Abstract
Carbenoxolone is a derivative of glycyrrhetinic acid found in the root of Glycyrrhiza glabra, colloquially known as licorice. It has been used as a treatment for peptic and oral ulcers. In recent years, carbenoxolone has been utilized in basic research for its ability to block gap junctional communication. Better understanding the distribution of carbenoxolone after systemic administration can lead to a better understanding of its potential sites of action. Presented is an ultra high-performance liquid chromatography tandem mass spectrometer (UHPLC–MS/MS) method for the identification and quantification of carbenoxolone in mouse blood and brain tissue. Twenty mice were injected intraperitoneally with 25 mg/kg carbenoxolone and brain tissue and blood were collected for analysis. Blood concentrations (mean ± SD) at 15, 30, 60 and 120 min were determined to be (n = 5) 5394 ± 778, 2636 ± 836, 1564 ± 541 and 846 ± 252 ng/mL, respectively. Brain concentrations (mean ± SD) at 15, 30, 60 and 120 mins were determined to be (n = 5) 171 ± 62, 102 ± 35, 55 ± 10 and 27 ± 9 ng/g, respectively. The analysis of these specimens at the four different time points resulted in blood and brain half-lives in mice of ~43 and 41 min, respectively. The UHPLC–MS/MS method was determined to be sensitive and robust for quantification of carbenoxolone.
Keywords: blood, brain, carbenoxolone, UHPLC–MS/MS
1 ∣. INTRODUCTION
Carbenoxolone is a derivative of glycyrrhetinic acid found in the root of Glycyrrhiza glabra, colloquially known as licorice. It has been used as a treatment for peptic and oral ulcers (Turpie & Thomson, 1965). Carbenoxolone is a classic blocker of gap junction channels and hemichannels. In recent years, carbenoxolone has been utilized in basic research for its ability to block gap junctional communication. Gap junctions are the channel-forming structures between the membranes of two adjoining cells. Gap junctions allow direct electrical communication between cells; hence they are also referred to as electrical synapses. Gap junction channels are formed by two hemichannels or connexons, one contributed by each cell at the synapse (McCracken & Roberts, 2006; Söhl, Maxeiner, & Willecke, 2005). Single hemichannels can exist on the cell membrane providing a pathway for exchange of intracellular components, including ions and adenosine triphosphate, with the extracellular solution. Gap junctions found on neurons as well as on glial cells have been found to affect the firing rate of neurons (Blenkinsop & Lang, 2006; Pannasch et al., 2011).
Carbenoxolone and gap junction blockers, are commonly used to investigate of gap junctions and their function in the nervous system. In blood, carbenoxolone is >99.95% bound to plasma proteins. The apparent volume of distribution is ~0.1 L/kg (Pinder et al., 1976). There are several in vivo studies of the possible effect of carbenoxolone in the central nervous system of animals after systemic administration. For instance, carbenoxolone administered systemically reduced seizures in epilepsy-prone rats (Gareri et al., 2004). Apomorphine-induced stereotypies were selectively blocked by systemic administration of carbenoxolone (Moore & Grace, 2002). Intracerebroventricular administration of carbenoxolone was shown to significantly decrease naloxone-precipitated morphine withdrawal signs (Moradi et al., 2013). Additionally, carbenoxolone has effects in the periphery. Systemic carbenoxolone blocked morphine-related colonic inflammation and constipation when administered intraperitoneally (Bhave et al., 2017).
The carbenoxolone molecule is polar and relatively large, which raises a question as to its ability to cross the blood-brain barrier. It was determined by analyzing cerebrospinal fluid that a negligible amount of carbenoxolone crosses the blood-brain barrier (Leshchenko, Likhodii, Yue, Burnham, & Velazquez, 2003). A limitation suggested by the researchers of that study was that carbenoxolone residing in brain tissue cannot be ruled out by their analyses. Better understanding of the distribution of carbenoxolone in blood and brain tissue after systemic administration can lead to a better understanding of its potential sites of action. After a 25 mg/kg i.p. injection of carbenoxolone, samples were collected at 15, 30, 60 and 120 min. Brain tissue was chosen for the study as some in vivo studies suggest possible effects in the central nervous system after systemic administration. Previous studies have suggested that carbenoxolone does not penetrate the blood–brain barrier after i.p. administration (Leshchenko et al., 2003).
An ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) method for the identification and quantification of carbenoxolone in mouse blood and brain tissue was developed. The method was validated in mouse brain homogenates and was significantly sensitive to quantitate the concentrations of carbenoxolone.
