Abstract
Feminine hygiene products (FHPs) are used on highly permeable and sensitive vaginal and vulvar tissues by many women. These products contain a variety of chemicals, and few regulations require disclosure of their ingredients. The objectives of this study are to identify volatile organic compounds (VOCs) that may be present in these products and to evaluate the potential for exposure and health risk associated with product use. We collected 79 commercially available FHPs, including washes, tampons, menstrual pads, wipes, sprays, powders and moisturizers, and analyzed their composition using purge and trap sampling, thermal desorption, gas chromatography and mass spectroscopy. Exposures and risks were modeled using reasonable upper bound exposure scenarios. The highest VOC concentrations (as total target VOCs) were found in washes, sprays and powders, with median concentrations from 25,000 to 34,000 ng/g. Benzene (maximum: 3,604 ng/g) was detected in 83% of the collected products, and 1,4-dioxane (maximum: 24,354 ng/g) in 50% of the products. VOC composition depended on the FHP type, manufacturer and brand. Products labeled as “organic,” “natural,” or “for sensitive skin” did not necessarily have lower VOC concentrations. For most FHPs, calculated risks were low; however, menstrual pads had hazard ratios of up to 11, sprays and powders had hazard ratios of up to 2.2 and excess cancer risks of up to 2.1 × 10−6, and washes had excess cancer risks of up to 3.3 × 10−6. Our data suggest that all tested FHPs contained some toxic VOCs, and that risks of using some products should be addressed. We recommend the elimination of toxic ingredients and the disclosure of all chemicals that are used in these products.
Keywords: Feminine hygiene products, Volatile organic compounds, Exposure, Health risk
1. Introduction
Feminine hygiene products (FHPs) include tampons, menstrual pads (also known as sanitary napkins), washes, sprays, powders and wipes. These products are widely used across the world, and the US sales of these products are over $3 billion annually (Statista.com, 2020). A woman may use more than 10,000 pads or tampons across her menstrual life cycle, which can be as long as 40 years (ages from 12 to 51 years). While menstrual pads and tampons are regarded as “medical devices” by the US Food & Drug Administration (US CDRH, 2005), few regulations require disclosure of the composition of FHPs (Kohen, 2001). In 2020, New York State will require disclosure of FHP ingredients, the first such requirement of its kind in the US (AP News, 2019).
FHPs are used on highly permeable and sensitive vaginal and vulvar tissues that have high uptake rates and sensitivity to chemicals and irritants. The permeability is a result of the structure, occlusion, hydration and susceptibility to friction of vaginal and vulvar tissues (Farage and Maibach, 2004; Hussain and Ahsan, 2005). Arteries, blood vessels and lymphatic vessels are abundant in the vaginal wall, which allows for the direct transfer of chemicals to the blood through peripheral circulation (Mirza et al., 2016; Richardson and Illum, 1992). Due to these characteristics, several researchers have suggested using uncertainty factors up to 10 when extrapolating chemical uptake from exposed skin to vulvar tissues, and factors up to 20 for extrapolating from skin to vaginal and other mucosal tissues (Farage and Maibach, 2004).
The possibility of chemical exposure, especially volatile organic compounds (VOCs), through the use of FHPs was raised in August 2014 when the group Women’s Voices for Earth reported that a well-known brand of menstrual pads contained styrene, chloromethane and chloroform (Women’s Voices for the Earth, 2014). In 2017, over 200 VOCs, including benzene, styrene, and trichloroethylene, were found in ten types of sanitary napkins and panty liners sold in South Korea (Soyun, 2017). Such reports are qualitative in nature, identifying chemicals, but not quantitative levels. Recently, phthalates and VOCs were detected in 11 sanitary pads from Korea, Japan, Finland, France, Greece and the US, although the calculated toluene and xylene exposures fell below the reference dose (RfD) (Park et al., 2019). In addition, a recent study using the National Health and Nutrition Examination Survey (NHANES) 2001–2004 data reported a statistically significant and positive relationship between the frequency of vaginal douching (presumably using feminine washes) and whole blood concentrations of 1,4-dichlorobenzene among women aged 20–49 years (Ding et al., 2020), a concern given the toxicity of this VOC.
VOCs are components of FHPs that are added as fragrances, adsorbents, moisture barriers, adhesives, and binders. VOCs may also be inadvertent contaminants in the raw materials or packaging. Exposure to VOCs that may be present in FHPs and other consumer products can occur through inhalation (Bello et al., 2009; Bridges, 2002; Singer et al., 2006), dermal permeation (Weisel and Jo, 1996; Xu and Weisel, 2005), and ingestion pathways. Exposure to high concentrations or long-term exposure of VOCs has been associated with many known or suspected effects including irritation to eyes, skin and nose; damage to the respiratory system, liver and kidney; reproductive effects; and carcinogenicity (Wolkoff et al., 2000, 2006; Anderson et al., 2007). Examples of notable VOCs include benzene, a known carcinogen (US EPA, 1998), 1,4-dioxane, a likely carcinogen (US EPA, 2013), and naphthalene, a possible carcinogen due to possible genetic toxicity (Schreiner, 2003; US EPA, 1999). Health risks (carcinogenic or non-carcinogenic) related to the use of FHPs over the lifecourse remain unanswered.
The widespread use of FHPs, the sensitivity and permeability of vaginal and vulvar tissues, prior reports of toxic VOCs in some FHPs, and gaps in our understanding of exposures and effects warrant an examination of the chemicals and potential risks associated with current FHPs. In this study, our objectives are to analyze the VOC composition in FHPs in the US market and to estimate the potential for health risks associated with their use.
2. Methods
2.1. Products selection
A broad range of FHPs were selected that included popular brands and products from Statista.com and Amazon.com. For menstrual pads and tampons, we sampled all products of at least the five best-selling brands in the US. For other products, selection was based on the “best sellers” list from Amazon.com. We also collected bestselling products from several store brands and included at least one “organic” or “natural” labeled product of each type of FHP. All products were purchased from local (Michigan) stores or online (Amazon.com). In total, we obtained 79 products: 13 types of feminine washes; 22 types of tampons; 22 types of menstrual pads; 12 types of wipes; 5 types of sprays and powders; and 5 types of moisturizers (Table 1).
Table 1.
Characteristics of the 79 feminine hygiene products collected in this study.
| Products | Number of Products | Mass (g) /unitb | No. of target VOCs detected | |||
|---|---|---|---|---|---|---|
| Tested | Store brand | “Organic” | Labeled with datea | |||
| Feminine wash | 13 | 4 | 2 | 0 | – | 41 |
| Tampon | 22 | 5 | 2 | 1 | 1.9 ± 0.2 | 52 |
| Menstrual pad | 22 | 6 | 1 | 3 | 5.3 ± 2.8 | 48 |
| Feminine wipe | 12 | 4 | 2 | 2 | 4.9 ± 1.2 | 57 |
| Feminine spray and powder | 5 | 0 | 1 | 0 | – | 42 |
| Feminine moisturizer | 5 | 2 | 2 | 2 | – | 35 |
Number of products that had dates of manufacture or expiration dates.
Mean ± standard deviation.
2.2. VOCs sampling - purge and trap method
Purge and trap methods (Kwon et al., 2008; Rosell et al., 2003) were used to sample VOCs (Figure S1). For feminine washes and moisturizers, 0.1 g of each product was transferred into a 40 mL glass vial, which was immediately sealed using a Teflon septum and a screw-on cap. After heating to 60C for 10 min in a dry bath, the vial was purged with pure N2 injected into the bottom of vial via a long needle that pieced the septum; flow exiting the vial passed through a 10 cm long stainless-steel adsorbent sampling tube (Scientific Instrument Services, Inc., Ringoes, New Jersey, USA) equipped with a needle inlet that also pierced the septum. The sampling tubes were packed with 150 mg anhydrous sodium sulfate (Fisher Scientific, Fair Lawn, New Jersey, USA) to remove water vapor, and 160 mg of 60/80 mesh Tenax-GR (Scientific Instrument Services, Inc., Palmer, Massachusetts, USA). The purge used 1000 mL of N2 over a 30 min period and the temperature was kept at 60C.
