Abstract
Background
Antibody detection is the main method for diagnosis of coccidioidomycosis, but it has limitations. The Coccidioides antigen enzyme immunoassay is recommended for testing cerebrospinal fluid in suspected meningitis. Reports on urine and serum antigen detection evaluated small numbers of patients who were mostly immunocompromised. The purpose of this study was to assess the accuracy of combined antibody and antigen detection for diagnosis.
Methods
A retrospective study, including all patients in whom Coccidioides antigen detection in serum was performed between January 2013 and May 2017, was conducted at Valleywise Health Medical Center (formerly Maricopa Integrated Health System). Sensitivity and specificity of antigen and antibody were evaluated in 158 cases and 487 controls.
Results
The sensitivity of antibody detection by immunodiffusion (ID) was 84.2%. The sensitivity of antigen detection was 57.0% if both urine and serum were tested and 36.7% if urine alone was tested. The sensitivity of combining antigen and ID antibody detection was 93.0%. The sensitivity of urine and serum antigen detection was 55.4% in proven and 58.7% in probable cases, 79.1% in disseminated and 41.6% in pulmonary cases, and 74.7% in immunocompromised and 40.0% in immunocompetent patients. Specificity was 99.4% for antigen detection and 96.5% for ID antibody detection. Diagnostic accuracy was 95.4% for ID antibody and antigen detection, 93.6% for ID antibody alone, and 89.1% for pathology or culture.
Conclusions
These findings support combined antibody and antigen detection for diagnosis of progressive coccidioidomycosis. The diagnosis may have been missed if antigen detection was not performed.
Keywords: coccidioidomycosis, antigen, antibody, immunoassay
Combined Coccidioides antigen and antibody testing is the most sensitive method for diagnosis of progressive disseminated or pulmonary coccidioidomycosis. Sensitivity of antibody detection by immunodiffusion or enzyme immunoassay is inadequate for screening. More-sensitive antibody testing methods are needed.
(See the Editorial Commentary by Fierer on pages 976–8.)
The incidence of coccidioidomycosis is increasing in the United States [1]. Incidence in Arizona increased from 6 per 100 000 in 2014 to 19 per 100 000 in 2017 [2]. A survey of healthcare providers found that 40% were uncertain how to evaluate the diagnostic tests because multiple methods were used and agreement between methods was limited [3].
Another study assessed the effect of delayed diagnosis on costs [4]. Diagnosis was delayed > 30 days in 43% of cases and accounted for $590 000 additional healthcare costs. The authors concluded that diagnostic tests for coccidioidomycosis must be more rapid and reliable.
Tests for antibodies are the most common methods for diagnosis [5, 6]. If the diagnostic algorithm includes screening with one test followed by verification and quantification using another, the screening test must be optimized for sensitivity. Saubolle noted that serologic tests are less sensitive than previously thought, reporting sensitivities of 83% for enzyme immunoassay (EIA), 71% for immunodiffusion (ID), and 56% for complement fixation (CF) [7]. Malo et al opined that serologic tests “may be insensitive” to early infection [8]. Several publications support these opinions. ID antibodies were detected in only 53% of immunocompromised and 73% of immunocompetent patients in one study [6] and 50% and 63%, respectively, in another [7]. Immunoglobulin G (IgG) antibodies were detected by ID in 74% and immunoglobulin M (IgM) antibodies in 39% of people living with AIDS (PLWA) [9]. Some clinicians regard a positive ID test as proof of coccidioidomycosis; however, positive results occurred in 2% of healthy subjects from highly endemic areas [10].
Antibody detection by EIA is considered the most sensitive method for diagnosis [6, 8], but EIA is less sensitive than presumed. One study evaluated specimens that were positive ID or CF from 150 patients [11]. Sensitivity for IgG EIA was 64% using one commercial kit and 75% using another, and sensitivity for IgM EIA was 68% for one kit and 72% for the other. IgM specificity was 98%–100% at 2 laboratories but 74% at a third.
A second study evaluated 49 cases and 201 controls [12]. Sensitivity for IgG antibodies was 69% for one EIA and 53% for the other [12], and that for IgM was 57% for one and 35% for the other. Specificity for IgG was 95% for one and 97% for the other whereas specificity for IgM was 70% for one and 85% for the other.
A third study evaluated 103 cases from Tucson, Arizona, and 112 controls from Bakersfield, California or Tucson (J. Malo, unpublished data). Sensitivity for IgG antibodies was 47% in one EIA and 71% in the other, and sensitivity for IgM was 22.3% in one EIA and 29.1% in the other. Specificity for IgG was 95% in one assay and 96% in the other and specificity for IgM was 98% in one and 99% in the other. The study also evaluated a new antibody EIA developed at MiraVista Diagnostics [10]. Sensitivity was 87% for IgG and 61% for IgM; specificity was 90% for IgG and 95% for IgM.
