Abstract
Background
Having a penicillin allergy label is associated with the use of less appropriate and more expensive antibiotics and increased healthcare utilization. Penicillin allergy testing results in delabeling most allergy claimants and may be cost-saving. This study aimed to project whether penicillin allergy testing in patients reporting a penicillin allergy is cost-saving.
Methods
In this economic evaluation study, we built decision models to project the economic impact of 2 strategies for a patient with a penicillin allergy label: (1) perform diagnostic testing (drug challenges, with or without skin tests); and (2) do not perform diagnostic testing. The health service perspective was adopted, considering costs with penicillin allergy tests, and with hospital bed-days/outpatient visits, antibiotic use, and diagnostic testing. Twenty-four base case decision models were built, accounting for differences in the diagnostic workup, setting (inpatient vs outpatient) and geographic region. Uncertainty was explored via probabilistic sensitivity analyses.
Results
Penicillin allergy testing was cost-saving in all decision models built. For models assessing the performance of both skin tests and drug challenges, allergy testing resulted in average savings (in United States [US] dollars) of $657 for inpatients (US: $1444; Europe: $489) and $2746 for outpatients (US: $256; Europe: $6045). 75% of simulations obtained through probabilistic sensitivity analysis identified testing as the less costly option.
Conclusions
Penicillin allergy testing was projected to be cost-saving across different scenarios. These results are devised to inform guidelines, supporting the adoption of policies promoting widespread testing of patients with a penicillin allergy label.
Keywords: drug allergy, drug challenge, economic evaluation, penicillin allergy
Penicillin allergy testing is projected to be cost-saving across different scenarios (including among inpatients and outpatients in the United States and Europe), economically supporting its generalized implementation across healthcare systems.
(See the Editorial Commentary by Mattingly and Heil on pages 939-41.)
A penicillin allergy is more commonly reported than an allergy to any other drug class [1]. Studies performed in general populations identify that up to 5% of individuals claim to be allergic to penicillins, and this percentage often rises to >10% in studies assessing outpatients or inpatients [2–4]. Most penicillin allergy claimants do not have a true penicillin allergy [5, 6]. Multiple studies have shown that performing diagnostic tests results in delabeling most (> 90%) patients reporting penicillin allergies, allowing them to be safely treated with penicillins. This is particularly important, as patients with a penicillin allergy label often receive less adequate antibiotics (with penicillin allergy testing recommended as part of antibiotic stewardship [7]), which not only results in worse clinical outcomes, but also in an increased healthcare burden. Patients with a penicillin allergy label have been reported to receive more expensive antibiotics [8, 9], have longer hospital stays [10, 11], are more frequently readmitted [12], and have a higher frequency of outpatient visits [13]. There is perception that penicillin allergy testing may be cost-saving in specific populations [14–16], though supportive data are sparse. Studies to date have largely assessed inpatients and did not explore the uncertainties associated with their estimates [8, 10].
Therefore, in this economic evaluation study, we used different sources to build decision models aiming to assess whether penicillin allergy testing is cost-saving. We explored the uncertainty of costs and consequences estimates by simulation methods and by building distinct models accounting for regional, patient setting, and test performance differences.
METHODS
Model Structure
This is a partial economic evaluation study, because it is primarily focused on costs, and it follows Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines [17]. We built decision models projecting whether penicillin allergy testing would be cost-saving. That is, we projected whether the costs of testing patients claiming to be allergic to penicillins would be lower than the underlying potential savings in hospital bed-days, outpatient visits, and antibiotic use. We adopted the health system perspective, considering direct medical costs. Estimates are expressed in United States (US) dollars ($) as of December 2017.
The population of this study consisted of patients with a penicillin allergy label, for whom 2 alternative scenarios were compared: performing vs not performing penicillin allergy diagnostic testing. In the first scenario, all patients are subject to a diagnostic workup—if positive, patients are treated as truly allergic; if negative, patients are considered to be tolerant to penicillins and treated as nonallergic (such assumption was explored in univariate sensitivity analyses). In the second scenario, testing for penicillin allergy is not performed, with all patients being treated as allergic to penicillins irrespective of whether they have a true allergy.
Patients whose initial reaction consisted of a severe cutaneous adverse reaction (< 1 in 10 000 exposures [18]), where any testing or re-exposure is contraindicated [19], were excluded from these scenarios.
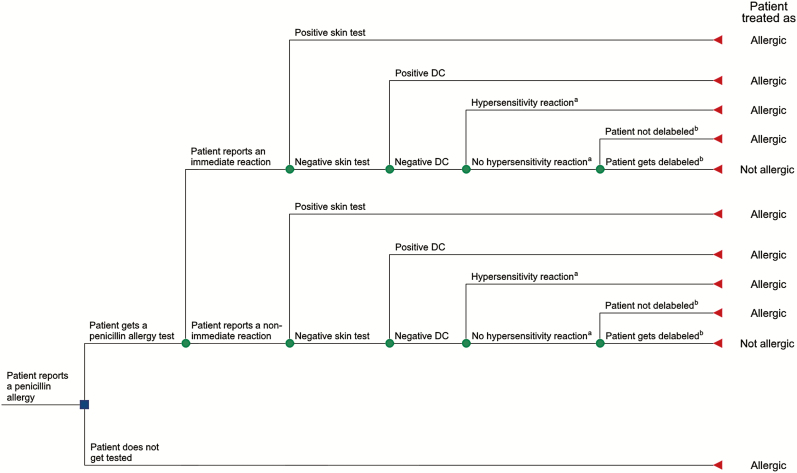
We built and analyzed 24 different base case (“main”) decision models (see Figure 1 for an example), accounting for differences/variants in the diagnostic workup, setting (inpatients vs outpatients), and geographical region (Figure 2). The diagnostic workflows encompassed sequential performance of skin testing and drug challenge (DC; ie, drug provocation testing), or direct performance of DC without prior skin tests. The former workflow was based on current recommendations [18, 19] and consisted of skin tests being performed to all individuals—if positive, patients were considered to be truly allergic to penicillins; if negative, individuals were subject to a subsequent DC (a positive DC indicated that the patient is truly allergic to penicillins, while a negative DC indicated penicillin tolerance). On the other hand, models involving direct performance of DC (which is recommended in American and British pathways for low-risk patients [20]) consisted of (1) sole performance of DC for all patients, or (2) direct performance of DC only among patients reporting a nonimmediate reaction, with the remaining individuals receiving sequential testing (skin tests followed by DC).
Figure 1.
Example of a decision tree: comparison between penicillin testing and not testing among inpatients. aHypersensitivity reaction to penicillins during treatment (ie, perceived false-negative drug challenge. bIn the base case scenario, we assumed that all patients with negative results were delabeled. Abbreviation: DC, drug challenge.
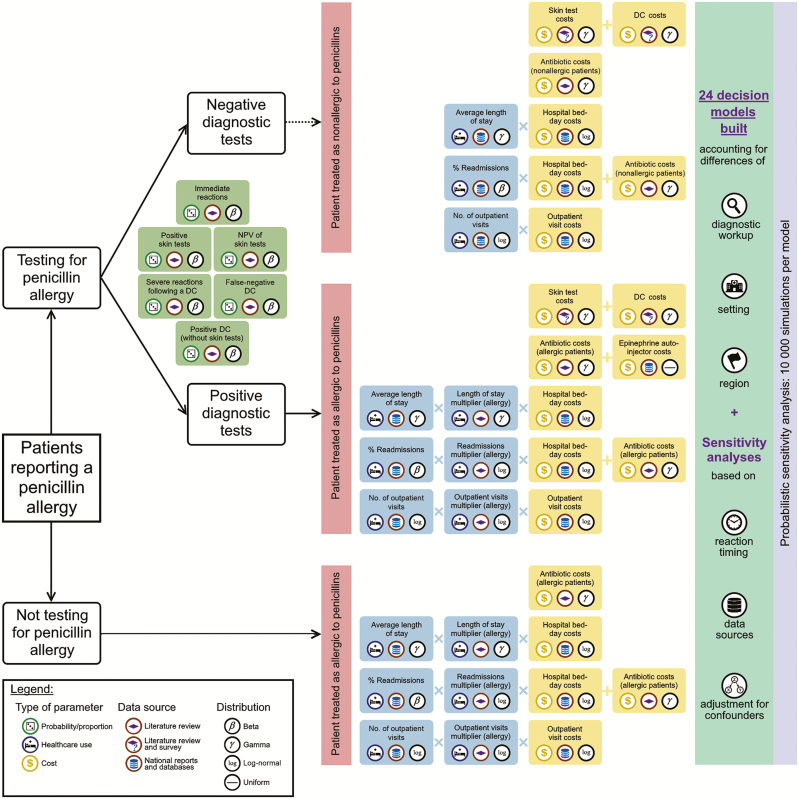
Figure 2.