For tampons, we measured the weight of a whole tampon, placed it in a 40 mL vial, added 5 mL of LC-MS grade deionized water (MilliporeSigma, Burlington, Massachusetts, USA), capped the vial, and heated it to 40C for 10 min. These samples were maintained at 40C and purged with 400 mL of N2 for 20 min to sample VOCs, as described previously for washes and moisturizers. For menstrual pads, the protocol was similar, except that we cut and weighed a small section from the middle of the pad (approximately 1.0 ± 0.2 g).
For wipes, each whole wipe was weighed, inserted into a 40 mL vial, heated to 40C, and sampling was conducted using a 600 mL N2 purge. For feminine sprays and powders, a measured weight (0.05–0.1 g) of the product was placed in a vial; 100 μL water was added; the vial was capped, heated to 60C for 10 min, and then purged with 1000 mL N2. These protocols, including purge duration, volume and temperature, were developed to capture 90% or more of VOCs in the FHPs, based on repeated tests of the same sample (i.e., the second test contained < 10% of the concentration of the first). After sampling was complete, the sodium sulfate was removed from the sorbent tube, which was then capped until analysis.
2.3. VOC analysis
Sample analyses followed well-developed protocols. Each adsorbent tube was injected with internal standards (fluorobenzene, p-bromofluorobenzene, and 1,2-dichlorobenzene-d4), and analyzed using a short-path automated thermal desorption system (ATD, Scientific Instrument Services, Inc., Ringoes, New Jersey, USA) coupled to a gas chromatography – mass spectrometer (GC–MS, Model 6890/5973, Agilent Technologies, Santa Clara, California, USA) equipped with a cryotrap/focuser (−140C to focus, 250C to inject) (Zhong et al., 2017). Chromatographic separation was performed using a DB-VRX capillary column (60 m × 0.25 mm, 1.4 μm film thickness) with helium as the carrier gas and a temperature program that started at 45C (10 min hold), ramped at 8C/min to 140C (10 min hold), and finally ramped at 30C/min to 225C (hold for 13 min). The MS detector, transfer line, EI ion source, and quadrupole temperatures were set at 250, 300, 230 and 150C, respectively. The MS was operated in full scan mode from 27–270 atomic mass unit (AMU). Peak areas were extracted by a ChemStation macro program (G1701BA Version B.01.00, Agilent, Santa Clara, USA), adjusted for internal standards and transferred to a spreadsheet.
The sum of target VOCs is designated as total target VOCs (TTVOCs). We investigated the larger non-target peaks in a subset of products using the MS fragmentation pattern and elution time. We provided a tentative identification of chemicals if their match quality values in the NIST 98 spectral library (NIST, 2008) exceeded 90%. Their masses were not quantified.
2.4. VOC calibration and quality control
Samples were analyzed for 98 target VOCs. Multipoint calibrations (1, 3, 10, 30, and 100 ng) were performed for each target VOC using authentic standards in mixtures or individual compounds (MilliporeSigma, Burlington, Massachusetts, USA). Calibration ranges were extended for several VOCs that were very prevalent in the FHPs. The range was extended in cases: to 400 ng for butanal, benzene, toluene, hexanal, heptanal, styrene and p-isopropyl toluene; 1000 ng for n-nonane, n-butyl acetate; 2000 ng for n-octane; 4000 ng for n-hexane, isopropyl acetate, n-propyl acetate, n-undecane, n-dodecane, and n-tetradecane, 10,000 ng for 2-butanone; 20,000 ng for α-pinene; 100,000 ng for limonene; 200,000 ng for ethyl acetate; and 400,000 ng for n-heptane. For ethyl acetate, n-undecane, n-dodecane and n-tetradecane, we used piece-wise linear and nonlinear calibrations; for other extended chemicals, piece-wise linear calibrations were used. Correlation coefficients of calibration curves exceeded 0.99. Recovery rates for most compounds ranged between 80% and 120%. Method detection limits (MDLs) for the target VOCs, determined as the standard deviation of seven replicate low concentration injections multiplied by 3.707 (Environmental Monitoring Systems Laboratory, 1996), ranged from 0.02 to 2.5 ng. The 98 target VOCs were divided into eight chemical groups: 6 aldehydes, 12 alkanes, 19 aromatics, 40 halohydrocarbons, 2 terpenes, 4 ketones, 7 esters, and 8 others. Table S1 shows target VOCs, chemical groups, internal standards, target ions, and MDLs. The results below MDL were set as 0, and shown as “ < MDL.”
Quality assurance activities included preparation and analysis of blanks and duplicates (47% of samples). The coefficient of variation of true duplicates averaged 40%. Duplicates were averaged for data analysis. A freshly loaded adsorbent tube injected with 10 ng of standards was analyzed daily. Differences between daily checks and calibration results were within 30%. Trace level contamination (< 5 ng) was detected in blanks for 11 compounds (n-hexane, benzene, pentanal, toluene, styrene, hexanal, nonanal, n-dodecane, naphthalene, n-tridecane, and n-tetradecane); blank-corrected results were used for these cases.
2.5. Exposure and risk assessment
Exposures were calculated assuming a reasonable upper level exposure scenario. We assumed that all VOCs in products were absorbed via dermal exposure by body for tampons, menstrual pads, feminine spays, powders and moisturizers (Kim et al., 2019) since dermal absorption might be larger than inhalation intake for gas phase chemicals (Gong et al., 2015, 2014); for washes and wipes, contact time is too short to absorb all VOCs into the skin; the remainder of VOCs exposure occurred by inhalation (Logue et al., 2011). Thus, we assumed dermal and inhalation exposure pathways for feminine washes and wipes, and dermal exposure for tampons, menstrual pads, feminine spays, powders and moisturizers. Risks were calculated as the sum of dermal and inhalation exposures for feminine washes and wipes.
We used eq. (1) to calculate daily dermal exposure dose (mg/kg-day) for FHPs other than washes, eq. (2) to estimate for non-cancer risks (hazard ratio) of dermal exposure, and eq. (3) for cancer risks of dermal exposure.
| (1) |
| (2) |
| (3) |
where Ci is the concentration (ng/g) of individual VOC in product; M is the residual mass (g) of product on skin each time for wipes, sprays, powders and moisturizers, or the measured mass of each unit for tampons and pads (Table S2); Freq/day is the times of the product used per day (Table S2); 10−6 is the conversion from ng to mg; RfD is the reference dose (mg/kg-day); and CSF is the cancer slope factor (kg-day/mg). Because feminine washes are washed away after application, daily dermal exposures of feminine washes were estimated using the water solution scenario in IH SkinPerm v2.0 (Tibaldi et al., 2014), which simulates the dynamics of evaporation, uptake in the stratum corneum, and permeation through the skin (parameters in Table S2). To account for the rapid permeation of VOCs through vaginal and vulvar tissues, model results were increased 20-fold (Farage and Maibach, 2004). For other FHPs, we assumed complete absorption of VOCs into the body given the long contact time. Potential risks associated with dermal doses were evaluated using RfDs (Table S3) and CSFs (Table S3) for dermal exposure from CalTOX (McKone and Maddalena, 2002), or were calculated based on oral exposure and gastrointestinal absorption factors (GIABS) (North Carolina Department of Environmental Quality, 2017).