Antigen testing was introduced in 2007. The sensitivity in urine was 71%, and 75% of patients were immunocompromised [13]. Serum testing incorporating ethylenediaminetetraacetic acid–heat pretreatment increased the sensitivity for detection of antigen; sensitivity was 73.1% in serum, 50% in urine, and 71.4% if serum and urine were tested [14]. Antigen was detected in cerebrospinal fluid (CSF) in 93% of patients with meningitis [15]. Antigen was detected in the serum in 16 of 19 (84%), urine in 20 of 28 (71%), and serum or urine in 29 of 30 (97%) of the meningitis cases, in 73% of immunocompetent patients, and in 95% of immunocompromised patients. These findings support the value of antigen detection in immunocompromised and immunocompetent patients and the importance of testing urine and serum.
The purpose of this study was to determine if combined urine and serum antigen and antibody detection improved the accuracy for diagnosis of progressive coccidioidomycosis.
METHODS
Patients
A study to assess the sensitivity and specificity of the Coccidioides antigen EIA and commercial antibody detection was implemented at Valleywise Health Medical Center (VWHMC) in 2017. Inclusion in the study required that medical records contained enough information to determine whether the patient had coccidioidomycosis. The protocol was reviewed and approved by the institutional review board at VWHMC (Maricopa Integrated Health System institutional review board [IRB], protocol number 2016–064) and MiraVista Diagnostics (MiraVista Diagnostics IRB, protocol number 00085) prior to implementation.
Medical Records Review
The investigators at VWHMC were provided with a secure, encrypted list of patients in whom serum, urine, and/or CSF was stored at MiraVista. Clinical data, radiographic imaging, laboratory findings, diagnosis, and treatment were reviewed to determine the presence of an active coccidioidomycosis diagnosis. Three concurrent controls in whom coccidioidomycosis was suspected but was not diagnosed or treated were evaluated for each case.
Patients with prior diagnosis of coccidioidomycosis but no evidence of active disease were excluded from the analysis. Patients were also excluded if serum was not tested for antigen. Patients with inadequate documentation to determine if coccidioidomycosis was present or absent were excluded.
Case report forms were deidentified according to an IRB-approved protocol before transferring them to MiraVista Diagnostics.
Diagnostic Classification
Diagnostic classification was as follows:
1. Proven coccidioidomycosis: Coccidioides isolated from fungal culture, or spherules seen by fungal stain in clinical specimens [16].
2. Probable coccidioidomycosis: Coccidioides antigen in urine or serum or antibodies detected by ID or CF.
3. No coccidioidomycosis: Alternative diagnosis established and no clinical evidence or treatment for coccidioidomycosis.
Clinical Classification
Clinical classification was as follows:
1. Disseminated coccidioidomycosis: Identification of Coccidioides in extrapulmonary sites by pathology or culture or clinical findings consistent with extrapulmonary involvement.
2. Pulmonary coccidioidomycosis: Respiratory complaints and radiographic findings consistent with coccidioidomycosis.
Monitoring of the Study
The MiraVista study monitor reviewed records supplied by VWHMC research personnel and requested additional information or clarification when necessary.
Coccidioides Antigen EIA and Antibody Detection
The antigen EIA has been described previously [13, 14]. ID and CF antibody testing was performed at the Coccidioidomycosis Serology Laboratory of the University of California, Davis (UCD laboratory) using proprietary reagents [17]. A commercial IgG and IgM antibody EIA was performed at a national reference laboratory.
Statistical Analysis
Statistical analysis was performed using MedCalc for Windows version 12.3.0 (Ostend, Belgium). A χ 2 analysis was used to compare subgroups. Student t test was performed to compare antigen concentrations between the groups. P values < .05 were considered significant. Values that were above the quantification limit were designated 8.2 ng/mL to reflect the maximum quantification limit. The Mann-Whitney test was used for comparison of median values between groups.
RESULTS
Patients
Seven hundred ten patients met study criteria, of whom 72 were excluded. Of the excluded patients, 51 had a prior diagnosis of coccidioidomycosis with no evidence of relapse. Serum was unavailable in 15 patients with suspected Coccidioides meningitis in whom CSF was stored. The diagnosis could not be confirmed in 6 patients.
One hundred fifty-two patients were diagnosed with an initial episode of coccidioidomycosis and 6 were relapses, for a total of 158 case episodes. A total of 486 control patients were diagnosed with other illnesses, 1 of whom was evaluated during a second illness for a total of 487 control episodes. The case and control episodes will be described as cases and controls for evaluation of diagnostic tests but as individual patients for evaluation of demographics and underlying conditions.
Demographic Characteristics and Underlying Conditions
Characteristics more common in the cases were immunocompromising conditions, male gender, and Asian race/ethnicity (Table 1). The mean age in the cases was 44.2 years and 48.1 years in controls (P = .0039). The median age was 43.5 years in the 60 PLWA and 44.5 years in the 92 patients without AIDS. AIDS was more common in cases than controls (Table 2). The proportion of cases with an immunosuppressive condition other than AIDS, treatment with immunosuppressive medications, and other chronic conditions was not different in cases and controls.