Graphical description of the methods and sources used for building decision models. Abbreviations: DC, drug challenge; NPV, negative predictive value.
Both for models involving sequential testing and for models consisting of direct DC, we built series of models assessing inpatients and series assessing outpatients. For inpatients, we compared the costs of the 2 alternatives considering the hospitalization period and the possibility of 30-day readmissions. For outpatients, we took into account the frequency of ambulatory visits and antibiotic use for a period of 5 years after penicillin allergy testing. To account for regional differences, we developed variants for each model specifically including data from the US, Europe, and Portugal (an example European country for which we had access to the most information).
Model Inputs
A full description on model inputs can be found in the Supplementary Methods. This includes information sources, statistical methods, and assumptions on how proportions/probabilities, healthcare use, and costs were estimated. In addition, Figure 2 and Table 1 summarize variables tested with their corresponding data sources.
Table 1.
Decision Models’ Inputs and Respective Distributions
| Variable | Distribution Parameters | Distribution | Source | ||
|---|---|---|---|---|---|
| Mean | SD | Median | |||
| A. Proportions | |||||
| Immediate reactions | |||||
| Inpatients | |||||
| All regions | 0.220 | 0.0539 | … | Beta | [21] |
| US | 0.210 | 0.0837 | … | Beta | [21] |
| Europe | 0.146a | 0.1897a | … | Beta | [21] |
| Portugal | 0.146b | 0.1897b | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.271 | 0.1889 | … | Beta | [21] |
| US | 0.224 | 0.1058 | … | Beta | [21] |
| Europe | 0.306a | 0.2168a | … | Beta | [21] |
| Portugal | 0.329a | 0.1750a | … | Beta | [21] |
| Positive skin tests | |||||
| Nondiscriminated reaction timing | |||||
| Inpatients | |||||
| All regions | 0.065 | 0.0548 | … | Beta | [21] |
| US | 0.058 | 0.0592 | … | Beta | [21] |
| Europe | 0.077a | 0.2718a | … | Beta | [21] |
| Portugal | 0.077b | 0.2718b | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.115 | 0.0995 | … | Beta | [21] |
| US | 0.109a | 0.1261a | … | Beta | [21] |
| Europe | 0.106 | 0.0775 | … | Beta | [21] |
| Portugal | 0.105 | 0.0400 | … | Beta | [21] |
| Immediate reactions | |||||
| Inpatients | |||||
| All regions | 0.044 | 0.0316 | … | Beta | [21] |
| US | 0.041a | 0.0316a | … | Beta | [21] |
| Europe | 0.044c | 0.0316c | … | Beta | [21] |
| Portugal | 0.044c | 0.0316c | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.144 | 0.0917 | … | Beta | [21] |
| US | 0.041a | 0.0316a | … | Beta | [21] |
| Europe | 0.176 | 0.0943 | … | Beta | [21] |
| Portugal | 0.176b | 0.0943b | … | Beta | [21] |
| Nonimmediate reactions | |||||
| Inpatients | |||||
| All regions | 0.128a | 0.2398a | … | Beta | [21] |
| US | 0.128c | 0.2398c | … | Beta | [21] |
| Europe | 0.128c | 0.2398c | … | Beta | [21] |
| Portugal | 0.128c | 0.2398c | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.051 | 0.0412 | … | Beta | [21] |
| US | 0.051c | 0.0412c | … | Beta | [21] |
| Europe | 0.068 | 0.0510 | … | Beta | [21] |
| Portugal | 0.068b | 0.0510b | … | Beta | [21] |
| Negative predictive value of skin tests | |||||
| Nondiscriminated reaction timing | |||||
| Inpatients | |||||
| All regions | 0.992 | 0.0020 | … | Beta | [21] |
| US | 0.993 | 0.0010 | … | Beta | [21] |
| Europe | 0.962a | 0.0533a | … | Beta | [21] |
| Portugal | 0.962b | 0.0533b | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.959 | 0.0265 | … | Beta | [21] |
| US | 0.975 | 0.0173 | … | Beta | [21] |
| Europe | 0.955 | 0.0283 | … | Beta | [21] |
| Portugal | 0.970 | 0.0105 | … | Beta | [21] |
| Immediate reactions | |||||
| Inpatients | |||||
| All regions | 0.937 | 0.0566 | … | Beta | [21] |
| US | 0.941 | 0.0548 | … | Beta | [21] |
| Europe | 0.937c | 0.0566c | … | Beta | [21] |
| Portugal | 0.937c | 0.0548c | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.940 | 0.0400 | … | Beta | [21] |
| US | 0.941 | 0.0548 | … | Beta | [21] |
| Europe | 0.940 | 0.0469 | … | Beta | [21] |
| Portugal | 0.940b | 0.0469b | … | Beta | [21] |
| Nonimmediate reactions | |||||
| Inpatients | |||||
| All regions | 0.990 | 0.0059 | … | Beta | [21] |
| US | 0.990c | 0.0059c | … | Beta | [21] |
| Europe | 0.990c | 0.0059c | … | Beta | [21] |
| Portugal | 0.990c | 0.0059c | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.951 | 0.0300 | … | Beta | [21] |
| US | 0.990 | 0.0059 | … | Beta | [21] |
| Europe | 0.947 | 0.0265 | … | Beta | [21] |
| Portugal | 0.929 | 0.0742 | … | Beta | [21] |
| Severe reactions following a DC | |||||
| Nondiscriminated reaction timing | |||||
| Outpatients | |||||
| All regions | 0.0016 | 0.0007 | … | Beta | [21] |
| US | 0.002 | 0.0010 | … | Beta | [21] |
| Europe | 0.0014 | 0.0006 | … | Beta | [21] |
| Portugal | 0.0014b | 0.0006b | … | Beta | [21] |
| Immediate reactions | |||||
| Outpatients | |||||
| All regions | 0.0006 | 0.0005 | … | Beta | [21] |
| US | 0.0006c | 0.0005c | … | Beta | [21] |
| Europe | 0.0005 | 0.0004 | … | Beta | [21] |
| Portugal | 0.0005b | 0.0004b | … | Beta | [21] |
| Nonimmediate reactions | |||||
| Outpatients | |||||
| All regions | 0.0007 | 0.0006 | … | Beta | [21] |
| US | 0.0007c | 0.0006c | … | Beta | [21] |
| Europe | 0.0007 | 0.0006 | … | Beta | [21] |
| Portugal | 0.0007b | 0.0006b | … | Beta | [21] |
| False-negative DC | |||||
| Nondiscriminated reaction timing | |||||
| Inpatients | |||||
| All regions | 0.0097 | 0.0066 | … | Beta | [21] |
| US | 0.012 | 0.0100 | … | Beta | [21] |
| Europe | 0.0097c | 0.0066c | … | Beta | [21] |
| Portugal | 0.0097c | 0.0066c | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.017 | 0.0100 | … | Beta | [21] |
| US | 0.011 | 0.0029 | … | Beta | [21] |
| Europe | 0.007 | 0.0024 | … | Beta | [21] |
| Portugal | 0.017a | 0.0235a | … | Beta | [21] |
| Immediate reactions | |||||
| Inpatients | |||||
| All regions | 0.007 | 0.0066 | … | Beta | [21] |
| US | 0.007c | 0.0066c | … | Beta | [21] |
| Europe | 0.005 | 0.0045 | … | Beta | [21] |
| Portugal | 0.005b | 0.0045b | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.012 | 0.0100 | … | Beta | [21] |
| US | 0.012c | 0.0100c | … | Beta | [21] |
| Europe | 0.011 | 0.0100 | … | Beta | [21] |
| Portugal | 0.011b | 0.0100b | … | Beta | [21] |
| Nonimmediate reactions | |||||
| Inpatients | |||||
| All regions | 0.041 | 0.0283 | … | Beta | [21] |
| US | 0.041c | 0.0283c | … | Beta | [21] |
| Europe | 0.037 | 0.0332 | … | Beta | [21] |
| Portugal | 0.037b | 0.0332b | … | Beta | [21] |
| Outpatients | |||||
| All regions | 0.048 | 0.0300 | … | Beta | [21] |
| US | 0.048c | 0.0300b | … | Beta | [21] |
| Europe | 0.048 | 0.0400 | … | Beta | [21] |
| Portugal | 0.048c | 0.0400c | … | Beta | [21] |