2 ∣. METHODS
2.1 ∣. Experimental
Twenty male Swiss Webster mice (Harlan Laboratories, Indianapolis, IN, USA) weighing 25–30 g were housed five to a cage in animal care quarters and maintained at 22 ± 2°C on a 12 h light-dark cycle. Food and water were available ad libitum. The mice were brought to the test room (22 ± 2°C, 12 h light-dark cycle), marked for identification, and allowed to recover for 18 h from transport and handling. The animal study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The mice were injected i.p. with 25 mg/kg carbenoxolone. Five mice were sacrificed at each time point (15, 30, 60 and 120 min). Immediately after death, blood and brain tissue samples were collected. The specimens were frozen at −80°C until analyzed.
2.2 ∣. Reagents
The primary reference material for carbenoxolone was purchased from Cayman Chemical Company (Ann Arbor, MI, USA) as a sodium salt and the internal standard (ISTD) 11-Nor-9-carboxy-delta-9-tetrahydrocannabinol-d3 (THCOOH-d3) was purchased from Cerilliant Corporation (Round Rock, TX, USA). Acetonitrile, ammonium format, formic acid, methanol and deionized water were purchased from Fisher Scientific (Hanover Park, IL, USA). All reagents were ACS grade or better. Medical-grade nitrogen was purchased from National Welders Supply Company (Richmond, Virginia).
2.3 ∣. Preparation of calibrators and quality control specimens
Carbenoxolone-free mouse brain homogenate, 1:3 mouse brain tissue–water and carbenoxolone-free mouse blood provided the matrices for all of the prepared calibrators. Appropriate volumes of the working carbenoxolone solution were added to pooled blood or brain homogenate to obtain seven-point calibration curves with a range of 10–1000 ng/mL in blood and 10–1000 ng/g in brain each day of the analysis. Carbenoxolone-free in-house mouse blood or brain homogenate provided the matrix for all prepared quality control (QC) specimens. As a result of limited availability of in-house carbenoxolone-free mouse blood only a single set of quality control samples were prepared at low control (LQC), with a target concentration of 30 ng/mL; medium control (MQC), with a target concentration of 300 ng/mL; and high control (HQC), with a target concentration of 750 ng/mL.
The following batch-prepared quality control brain homogenate specimens were prepared and used for all brain homogenate validation experiments; low control (LQC), with a target concentration of 30 ng/g; medium control (MQC), with a target concentration of 300 ng/g; and high control (HQC), with a target concentration of 750 ng/g. A drug-free control (negative control) that did not contain carbenoxolone, but did contain the THCOOH-d3, ISTD, and a double-negative control that did not contain carbenoxolone or ISTD prepared in mouse blood or brain homogenate were also analyzed each day of the analysis. All QC samples were stored at −20°C until analysis.
2.3.1 ∣. Specimen preparation and extraction
Carbenoxolone was isolated from blood or brain homogenate by liquid–liquid extraction with acetonitrile using a modified previously described method for tetrahydrocannabinol and other cannabinoids and their metabolites (Poklis, Thompson, Long, Lichtman, & Poklis, 2010). No deuterated form of carbenoxolone was located for use as an ISTD. THC-COOH-d3 was used as the ISTD. THC-COOH-d3 was successfully used in the described sample preparation technique and had a similar pKa (4.21) to the pKa (4.04) of carbenoxolone. In brief, brain tissue specimens were homogenized 1:3 with water. The ISTD, 10 ng THC-COOH-d3, was added to aliquots of 100 μL of blood or 400 μL of homogenized brain tissue calibrators, controls and samples. These samples were mixed and allowed to equilibrate. A 1 mL aliquot of ice-cold acetonitrile was added drop by drop to each sample while vortex mixing. The samples were then centrifuged at 3500 rpm for 10 min. After centrifugation, the samples were placed in a −40°C freezer for at least 2 h. The top layer containing the acetonitrile was removed via a disposable glass pipette and placed in a clean test tube. Samples were dried using a Savant AES1000 (Thermo Savant, Holbrook, NY, USA). The samples were reconstituted with 100 μL of 1:10 water-methanol and placed in autosampler vials for analysis.
2.4 ∣. Instrumental analysis
A Shimadzu ultra-performance liquid chromatography (UHPLC) system (Shimadzu, Kyoto, Japan) was used for the chromatographic separation of carbenoxolone and the ISTD. The analytical column was a Zorbax Eclipse XDB-C18 column, 4.6 × 75 mm, 3.5 μm (Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of water–methanol (10:90, v/v) with 0.1 mM ammonium formate and was delivered at an isocratic flow rate of 1 mL/min. The sample injection volume was 2 μL.