Eq. (4) was used to calculate daily inhalation exposure concentrations (μg/m3), eq. (5) for non-cancer risks (hazard ratio) of inhalation exposure, and eq. (6) for cancer risks of inhalation exposures.
| (4) |
| (5) |
| (6) |
where Ci is the concentration (ng/g) of individual VOC in product; M is the mass (g) of the product used each time, 10 g for washes, specific mass measured of each unit for wipes (details in Table S2); Freq/day is the times of the product used per day (Table S2); HDF is the house dilution factor to estimate the average VOCs concentration based on the house size, the exchange with the outdoor and time spent indoor (Batterman, 2017); 10−3 is the conversion from ng to μg; RfC is the reference concentration (μg/m3); and UR is the unit risk (m3/μg). Inhalation exposures assumed FHPs were used in a typical bathroom setting (volume = 10 m3, air change rate from bathroom to house = 1.8 hr−1) in a house setting (volume = 191 m3, air change rate from indoor to outdoor = 0.63 hr−1), and a fully-mixed box model with total exposure time of 948 min/day along with a decay model, which was used to estimate the house dilution factor (Batterman, 2017). Values for indoor time, air change rates and house size were taken from the US Environmental Protection Agency (EPA) Exposure Factors Handbook (US EPA, 2011) (Table S2). Estimated airborne concentrations were compared to RfCs (Table S3) and URs (Table S3) for inhalation exposure obtained from the US EPA (US EPA, 2019a) and the state of Michigan (Michigan EGLE, 2019) to evaluate the potential risks.
2.6. Data analysis
Descriptive statistics in the text are presented as the median VOC mass found for each type of FHP, and with additional statistics (mean, standard deviation, and range) in the tables. Differences between product groups were evaluated using Mann-Whitney U tests (two samples) and Kruskal-Wallis tests (three or more samples). Influence of different factors (FHP type, brand type, “organic,” “for sensitive skin,” “scent,” and interaction term between FHP type and the other four individual factors) on VOC composition were evaluated using permutational multivariate analysis of variance (PERMANOVA) with 9999 permutations (Anderson, 2017). All statistical tests were two-sided with a type-I error rate of 0.05. Analyses used SPSS (SPSS, Inc., Chicago, Illinois, USA) and R version 3.6.0 (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
3. Results
3.1. Product characteristics
Details of the 79 tested FHPs are listed in Table 1. All FHPs other than sprays and powders had store-brand alternatives, and “organic” products were found for each type of FHP. Only 10% of the products indicated the manufacturing or expiration dates, but 81% listed the manufacturing location. Most tampons and menstrual pads collected for the study were labeled as regular-sized. Tampons had fairly similar weights, 1.9 ± 0.2 g/unit (N = 22) as did feminine wipes, 4.9 ± 1.2 g/unit (N = 12); weights of menstrual pads had greater variation, 5.3 ± 2.8 g/unit (N = 22).
3.2. VOCs in different feminine hygiene products
We identified from 11 to 45 target VOCs in the various FHPs, representing 25 to 80% of the 40 to 170 distinct peaks seen on the chromatograms. Most of the larger non-target peaks were identified as fragrances, such as linalool, eucalyptol and benzyl acetate, and several others were identified as adhesives in the menstrual pads, most likely used on the bottom of the pad to help it stick to underwear (Table S4).
By product type, washes, sprays and powders contained the highest TTVOC concentrations while tampons and moisturizers had the lowest (p < 0.002; Table 2). The VOC composition based on chemical groups (aldehydes, alkanes, aromatics, halohydrocarbons, terpenes, ketones, esters and others, Table S1) showed large differences (Table 2, Figure S2). All product types except wipes contained a high proportion of alkanes. Wipes had the highest percentages of esters and ketones, while washes, tampons, menstrual pads, sprays and powders were dominated by terpenes and alkanes. In moisturizers, aldehydes was the principal VOC group.
Table 2.
Concentrations (ng/g) of VOC groups in different types of feminine hygiene products.
| Product | VOC | Median | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Feminine wash (N = 13) | Aldehydes | 69 | 141 | 233 | 4.3 | 897 |
| Alkanes | 13,875 | 11,968 | 8344 | 63 | 28,745 | |
| Aromatics | 298 | 606 | 861 | 14 | 3088 | |
| Halohydrocarbons | < MDL | 24 | 52 | < MDL | 156 | |
| Terpenes | 4649 | 18,292 | 29,140 | 15 | 91,404 | |
| Ketones | 48 | 67 | 65 | < MDL | 200 | |
| Esters | < MDL | 1522 | 5465 | < MDL | 19,709 | |
| Others | 1177 | 3067 | 6473 | < MDL | 24,354 | |
| TTVOCs | 24,619 | 35,687 | 34,736 | 97 | 112,616 | |
| Aldehydes | 52 | 56 | 31 | 13 | 138 | |
| Alkanes | 28 | 138 | 506 | 6.9 | 2402 | |
| Aromatics | 3.7 | 5.0 | 4.9 | 0.9 | 23 | |
| Halohydrocarbons | 1.9 | 2.4 | 2.1 | 0.1 | 7.2 | |
| Terpenes | 3.5 | 67 | 292 | 0.5 | 1374 | |
| Ketones | 1.4 | 3.5 | 4.9 | < MDL | 23 | |
| Esters | 0.4 | 0.8 | 1.0 | < MDL | 3.1 | |
| Others | < MDL | 0.1 | 0.2 | < MDL | 1.0 | |
| TTVOCs | 102 | 272 | 573 | 47 | 2472 | |
| Aldehydes | 14 | 20 | 14 | 4.4 | 53 | |
| Alkanes | 337 | 8407 | 18,431 | 4.3 | 75,266 | |
| Aromatics | 2.5 | 5.6 | 8.0 | 0.4 | 32 | |
| Halohydrocarbons | 0.5 | 2.3 | 5.5 | < MDL | 26 | |
| Terpenes | 9.5 | 35 | 97 | 1.0 | 459 | |
| Ketones | 0.1 | 0.7 | 1.1 | < MDL | 3.8 | |
| Esters | 0.2 | 2.3 | 5.5 | < MDL | 25 | |
| Others | < MDL | < MDL | 0.1 | < MDL | 0.7 | |
| TTVOCs | 85 | 8473 | 18,440 | 20 | 75,322 | |
| Aldehydes | 15 | 20 | 22 | 0.8 | 84 | |
| Alkanes | 5.0 | 39 | 97 | 0.9 | 342 | |
| Aromatics | 4.2 | 25 | 46 | 0.3 | 150 | |
| Halohydrocarbons | 0.2 | 0.9 | 1.9 | < MDL | 6.7 | |
| Terpenes | 54 | 680 | 1704 | 0.9 | 5943 | |
| Ketones | 27 | 484 | 1336 | 2.5 | 4711 | |
| Esters | 18 | 816 | 1924 | < MDL | 6641 | |
| Others | 0.7 | 1.3 | 1.4 | 0.1 | 4.3 | |
| TTVOCs | 356 | 2067 | 3800 | 38 | 13,007 | |
| Aldehydes | 114 | 298 | 341 | 70 | 874 | |
| Alkanes | 18,158 | 38,370 | 56,141 | 42 | 134,732 | |
| Aromatics | 1295 | 3486 | 4591 | 39 | 11,232 | |
| Halohydrocarbons | 12 | 16 | 19 | < MDL | 43 | |
| Terpenes | 5316 | 9454 | 13,576 | 51 | 33,364 | |
| Ketones | 1299 | 968 | 898 | 5.9 | 2047 | |
| Esters | 26 | 73 | 104 | < MDL | 245 | |
| TTVOCs | 34,340 | 52,665 | 55,786 | 607 | 146,376 | |
| Aldehydes | 64 | 190 | 271 | 44 | 671 | |
| Alkanes | 140 | 152 | 120 | 24 | 286 | |
| Aromatics | 26 | 57 | 55 | 12 | 125 | |
| Halohydrocarbons | < MDL | 0.6 | 1.4 | < MDL | 3.2 | |
| Terpenes | 3.3 | 32 | 43 | < MDL | 88 | |
| Ketones | 23 | 22 | 5.7 | 13 | 28 | |
| Esters | < MDL | 0.6 | 1.4 | < MDL | 3.2 | |
| TTVOCs | 236 | 454 | 456 | 103 | 1200 |
We examined VOCs that are known or suspected carcinogens (chloroform, benzene, 1,4-dioxane, etc.). Chloroform, a likely human carcinogen, was found in 62% of the analyzed products with the highest level, 149 ng/g, in a wash (product W7). Benzene, another carcinogen, was found in all washes, moisturizers, sprays and powders (detected in 83% of products), and the highest level was found in a spray, 3604 ng/g (S4). 1,4-dioxane, classified as a likely carcinogen, was found in 50% products (92% of the washes, and 75% of the wipes), with the highest level in a wash, 24,354 ng/g (W12). 1,4-Dichlorobenzene was found in 59% of tampons (mean and median concentrations of 0.2 and 0.4 ng/g). Naphthalene was found in most (87%) products at very low concentrations; a wash (W2) had the highest level, 69 ng/g.