Table 1.
Demographic Characteristics Among Cases and Controls
| Characteristic | Case Patients | Control Patients | P Value |
|---|---|---|---|
| (n = 152) | (n = 486) | ||
| Male gender | 115/152 (75.7) | 330/486 (67.9) | .0843 |
| Race/ethnicity | |||
| Hispanic | 65/152 (42.8) | 232/486 (47.7) | .3351 |
| White, non-Hispanic | 39/152 (25.7) | 172/486 (35.4) | .0340 |
| African American | 28/152 (18.4) | 63/486 (13.0) | .1266 |
| Asian | 11/152 (7.2) | 7/486 (1.4) | .0004 |
| Native American | 4/152 (2.6) | 6/486 (1.0) | .2815 |
| Hawaiian | 1/152 (0.2) | 0/486 (0) | .2530 |
| African | 0/152 (0) | 1/486 (0.2) | .5224 |
| Unknown | 3/152 (2.0) | 5/486 (1.0) | .5804 |
Data are expressed as present/total, no. (%).
Table 2.
Underlying Conditions Among Cases and Controls
| Condition | Case Patients | Control Patients | P Value |
|---|---|---|---|
| (n = 152) | (n = 486) | ||
| AIDS | 60/152 (39.5) | 140/486 (28.8) | .0110 |
| Hematologic malignancy | 5/152 (3.3) | 13/486 (2.7) | .9132 |
| Corticosteroids | 5/152 (3.3) | 17/486 (3.5) | .8914 |
| Autoimmune | 10/152 (6.6) | 44/486 (9.0) | .4457 |
| Cancer | 4/152 (2.6) | 17/486 (3.5) | .7773 |
| Lung disease | 6/152 (3.9) | 69/486 (14.2) | .0010 |
| Diabetes | 28/152 (18.4) | 73/486 (15.0) | .3812 |
| Hypertension | 22/152 (14.5) | 93/486 (19.1) | .2430 |
| Liver disease | 17/152 (11.2) | 51/486 (10.5) | .9254 |
| Tuberculosis | 5/152 (3.3) | 30/486 (6.2) | .2443 |
| Miscellaneous | 9/152 (5.9) | 38/486 (7.8) | .5447 |
| None | 69/152 (45.4) | 162/486 (33.3) | .0090 |
Data are expressed as present/total, no. (%). More than 1 condition was present in some patients.
Disseminated disease was more common in nonwhite compared with white patients (53.1% [60/113] vs 15.0% [6/40]; P < .0001). AIDS was not a risk factor in nonwhite patients: 54.8% (23/42) of PLWA and 52.1% (37/71) of those without AIDS had disseminated disease (P = .8466). AIDS also was not a risk factor in white patients: 29.4% (5/17) of those with AIDS and 5.6% (1/17) of those without AIDS (P = .1748) had disseminated disease.
Diagnostic Test Results in Cases and Controls
Antibody detection did not follow a specific algorithm. Serum was usually tested by ID and CF. IgG and IgM EIAs were performed at a reference laboratory in 34 cases and were the sole basis for diagnosis in 3 cases.
Antibody was detected by ID in 84.2% (123/146) and CF in 68.2% (86/126) of cases (P = .0023; Table 3). Both were performed in 122 cases and both were positive in 66.4%, ID alone in 27.9%, and CF alone in none. ID antibody was detected in 84.2% (123/146) and antigen in 57.0% (90/158) of cases (P < .0001).
Table 3.
Results in Cases and Controls
| Test | Cases | Controls |
|---|---|---|
| (n = 158) | (n = 487) | |
| Antibody (ID IgG) | 113/145 (77.9) | 10/462 (2.2) |
| Antibody (ID IgM) | 38/145 (26.2) | 8/162 (1.7) |
| Antibody (ID IgG or ID IgM) | 123/146 (84.2)a,b,c | 16/462 (3.5) |
| Antibody (CF) | 86/126 (68.2)a | 0/52 (0) |
| Antibody (ID or CF) | 129/152 (84.9) | 16/464 (3.4) |
| Antigen (serum) | 81/158 (51.3)d | 2/487 (0.4) |
| Antigen (urine) | 51/139 (36.7)d | 3/175 (0.6) |
| Antigen (serum or urine) | 90/158 (57.0)b | 3/487 (0.6) |
| Antibody (ID) or antigen (serum or urine) | 147/158 (93.0)c | 19/487 (3.1) |
| Cytopathology or histopathology | 63/115 (54.8) | 1/237 (0.4) |
| Culture | 60/100 (60.0) | 0/158 (0) |
| Pathology or culture | 83/125 (66.4) | 1/269 (0.4) |
Data are expressed as present/total, no. (%).
Abbreviations: CF, complement fixation; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M.