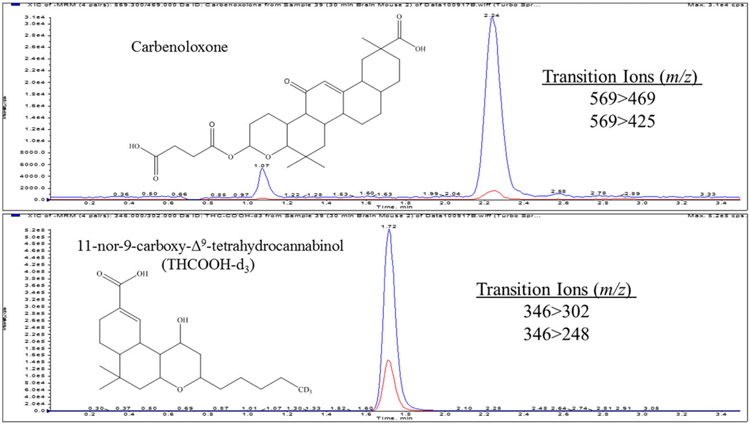
The UHPLC system attached to a a tandem mass spectrometer (MS/MS), a Sciex 6500 QTRAP system with an IonDrive Turbo V source for TurbolonSpray® (Sciex, Ontario, Canada). This system was controlled by Analyst software (Sciex, Ontario, Canada). The source temperature was set at 650°C, and curtain gas had a flow rate of 30 mL/min. The ionspray voltage was −4500 V, with the ion source gases 1 and 2 having flow rates of 60 mL/min. The declustering potential was −160 V for carbenoxolone and −110 V for THC-COOH-d3. The acquisition mode used was multiple reaction monitoring. The following transition ions (m/z) with corresponding collision energies in parentheses were monitored in negative ion mode: 569 → 469 (−40) and 569 → 425 (−60) for carbenoxolone and 346 → 302 (−28) and 346 → 289 (−38) for THC-COOH-d3. The retention times for carbenoxolone and ISTD were 1.7 and 2.3, respectively. The total run time for the analytical method was 3.5 min (Figure 1).
FIGURE 1.
A typical chromatograph of a mouse brain homogenate sample extract
3 ∣. METHOD VALIDATION
The evaluation of the brain homogenate assay was conducted over five separate analytical runs. Sample batches were analyzed as recommended for biomedical assay validation (SWGTOX, 2013) for linearity, lower limit of quantification (LOQ), lower limit of detection (LOD), precision, bias, carryover, selectivity and stability. Absolute recovery and ion suppression were also accessed.
3.1 ∣. Linearity, limit of quantification and limit of detection
Linearity was verified from seven-point calibration curves prepared with concentrations of 10, 20, 50, 100, 200, 500 and 1000 ng/g in brain homogenate over five analytical runs. A linear regression of the ratio of the peak area counts of carbenoxolone and the ISTD vs. concentration was used to construct the calibration curves. The linear regression correlation coefficients (r2) for the carbenoxolone calibration curves for all five batches ranged from 0.9934 to 1.0000. The LOD and LOQ were administratively set at 10 ng/g, the concentration of the lowest calibrator. Concentrations were not detected below 10 ng/mL or 10 ng/g in any of the positive samples and therefore it was not necessary to determine a true LOD. LOD/LOQ samples were prepared using three different lots of brain homogenate on three different days. The LOD/LOQ samples were determined to be within ±20% of the target value and had responses at least 10 times greater than the signal-to-noise ratio of drug-free brain tissue homogenates.
3.2 ∣. Precision and bias
Precision and bias were determined using the prepared brain homogenate QC samples. The largest calculated inter-run percentage coefficient of variation (CV) for each concentration (n = 3) over the five validation runs was used to assess intra-run precision. The determined CVs were −16, −13 and −11% for the LQC, MQC and HQC, respectively. The inter-run precision and bias were calculated for each concentration over the five validation runs using the combined QC values (n = 3) over 5 days for a total of 15 replicates at each concentration. The determined CVs were 9, 13 and 10% for the LQC, MQC and HQC, respectively. The determined percentage biases were −10, −5 and <1 for the LQC, MQC and HQC, respectively (Table 1).
TABLE 1.