| Positive DC without prior skin tests | |||||
| Nondiscriminated reaction timing | |||||
| Inpatients | |||||
| All regions | 0.048a | 0.8246a | … | Beta | [22–37] |
| US | 0.016a | 0.2164a | … | Beta | [22, 25, 27, 28, 30, 36] |
| Europe | 0.045a | 0.1507a | … | Beta | [26, 32, 34, 35, 37] |
| Portugal | 0.045b | 0.1507b | … | Beta | b |
| Outpatients | |||||
| All regions | 0.056a | 0.7708a | … | Beta | [23–26, 28–31, 33–37] |
| US | 0.017a | 0.3132a | … | Beta | [25, 28, 30, 36] |
| Europe | 0.055a | 0.6976a | … | Beta | [26, 34, 35, 37] |
| Portugal | 0.055b | 0.6976b | … | Beta | b |
| Severe reactions following a DC without prior skin tests | |||||
| Nondiscriminated reaction timing | |||||
| Inpatients | |||||
| All regions | 0.006a | 1.3256a | … | Beta | [22–37] |
| US | 0.003a | 0.5153a | … | Beta | [22, 25, 27, 28, 30, 36] |
| Europe | 0.009a | 0.6929a | … | Beta | [26, 32, 34, 35, 37] |
| Portugal | 0.009b | 0.6929b | … | Beta | b |
| Outpatients | |||||
| All regions | 0.006a | 1.2244a | … | Beta | [23–26, 28–31, 33–37] |
| US | 0.004a | 0.7044a | … | Beta | [25, 28, 30, 36] |
| Europe | 0.005a | 0.6303a | … | Beta | [26, 34, 35, 37] |
| Portugal | 0.005b | 0.6303b | … | Beta | b |
| B. Health services use | |||||
| Baseline average length of stay, d | |||||
| All regions | 6.3 | 16.7 | … | Gamma | [38–42] |
| US | 4.5 | 9.8 | … | Gamma | [38] |
| Europe | 6.7 | 19.2 | … | Gamma | [39–42] |
| Portugal | 7.6 | 15.5 | … | Gamma | [42] |
| Increase in LOS (multiplier) for patients with a penicillin allergy labeld | |||||
| All regions | 1.066 | 0.0578 | … | Gamma | [10–12, 43–54] |
| US | 1.099 | 0.0786 | … | Gamma | [10, 43–46, 48, 53] |
| Europe | 1.084 | 0.0066 | … | Gamma | [11, 12, 52] |
| Portugal | 1.088 | 0.0067 | … | Gamma | [11, 52] |
| Baseline frequency of readmissions (proportion) | |||||
| All regions | 0.095 | 0.0316 | … | Beta | [38, 55–58] |
| US | 0.139 | 0.024e | … | Beta | [38] |
| Europe | 0.080 | 0.0141 | … | Beta | [55–58] |
| Portugal | 0.068 | 0.0173e | … | Beta | [56] |
| Increase in readmissions rate (multiplier) for patients with a penicillin allergy label (log-normal scale)d | |||||
| All regions | 0.238 | 0.0541 | … | Log-normal | [12, 45, 46, 50, 59] |
| US | 0.224 | 0.0858 | … | Log-normal | [45, 46] |
| Europe | 0.189 | 0.0800 | … | Log-normal | [12] |
| Portugal | 0.189b | 0.0800b | … | Log-normal | b |
| Baseline No. of outpatient visits within 5 y | |||||
| All regions | 32.90 | … | 38.50 | Log-normal | [60–63] |
| US | 20.67 | … | 23.15e | Log-normal | [62, 63] |
| Europe | 38.34 | … | 41.17 | Log-normal | [61, 63] |
| Portugal | 18.15 | … | 20.33e | Log-normal | [61, 63] |
| Increase in outpatient visits’ frequency (multiplier) for patients with a penicillin allergy labeld | |||||
| All regions | 0.585f | 0.5098f | … | Log-normal | [13, 15, 64, 65] |
| US | 0.160f | 0.0877f | … | Log-normal | [15, 64, 65] |
| Europe | 5.28f | … | 1.67f | Log-normal | [13] |
| Portugal | 5.28b | … | 1.67 b | Log-normal | b |
| C. Costs (2017 US dollars) | |||||
| Hospital bed-day (“daily hotel costs”) | |||||
| All regions | 684 | … | 543 | Log-normal | [63, 66–69] |
| US | 1728 | … | 1167e | Log-normal | [63, 66, 67] |
| Europe | 433 | … | 256 | Log-normal | [63, 66, 68] |
| Portugal | 322 | … | 217e | Log-normal | [63, 66, 68] |
| Outpatient visit | |||||
| All regions | 58 | … | 61 | Log-normal | [63, 66] |
| US | 103 | … | 96e | Log-normal | [63, 66] |
| Europe | 40 | … | 32 | Log-normal | [63, 66] |
| Portugal | 32 | … | 30e | Log-normal | [63, 66] |
| Antibiotics for hospitalized patients with no penicillin allergy labeld | |||||
| All regions | 245 | 139 | … | Gamma | [8, 9, 47, 48, 51, 59, 70–77] |
| US | 260 | 167 | … | Gamma | [8, 9, 48, 71, 73, 76] |
| Europe | 212 | 61 | … | Gamma | [70, 72, 75, 77] |
| Portugal | 212b | 61b | … | Gamma | b |
| Antibiotics for hospitalized patients with penicillin allergy labeld | |||||
| All regions | 461 | 216 | … | Gamma | [8, 9, 47, 48, 51, 59, 70–77] |
| US | 513 | 217 | … | Gamma | [8, 9, 48, 71, 73, 76] |
| Europe | 432 | 177 | … | Gamma | [70, 72, 75, 77] |
| Portugal | 432b | 177b | … | Gamma | b |
| Antibiotics for outpatients with no penicillin allergy labeld,g | |||||
| All regions | 37 | 32 | … | Gamma | [64, 65, 78, 79] |
| US | 37 | 32 | … | Gamma | [64, 65, 78, 79] |
| Europe | 37c | 32c | … | Gamma | c |
| Portugal | 37c | 32c | … | Gamma | c |
| Antibiotics for outpatients with penicillin allergy labeld,g | |||||
| All regions | 59 | 45 | … | Gamma | [64, 65, 78, 79] |
| US | 59 | 45 | … | Gamma | [64, 65, 78, 79] |
| Europe | 59c | 45c | … | Gamma | c |
| Portugal | 59c | 45c | … | Gamma | c |
| Epinephrine auto-injector | |||||
| All regions | Uniform distribution (range, $62–$730) | [80–82] | |||
| US | Discrete values: $375; $494; $730 | [80] | |||
| Europe | Uniform distribution (range, $50–$100) | [81, 82] | |||
| Portugal | Discrete values: $62; $6 | [82] | |||
| Skin tests | |||||
| Literature search-retrieved amounts | |||||
| Inpatients | |||||
| All regions | 86 | 39 | … | Gamma | [10, 21, 71, 83–85] |
| US | 102 | 39 | … | Gamma | [10, 71, 83, 84] |
| Europe | 64 | 27 | … | Gamma | [21, 85] |
| Portugalh | 74 | 29 | … | Gamma | [86] |
| Outpatients | |||||
| All regions | 106 | 41 | … | Gamma | [10, 21, 71, 83–85] |
| US | 120 | 40 | … | Gamma | [10, 71, 83, 84] |
| Europe | 87 | 33 | … | Gamma | [85, 86] |
| Portugalh | 96 | 38 | … | Gamma | [86] |
| Survey-retrieved amounts | |||||
| Inpatients | |||||
| All regions | 77 | 55 | … | Gamma | [86] |
| US | 135 | 53 | … | Gamma | [86] |
| Europe | 72 | 53 | … | Gamma | [86] |
| Portugal | 74 | 75 | … | Gamma | [86] |
| Outpatients | |||||
| All regions | 83 | 65 | … | Gamma | [86] |
| US | 107 | 37 | … | Gamma | [86] |
| Europe | 81 | 67 | … | Gamma | [86] |
| Portugal | 94 | 108 | … | Gamma | [86] |
| Drug challenges | |||||
| Literature search-retrieved amounts | |||||
| Inpatients | |||||
| All regions | 80 | 13 | … | Gamma | [10, 83–86] |
| US | 78 | 18 | … | Gamma | [10, 83, 84] |
| Europe | 81 | 5 | … | Gamma | [85, 86] |
| Portugalh | 84 | 1 | … | Gamma | [86] |
| Outpatients | |||||
| All regions | 94 | 16 | … | Gamma | [10, 83–86] |
| US | 91 | 19 | … | Gamma | [10, 83, 84] |
| Europe | 101 | 2 | … | Gamma | [85, 86] |
| Portugalh | 102 | 1 | … | Gamma | [86] |
| Survey-retrieved amounts | |||||
| Inpatients | |||||
| All regions | 171 | 142 | … | Gamma | [86] |
| US | 173 | 38 | … | Gamma | [86] |
| Europe | 171 | 148 | … | Gamma | [86] |
| Portugal | 127 | 150 | … | Gamma | [86] |
| Outpatients | |||||
| All regions | 273 | 245 | … | Gamma | [86] |
| US | 208 | 59 | … | Gamma | [86] |
| Europe | 279 | 255 | … | Gamma | [86] |
| Portugal | 193 | 228 | … | Gamma | [86] |
Abbreviations: DC, drug challenge; LOS, length of stay; SD, standard deviation; US, United States.