Statistical analyses for comparison of results and determination of P values are as follows: aP = .0023. bP < .0001. cP = .0177. dP = .0139.
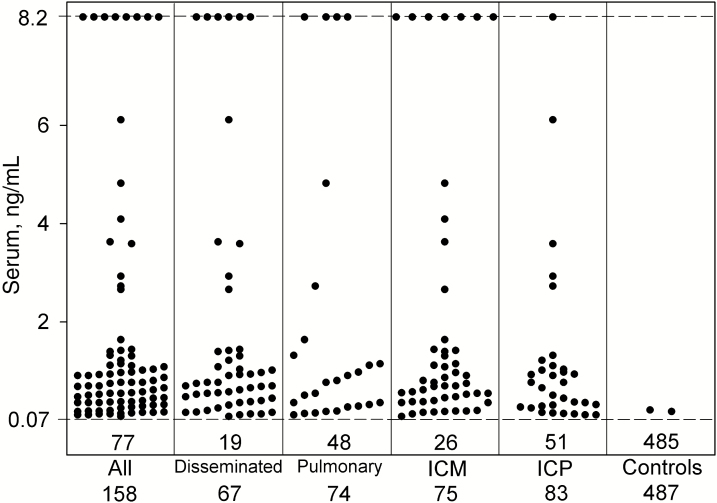
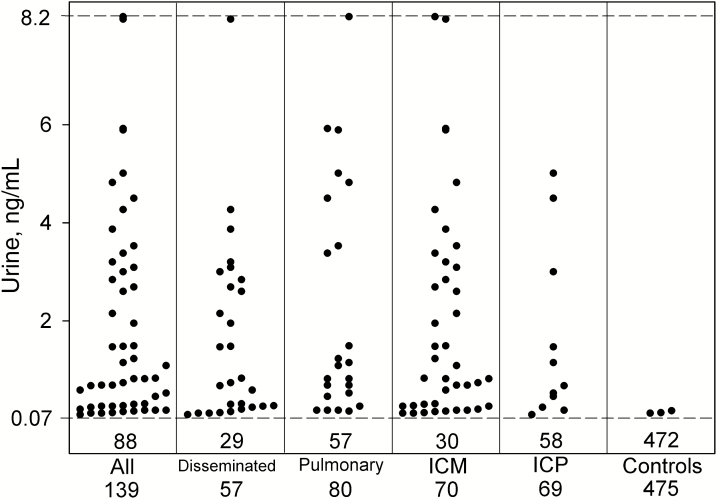
Antigen was detected in serum in 51.3% (81/158) and urine in 36.7% (51/139) of cases (P = .0139). The sensitivity of combined antigen or ID antibody detection was 93.0% (147/158) and of antibody alone was 84.2% (123/146) (P = .0177). Specificity was higher by antigen than ID antibody detection (99.4% [484/487] vs 96.5% [446/462], respectively; P = .0032). Serum and urine antigen concentrations for individual patients are presented in Figures 1 and 2, respectively.
Figure 1.
Serum antigen concentrations in case and control episodes with different characteristics. The dashed line at 0.07 ng/mL represents the cutoff for positivity. Each point above the dashed cutoff line represents a positive result. The number in the box below the dashed line represents the number of patients with negative results, and the number below each column represents the total number of patients tested. Results shown at the hashed line at the top of the figure are above the limit of quantification of the assay and are expressed as 8.2 ng/mL. Abbreviations: ICM, immunocompromised; ICP, immunocompetent.
Figure 2.
Urine antigen concentrations in case and control episodes with different characteristics. The dashed line at 0.07 ng/mL represents the cutoff for positivity. Each point above the dashed cutoff line represents a positive result. The number in the box below the dashed line represents the number of patients with negative results, and the number below each column represents the total number of patients tested. Results shown at the hashed line at the top of the figure are above the limit of quantification of the assay and are expressed as 8.2 ng/mL. Abbreviations: ICM, immunocompromised; ICP, immunocompetent.
Diagnostic Accuracy
The large numbers of cases and controls supported analysis of diagnostic accuracy [9]. Diagnostic accuracy was determined for ID antibody detection alone, antigen or antibody detection, and pathology or culture. The prevalence of progressive coccidioidomycosis at VWHMC during the study was estimated to be 10.3%. The sensitivity for antibody alone was 84.2%, specificity 96.5%, positive predictive value (PPV) 88.5%, negative predictive value (NPV) 95.1%, and diagnostic accuracy 93.6%. The sensitivity for combined antibody or antigen detection was 93.0%, specificity 96.1%, PPV 88.6%, NPV 97.7%, and diagnostic accuracy 95.4%. For culture- or pathology-confirmed cases, sensitivity was 66.4%, specificity 99.6%, PPV 98.8%, NPV 86.4%, and diagnostic accuracy 89.1%.