Intra- and inter-day accuracy and precision for carbenoxolone in mouse brain homogenates
| Carbenoxolone | Control | Mean concentration (ng/g) |
CV (%) |
Bias (%) |
|---|---|---|---|---|
| Intra-day (n = 3) | LQC (30 ng/g) | 28 | 15 | −7 |
| MQC (300 ng/g) | 314 | 15 | 5 | |
| HQC (750 ng/g) | 800 | 9 | 7 | |
| Inter-day (n = 15) | LQC (30 ng/g) | 27 | 9 | −10 |
| 5 days | MQC (300 ng/g) | 285 | 13 | −5 |
| HQC (750 ng/g) | 754 | 10 | > 1 |
LQC, Low quality control; MQC, medium quality control; HQC, high quality control.
3.3 ∣. Carryover
Sample carryover was evaluated in each of the five validation runs using two different procedures. First, immediately following the injection of the 1000 ng/g carbenoxolone calibrator, a negative control was injected. Second, an injection of the HQC (750 ng/g) sample was immediately followed by injection of the LQC (3 ng/g) sample. This procedure was routinely applied each time that the 1000 ng/g calibrator, HQC and LQC samples were analyzed. Lack of carryover was confirmed as carbenoxolone was not detected in the negative controls and the LQC samples did not demonstrate a significant quantified bias of >20%.
3.4 ∣. Selectivity
The selectivity of the assay was determined using six different lots of carbenoxolone-free brain homogenate. Each individual lot was analyzed with and without ISTD. No peaks were detected that co-eluted with the carbenoxolone or with the ISTD. This ensured that endogenous brain homogenate components did not interfere with the assay.
3.5 ∣. Stability
Carbenoxolone in brain homogenate was determined for frozen and thawed and “post-preparative” stability. The experiments were performed using the three of the carbenoxolone control specimens: LQC (30 ng/g), MQC (300 ng/g) and HQC (750 ng/g). All studies included three replicate analyses of each QC specimen. Since specimens are often stored frozen and thawed for re-analysis, the stability of carbenoxolone in brain homogenate was determined after three freeze–thaw cycles. The QC specimens were stored at −20°C, then twice removed and allowed to thaw unassisted. Once thawed, they were re-frozen for 24 h. The specimens were removed a third time, allowed to thaw and then analyzed. The freeze–thaw QC samples were extracted and quantified against freshly prepared calibrators. The “post-preparative” stability of the carbenoxolone was evaluated by allowing extracts to sit in the UHPLC–MS/MS's autosampler. A batch of the extracted LQC, MQC and HQC was quantified against a freshly prepared calibration. The extracted controls were then allowed to sit in the autosampler for 72 h at 5°C after which they were re-injected and quantified from the initial calibration. The results of the initial analysis were compared with those of the re-injected samples. Carbenoxolone was considered stable under the conditions of the freeze–thaw and post-preparative analysis, if the concentrations of the QC samples were within ±20% of the target concentration samples. Under these tested conditions, carbenoxolone was determined to be stable (Table 2).
TABLE 2.
Stability of carbenoxolone in brain homogenates
| Carbenoxolone | Control | Mean concentration (ng/g) |
CV (%) |
Bias (%) |
|---|---|---|---|---|
| Post-preperative (n = 3) | LQC (30 ng/g) | 25 | 3 | −17 |
| MQC (300 ng/g) | 246 | 3 | −14 | |
| HQC (750 ng/g) | 737 | 7 | −2 | |
| Freeze–thaw (n = 3) | LQC (30 ng/g) | 28 | 15 | −6 |
| MQC (300 ng/g) | 326 | 13 | 9 | |
| HQC (750 ng/g) | 789 | 13 | 5 |
3.6 ∣. Absolute recovery and ion suppression
The absolute percentage recovery and percentage ion suppression of the assay for the carbenoxolone and the ISTD were determined at 100 ng/g. Matrix samples were prepared by extracting six different lots of drug-free brain homogenate and then adding carbenoxolone and the ISTD. Extracted samples were then prepared by fortifying six different lots of drug-free brain homogenate and then extracting. Six neat samples were prepared in mobile phase. The absolute recovery of the assay was determined by comparing the mean absolute peak area of the extracted samples with the mean absolute peak area of the matrix samples. The ion suppression of the assay was determined by comparing the mean absolute area of the matrix samples with the mean absolute area of the neat samples. The absolute percentage recovery at 100 ng/g carbenoxolone and THCOOH-d3 was determined to be 103 ± 6 and 111 ± 10%, respectively. The percentage ion suppression for 100 ng/g carbenoxolone and THCOOH-d3 was determined to be 24 ± 7 and 24 ± 15%, respectively.