aBeta distribution parameters estimated by means of program evaluation and review technique methods.
bIn the absence of satisfactory specific Portuguese data, parameters from existent European studies were used.
cIn the absence of satisfactory specific regional data, parameters from all regions were used.
d Supplementary Figure 1 illustrates the selection process resulting in the identification of the included studies.
eEstimates based on the average ratios obtained for the remaining regions.
fData for all regions and for US models were obtained by means of meta-analysis of several studies. Regarding Europe, only 1 study was available.
gOne-year estimate.
hCorresponds to estimates obtained by means of formal cost assessments; survey-obtained data were solely used in the context of “survey-retrieved amounts.”
Data Analysis
For each decision model, we projected the respective incremental net benefit, defined as the cost difference between testing and not testing for penicillin allergy. An incremental net benefit > 0 identified testing as the less costly alternative.
These analyses were based on base case input values (“most likely input values”) for all variables in every decision model (Table 1). However, all these estimates have an associated uncertainty, which we addressed through sensitivity analyses. We performed univariate/1-way deterministic sensitivity analyses on the percentage of patients with negative tests who must be treated as nonallergic for penicillin allergy testing to become cost-saving, because, in clinical practice, a substantial percentage of patients with negative testing are not appropriately delabeled in medical records, get relabeled, and/or are incorrectly treated as being penicillin allergic [9, 87]. In addition, we performed probabilistic sensitivity analyses via Monte Carlo simulation methods. That is, for each mode, we ran 10 000 simulations with variables not assuming base case input values, but rather any allowed value according to their distribution of probabilities (Table 1). From the probabilistic sensitivity analyses performed, we retrieved the proportion of simulations identifying penicillin allergy testing as cost-saving, and the average and median incremental net benefits. Therefore, for each model, we determined whether penicillin allergy testing was cost-saving under base case assumptions, and how many simulations identified penicillin allergy testing as cost-saving when the values of variables varied under a distribution of probabilities. To identify the variables more strongly associated with higher or lower incremental net benefits, we performed univariable linear regressions with the dependent variable corresponding to the incremental net benefits obtained in the set of simulations resulting from each decision model. Decision models and sensitivity analyses were performed using TreeAgePro 2019 (TreeAge Software, Williamstown, Massachusetts), while all other statistical analyses were performed using software R (version 3.5.0).
Additional Sensitivity Analyses
In addition to our base case models, we performed additional sensitivity analyses with models (1) not discriminating allergic reaction timing (ie, not distinguishing immediate from nonimmediate reactions), (2) taking into account the possibility of delabeling patients solely based on the clinical history, (3) using alternative data sources [88], or (4) restricting inputs to studies that controlled for confounding by matching or multivariable analyses (Supplementary Methods).
RESULTS
In all decision models built, under base case analyses, performing penicillin allergy testing was associated with lower costs than not testing (incremental net benefits > 0) (Tables 2 and 3 and Figure 3). For models in which the penicillin allergy diagnostic workup encompassed the sequential performance of skin testing and DC, the average incremental net benefit for inpatients was $657 ($1444 for the US vs $489 for Europe), while for outpatients it was $2746 (US: $256; Europe: $6045). For inpatients, an average minimum of 23.0% individuals with negative tests need to be effectively delabeled so that penicillin allergy testing becomes cost-saving (US: 10.4%; Europe: 21.3%); for outpatients, this percentage decreases to 16.2% (US: 46.4%; Europe: 2.9%).
Table 2.
Results of Decision Models Testing Performing Versus Not Performing Sequential Diagnostic Testinga in Patients With a Penicillin Allergy Label
| Model Characteristics | Cost Associated With Performing Allergy Testsb,c | Cost Associated With Not Performing Allergy Testsb,c | Incremental Net Benefitb,d | Minimum % Delabeled So That Testing Is Cost- savinge | Probabilistic Sensitivity Analysis Results | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting/Type of Patients | Region | % of Simulations With Testing as Best Strategy | Average Incremental Net Benefit | Median Incremental Net Benefit (25th–75th Percentile) | % Delabeled So That Testing is the Best Strategy in > 50% of Simulationsf | ||||
| Inpatients | All | $5004 | $5379 | $375 | 28.9% | 66.8% | $361 | $115 (−$53 to $356) | 56% |
| US | $14 005 | $15 449 | $1444 | 10.4% | 78.0% | $1492 | $277 ($29 to $892) | 37% | |
| Europe | $5825 | $6314 | $489 | 21.3% | 72.2% | $491 | $116 (−$14 to $318) | 53% | |
| Portugal | $4218 | $4536 | $319 | 31.2% | 72.4% | $328 | $124 (−$13 to $333) | 54% | |
| Outpatients | All | $3580 | $5887 | $2308 | 8.2% | 83.1% | $2368 | $1178 ($269–$3035) | 15% |
| US | $3049 | $3307 | $256 | 46.4% | 72.6% | $258 | $166 (−$20 to $426) | 57% | |
| Europe | $3493 | $9538 | $6045 | 2.9% | 60.1% | $6291 | $513 (−$456 to $3882) | 29% | |
| Portugal | $1662 | $4037 | $2375 | 7.4% | 56.7% | $2375 | $157 (−$300 to $1610) | 51% |
Abbreviations: $, United States dollars; US, United States.
aCorresponding to skin testing followed, if negative, by a drug challenge.
bBase case analyses results.
cSum of costs involving (1) performance of penicillin allergy tests and (2) consequences (as expressed in monetary units) resulting from healthcare use.
dAn incremental net benefit > 0 indicates that penicillin allergy testing is cost-saving.
eCorresponds to the minimum percentage of patients with negative penicillin allergy testing that needs to be effectively treated as nonallergic so that testing becomes cost-saving (incremental net benefit > 0).
fCorresponds to the minimum percentage of patients with negative penicillin allergy testing that needs to be effectively treated as nonallergic so that at least half of simulations performed by probabilistic sensitivity analysis identify penicillin allergy testing as cost-saving.
Table 3.