Proven and Probable Cases
The sensitivity of antibody detection by ID was 87.2% (68/78) in proven cases and 76.1% (54/71) in probable cases (P = .0911; Table 4). The sensitivity of antibody detection by CF was 75.7% (53/70) in proven cases and 57.9% (33/75) in probable cases (P = .0375). Sensitivity of antigen detection was 55.4% (46/83) in proven cases and 58.7% (44/75) in probable cases (P = .7484). Sensitivity of antibody or antigen detection was 94.0% (78/83) in proven cases and 94.7% (71/75) in probable cases (P = .2620).
Table 4.
Results in Proven and Probable Cases
| Test | Proven | Probable | P Value |
|---|---|---|---|
| (n= 83) | (n= 75) | ||
| Antibody (ID IgG) | 65/77 (84.4) | 48/68 (70.6) | .06992 |
| Antibody (ID IgM) | 22/78 (28.2) | 17/68 (25.0) | .7104 |
| Antibody (ID IgG or ID IgM) | 68/78 (87.2)a,b,c | 54/71 (76.1)a,b,c | .0911 |
| Antibody (CF) | 53/70 (75.7)a | 33/57 (57.9)a | .0375 |
| Antibody (ID or CF) | 71/81 (87.6) | 58/72 (80.6) | .2690 |
| Antigen (serum) | 45/83 (54.2)d | 36/75 (48.0)d | .5240 |
| Antigen (urine) | 28/76 (36.8)d | 23/63 (36.5)d | 1.000 |
| Antigen (serum or urine) | 46/83 (55.4)b | 44/75 (58.7)b | .7484 |
| Antibody (ID) or antigen (serum or urine) | 78/83 (94)c | 71/75 (94.7)c | .2620 |
| Cytopathology or histopathology | 61/79 (77.2) | 0/34 (0) | ND |
| Culture | 58/70 (82.9) | 0/28 (0) | ND |
| Pathology or culture | 83/83 (100) | 0/42 (0) | ND |
Data are expressed as present/total, no. (%).
Abbreviations: CF, complement fixation; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M; ND, not done.
P values for comparison between columns are presented in the P value column. Statistical analyses for comparison of results within columns are as follows:
a P = .0890 (proven); P = .0363 (probable).
b P = .0001 (proven); P = .0341 (probable).
c P = .1778 (proven); P = .0017 (probable).
d P = .0381 (proven); P = .2266 (probable).
The sensitivity of antibody detection in proven cases was 87.2% (68/78) by ID and 75.7% by CF (53/70) (P = .0890; Table 4). Sensitivity was 87.2% (68/78) for antibody and 55.4% (46/83) for antigen detection (P < .0001). The sensitivity for antigen or antibody detection was 91.6% (78/83) and 87.2% (68/78) by antibody detection alone (P = .1778).
The sensitivity of antibody detection in probable cases was 76.1% (50/71) by ID and 57.9% (33/57) by CF (P = .0363; Table 4). Sensitivity for antibody detection was 76.1% (50/71) and 58.7% (44/75) for antigen detection (P = .0341). The sensitivity for antigen or antibody detection was 94.7% (71/75) and 76.1% (54/71) for antibody detection alone (P = .0017).
Disseminated and Pulmonary Cases
The sensitivity of antibody detection by ID was 91.7% (55/60) in disseminated cases and 79.8% (67/84) in pulmonary cases (P = .1108; Table 5). The sensitivity of antibody detection by CF was 78.2% (43/55) in disseminated cases and 59.2% (42/71) in pulmonary cases (P = .0388). Sensitivity of antigen detection was 79.1% (53/67) in disseminated cases and 41.6% (37/89) in pulmonary cases (P = .0001). Sensitivity of ID antibody or antigen detection was 98.5% (66/67) in disseminated cases and 89.9% (80/89) in pulmonary cases (P = .0656).
Table 5.
Results in Disseminated and Pulmonary Cases
| Test | Disseminated | Pulmonary | P Value |
|---|---|---|---|
| (n = 67) | (n = 89) | ||
| Antibody (ID IgG) | 54/60 (90.0) | 58/83 (69.9) | .0075 |
| Antibody (ID IgM) | 8/60 (13.3) | 30/83 (36.1) | .0043 |
| Antibody (ID IgG or ID IgM) | 55/60 (91.7) a,b,c | 67/84 (79.8)a,b,c | .1108 |
| Antibody (CF) | 43/55 (78.2)a | 42/71 (59.2)a | .0388 |
| Antibody (ID or CF) | 59/64 (92.2) | 69/86 (80.2) | .0716 |
| Antigen (serum) | 48/67 (71.6)d | 33/89 (37.1)d | .0001 |
| Antigen (urine) | 28/57 (49.1)d | 23/80 (28.8)d | .0249 |
| Antigen (serum or urine) | 53/67 (79.1)b | 37/89 (41.6)b | .0001 |
| Antibody (ID) or antigen (serum or urine) | 66/67 (98.5)c | 80/89 (89.9)c | .0656 |
| Cytopathology or histopathology | 26/43 (60.5) | 35/70 (50.0) | .3719 |
| Culture | 29/44 (65.9) | 29/52 (55.8) | .5175 |
| Pathology or culture | 38/51 (74.5) | 45/73 (61.6) | .1908 |
Data are expressed as present/total, no. (%).