3.7 ∣. Application of the method
Applying the present method, carbenoxolone was detected and quantitated in 20 mouse blood and brain tissue specimens collected after receiving an i.p. dose of 25 mg/kg of carbenoxolone. The five blood and brain specimens were collected at 15, 30, 60 and 120 min. Sample analysis include a set of matrix-matched LQC, MQC and HQC, a negative control and a double negative control. No sample preparation was needed for the blood specimens prior to the liquid–liquid extraction. Brain specimens were homogenized at 1:3 mouse brain tissue–water. Matrix-matched calibrators were prepared in mouse blood or brain tissue homogenate. The linear regression correlation coefficient (r2) values for both blood and brain calibrations curves were >0.9980. All blood and brain tissue homogenate QC specimens were determined to be ±15% of their expected concentrations and carbenoxolone was not detected in any of the negative controls nor THCCOOH-d3 in the double-negative controls.
4 ∣. RESULTS
The carbenoxolone concentrations (mean ± SD) determined for blood (n = 5) at 15, 30, 60 and 120 min were 5394 ± 778, 2636 ± 836, 1564 ± 541 and 846 ± 252 ng/mL, respectively. The carbenoxolone concentrations (mean ± SD) determined for brain tissue (n = 5) at 15, 30, 60 and 120 mins were 171 ± 62, 102 ± 35, 55 ± 10 and 27 ± 9 ng/g, respectively. The determined blood to brain ratio for each of the 20 time points was 31 ± 10 to 1 with the mean blood to brain ratio at each time point ranging from 26:1 to 32:1. The elimination rates of carbenoxolone in blood and brain were also similar (Figure 2) and based on the mean concentrations the half-life of carbenoxolone in mouse blood and brain were estimated to be 43 and 41 min, respectively.
FIGURE 2.
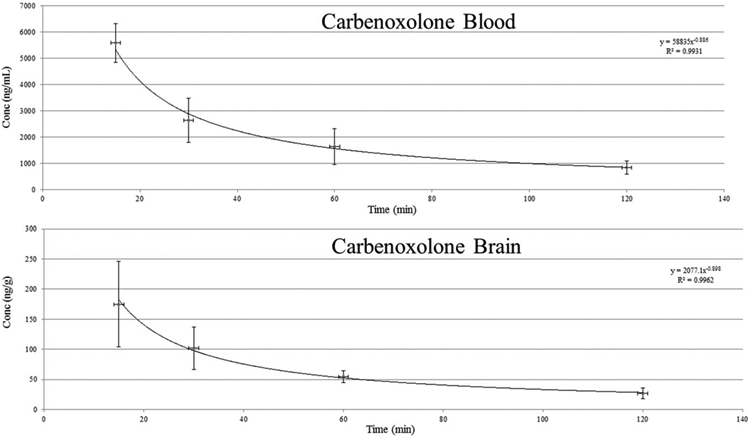
Change in carbenoxolone concentrations in brain and blood as a function of time
5 ∣. DISCUSSION
The presented UHPLC–MS/MS method for the determination of carbenoxolone in mouse brain tissue was sensitive and robust for quantification. The method uses a well-established, reliable liquid–liquid extraction procedure with a simple isocratic HPLC method that resolved carbenoxolone and the ISTD. The high blood to brain ratio was not unexpected based on the reported apparent volume of distribution, 0.1 L/kg. A previous study suggested that carbenoxolone does not penetrate the blood-brain barrier after i.p. administration (Leshchenko et al., 2003). The authors did note that the possibility of a transient residence into brain tissue could not be ruled out by their analyses. Their study involved the i.p. injection of carbenoxolone (50 mg/kg) in 16 adult rats, followed by blood taken from the vein in the tail and cerebrospinal fluid collected from the cisterna magna at 20, 40, 60 and 90 min. These samples were analyzed using a high-performance liquid chromatography method (Zhang, 2002) and the cerebrospinal fluid concentrations were determined to be <1 μM or 570 ng/mL carbenoxolone. This concentration was well above the LOD/LOQ of 10 ng/g for brain in the presented method. Even with the ability to detect and quantitate the brain concentrations after an i.p. administration, caution should be exercised in the interpretation of direct effect of carbenoxolone on the central nervous system. The evaluation of effects in the central nervous system may be more appropriately studied through intracranial administration. A study examining carbenoxolone metabolism noted that carbenoxolone hydrolysis in mammalian tissues does not appear to occur and most of carbenoxolone metabolism occurs owing to the microflora of the stomach and small intestine (Iveson, Lindijp, & Parke, 1971). This suggests that active metabolites should not be a concern in i.p. administration; however, further studies should examine carbenoxolone's metabolites, their possible effects and ability to cross the blood'brain barrier.