Results of Decision Models Testing Performing Versus Not Performing Direct Drug Challengesa in Patients With a Penicillin Allergy Label
| Model Characteristics | Cost Associated With Performing Allergy Testsb,c | Cost Associated With Not Performing Allergy Testsb,c | Incremental Net Benefitb,d | Minimum % Delabeled So That Testing is Cost-savinge | Probabilistic Sensitivity Analysis Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Type | Cases in Which a Direct DC Was Performed | Region | % of Simulations With Testing as Best Strategy | Average Incremental Net Benefit | Median Incremental Net Benefit (25th–75th Percentile) | % Delabeled So That Testing Is the Best Strategy in > 50% of Simulationsf | ||||
| Inpatients | NIR | All | $4888 | $5379 | $492 | 16.6% | 76.4% | $492 | $197 ($10–$472) | 33% |
| US | $13 657 | $15 449 | $1792 | 5.2% | 86.0% | $1764 | $418 ($134–$1063) | 19% | ||
| Europe | $5719 | $6314 | $595 | 14.0% | 81.3% | $582 | $183 ($39–$406) | 35% | ||
| Portugal | $4123 | $4536 | $414 | 19.9% | 82.2% | $410 | $192 ($44–$424) | 36% | ||
| All | All | $4851 | $5362 | $511 | 13.5% | 78.4% | $521 | $224 ($29–$501) | 26% | |
| US | $9291 | $10 634 | $1343 | 5.5% | 86.4% | $1339 | $412 ($150–$934) | 16% | ||
| Europe | $3495 | $3924 | $429 | 15.9% | 83.7% | $432 | $187 ($50–$388) | 31% | ||
| Portugal | $2870 | $3211 | $341 | 19.8% | 84.0% | $342 | $196 ($53–$394) | 30% | ||
| Outpatients | NIR | All | $3518 | $5887 | $2369 | 5.4% | 84.6% | $2368 | $1292 ($323–$3020) | 10% |
| US | $2952 | $3307 | $355 | 27.8% | 84.0% | $358 | $260 ($70–$537) | 35% | ||
| Europe | $3360 | $9538 | $6178 | 2.0% | 61.4% | $5809 | $552 (−$395 to 3867) | 20% | ||
| Portugal | $1542 | $40 367 | $2495 | 5.1% | 59.4% | $2670 | $246 (−$253 to $1842) | 40% | ||
| All | All | $3480 | $6124 | $2644 | 3.8% | 85.7% | $2665 | $1443 ($380–$3466) | 7% | |
| US | $2890 | $3307 | $417 | 18.8% | 88.1% | $410 | $309 ($115–$578) | 23% | ||
| Europe | $2793 | $9538 | $6745 | 1.5% | 62.8% | $6566 | $664 (−$393 to $4520) | 15% | ||
| Portugal | $1355 | $4037 | $2682 | 3.8% | 61.5% | $2767 | $291 (−$232 to $1948) | 28% |
In cases not reporting a nonimmediate reaction, skin tests were performed and, if negative, followed by a drug challenge.
Abbreviations: $, United States dollars; NIR, nonimmediate reaction; US, United States.
aDirect drug challenges correspond to drug challenges without prior skin tests.
bBase case analyses results.
cSum of costs involving (1) performance of penicillin allergy tests and (2) consequences (as expressed in monetary units) resulting from healthcare use.
dAn incremental net benefit > 0 indicates that penicillin allergy testing is cost-saving.
eCorresponds to the minimum percentage of patients with negative penicillin allergy testing that needs to be effectively treated as nonallergic so that testing becomes cost-saving (incremental net benefit > 0).
fCorresponds to the minimum percentage of patients with negative penicillin allergy testing that needs to be effectively treated as nonallergic so that at least half of simulations performed by probabilistic sensitivity analysis identify penicillin allergy testing as cost-saving.
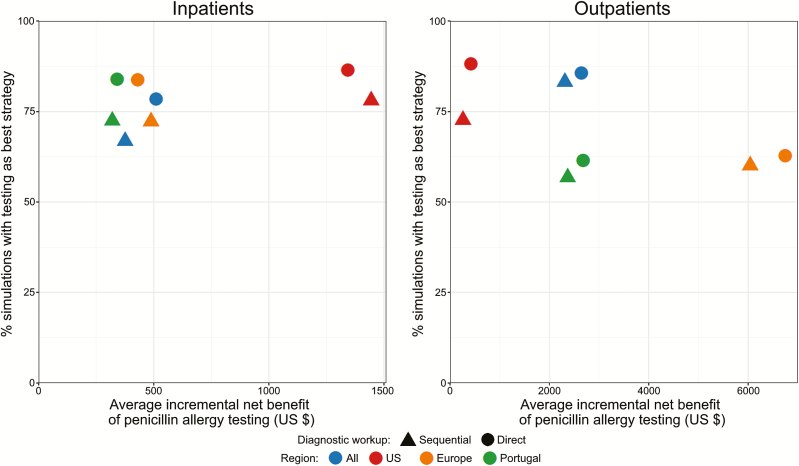
Figure 3.
Summary of the results of economic decision models by setting and region. Incremental net benefits (difference of net benefits, with an incremental net benefit > 0 indicating that penicillin allergy testing is cost-saving) obtained through use of fixed values were plotted against the percentage of simulations (obtained via probabilistic sensitivity analysis) identifying penicillin allergy testing as cost-saving. Sequential diagnostic workup corresponds to the performance of skin tests followed, if negative, by drug challenges. Direct diagnostic testing corresponds to the direct performance of drug challenges without prior skin tests (either in all patients or only in those reporting nonimmediate reactions). Abbreviation: US, United States.
In probabilistic sensitivity analyses, 70.2% of all simulations identified penicillin allergy testing as the less costly strategy (Table 2; Supplementary Figure 2). In all decision models, testing was cost-saving in more than half of simulations.
For models in which skin tests were only performed in patients reporting immediate reactions (with direct DC performed in the remaining patients) (Table 3), the average incremental net benefit was $823 for inpatients (US: $1792; Europe: $595) and $2849 for outpatients (US: $355; Europe: $6178). In probabilistic sensitivity analyses, penicillin allergy testing was the less expensive option in 76.9% of simulations.
For models in which DC were directly performed in all patients irrespective of the reported reaction timing (Table 3), the average incremental net benefit was $656 for inpatients (US: $1343; Europe: $429) and $3122 for outpatients (US: $417; Europe: $6745). The minimum percentage of patients needing to be delabeled so that penicillin allergy testing becomes cost-saving was 13.7% for inpatients (US: 5.5%; Europe: 15.9%) and 7.0% for outpatients (US: 18.8%; Europe: 1.5%). In probabilistic sensitivity analyses, penicillin allergy testing was found to be the less costly strategy in 78.8% of simulations.
Results were similar when performing additional sensitivity analyses (1) not distinguishing immediate from nonimmediate reactions, (2) considering the possibility of delabeling patients solely based on the clinical history, (3) using alternative sources [86, 89], or (4) restricting inputs to studies that controlled for confounding (Supplementary Tables 1–4).
Supplementary Figure 3 shows the results of the linear regression models performed to identify the variables whose increase was more strongly associated with higher incremental net benefits.
DISCUSSION
In this economic evaluation study, we projected that penicillin allergy testing would be cost-saving for both inpatients and outpatients in the US and Europe, with an incremental net benefit ranging between $256 and $6745. In addition, in probabilistic sensitivity analyses with variables varying according to a distribution of probabilities, more than three-fourths of simulations identified penicillin allergy testing as cost-saving. However, these results are based on existing evidence and on model-based simulation methods. While those are representative scenarios of the most usual practices, unstudied scenarios cannot not be excluded, especially since penicillin allergy testing is highly heterogeneous [86] and antibiotic utilization patterns vary globally. On account of that, it is expected that in some institutions (eg, those in which patients reporting a penicillin allergy are systematically treated with cephalosporins), penicillin allergy testing does not result in such substantial inpatient savings. While highlighting the need for complementary context-based economic assessments, this does not diminish the importance of our study, as, in current clinical practice, aztreonam and non-β-lactams are more frequently chosen in patients reporting a penicillin allergy [9, 59, 75].
The proportion of cost-saving simulations was higher for models using US-based data, whereas European models tended to result in higher incremental net benefits. This difference reflects the higher hospitalization costs and the lower frequency of ambulatory visits observed in the US. In addition, it reflects the uncertainty related to the relative lack of European studies assessing the consequences of having a penicillin allergy label. Indeed, the particularly high incremental net benefits obtained with models assessing European outpatients are likely explained by the existence of only a single Dutch study that compared the frequency of outpatient visits among patients with and without a penicillin allergy label, and which found large differences [13]. When using American data for that variable, European models yield lower incremental net benefits, but higher percentages of simulations identifying testing as the best strategy—for sequential performance of skin testing and DC, an incremental net benefit of $128 was observed, with 61% of simulations identifying testing as the best strategy; these values go up to $183 and 72% when direct DC is performed in outpatients reporting nonimmediate reactions, and to $237 and 78% when direct DC is performed in all outpatients.