Abbreviations: CF, complement fixation; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M.
P values for comparison between columns are presented in the P value column. Statistical analyses for comparison of results within columns are as follows:
a P = .0639 (disseminated); P = .0077 (pulmonary).
b P = .0791 (disseminated); P < .0001 (pulmonary).
c P = .0997 (disseminated); P = .0876 (pulmonary).
d P = .0158 (disseminated); P = .2581 (pulmonary).
The sensitivity of antibody detection in disseminated cases was 91.7% (55/60) by ID and 78.2% (43/55) by CF (P = .0639). Sensitivity was 91.7% (55/60) for antibody and 79.1% (53/67) for antigen detection (P = .0791). The sensitivity was 98.5% (66/67) for combined antigen or antibody detection and 91.7% (55/60) by antibody detection alone (P = .0997).
The sensitivity of antibody detection in pulmonary cases was 79.8% (67/84) by ID and 59.2% (42/71) by CF (P = .0077; Table 5). Sensitivity was 79.8% (67/84) for antibody and 41.6% (37/89) for antigen detection (P < .0001). The sensitivity was 89.9% (80/89) for combined antigen or antibody detection and 79.8% (67/84) by antibody detection alone (P = .0876).
Immunocompromised and Immunocompetent Cases
The sensitivity of antibody detection by ID was 73.2% (52/71) in immunocompromised cases and 93.4% (71/76) in immunocompetent cases (P = .0020).
Sensitivity of antigen detection was 74.7% (56/75) in immunocompromised cases and 41% (34/83) in immunocompetent cases (P = .0001). Sensitivity of combined antibody or antigen detection was 93.3% (70/75) in immunocompromised cases and 92.8% (77/83) in immunocompetent cases (P = .8496).
The sensitivity of antibody detection in immunocompromised cases was 73.2% (52/71) by ID and 70.9% (39/55) by CF (P = .8420; Table 6). Sensitivity was 73.2% (52/71) for ID antibody detection and 74.7% (56/75) for antigen detection (P = .8528). The sensitivity was 93.3% (70/75) for combined antigen or antibody detection and 73.2% (52/71) by antibody detection alone (P = .0014).
Table 6.
Results in Immunocompromised and Immunocompetent Cases
| Test | Immunocompromised | Immunocompetent | P Value |
|---|---|---|---|
| (n = 75) | (n = 83) | ||
| Antibody (ID IgG) | 49/69 (71.0) | 64/76 (84.2) | .0867 |
| Antibody (ID IgM) | 12/69 (17.4) | 26/76 (34.2) | .0350 |
| Antibody (ID IgG or ID IgM) | 52/71 (73.2)a,b,c | 71/76 (93.4)a,b,c | .0020 |
| Antibody (CF) | 39/55 (70.9)a | 47/72 (65.3)a | .6331 |
| Antibody (ID or CF) | 55/73 (75.3) | 74/79 (93.7) | .0033 |
| Antigen (serum) | 49/75 (65.3)d | 32/83 (38.6)d | .0014 |
| Antigen (urine) | 40/70 (57.1)d | 11/69 (15.9)d | .0001 |
| Antigen (serum or urine) | 56/75 (74.7)b | 34/83 (41.0)b | .0001 |
| Antibody (ID) or antigen (serum or urine) | 70/75 (93.3)c | 77/83 (92.8)c | .8496 |
| Cytopathology or histopathology | 27/46 (58.7) | 34/66 (51.5) | .5756 |
| Culture | 26/46 (56.5) | 32/52 (61.5) | .7667 |
| Pathology or culture | 37/56 (66.1) | 46/60 (76.7) | .8979 |
Data are expressed as present/total, no. (%).
Abbreviations: CF, complement fixation; ID, immunodiffusion; IgG, immunoglobulin G; IgM, immunoglobulin M.
P values for comparison between columns are presented in the P value column. Statistical analyses for comparison of results within columns are as follows:
a P = .8420 (immunocompromised); P < .0001 (immunocompetent).
b P = .8528 (immunocompromised); P < .0001 (immunocompetent).
c P = .0014 (immunocompromised); P = 1.000 (immunocompetent).
d P = .3936 (immunocompromised); P = .0021 (immunocompetent).
The sensitivity of antibody detection in immunocompetent cases was 93.4% (71/76) by ID and 65.3% (47/72) by CF (P < .0001; Table 4). Sensitivity was 93.4% (71/76) for antibody detection and 40.0% (30/83) for antigen detection (P < .0001). The sensitivity for combined antigen or antibody detection was 92.8% (77/83) and 93.4% for antibody detection alone (P = 1.0000).