3.3. VOC difference by brand and label
Our samples included several scented tampons and menstrual pads. These products tended to have higher aromatic and terpenoid content than the unscented products. As examples, terpene levels (median of 692 ng/g, N = 2, mostly limonene) in scented tampons far exceeded the 2.9 ng/g level (N = 20) in unscented tampons (p = 0.04). Aromatics were 14 ng/g in scented tampons, slightly higher than 3.4 ng/g in unscented tampons (p = 0.08). Similarly, scented menstrual pads (N = 3) contained higher levels of aromatic compounds (including benzene, toluene, ethylbenzene, xylene, styrene, etc.) than unscented pads (N = 19), e.g., 26 versus 2.4 ng/g (p = 0.09), and 108 versus 7.8 ng/g for terpenoid VOCs (p = 0.07).
We compared VOC levels between store brands and non-store brands. Often, non-store brands had higher VOC levels than store brands (Fig. 1 and Table S5). For washes, the median concentrations in non-store brands of aromatic and terpenoid compounds (especially limonene) exceeded levels in store brands (p < 0.02). For tampons, non-store brands had higher levels of octanal, p-isopropyl toluene, and limonene (p < 0.05), but lower levels of butanal (p = 0.03). For menstrual pads, non-store brands had significantly higher terpene levels, although chloroform levels were lower (p = 0.02). For wipes, non-store brands had much higher levels of several VOCs, and TTVOC level was 1206 ng/g, six times higher than store brands (p = 0.008). For moisturizers, VOC differences were not significant, probably due to the limited sample size.
Fig. 1.
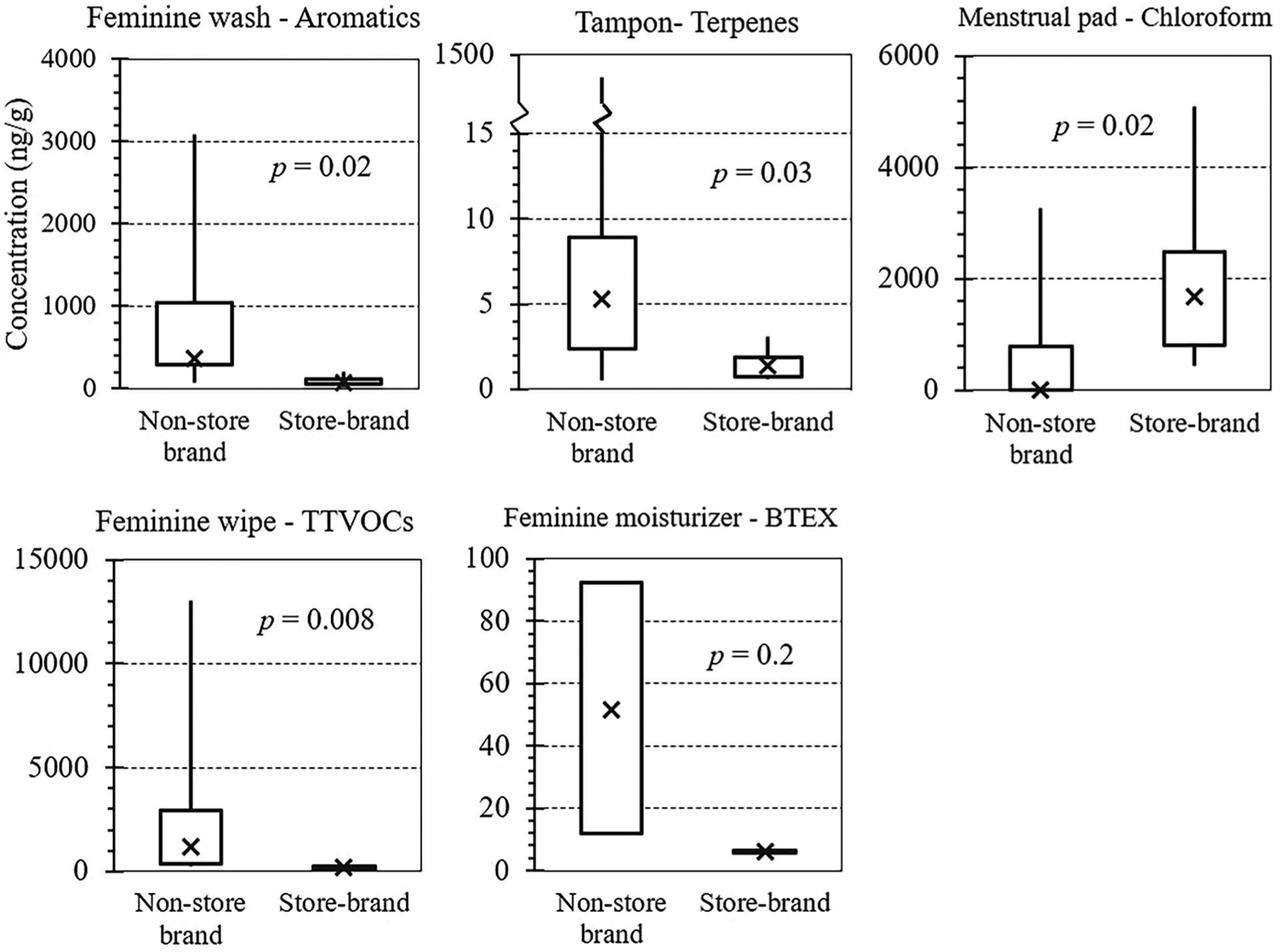
Boxplots of specific VOC concentrations (ng/g) in feminine hygiene products between non-store brand and store brand. Shows maximum, 75th percentile, median (shown as cross), 25th percentile, and minimum concentrations. P-values are from Mann-Whitney U tests.
Product labels were not a consistent indicator of VOC content (Table S5). As with the “organic” designation, there was no clear distinction with respect to VOC levels. For products labeled as “organic,” tampons had lower halohydrocarbons (including chloroform) compared to products without such labels (p < 0.08). Organic washes had higher concentrations of aldehydes, terpenes, limonene, n-nonane and n-undecane; organic wipes had more BTEX (benzene, toluene, ethylbenzene, and xylene), tetrahydrofuran and α-pinene and organic menstrual pads had slightly higher levels of p-, m-xylene and ketones (p = 0.09). We saw no significant differences for sprays, powders, and moisturizers, but sample sizes were small for these types of products.
Some of the washes, menstrual pads and wipes were labeled “for sensitive skin.” Several differences between these and conventional products are suggested (Table S5). For washes, these products had lower levels of terpenes, but higher n-decane. For pads, n-undecane was lower, but pentanal was higher. For wipes, concentrations of isopropyl acetate and n-propyl acetate were higher.
Influences of the five factors and four interaction terms on VOC composition are shown in Table S6 (results of PERMANOVA). Each factor (FHP type, brand type, “organic,” “for sensitive skin,” and “scent”) significantly influenced VOC composition. In addition, the interaction term between FHP type and brand type (store brand versus non-store brand) was significant, which indicates that the brand effect depends on the FHP type. The most important factor affecting VOC composition was the product type (as suggested earlier in Figure S2). After this factor, the label “for sensitive skin” had the most influence; although this explained only a small fraction of the variation in composition (see R2 in Table S6).