As expected, since direct DC is less costly than a full diagnostic evaluation [83], the percentage of cost-saving simulations was higher for models performing direct DC. We estimated that, for each false-positive skin test, an average of $1022 for inpatients and $3601 for outpatients would be saved if DC had been performed instead. These estimates are lower than those of studies assessing specific populations [16], reflecting the incorporation of information from multiple sources. However, direct DC is currently only advisable for “low-risk patients.” These correspond to individuals reporting mild reactions or a clinical history poorly compatible with a true allergic reaction, in whom the frequency of severe events following a direct DC is deemed to be < 1% [89]. Nevertheless, there is accumulating evidence suggesting that direct DC can be safely performed in broader contexts [22, 36].
In addition to skin tests and DC, the European Network for Drug Allergy recommends the performance of in vitro tests for patients reporting immediate reactions. In our decision models, penicillin allergy testing remained cost-saving even considering the additional quantification of specific immunoglobulin E (IgE). In fact, 68.9% of simulations identified testing as the least expensive strategy when considering a percentage of positive IgE results of 13.5%, and average quantification costs of $46 for inpatients and $71 for outpatients [21, 85]. However, data on the accuracy and costs of IgE quantification are limited [90].
The base case scenario in our model assumed that all patients with a negative diagnostic workup would be treated as nonallergic. Nevertheless, current evidence suggests that as many as half of the patients with negative results may not get correctly delabeled (or get erroneously relabeled) [9, 87]. For the sequential performance of skin tests and DC to be cost-saving, a minimum of 30%–60% of inpatients (depending on whether base case input values or the results of probabilistic sensitivity analyses are being considered) and 10%–50% of outpatients would need to be effectively delabeled. These percentages notably decrease to 15%–30% in inpatients and 5%–30% in outpatients when considering direct DC only. It is thus possible that penicillin allergy testing is cost-saving even when less than half of the patients with negative tests are treated as nonallergic, although this depends on the setting in which testing is performed. This issue highlights the importance of delabeling patients with a negative diagnostic workup, and of opting for direct DC at least in “low risk” patients.
This study has some limitations. We adopted the health services perspective, as we were not able to assess such costs as those related to transportation, patient and caregiver time, and productivity. Productivity costs would be particularly difficult to measure accurately, not least on account of the paucity of information regarding the sociodemographic composition of patients with a penicillin allergy label seeking healthcare (average wages probably not being good indicators of productivity losses among those patients [3]). In addition, the lifelong burden of having a penicillin allergy label was not fully considered. In fact, for inpatients, our models only took into account that specific hospitalization and any possible readmission within the next month after discharge, ignoring potential savings in subsequent hospitalizations or in the use of ambulatory care. For outpatients, we assessed a 5-year time horizon and did not consider the possibility of hospitalizations during that period. These choices reflected the time horizon of primary studies that evaluated the healthcare use of patients with a penicillin allergy label, with only a few having a follow-up period of more than a year [15]. However, demonstrating savings adopting the health services perspective and with these short follow-up periods is a conservative strategy. True savings are likely far greater than those projected, particularly if patient productivity/time gains are accounted for (ie, for most outpatients, productivity/time loss for testing would be compensated by larger future gains related to decreased healthcare use), transportation savings, and a potential lifelong delabeling. Such savings were also greater when considering—as indicated in some pathways [20] – the possibility of delabeling some patients based on clinical history alone, notably those whose clinical history is incompatible with that of a true allergic reaction (Supplementary Table 2).
Another important limitation results from the fact that literature-based evidence was obtained by studies that often adopted different methodologies and definitions. Such heterogeneity mirrors the diversity in the practice of penicillin allergy testing and may explain differences in the results obtained across different studies. We tried to minimize the impact of that variability by defining distributions of probabilities for each variable, based on which we performed probabilistic sensitivity analysis. In addition, most evidence was obtained by retrospective studies for which confounding may have been incompletely controlled. Patients with a penicillin allergy label are known to be demographically different and have more comorbidities than those without such label [3]. Additionally, among patients with a penicillin allergy label, those who get tested tend to have more morbidity than the remainder and tend to be those who need testing prior to planned/required antibiotic use. Therefore, it is not possible to causally prove that increased healthcare use among patients with a penicillin allergy label is necessarily because of such label. Nevertheless, we performed sensitivity analyses restricted to studies that at least partially addressed confounding. In those analyses, penicillin allergy testing was still projected to be cost-saving, although with smaller incremental net benefits for US patients. Further prospective follow-up studies are needed to more accurately assess the impact of delabeling patients claiming a penicillin allergy label.
This study has also several strengths. This is the first economic evaluation study based on decision analytic modeling assessing whether penicillin allergy testing is cost-saving across methods of testing, settings, and regions. Our input parameters were obtained from diverse information sources, including scientific literature, administrative databases, and technical reports. In addition, when estimating parameters based on the literature, we performed systematic or comprehensive searches of the literature with the aim of obtaining data from the largest number of relevant studies. Data from these different studies were then pooled by means of meta-analysis, so that the more precise studies provided a larger contribution to our estimates. We performed probabilistic sensitivity analyses to explore the uncertainty associated with our estimates. Finally, we performed ancillary sensitivity analyses, with penicillin allergy testing projected to be cost-saving even under more conservative assumptions.
In conclusion, penicillin allergy testing was projected to be cost-saving across an array of testing strategies and scenarios. While not precluding the need for context-based assessments, this study provides evidence that verification of reported penicillin allergy has economic advantages. If patients are successfully delabeled and are treated as not penicillin-allergic, advantages may be even higher than the presented incremental net benefits. These results are devised to inform guidelines, supporting the adoption of policies promoting generalized penicillin allergy testing on economic grounds, in addition to clinical grounds.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This work was supported by the Portuguese Ministry for Science, Technology and Higher Education (Ministério da Ciência, Tecnologia e Ensino Superior), through the Portuguese Foundation for Science and Technology (Fundação para a Ciência e Tecnologia).
Disclaimer. The funding sources had no involvement in study design, data collection, analysis, interpretation, report writing, or the decision to submit the article for publication. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) the American Academy of Allergy, Asthma and Immunology (AAAAI) Foundation, or the Massachusetts General Hospital (MGH).
Financial support. This work was supported by the Portuguese Foundation for Science and Technology (grant number PD/BD/129836/2017 to B. S.-P.); the NIH (grant number K01AI125631 to K. G. B.); the AAAAI Foundation (support to K. G. B.); and the MGH Claflin Distinguished Scholar Award (to K. G. B.).