Among 60 cases in PLWA, sensitivity was 93.3% (56/60) by combined antigen or ID antibody detection and 75.0% (45/60) by ID antibody detection alone (P = .0125). Antigen alone was positive in 18.3% (11/60) of cases. Coccidioides was isolated from blood in 13.6% (5/38) of PLWA in whom fungal blood cultures were performed.
Antigen was positive but antibody was negative in 16.3% (24/147) of cases including 12.0% (10/83) of proven, 22.7% (17/75) of probable, 16.4% (11/67) of disseminated, 15.7% (14/89) of pulmonary, 24.0% (18/75) of immunocompromised, and 7.2% (6/83) of immunocompetent patients, and 18.3% (11/60) of PLWA.
Median antigen concentrations for the different groups are presented in Table 7. Concentrations were similar except for a trend toward higher urine concentration in the proven than the probable group (1.7200 ng/mL and 0.6900 ng/mL, respectively; P = .0512).
Table 7.
Analysis of the Antigen Concentration in Cases
| Group (No. of Cases) | Median Serum | P Value | Median Urine | P Value |
|---|---|---|---|---|
| Proven (45) | 0.7700 | .1755 | 1.7200 | .0512 |
| Probable (36) | 0.6750 | 0.6900 | ||
| Disseminated (32) | 0.7150 | .4950 | 0.6800 | .4074 |
| Pulmonary (33) | 0.7700 | 1.0900 | ||
| Immunocompromised (49) | 0.7500 | .1887 | 0.9600 | .5984 |
| Immunocompetent (32) | 0.7150 | 0.6800 | ||
| AIDS (38) | 0.9400 | .2472 | 0.9600 | .7596 |
| Other immunocompromised (9) | 0.5600 | 0.6900 |
The Mann-Whitney test was used for determination of P values.
DISCUSSION
Asian ethnicity was associated with increased risk for coccidioidomycosis and nonwhite race was associated with increased risk for disseminated disease. Age was not a risk factor for coccidioidomycosis.
The highest sensitivity was achieved by testing serum and urine for antigen and serum for antibodies by ID: 93.0% overall, 91.6% for proven, 94.7% for probable, 98.5% for disseminated, 89.9% for pulmonary, 93.3% for immunocompromised, 92.8% for immunocompetent, and 93.3% for PLWA. Diagnostic accuracy was 95.4% by antibody and antigen detection, 93.6% by antibody detection alone (93.6%), and 89.1% by culture or pathology. Diagnosis may have been missed or delayed if antigen testing was not performed in 16.3% of cases overall and in 24.0% of those who were immunocompromised. These findings support urine and serum antigen and ID antibody testing in all patients suspected to have progressive coccidioidomycosis.
This study does not resolve uncertainty about antibody detection [3]. Antibody testing alone was not sensitive enough (84%) for screening (Table 3). Combined antigen and antibody detection improved sensitivity (93%). Antibody detection by EIA was performed in only 22% of cases and was not evaluated as a method for diagnosis. Commercial EIA kits are not sensitive enough for screening (47%–75%).
The antigen test has been described as insensitive and unnecessary except for CSF if meningitis is suspected. Sensitivity in urine was 71% in the initial study [13] and 37% in this study. Immunocompromise was greater in the initial study, and most patients had AIDS. The sensitivity of the Coccidioides antigen assay in urine was 76% in PLWA in this study, comparable to the initial study. The sensitivity in serum was 51% in this and the initial study [14].
By comparison, antigen was detected in 95%–100% of PLWA with histoplasmosis in the Histoplasma antigen assay [18, 19]. Lower sensitivity could be inherent to the assay or lower fungal burden in coccidioidomycosis. The lower limit of detection (LLOD) in the Histoplasma antigen EIA is 65 pg/mL. The cutoff for positivity in the Coccidioides antigen assay is 70 pg/mL, as established by receiver operating characteristic curve analysis, but LLOD was not determined.
Fungal burden is higher in histoplasmosis, an infection characterized by diffuse pulmonary involvement, widespread proliferation in reticuloendothelial tissues, and fungemia in 64% of PLWA [20]. Coccidioidomycosis is more localized, and fungemia occurred in only 13% of PLWA (5/38) in this and 3% (4/153) in another study [21]. Analytical sensitivity is not the cause for lower clinical sensitivity.
Evaluation of antigen results in 5000 patients tested in 2017 and 2018 support the importance of testing urine and serum (L. W., unpublished data). Urine alone was tested in 46%, serum alone in 36%, and both in 18% of patients. Among 52 patients in whom both were tested and at least 1 was positive, 21% would have been missed by testing serum only and 23% by testing urine only. Both should be tested.
This study has several strengths. It is the largest study that evaluated diagnostic tests for progressive coccidioidomycosis and includes antigen and antibody testing. Second, the physicians were experienced in diagnosis and management of coccidioidomycosis. Third, testing was performed at experienced laboratories (antibody at the UCD laboratory and antigen at MiraVista Diagnostics).