3.4. Health risks
The potential for chronic non-cancer health effects, expressed as estimated hazard ratios (HRs), is shown in Fig. 2A and B. Menstrual pads had the highest HRs, up to 11, largely due to n-heptane (HR = 11) which is associated with irritation to skin, respiratory tract and effects on the central nervous system (ILO, 2019a). One of the pads (MP11) contributed to the highest dermal dose of n-heptane (0.0033 mg/kg-day), eleven times the reference dose (0.0003 mg/kg-day). The second contributor was carbon tetrachloride, which is associated with skin and eye irritation, and even dermatitis through its defatting action (O’Neil et al., 2013). Sprays and powders had the second highest HRs, up to 2.2, also largely due to n-heptane. The next largest contributors were n-nonane and 1,2,4-trimethylbenzene, which are associated with eye, skin and respiratory tract irritation and central nervous system effects (ILO, 2019b; c). These were followed by n-hexane in sprays and powders; its most sensitive endpoint is possible reproductive effects (ILO, 2019d). The other FHPs had HRs below 0.1, indicating negligible potential for non-cancer health effects. 1,4-dioxane contributed 73% of the HR of inhalation exposure due to washes (HR = 0.03). Long-term exposure to 1,4-dioxane defats the skin, which may cause dryness or cracking, and may affect on the central nervous system, kidneys and liver (ILO, 2019e).
Fig. 2.
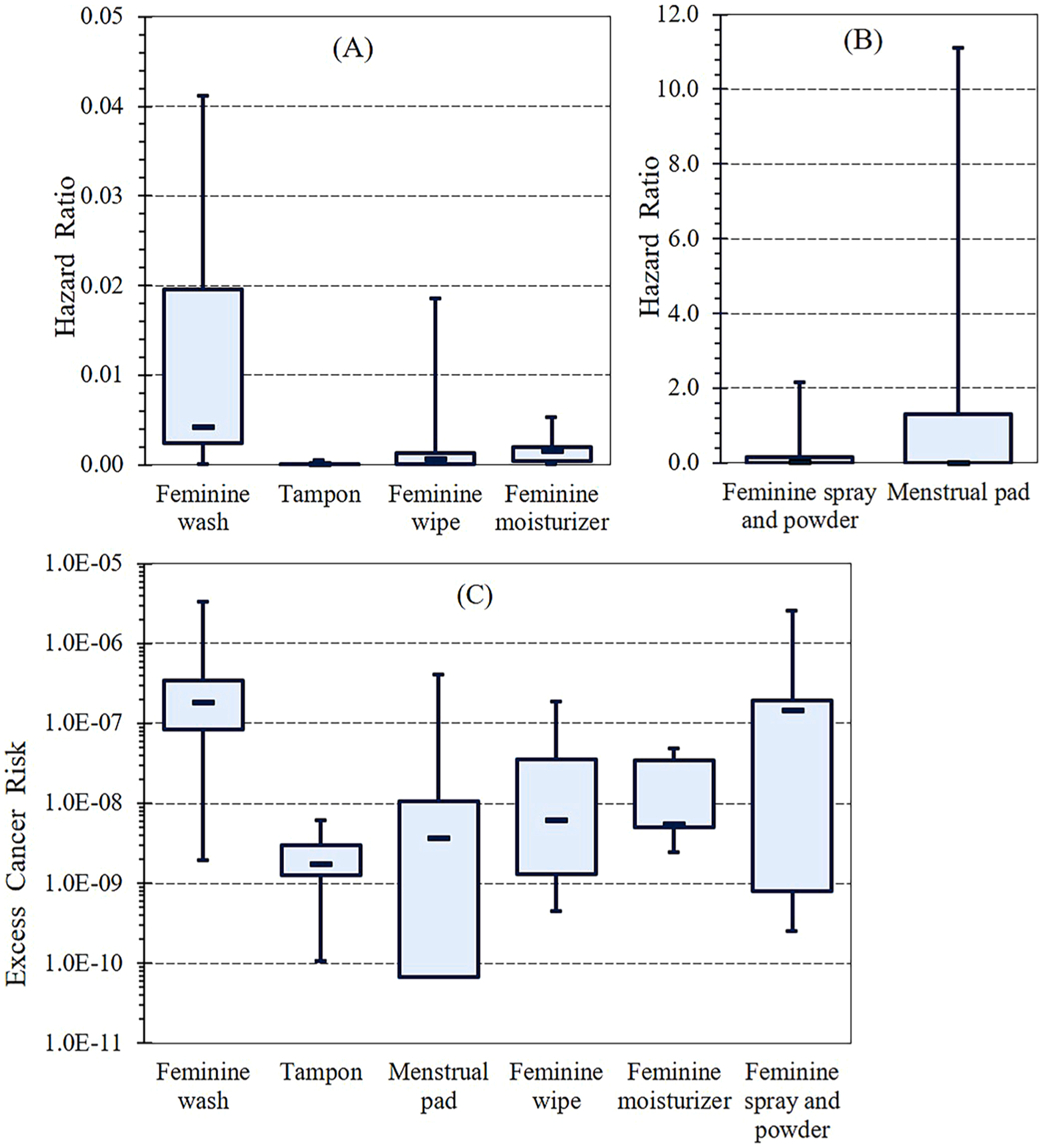
Boxplots of hazard ratio (A and B) and cancer risk (C) for different feminine hygiene products. Shows maximum, 75th percentile, median, 25th percentile, and minimum values.
The estimated excess cancer risks (CRs) associated with the use of individual FHPs are shown in Fig. 2C. For washes, the CR reached 3.3 × 10−6, largely (greater than 99%) due to the inhalation of 1,4-dioxane. This chemical is suspected of causing malignant tumors in the nasal cavity and liver (Kasai et al., 2009, 2008). For sprays and powders, the CRs was as high as 2.1 × 10−6, largely (99%) due to dermal exposure of benzene, which is associated with multi-organ carcinogenicity (e.g., leukemia, malignant lymphoma, and lung cancer) (Snyder et al., 1993; Yin et al., 1996), genetic damage and fetotoxicity (Green et al., 1978; ILO, 2019f). Cancer risks associated with all other products fell below 10−6, a commonly used and protective reference level.
4. Discussion
Like many other consumer products, FHPs contain generally small quantities of VOCs that may be inadvertent or residual components in the product ingredients, used in manufacturing or packaging these products, or added for specific purposes, e.g., as fragrances, binders, and adhesives. To a large degree, VOC composition and concentration depended on the type of product, and levels were brand-specific. Labels indicating “organic” and “for sensitive skin” were not indicative of VOC concentration. All of the FHPs tested contained several VOCs that are considered toxic, but at widely varying concentrations. Using generally conservative exposure assumptions, the estimated non-cancer and cancer risks due to VOC exposure approached or sometimes exceeded health protective reference levels for certain products, including several of menstrual pads, washes, sprays and powders; however, risks for most products appeared negligible.
4.1. VOC composition
VOC composition depended on the product type. Alkanes were the dominant VOC group in all FHPs, except wipes. Detected target alkanes include many commonly used solvents and lubricants (US EPA, 2019b), e.g., n-hexane, n-heptane, n-nonane, n-decane, n-undecane, n-tridecane, n-tetradecane. Long term exposure to these substances may induce irritation to skin, mucous membrane and respiratory tracts (Bingham et al., 2001), and some have reproductive effects (ILO, 2019d). Menstrual pads contained several nontargeted alkane VOCs, e.g. 3-methylhexane, 2-methylhexane and methylcyclohexane, which are commonly used in solvents and adhesives (ECHA, 2019). These chemicals have the potential for health impacts, including irritation to skin, eyes, and respiratory tract (ECHA, 2019; US EPA, 2019c). Generally, alkanes have low toxicity and little if any cancer risk. However, several products had relatively high concentrations of alkanes, e.g., n-heptane in a feminine spray (S2) tested at 90,482 ng/g, conferring a non-cancer HR of 2.1.