Potential conflicts of interest. K. G. B. has a clinical decision support tool for inpatient β-lactam allergy evaluation used at Partners HealthCare Systems and licensed to Persistent Systems. E. M. has received research grants from ALK (the sellers of Pre-Pen in the United States) and has consulted for and is serving on a data and safety monitoring board for Audentes. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sousa-Pinto B, Fonseca JA, Gomes ER. Frequency of self-reported drug allergy: a systematic review and meta-analysis with meta-regression. Ann Allergy Asthma Immunol 2017; 119:362–73.e2. [DOI] [PubMed] [Google Scholar]
- 2. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy 2004; 34:1597–601. [DOI] [PubMed] [Google Scholar]
- 3. Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med 2009; 122:778.e1–7. [DOI] [PubMed] [Google Scholar]
- 4. Zhou L, Dhopeshwarkar N, Blumenthal KG, et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016; 71:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harandian F, Pham D, Ben-Shoshan M. Positive penicillin allergy testing results: a systematic review and meta-analysis of papers published from 2010 through 2015. Postgrad Med 2016; 128:557–62. [DOI] [PubMed] [Google Scholar]
- 6. Torres MJ, Romano A, Celik G, et al. Approach to the diagnosis of drug hypersensitivity reactions: similarities and differences between Europe and North America. Clin Transl Allergy 2017; 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet 2019; 393:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with β-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol 2016; 117:67–71. [DOI] [PubMed] [Google Scholar]
- 9. Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med 2013; 8:341–5. [DOI] [PubMed] [Google Scholar]
- 10. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 11. Sousa-Pinto B, Cardoso-Fernandes A, Araujo L, Fonseca JA, Freitas A, Delgado L. Clinical and economic burden of hospitalizations with registration of penicillin allergy. Ann Allergy Asthma Immunol 2018; 120:190–4.e2. [DOI] [PubMed] [Google Scholar]
- 12. van Dijk SM, Gardarsdottir H, Wassenberg MW, Oosterheert JJ, de Groot MC, Rockmann H. The high impact of penicillin allergy registration in hospitalized patients. J Allergy Clin Immunol Pract 2016; 4:926–31. [DOI] [PubMed] [Google Scholar]
- 13. Su T, Broekhuizen BDL, Verheij TJM, Rockmann H. The impact of penicillin allergy labels on antibiotic and health care use in primary care: a retrospective cohort study. Clin Transl Allergy 2017; 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mattingly TJ 2nd, Meninger S, Heil EL. Penicillin skin testing in methicillin-sensitive Staphylococcus aureus bacteremia: a cost-effectiveness analysis. PLoS One 2019; 14:e0210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macy E, Shu YH. The effect of penicillin allergy testing on future health care utilization: a matched cohort study. J Allergy Clin Immunol Pract 2017; 5:705–10. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Shahabi-Sirjani A, Figtree M, Hoyle P, Fernando SL. Safety of direct drug provocation testing in adults with penicillin allergy and association with health and economic benefits. Ann Allergy Asthma Immunol 2019; 123:468–75. [DOI] [PubMed] [Google Scholar]
- 17. Husereau D, Drummond M, Petrou S, et al. CHEERS Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013; 346:f1049. [DOI] [PubMed] [Google Scholar]
- 18. Joint Task Force on Practice Parameters, American Academy of Allergy Asthma and Immunology, American College of Allergy Asthma and Immunology, Joint Council of Allergy Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 19. Demoly P, Adkinson NF, Brockow K, et al. International consensus on drug allergy. Allergy 2014; 69:420–37. [DOI] [PubMed] [Google Scholar]
- 20. Chiriac AM, Banerji A, Gruchalla RS, et al. Controversies in drug allergy: drug allergy pathways. J Allergy Clin Immunol Pract 2019; 7:46–60.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sousa-Pinto B, Tarrio I, Blumenthal KG, et al. Accuracy of penicillin allergy diagnostic tests: A systematic review and meta-analysis. 2019. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep 2019; 19:27. [DOI] [PubMed] [Google Scholar]
- 23. Choi J, Lee JY, Kim KH, Choi J, Ahn K, Kim J. Evaluation of drug provocation tests in Korean children: a single center experience. Asian Pac J Allergy Immunol 2016; 34:130–6. [DOI] [PubMed] [Google Scholar]
- 24. Confino-Cohen R, Rosman Y, Meir-Shafrir K, et al. Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract 2017; 5:669–75. [DOI] [PubMed] [Google Scholar]
- 25. Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract 2019; 7:236–43. [DOI] [PubMed] [Google Scholar]
- 26. Ibáñez MD, Rodríguez Del Río P, Lasa EM, et al. Penicillin Allergy in Children (APENIN) Task Force; Pediatric Allergy Committee, Spanish Society of Allergy and Clinical Immunology (SEAIC) . Prospective assessment of diagnostic tests for pediatric penicillin allergy: from clinical history to challenge tests. Ann Allergy Asthma Immunol 2018; 121:235–44.e3. [DOI] [PubMed] [Google Scholar]
- 27. Kao L, Rajan J, Roy L, Kavosh E, Khan DA. Adverse reactions during drug challenges: a single US institution’s experience. Ann Allergy Asthma Immunol 2013; 110:86–91.e1. [DOI] [PubMed] [Google Scholar]
- 28. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc 2019; 40:57–61. [DOI] [PubMed] [Google Scholar]
- 29. Labrosse R, Paradis L, Lacombe-Barrios J, et al. Efficacy and safety of 5-day challenge for the evaluation of nonsevere amoxicillin allergy in children. J Allergy Clin Immunol Pract 2018; 6:1673–80. [DOI] [PubMed] [Google Scholar]
- 30. Lindsey D, Banks T. Efficient identification and clearance of low-risk penicillin allergy patients. Ann Allergy Asthma Immunol 2018; 121:S15. [Google Scholar]
- 31. Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016; 170:e160033. [DOI] [PubMed] [Google Scholar]
- 32. Murphy K, Scanlan B, Coghlan D. Does this child really have a penicillin allergy? Ir Med J 2015; 108:103, 105–6. [PubMed] [Google Scholar]
- 33. Noh SR, Yoon J, Cho HJ, et al. Outcomes of drug provocation tests in Korean children with suspected drug hypersensitivity reaction. Allergy Asthma Res Dis 2018; 6:26–33. [Google Scholar]
- 34. García Rodriguez R, Moreno Lozano L, Extremera Ortega A, Borja Segade J, Galindo Bonilla P, Gómez Torrijos E. Provocation tests in nonimmediate hypersensitivity reactions to beta-lactam antibiotics in children: are extended challenges needed? J Allergy Clin Immunol Pract 2019; 7:265–9. [DOI] [PubMed] [Google Scholar]
- 35. Savic L, Gurr L, Kaura V, et al. Penicillin allergy de-labelling ahead of elective surgery: feasibility and barriers. Br J Anaesth 2018; 123:e110–6. [DOI] [PubMed] [Google Scholar]
- 36. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017; 5:813–5. [DOI] [PubMed] [Google Scholar]
- 37. Vezir E, Dibek Misirlioglu E, Civelek E, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol 2016; 27:50–4. [DOI] [PubMed] [Google Scholar]
- 38. Agency for Healthcare Research and Quality. HCUPnet—Healthcare Cost and Utilization Project. Available at: https://hcupnet.ahrq.gov. Accessed 1 February 2020.
- 39. NHS Digital. Hospital episode statistics. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity. Accessed 1 February 2020.
- 40. Spanish National Statistics Institute. Hospital morbidity survey. Available at: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176778&menu=ultiDatos&idp=1254735573175. Accessed 1 February 2020.
- 41. German Federal Statistical Office. Hospital statistics—basic data of the hospitals and the prevention or rehabilitation facilities [in German]. Available at: http://www.gbe-bund.de/gbe10/. Accessed 1 February 2020.
- 42. Portuguese Central Administration of the Healthcare System. National database of diagnosis-related groups. Available at: https://dados.gov.pt/pt/datasets/morbilidade-e-mortalidade-hospitalar/. Accessed 1 February 2020. [Google Scholar]
- 43. Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis 2018; 66:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blumenthal KG, Shenoy ES, Huang M, et al. The impact of reporting a prior penicillin allergy on the treatment of methicillin-sensitive Staphylococcus aureus bacteremia. PLoS One 2016; 11:e0159406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Conway EL, Lin K, Sellick JA, et al. Impact of penicillin allergy on time to first dose of antimicrobial therapy and clinical outcomes. Clin Ther 2017; 39:2276–83. [DOI] [PubMed] [Google Scholar]
- 46. Huang KG, Cluzet V, Hamilton K, Fadugba O. The impact of reported beta-lactam allergy in hospitalized patients with hematologic malignancies requiring antibiotics. Clin Infect Dis 2018; 67:27–33. [DOI] [PubMed] [Google Scholar]
- 47. Irawati L, Hughes JD, Keen NJ, Golledge CL, Joyce AW. Influence of penicillin allergy on antibiotic prescribing patterns and costs. J Pharm Pract Res 2006; 36:286–90. [Google Scholar]
- 48. Jones BM, Avramovski N, Concepcion AM, Crosby J, Bland CM. Clinical and economic outcomes of penicillin skin testing as an antimicrobial stewardship initiative in a community health system. Open Forum Infect Dis 2019; 6:ofz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leis JA, Palmay L, Ho G, et al. Point-of-care β-lactam allergy skin testing by antimicrobial stewardship programs: a pragmatic multicenter prospective evaluation. Clin Infect Dis 2017; 65:1059–65. [DOI] [PubMed] [Google Scholar]
- 50. Lucas M, Arnold A, Sommerfield A, et al. Antibiotic allergy labels in children are associated with adverse clinical outcomes. J Allergy Clin Immunol Pract 2019; 7:975–82. [DOI] [PubMed] [Google Scholar]
- 51. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy 2003; 33:501–6. [DOI] [PubMed] [Google Scholar]
- 52. Sousa-Pinto B, Araújo L, Freitas A, Delgado L. Hospitalizations in children with a penicillin allergy label: an assessment of healthcare impact. Int Arch Allergy Immunol 2018; 176:234–8. [DOI] [PubMed] [Google Scholar]
- 53. Covington EW, Baldwin BJ, Warren E. Pharmacy-led β-lactam allergy interview (BLAI) reduces duration of fluoroquinolones within a community hospital. Ann Pharmacother 2019; 53:588–95. [DOI] [PubMed] [Google Scholar]
- 54. MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study. Clin Infect Dis 2016; 63:904–10. [DOI] [PubMed] [Google Scholar]
- 55. Friebel R, Hauck K, Aylin P, Steventon A. National trends in emergency readmission rates: a longitudinal analysis of administrative data for England between 2006 and 2016. BMJ Open 2018; 8:e020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sousa-Pinto B, Gomes AR, Oliveira A, et al. Hospital readmissions in Portugal over the last decade [in Portuguese]. Acta Med Port 2013; 26:711–20. [PubMed] [Google Scholar]
- 57. van der Laan J, Penning C, Bruin A. Hospital readmission ratio: methodological report of the 2015 models. The Hague: Statistics Netherlands, 2017. Available at: https://www.cbs.nl/-/media/_pdf/2017/25/2017mr03%20hospital%20readmission%20ratio.pdf. Accessed 1 February 2020. [Google Scholar]
- 58. van der Laan J, Penning C, Bruin A. Hospital readmission ratio: methodological report of the 2016 models. The Hague: Statistics Netherlands,2018. Available at: https://www.cbs.nl/en-gb/our-services/methods/surveys/aanvullende%20onderzoeksbeschrijvingen/hospital-readmission-ratio-models-2016. Accessed 1 February 2020. [Google Scholar]
- 59. Knezevic B, Sprigg D, Seet J, et al. The revolving door: antibiotic allergy labelling in a tertiary care centre. Intern Med J 2016; 46:1276–83. [DOI] [PubMed] [Google Scholar]
- 60. Organisation for Economic Co-operation and Development. OECD data: doctors’ consultations. Available at: https://data.oecd.org/healthcare/doctors-consultations.htm. Accessed 1 February 2020.