The study has limitations. Fifty-two percent of cases were immunocompromised, mostly with AIDS. Findings may differ at institutions with fewer immunocompromised patients. Second, it was retrospective. Third, incorporation bias could have influenced findings in the probable cases. However, probable cases were patients diagnosed and treated for coccidioidomycosis by their physicians, and findings were similar in proven cases. Third, the UCD laboratory used proprietary antigens and testing methods; its results may not agree with those performed elsewhere [7, 22].
In summary, combined ID antibody and antigen detection was the most sensitive method for diagnosis of coccidioidomycosis. This and earlier studies do not support antibody detection alone for initial screening. More-sensitive antibody tests are needed.
Notes
Acknowledgments. The authors thank Mary Mulrow, Aseel Abdulahad, and Viola Zepeda for managing the study at Maricopa Integrated Health System; Suphansa Gunn for managing the study at MiraVista Diagnostics; Wesley Keown at MiraVista Diagnostics for statistical analysis and preparation of images; and Chadi Hage, Indiana University School of Medicine, for advice in preparing the manuscript.
Potential conflicts of interest. L. W. is the owner and president of MiraVista Diagnostics, and M. D. and E. H. are employees of MiraVista Diagnostics. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol 2019; 57(Suppl 1):S30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson L, Ting J, Lin H, et al. The rise of valley fever: prevalence and cost burden of coccidioidomycosis infection in California. Int J Environ Res Public Health 2019; 16. doi:10.3390/ijerph16071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Erhart LM, Anderson S, et al. Coccidioidomycosis: knowledge, attitudes, and practices among healthcare providers—Arizona, 2007. Med Mycol 2011; 49:649–56. [DOI] [PubMed] [Google Scholar]
- 4. Donovan FM, Wightman P, Zong Y, et al. Delays in coccidioidomycosis diagnosis and associated healthcare utilization, Tucson, Arizona, USA. Emerg Infect Dis 2019; 25:1745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donovan FM, Zangeneh TT, Malo J, Galgiani JN. Top questions in the diagnosis and treatment of coccidioidomycosis. Open Forum Infect Dis 2017; 4:ofx197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016. PMID: PM27470238. [DOI] [PubMed] [Google Scholar]
- 7. Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci 2007; 1111:301–14. [DOI] [PubMed] [Google Scholar]
- 8. Malo J, Luraschi-Monjagatta C, Wolk DM, Thompson R, Hage CA, Knox KS. Update on the diagnosis of pulmonary coccidioidomycosis. Ann Am Thorac Soc 2014; 11:243–53. [DOI] [PubMed] [Google Scholar]
- 9. Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC 2009; 19:203–11. Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine 1990; 69:384-91. PMID: 2146461. [DOI] [PubMed] [Google Scholar]
- 10. Malo J, Holbrook E, Zangeneh T, et al. Enhanced antibody detection and diagnosis of coccidioidomycosis with the MiraVista IgG and IgM detection enzyme immunoassay. J Clin Microbiol 2017; 55:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan S, Saubolle MA, Oubsuntia T, et al. Interlaboratory agreement of coccidioidomycosis enzyme immunoassay from two different manufacturers. Med Mycol 2019; 57:441–6. [DOI] [PubMed] [Google Scholar]
- 12. Grys TE, Brighton A, Chang YH, Liesman R, Bolster LC, Blair JE. Comparison of two FDA-cleared EIA assays for the detection of Coccidioides antibodies against a composite clinical standard. Med Mycol 2018. doi:10.1093/mmy/myy094. [DOI] [PubMed] [Google Scholar]
- 13. Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis 2008; 47:e69–73. [DOI] [PubMed] [Google Scholar]
- 14. Durkin M, Estok L, Hospenthal D, et al. Detection of Coccidioides antigenemia following dissociation of immune complexes. Clin Vaccine Immunol 2009; 16:1453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kassis C, Zaidi S, Kuberski T, et al. Role of coccidioides antigen testing in the cerebrospinal fluid for the diagnosis of coccidioidal meningitis. Clin Infect Dis 2015; 61:1521–6. [DOI] [PubMed] [Google Scholar]
- 16. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2019. pii: ciz1008. PMID: 31802125. doi: 10.1093/cid/ciz1008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappagianis D, Zimmer BL. Serology of coccidioidomycosis. Clin Microbiol Rev 1990; 3:247–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53:448–54. [DOI] [PubMed] [Google Scholar]
- 19. Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol 2007; 14:1587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wheat J, Hafner R, Korzun AH, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trials Group. Am J Med 1995; 98:336–42. [DOI] [PubMed] [Google Scholar]
- 21. Woods CW, McRill C, Plikaytis BD, et al. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994–1997: incidence, risk factors, and prevention. J Infect Dis 2000; 181:1428–34. [DOI] [PubMed] [Google Scholar]
- 22. Saubolle MA, McKellar PP, Sussland D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol 2007; 45:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]