6 ∣. CONCLUSION
The presented UHPLC–MS/MS method for the determination of carbenoxolone was sensitive and robust for quantification of carbenoxolone in mouse blood and brain tissue. The method uses a well-established, reliable liquid–liquid extraction procedure with a simple isocratic separation that resolved carbenoxolone and the ISTD. The analysis of the brain and blood specimens at the four different time points resulted in a relatively consistent blood and brain ratio and the half-life in mice was determined to be ~43 and 41 min, respectively.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health (DA033934).
REFERENCES
- Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, & Akbarali HI (2017). Connexin–purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. The FASEB Journal, 31, 2649–2660. 10.1096/fj.201601068R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, & Lang EJ (2006). Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. Journal of Neuroscience, 26, 1739–1748. 10.1523/JNEUROSCI.3677-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareri P, Condorelli D, Belluardo N, Russo E, Loiacono A, Barresi V, … De Sarro G (2004). Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats. Neuropharmacology, 47, 1205–1216. 10.1016/j.neuropharm.2004.08.021 [DOI] [PubMed] [Google Scholar]
- Iveson P, Lindijp WE, & Parke DV (1971). The metabolism of carbenoxolone in the rat. Xenobiotica, 1, 79–95. 10.3109/00498257109044381 [DOI] [PubMed] [Google Scholar]
- Leshchenko Y, Likhodii S, Yue W, Burnham WM, & Velazquez JLP (2003). Carbenoxolone does not cross the blood brain barrier: An HPLC study. BMC Neuroscience, 3, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken CB, & Roberts DCS (2006). Neuronal gap junctions: Expression, function, and implications for behavior. International Review of Neurobiology, 73, 125–151. 10.1016/S0074-7742(06)73004-5 [DOI] [PubMed] [Google Scholar]
- Moore H, & Grace AA (2002). A role for electrotonic coupling in the striatum in the expression of dopamine receptor-mediated sterotypies. Neuropsychopharmacology, 27, 980–992. 10.1016/S0893-133X(02)00383-4 [DOI] [PubMed] [Google Scholar]
- Moradi S, Charkhpour M, Ghavimi H, Motahari R, Ghaderi M, & Hassanzadeh K (2013). Gap junction blockers: A potential approach to attenuate morphine withdrawal symptoms. Journal of Biomedical Science, 20, 77. 10.1186/1423-0127-20-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Vargová L, Reingruber J, Ezan P, Holcman D, & Giaume C (2011). Astroglial networks scale synaptic activity and plasticity. National Academy of Sciences of the USA, 108, 8467–8472. 10.1073/pnas.1016650108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder RM, Brogden RN, Sawyer PR, Speight TM, Spencer R, & Avery GS (1976). Carbenoxolone: A review of its pharmacological properties and therapeutic efficacy in peptic ulcer disease. Drugs, 11, 245–307. 10.2165/00003495-197611040-00002 [DOI] [PubMed] [Google Scholar]
- Poklis JL, Thompson CC, Long KA, Lichtman AH, & Poklis A (2010). Disposition of cannabichromene, cannabidiol, and Δ9-tetrahydrocannabinol and its metabolites in mouse brain following marijuana inhalation determined by high-performance liquid chromatography–tandem mass spectrometry. Journal of Analytical Toxicology, 34, 516–520. 10.1093/jat/34.8.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G, Maxeiner S, & Willecke K (2005). Expression and functions of neuronal gap junctions. International Review of Neurobiology, 6, 191–200. [DOI] [PubMed] [Google Scholar]
- SWGTOX (2013). Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. Journal of Analytical Toxicology, 37, 452–474. [DOI] [PubMed] [Google Scholar]
- Turpie AG, & Thomson TJ (1965). Carbenoxolone sodium in the treatment of gastric ulcer with special reference to side-effects. Gut, 6, 591–594. 10.1136/gut.6.6.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y (2002). Determination of furazolidone, carbenoxolone sodium and berberine hydrochloride in wei kang tablets by reversed-phase high performance liquid chromatography (RP-HPLC). Chinese Journal of Chromatography, 20, 350–352. [PubMed] [Google Scholar]