- 61. World Health Organization. Outpatient contacts per person per year. Available at: https://gateway.euro.who.int/en/indicators/hfa_543-6300-outpatient-contacts-per-person-per-year/. Accessed 1 February 2020.
- 62. Talwalkar A, Hing E, Palso K. National ambulatory medical care survey: 2011 summary tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2011_namcs_web_tables.pdf. Accessed 1 February 2020.
- 63. The World Bank. World Bank open data. Available at: https://data.worldbank.org/. Accessed 1 February 2020.
- 64. Kraemer MJ, Caprye-Boos H, Berman HS. Increased use of medical services and antibiotics by children who claim a prior penicillin sensitivity. West J Med 1987; 146:697–700. [PMC free article] [PubMed] [Google Scholar]
- 65. Macy E. Elective penicillin skin testing and amoxicillin challenge: effect on outpatient antibiotic use, cost, and clinical outcomes. J Allergy Clin Immunol 1998; 102:281–5. [DOI] [PubMed] [Google Scholar]
- 66. Stenberg K, Lauer JA, Gkountouras G, Fitzpatrick C, Stanciole A. Econometric estimation of WHO-CHOICE country-specific costs for inpatient and outpatient health service delivery. Cost Eff Resour Alloc 2018; 16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. American Hospital Association. AHA hospital statistics, 2018 ed. Chicago, IL: AHA, 2018. [Google Scholar]
- 68. Garel P, Notarangelo I. Hospitals in Europe: healthcare data. Hospital healthcare Europe 2017. Available at: https://hospitalhealthcare.com/latest-issue-2017/hospitals-in-europe-healthcare-data-8/. Accessed 11 March 2020.
- 69. Canadian Institute for Health Information. Hospital trends in Canada. Ottawa, Canada: CIHR, 2005. Available at: https://secure.cihi.ca/free_products/Hospital_Trends_in_Canada_e.pdf. Accessed 1 February 2020. [Google Scholar]
- 70. Borch JE, Andersen KE, Bindslev-Jensen C. The prevalence of suspected and challenge-verified penicillin allergy in a university hospital population. Basic Clin Pharmacol Toxicol 2006; 98:357–62. [DOI] [PubMed] [Google Scholar]
- 71. Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize antibiotic utilization. Am J Med 1999; 107:166–8. [DOI] [PubMed] [Google Scholar]
- 72. Li M, Krishna MT, Razaq S, Pillay D. A real-time prospective evaluation of clinical pharmaco-economic impact of diagnostic label of ‘penicillin allergy’ in a UK teaching hospital. J Clin Pathol 2014; 67:1088–92. [DOI] [PubMed] [Google Scholar]
- 73. Nootheti S, Kavosh E, Bielory L. Pharmoeconomics of penicillin skin testing (PST) on inpatient antibiotic use in patients with penicillin allergy (PA). J Allergy Clin Immun 2006; 117:S81. [Google Scholar]
- 74. Picard M, Bégin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract 2013; 1:252–7. [DOI] [PubMed] [Google Scholar]
- 75. Satta G, Hill V, Lanzman M, Balakrishnan I. β-lactam allergy: clinical implications and costs. Clin Mol Allergy 2013; 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health Syst Pharm 2017; 74:232–7. [DOI] [PubMed] [Google Scholar]
- 77. Manzaneque A, López-Cabezas C, Mensa M, et al. Potentially inappropriate prescription in patients with a history of allergy to β-lactam antibiotics: a health care challenge. J Investig Allergol Clin Immunol 2016; 26:55–6. [PubMed] [Google Scholar]
- 78. MacLaughlin EJ, Saseen JJ, Malone DC. Costs of beta-lactam allergies: selection and costs of antibiotics for patients with a reported beta-lactam allergy. Arch Fam Med 2000; 9:722–6. [DOI] [PubMed] [Google Scholar]
- 79. Vyles D, Chiu A, Routes J, et al. Antibiotic use after removal of penicillin allergy label. Pediatrics 2018; 141. doi:10.1542/peds.2017-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Westermann-Clark E, Pepper AN, Lockey RF. Economic considerations in the treatment of systemic allergic reactions. J Asthma Allergy 2018; 11:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Joint Formulary Committee. British National Formulary (BNF) 73 March 2017. XXX, London: Pharmaceutical Press; 2017. [Google Scholar]
- 82. Portugal Serviço Nacional de Saude. Autoridade Nacional do Medicamento e Produtos de Saúde (Infarmed). Available at: http://www.infarmed.pt/. Accessed 1 February 2020.
- 83. Blumenthal KG, Li Y, Banerji A, Yun BJ, Long AA, Walensky RP. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract 2018; 6:1019–27.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen JR, Tarver SA, Alvarez KS, Wei W, Khan DA. Improving aztreonam stewardship and cost through a penicillin allergy testing clinical guideline. Open Forum Infect Dis 2018; 5:ofy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferré-Ybarz L, Salinas Argente R, Gómez Galán C, Duocastella Selvas P, Nevot Falcó S. Analysis of profitability in the diagnosis of allergy to beta-lactam antibiotics. Allergol Immunopathol (Madr) 2015; 43:369–75. [DOI] [PubMed] [Google Scholar]
- 86. Sousa-Pinto B, Blumenthal KG, Macy E, et al. Diagnostic testing for penicillin allergy: a survey of practices and cost perceptions. Allergy 2019; 75:436–41. [DOI] [PubMed] [Google Scholar]
- 87. Lachover-Roth I, Sharon S, Rosman Y, Meir-Shafrir K, Confino-Cohen R. Long-term follow-up after penicillin allergy delabeling in ambulatory patients. J Allergy Clin Immunol Pract 2019; 7:231–5.e1. [DOI] [PubMed] [Google Scholar]
- 88. Au LYC, Siu AM, Yamamoto LG. Cost and risk analysis of lifelong penicillin allergy. Clin Pediatr (Phila) 2019; 58:1309–14. [DOI] [PubMed] [Google Scholar]
- 89. Macy E, Vyles D. Who needs penicillin allergy testing? Ann Allergy Asthma Immunol 2018; 121:523–9. [DOI] [PubMed] [Google Scholar]
- 90. Torres MJ, Blanca M, Fernandez J, et al. ENDA; EAACI Interest Group on Drug Hypersensitivity . Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy 2003; 58:961–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.