Terpenes were found in all FHPs, especially in washes and sprays. Our target terpenes, α-pinene and limonene, are frequently used as fragrances (US EPA, 2019b), but can cause skin and respiratory tract irritation at high concentrations, and, in reaction with ozone, can produce strong irritants and carcinogens, including formaldehyde, acrolein, methacrolein, and methyl vinyl ketone (Christensson et al., 2009; Wolkoff, 2013; Wolkoff et al., 2000). The US EPA’s Safer Choice Criteria program codes limonene with a yellow triangle designating that while functional, this ingredient has hazard profile issues (US EPA, 2019c). In terms of risk calculation, only limonene has a reference concentration for inhalation exposure (Michigan EGLE, 2019). Thus, the health risks via exposure to terpenoid VOCs may not be well characterized. In addition, we identified several nontarget terpenoid compounds in washes and wipes, e.g., eucalyptol, linalool, citral and camphor (Table S4). While these VOCs can be irritants (ECHA, 2019), we did not quantify concentrations and health risks. Terpenes are commonly used in consumer cleaning products (Bartzis et al., 2015; Dinh et al., 2015; Steinemann et al., 2011), and thus indoor levels of these compounds are often elevated (Tichenor and Mason, 1988; Zarogianni et al., 2018). More quantitative toxicity information of terpenes is required, including reference doses for dermal exposure, to present a more complete assessment.
Aromatic hydrocarbons, e.g., benzene, toluene and styrene, were present in many FHPs at low concentrations. The function of these VOCs in FHPs is unclear; they may be residual contaminants. Despite the low concentrations of these VOCs, several are potent carcinogens including benzene, cresol, and naphthalene (US EPA, 2019a). Feminine sprays and powders had the highest concentration of aromatic VOCs (Table 2; benzene reached 3604 ng/g; toluene reached 4538 ng/g), and these product types were associated with a higher cancer risk (highest CR = 2.1 × 10−6; largely due to benzene). Moisturizers had a lower TTVOC levels than menstrual pads, but were associated with a similar or higher cancer risk, largely due to the presence of aromatic VOCs (Figure S2).
Although menstrual pads and tampons are regarded as “medical devices” by the US FDA (US CDRH, 2005), we recommend that regulations should require the disclosure of ingredients in FHPs, since the chemicals in these products are complicated and potentially harmful to consumers. New York State will become the first state to require disclosure of FHP ingredients, starting in 2020 (AP News, 2019).
4.2. Product labels
Product labels declaring “organic,” “all natural,” and “for sensitive skin” did not relate to their VOC composition or concentration, and generally these products did not differ from conventional products. Although materials in FHPs labelled “organic” or “all natural” are extracted from plants, this has little if any significance for irritation and toxicity, and the “natural” materials used in “organic” products may be contaminated from global or local pollution more than synthetic materials (Thompson and Darwish, 2019). The term “organic” is clearly defined by the US Department of Agriculture (USDA) National Organic Program standards, which address food production, processing and distribution. For personal care products including FHPs, however, this term is not well defined or regulated. A number of FHPs show the USDA organic seal, which requires suppliers to become certified, change their labels, and reformulate their products. However, this does not require the stringency of the controls that are placed on production of food (USDA, 2008).
Interestingly, the national, non-store brands often had higher concentration of several VOCs than store brands. For tampons, most of store-brand products (80%) were produced in Israel; non-store brands were mainly produced in the US (67%) and Mexico (17%). Most of the other FHPs (including non-store and store brands) were produced in the US and Canada. Possibly, a single manufacturer may make FHPs sold for multiple store brands, with the major difference being packaging and labeling, and not the raw materials and ingredients. In contrast, the non-store brands may utilize their own factory and separately source ingredients with different specifications that lead to higher VOC levels.
4.3. Health risks
Using reasonable upper level exposure scenarios, VOC exposure from lifetime use of most menstrual pads and tampons was not associated with meaningful non-cancer or cancer health risks, except for several pads with high non-cancer risks. Two recent studies were conducted for VOCs in sanitary pads (Kim et al., 2019; Park et al., 2019). These studies used head-space methods or only collected air samples inside of packages to measure the VOC content in FHPs, which underestimated results, including exposures and health risks. In addition, a smaller set of target VOCs was considered, further implying underestimation. However, some findings were similar. Both non-cancer and cancer risks associated with sanitary pads sold in South Korea were below reference levels. Alkanes including heptane, decane, nonane and undecane contributed the majority of non-cancer risks, although levels remained low, similar to the present study (Kim et al., 2019). With the exception of carbon tetrachloride, which we also found in menstrual pads, the drivers of the cancer risk (bromodichloromethane, 1,2-dichloroethane, 1,2-dichloropropane, 1,4-dichlorobenzene) differed from the present study (Kim et al., 2019). Several of these halogenated VOCs may be disinfection byproducts or other inadvertent contaminants. Low VOC levels and health risks were found for sanitary pads sold in South Korea, Japan, Finland, France, Greece and elsewhere in the US (Park et al., 2019), e.g., the highest concentration of toluene was 5.5 ng/g (Brand 4) and 12.4 ng/g (MP12) in the present study.
Our risk estimates may be underestimated for several reasons. Risks were calculated for only target VOCs. (These VOCs used authentic standards and we confirmed the target VOCs to be present.) Many of the target VOCs lack reference exposure levels or CSFs for estimating the risks. We recommend additional studies that explore reproductive effects of these VOCs given their pervasiveness in FHPs. We identified additional (non-target) VOCs in the FHPs, but did not calculate possible risks associated with these compounds. FHPs may include toxic components other than VOCs, e.g., dioxins and pesticides (DeVito and Schecter, 2002; Nicole, 2014; Shin and Ahn, 2007). Finally, women may use several types of FHPs simultaneously, e.g., pads, tampons and washes. Such cases may be analyzed using an additive assumption, which may increase the risk above the reference level (Ding et al., 2020).
The association between self-reported use of FHPs and exposure to 1,4-dichlorobenzene and ethylbenzene (Ding et al., 2020) (noted earlier) was based on analysis of data from the NHANES 2001–2004, which measured VOCs in blood in 2432 US women between 2001 and 2004. In the present study, we did not find significant levels of these VOCs in the contemporary FHPs tested. Over the past several decades, reductions in VOCs in building materials, vehicle emissions and elsewhere have led to substantially lower indoor and outdoor exposures (Warneke et al., 2012; Weschler, 2009). Similar trends likely apply to consumer products and FHPs, although documentation for FHPs is incomplete. We suspect that some FHPs manufactured decades earlier had considerably higher VOC content, possibly including chlorinated compounds as residuals from fiber bleaching processes. However, we do not have first-hand information to confirm such speculations.
5. Study strengths and limitations
Strengths of our study include the analysis of 79 different FHPs that included the bestselling products and brands in the US, and the measurement of a wide range VOCs. To our knowledge, this is the first study measuring VOCs in such a wide range of FHPs; this both fills a data gap and provides an overall picture of VOC content in these consumer products. We utilized sensitive and validated methods, which included many toxic VOCs. Exposures and health risks were estimated using reasonable upper level exposure scenarios, and results provide conservative but realistic information relevant for consumers.
Our study has several limitations. Sample sizes were relatively small for some types of FHPs, e.g., feminine sprays, powders, and moisturizers and FHPs were drawn from only the US market. The assumed exposure scenarios, while conservative, may not accurately reflect all or typical situations. Risks were estimated only for target VOCs, e.g., we did not quantify non-target compounds, nor did we investigate inorganic, semi-volatile, or microbiological constituents. Uncertainties in the RfCs and CSFs can be large. Despite these limitations, the study reveals the presence of VOCs in FHPs that are undesirable constituents, and more comprehensive follow-up studies, particularly in other countries, appear warranted.
