storing COOH-terminal gastrin immunoreactivity. Histochemistry 58:23–31 [DOI] [PubMed] [Google Scholar]
- 10. Larsson LI, Sundler F, Alumets J, Håkanson R, Schaffalitzky de Muckadell OB, Fahrenkrug J 1977. Distribution, ontogeny and ultrastructure of the mammalian secretin cell. Cell Tissue Res 181:361–368 [DOI] [PubMed] [Google Scholar]
- 11. Polak JM, Bloom S, Coulling I, Pearse AG 1971. Immunofluorescent localization of enteroglucagon cells in the gastrointestinal tract of the dog. Gut 12:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz TW, Holst B 2010. An enteroendocrine full package solution. Cell Metab 11:445–447 [DOI] [PubMed] [Google Scholar]
- 13. Fridolf T, Böttcher G, Sundler F, Ahrén B 1991. GLP-1 and GLP-1(7–36) amide: influences on basal and stimulated insulin and glucagon secretion in the mouse. Pancreas 6:208–215 [PubMed] [Google Scholar]
- 14. Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB 2011. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab 13(Suppl 1):5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barker N, van de Wetering M, Clevers H 2008. The intestinal stem cell. Genes Dev 22:1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007 [DOI] [PubMed] [Google Scholar]
- 17. Cheng H, Leblond CP 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561 [DOI] [PubMed] [Google Scholar]
- 18. van der Flier LG, Clevers H 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260 [DOI] [PubMed] [Google Scholar]
- 19. Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G 2010. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest 120:1708–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sei Y, Lu X, Liou A, Zhao X, Wank SA 2011. A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am J Physiol Gastrointest Liver Physiol 300:G345–G356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bjerknes M, Cheng H 1981. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160:77–91 [DOI] [PubMed] [Google Scholar]
- 22. Aiken KD, Kisslinger JA, Roth KA 1994. Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn 201:63–70 [DOI] [PubMed] [Google Scholar]
- 23. Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM 2009. Characterization of a novel ghrelin cell reporter mouse. Regul Pept 155:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stegmann A, Hansen M, Wang Y, Larsen JB, Lund LR, Ritié L, Nicholson JK, Quistorff B, Simon-Assmann P, Troelsen JT, Olsen J 2006. Metabolome, transcriptome, and bioinformatic cis-element analyses point to HNF-4 as a central regulator of gene expression during enterocyte differentiation. Physiol Genomics 27:141–155 [DOI] [PubMed] [Google Scholar]
- 25. Hald A, Rønø B, Lund LR, Egerod KL 2012. LPS counter regulates RNA expression of extracellular proteases and their inhibitors in murine macrophages. Mediators Inflamm 2012:157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaffl MW 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen JV, Schwartz JC, Griep-Raming J, Nielsen ML, Damoc E, Denisov E, Lange O, Remes P, Taylor D, Splendore M, Wouters ER, Senko M, Makarov A, Mann M, Horning S 2009. A dual pressure linear ion trap Orbitrap instrument with very high sequencing speed. Mol Cell Proteomics 8:2759–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI 2008. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105:16767–16772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S 2011. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 140:903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chandra R, Samsa LA, Vigna SR, Liddle RA 2010. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res 341:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wierup N, Björkqvist M, Weström B, Pierzynowski S, Sundler F, Sjölund K 2007. Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab 92:3573–3581 [DOI] [PubMed] [Google Scholar]
- 32. Liddle RA 1997. Cholecystokinin cells. Annu Rev Physiol 59:221–242 [DOI] [PubMed] [Google Scholar]
- 33. Rehfeld JF 1978. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem 253:4022–4030 [PubMed] [Google Scholar]
- 34. Rehfeld JF, Bardram L, Hilsted L 1992. Ontogeny of procholecystokinin maturation in rat duodenum, jejunum, and ileum. Gastroenterology 103:424–430 [DOI] [PubMed] [Google Scholar]
- 35. Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E 2004. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann NY Acad Sci 1014:1–12 [DOI] [PubMed] [Google Scholar]
- 36. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM 2008. Glucose sensing in L cells: a primary cell study. Cell Metab 8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F 2009. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM 2012. Overlap between enteroendocrine K and L cells revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR 1998. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem 273:34970–34975 [DOI] [PubMed] [Google Scholar]
- 40. Roth KA, Kim S, Gordon JI 1992. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol 263:G174–G180 [DOI] [PubMed] [Google Scholar]
- 41. Rindi G, Ratineau C, Ronco A, Candusso ME, Tsai M, Leiter AB 1999. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 126:4149–4156 [DOI] [PubMed] [Google Scholar]
- 42. Althage MC, Ford EL, Wang S, Tso P, Polonsky KS, Wice BM 2008. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 283:18365–18376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mortensen K, Christensen LL, Holst JJ, Orskov C 2003. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196 [DOI] [PubMed] [Google Scholar]
- 44. De Smet B, Mitselos A, Depoortere I 2009. Motilin and ghrelin as prokinetic drug targets. Pharmacol Ther 123:207–223 [DOI] [PubMed] [Google Scholar]
- 45. Beucher A, Gjernes E, Collin C, Courtney M, Meunier A, Collombat P, Gradwohl G 2012. The homeodomain-containing transcription factors arx and pax4 control enteroendocrine subtype specification in mice. PLoS ONE 7:e36449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.