6. Conclusion
Feminine hygiene products are applied to sensitive and permeable tissues where chemical exposure is rapid and of special concern. All 79 feminine hygiene products tested contained multiple VOCs, but generally at low concentrations. Using conservative but realistic exposure assumptions, the use of FHPs is associated with calculated cancer and non-cancer risks that fall below health protective guidelines in most cases. However, several menstrual pads, washes, sprays and powders with higher levels of toxic VOCs, including benzene, n-heptane and 1,4-dioxane, can be associated with calculated risks that approach or exceed guidelines, and simultaneous use of several FHPs will increase risk levels. Product labels were not informative with respect to VOC composition and concentration. We recommend disclosure of ingredients and production dates and suggest the removal of VOCs that do not serve functional or aesthetic purposes.
Supplementary Material
Acknowledgements
Support for this research was provided by grant P30ES017885 from the National Institute of Environmental Health Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105740.
References
- Anderson MJ, 2017. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. 1–15. [Google Scholar]
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE, 2007. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci 97 (2), 355–363. [DOI] [PubMed] [Google Scholar]
- AP News, 2019. New York to be 1st to require disclosure of tampon materials [cited 2019Oct. 11]. Available from: https://apnews.com/d613da947c2943a4-ba1d8458745f6204.
- Bartzis J, Wolkoff P, Stranger M, Efthimiou G, Tolis EI, Maes F, Norgaard AW, Ventura G, Kalimeri KK, Goelen E, Fernandes O, 2015. On organic emissions testing from indoor consumer products’ use. J. Hazard. Mater 285, 37–45. [DOI] [PubMed] [Google Scholar]
- Batterman S, 2017. Review and Extension of CO2-Based Methods to Determine Ventilation Rates with Application to School Classrooms. Int. J. Env. Res. Public Health 14 (2), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A, Quinn MM, Perry MJ, Milton DK, 2009. Characterization of occupational exposures to cleaning products used for common cleaning tasks-a pilot study of hospital cleaners. Environ. Health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham E, Cohrssen B, Powell CH, 2001. Patty’s toxicology. Wiley, New York. [Google Scholar]
- Bridges B, 2002. Fragrance: emerging health and environmental concerns. Flavour Fragrance J. 17 (5), 361–371. [Google Scholar]
- Christensson JB, Forsstrom P, Wennberg AM, Karlberg AT, Matura M, 2009. Air oxidation increases skin irritation from fragrance terpenes. Contact Dermatitis 60 (1), 32–40. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Schecter A, 2002. Exposure assessment to dioxins from the use of tampons and diapers. Environ. Health Perspect 110 (1), 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Batterman S, Park SK, 2020. Exposure to Volatile Organic Compounds and Use of Feminine Hygiene Products Among Reproductive-Aged Women in the United States. J. Women’s Health 29 (1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TV, Kim SY, Son YS, Choi IY, Park SR, Sunwoo Y, Kim JC, 2015. Emission characteristics of VOCs emitted from consumer and commercial products and their ozone formation potential. Environ. Sci. Pollut. Res 22 (12), 9345–9355. [DOI] [PubMed] [Google Scholar]
- European Chemicals Agency (ECHA), 2019. [cited 2019 Oct. 30]. Available from: https://echa.europa.eu/.
- Environmental Monitoring Systems Laboratory (Cincinnati Ohio), 1996. Methods for the determination of metals and inorganic chemicals in environmental samples. Noyes Publications, xii, Westwood, N.J., pp. 535. [Google Scholar]
- Farage M, Maibach HI, 2004. The vulvar epithelium differs from the skin: implications for cutaneous testing to address topical vulvar exposures. Contact Dermatitis 51 (4), 201–209. [DOI] [PubMed] [Google Scholar]
- Gong M, Weschler CJ, Liu L, Shen H, Huang L, Sundell J, Zhang Y, 2015. Phthalate metabolites in urine samples from Beijing children and correlations with phthalate levels in their handwipes. Indoor Air 25 (6), 572–581. [DOI] [PubMed] [Google Scholar]
- Gong M, Zhang Y, Weschler CJ, 2014. Predicting dermal absorption of gas-phase chemicals: transient model development, evaluation, and application. Indoor Air 24(3), 292–306. [DOI] [PubMed] [Google Scholar]
- Green JD, Leong BKJ, Laskin S, 1978. Inhaled Benzene Fetotoxicity in Rats. Toxicol.Appl. Pharmacol 46 (1), 9–18. [DOI] [PubMed] [Google Scholar]
- Hussain A, Ahsan F, 2005. The vagina as a route for systemic drug delivery. J. Control.Release 103 (2), 301–313. [DOI] [PubMed] [Google Scholar]
- ILO International Chemical Safety Cards (ICSC), 2019a. n-Heptane [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0657.
- ILO International Chemical Safety Cards (ICSC), 2019b. Nonane [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=1245.
- ILO International Chemical Safety Cards (ICSC), 2019c. 1,2,4-Trimethylbenzene [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=1433.
- ILO International Chemical Safety Cards (ICSC), 2019d. n-Hexane [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0279.
- ILO International Chemical Safety Cards (ICSC), 2019e. 1,4-Dioxane [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0041.
- ILO International Chemical Safety Cards (ICSC), 2019f. Benzene [cited 2019 Dec. 3]. Available from: http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0015.
- Kasai T, Kano H, Umeda Y, Sasaki T, Ikawa N, Nishizawa T, Nagano K, Arito H, Nagashima H, Fukushima S, 2009. Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats. Inhal. Toxicol 21 (8–11), 889–897. [DOI] [PubMed] [Google Scholar]
- Kasai T, Saito M, Senoh H, Umeda Y, Aiso S, Ohbayashi H, Nishizawa T, Nagano K, Fukushima S, 2008. Thirteen-week inhalation toxicity of 1,4-dioxane in rats. Inhal. Toxicol 20 (10), 961–971. [DOI] [PubMed] [Google Scholar]
- Kim HY, Lee JD, Kim J-Y, Lee JY, Bae O-N, Choi Y-K, Baek E, Kang S, Min C, Seo K, Choi K, Lee B-M, Kim K-B, 2019. Risk assessment of volatile organic compounds (VOCs) detected in sanitary pads. J. Toxicol. Environ. Health, A 82 (11), 678–695. [DOI] [PubMed] [Google Scholar]
- Kohen JM, 2001. The History of the Regulation of Menstrual Tampons [cited 2019 Oct.28]. In: dash.harvard.edu. Available from: https://dash.harvard.edu/handle/1/8852185.
- Kwon KD, Jo WK, Lim HJ, Jeong WS, 2008. Volatile pollutants emitted from selected liquid household products. Environ. Sci. Pollut. Res 15 (6), 521–526. [DOI] [PubMed] [Google Scholar]
- Logue JM, McKone TE, Sherman MH, Singer BC, 2011. Hazard assessment of chemical air contaminants measured in residences. Indoor Air 21 (2), 92–109. [DOI] [PubMed] [Google Scholar]
- McKone TE, Maddalena RL, 2002. Eight years of model evaluation: The CalTOX experience. Epidemiology 13 (4), S118. [Google Scholar]
- Michigan Department of Environment, Great Lakes, and Energy, 2019. Michigan air toxics system initial threshold screening level/initial risk screening level (ITSL/IRSL) toxics screening level query [cited 2019 July 30]. Available from: http://www.deq.state.mi.us/itslirsl/.
- Mirza MA, Panda AK, Asif S, Verma D, Talegaonkar S, Manzoor N, Khan A, Ahmed FJ, Dudeja M, Iqbal Z, 2016. A vaginal drug delivery model. Drug Deliv. 23 (8), 3123–3134. [DOI] [PubMed] [Google Scholar]
- Nicole W, 2014. A Question for Women’s Health Chemicals in Feminine Hygiene Products and Personal Lubricants. Environ. Health Perspect 122 (3), A70–A75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Standards and Technology (NIST) Mass Spectrometry Data Center, 2008. NIST/EPA/NIH Mass Spectral Library (NIST 08) and NIST Mass Spectral Search Program (Version 2.0f).
- North Carolina Department of Environmental Quality, 2017. Risk Evaluation Equations and Calculations. [Google Scholar]
- O’Neil MJ, Heckelman PE, Dobbelaar PH, Roman KJ, Kenny CM, Karaffa LS, Royal Society of Chemistry (Great Britain), 2013. The Merck index : an encyclopedia of chemicals, drugs, and biologicals. 15th ed. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- Park CJ, Barakat R, Ulanov A, Li Z, Lin P-C, Chiu K, Zhou S, Perez P, Lee J, Flaws J, Ko CJ, 2019. Sanitary pads and diapers contain higher phthalate contents than those in common commercial plastic products. Reprod. Toxicol 84, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JL, Illum L, 1992. Penetration Enhancement for Polypeptides through Epithelia.D. Routes of Delivery - Case-Studies. 8. The Vaginal Route of Peptide and Protein Drug Delivery. Adv. Drug Del. Rev 8 (2–3), 341–366. [Google Scholar]
- Rosell M, Lacorte S, Ginebreda A, Barcelo D, 2003. Simultaneous determination of methyl tert.-butyl ether and its degradation products, other gasoline oxygenates and benzene, toluene, ethylbenzene and xylenes in Catalonian groundwater by purge-and-trap-gas chromatography-mass spectrometry (vol 995, pg 171, 2003). J. Chromatogr. A 1007 (1–2), 209–210. [DOI] [PubMed] [Google Scholar]
- Schreiner CA, 2003. Genetic toxicity of naphthalene: A review. J. Toxicol. Env. Heal. B6 (2), 161–183. [DOI] [PubMed] [Google Scholar]
- Shin JH, Ahn YG, 2007. Analysis of polychlorinated dibenzo-p-dioxins and dibenzofurans in sanitary products of women. Text. Res. J 77 (8), 597–603. [Google Scholar]
- Singer BC, Destaillats H, Hodgson AT, Nazaroff WW, 2006. Cleaning products and air fresheners: emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air 16 (3), 179–191. [DOI] [PubMed] [Google Scholar]
- Snyder R, Witz G, Goldstein BD, 1993. The Toxicology of Benzene. Environ. Health Perspect 100, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So-yun K, 2017. Right to Have Safe Periods [cited 2019 Oct. 28]. In: NewsH, Hanyang University, Seoul, Korea. Available from: https://www.hanyang.ac.kr/web/eng/special1?p_p_id=newsView_WAR_newsportlet&p_p_lifecycle=0&_newsView_WAR_newsportlet_action=view_message&_newsView_WAR_newsportlet_messageId=158944. [Google Scholar]
- Statista.com, 2020. Feminine Hygiene - United States [cited 2020 Jan 9]. Available from: https://www.statista.com/outlook/80040000/109/feminine-hygiene/united-states.
- Steinemann AC, MacGregor IC, Gordon SM, Gallagher LG, Davis AL, Ribeiro DS, Wallace LA, 2011. Fragranced consumer products: Chemicals emitted, ingredients unlisted. Environ. Impact Assess. Rev 31 (3), 328–333. [Google Scholar]
- Thompson LA, Darwish WS, 2019. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol 2019, 2345283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibaldi R, ten Berge W, Drolet D, 2014. Dermal Absorption of Chemicals: Estimation by IH SkinPerm. J. Occup. Env. Hyg 11 (1), 19–31. [DOI] [PubMed] [Google Scholar]
- Tichenor BA, Mason MA, 1988. Organic Emissions from Consumer Products and Building-Materials to the Indoor Environment. JAPCA. 38 (3), 264–268. [DOI] [PubMed] [Google Scholar]
- U.S. Center for Devices and Radiological Health (US CDRH), 2005. Menstrual Tampons and Pads: Information for Premarket Notification Submissions (510(k)s) - Guidance for Industry and FDA Staff [cited 2019 Oct. 28]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/menstrual-tampons-and-pads-information-premarket-notification-submissions-510ks-guidance-industry.
- U.S. Environmental Protection Agency (US EPA), 1998. Carcinogenic Effects of Benzene: An Update. Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA), 1999. Integrated Risk Information System (IRIS) on Naphthalene. Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA), 2011. Highlights Of The Exposure Factors Handbook (Final Report) (EPA/600/R-10/030). Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA), 2013. Toxicological review of 1,4-dioxane (with inhalation update). Washington, DC. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA), 2019a. Regional Screening Levels [cited 2019 July 30]. Available from: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables.
- U.S. Environmental Protection Agency (US EPA), 2019b. Chemical Data Reporting under the Toxic Substances Control Act [cited 2019 Nov. 1]. Available from:https://www.epa.gov/chemical-data-reporting.
- U.S. Environmental Protection Agency (US EPA), 2019c. Safer Chemical Ingredients List [cited 2019 Oct. 30]. Available from: https://www.epa.gov/saferchoice/safer-ingredients.
- U.S. Department of Agriculture (USDA), National Organic Program, 2008. Cosmetics, Body Care Products, and Personal Care Products. Available from: https://www.ams.usda.gov/sites/default/files/media/OrganicCosmeticsFactSheet.pdf.
- Warneke C, de Gouw JA, Holloway JS, Peischl J, Ryerson TB, Atlas E, Blake D, Trainer M, Parrish DD, 2012. Multiyear trends in volatile organic compounds in Los Angeles, California: Five decades of decreasing emissions. J. Geophys. Res. - Atmos 117, D00V17. [Google Scholar]
- Weisel CP, Jo WK, 1996. Ingestion, inhalation, and dermal exposures to chloroform and trichloroethene from tap water. Environ. Health Perspect 104 (1), 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, 2009. Changes in indoor pollutants since the 1950s. Atmos. Environ 43(1), 153–169. [Google Scholar]
- Wolkoff P, 2013. Indoor air pollutants in office environments: Assessment of comfort, health, and performance. Int. J. Hyg. Environ. Health 216 (4), 371–394. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD, 2000. Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air 10 (2), 82–91. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Wilkins CK, Clausen PA, Nielsen GD, 2006. Organic compounds in office environments - sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 16 (1), 7–19. [DOI] [PubMed] [Google Scholar]
- Women’s Voices for the Earth, 2014. Testing Reveals Toxic Chemicals in Procter & Gamble’s Always Pads [cited 2019 Oct. 28]. Available from: http://www.womensvoices.org/2014/10/13/testing-reveals-toxic-chemicals-in-procter-gambles-always-pads/.
- Xu X, Weisel CP, 2005. Dermal uptake of chloroform and haloketones during bathing. J. Expo. Anal. Environ. Epidemiol 15 (4), 289–296. [DOI] [PubMed] [Google Scholar]
- Yin SN, Hayes RB, Linet MS, Li GL, Dosemeci M, Travis LB, Zhang ZN, Li DG, Chow WH, Wacholder S, Blot WJ, Wang YZ, Jiang ZL, Dai TR, Zhang WY, Chao XY, Ye PZ, Kou QR, Chang XC, Lin XF, Meng JF, Ding CY, Zho JS, 1996. An expanded cohort study of cancer among benzene-exposed workers in China. Environ. Health Perspect 104, 1339–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarogianni AM, Loupa G, Rapsomanikis S, 2018. Fragrances and Aerosol during Office Cleaning. Aerosol. Air Qual. Res 18 (5), 1162–1167. [Google Scholar]
- Zhong LX, Su FC, Batterman S, 2017. Volatile Organic Compounds (VOCs) in Conventional and High Performance School Buildings in the US. Int. J. Env. Res. Public Health 14 (1), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


