Abstract
Background
Immune-related adverse events (irAEs) have been reported to be associated with the efficacy of immunotherapy. Herein, we conducted a meta-analysis to demonstrate that irAEs could predict the efficacy of immune checkpoint inhibitors (ICIs) in lung cancer patients.
Methods
Literature on the correlation between irAEs and the efficacy of immunotherapy in lung cancer patients were searched to collect the data on objective response rate (ORR), overall survival (OS), or progression-free survival (PFS) of the patients. These data were incorporated into the meta-analysis.
Results
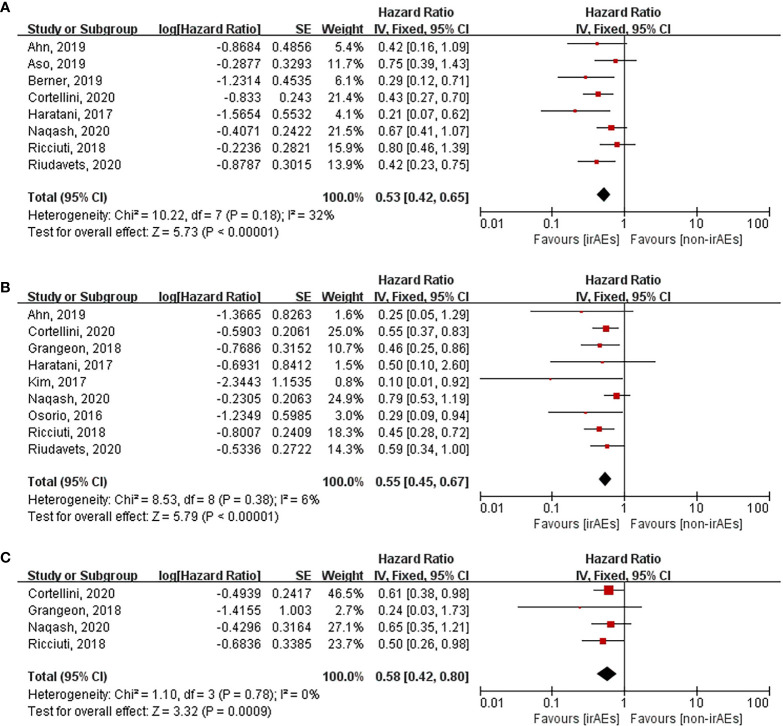
A total of 34 records encompassing 8,115 patients were examined in this study. The irAEs occurrence was significantly associated with higher ORR {risk ratio (RR): 2.43, 95% confidence interval (CI) [2.06–2.88], p < 0.00001} and improved OS {hazard ratio (HR): 0.51, 95% CI [0.43–0.61], p < 0.00001}, and PFS (HR: 0.50, 95% CI [0.44–0.57], p < 0.00001) in lung cancer patients undergoing ICIs. Subgroup analysis revealed that OS was significantly longer in patients who developed dermatological (OS: HR: 0.53, 95%CI [0.42–0.65], p < 0.00001), endocrine (OS: HR: 0.55, 95%CI [0.45–0.67], p < 0.00001), and gastrointestinal irAEs (OS: HR: 0.58, 95%CI [0.42–0.80], p = 0.0009) than in those who did not. However, hepatobiliary, pulmonary, and high-grade (≥3) irAEs were not correlated with increased OS and PFS.
Conclusion
The occurrence of irAEs in lung cancer patients, particularly dermatological, endocrine, and gastrointestinal irAEs, is a predictor of enhanced ICIs efficacy.
Keywords: immune-related adverse events (irAEs), efficacy, immune checkpoint inhibitors (ICIS), lung cancer, meta-analysis
Introduction
Immunotherapy has led to unprecedented improvements in the life expectancy of cancer patients, particularly those with lung cancer, by priming the immune system to fight against the tumor cells. ICIs target cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1), which are critical molecules that negatively regulate the T-cell activity and aid the tumor cells in evading immune surveillance. However, owing to the heightened immune response, the irAEs caused by ICIs are frequent and ineluctable. A meta-analysis showed that the probability of irAE incidence ranges from 54% to 76% and varies according to the types of ICIs (1). Any organ system could be involved, including the skin, endocrine glands, gastrointestinal tract, liver, pulmonary, and, less commonly, the cardiovascular and central nervous systems. Although the underlying mechanism has not been completely elucidated, increased T-cell activity, B-cell-mediated autoantibody production, and cross-reactive tumoral antigenicity have been suggested to be involved in the occurrence of irAEs (2).
The irAEs inevitably affect the treatment and prognosis of the patients. Once these events occur, clinicians adopt different management strategies according to the types and grades of irAEs. The National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO) have issued guidelines for the management of irAEs and have provided comprehensive general treatment algorithms for the clinicians (3, 4). In terms of prognosis, discontinuing ICIs may affect the life expectancy of the patients. However, the clinicians have noticed that patients who developed irAEs were more likely to benefit from ICIs. Meanwhile, theoretically, both antitumor and anti-self-adverse effects could result when the immunity is enhanced. Thus, there seems to exist a correlation between the occurrence of irAEs and the efficacy of immunotherapy.
A systemic review of melanoma has been conducted, which revealed that irAEs could predict survival and response in patients treated with ICIs (5), however, comprehensive meta-analyses focusing on lung cancer have not yet been performed. Herein, our work aimed to elucidate whether the occurrence of irAEs could predict the efficacy of ICIs in patients with lung cancer. Furthermore, we performed a subgroup analysis to decipher the association of organ-specific and grade-specific irAEs with the clinical outcomes of the patients.
Materials and Methods
Search Strategy
PubMed, Embase, and Cochrane Library databases from inception to October 15, 2020 were searched to locate eligible studies reporting the association between the efficacy of ICIs and irAEs in lung cancer patients undergoing immunotherapy. The search strategy was “[immune-related (Title/Abstract)] AND [adverse events (Title/Abstract)] AND {[lung cancer (Title)] OR [SCLC (Title)]}.” The citations of relevant articles were also reviewed in case of omission. The language was restricted to English, and conference abstracts were also included.
Inclusion and Exclusion Criteria
The studies that met the following criteria were included in the review:
Involving patients who were diagnosed with lung cancer.
Involving patients who were treated with CTLA-4, PD-1, or PD-L1 inhibitors.
Studies reporting the association between irAEs and ICIs efficacy.
Studies that provided data on ORR, OS, or PFS of the patients.
Studies that provided the HR of OS or PFS in patients who developed irAEs versus patients without irAEs and 95% CI.
Studies published in the English language.
The exclusion criteria of the studies to be included in the review were as follows:
Studies that provided OS and PFS, but not HR, or provided HR and P-value, but not 95% CI.
Duplicated data or overlapping study populations (the most recent report was included).
The adverse events were not caused by the use of ICIs.
Data Collection and Quality Assessment
DW and CC independently extracted the data from the included studies. The following data were extracted: author, year of publication, trial design, landmark analysis, sample size, irAE type and grade, ORR of patients with and without irAEs, HRs, and 95% CIs for OS or PFS of patients with global, organ-specific, and grade-specific irAEs. Several studies provided both multivariate and univariate HRs, and we selected the former. If studies provided HRs with and without landmark analysis, we chose the former. If studies provided the HRs of global as well as organ-specific irAEs, we opted for the former. If studies provided the HRs of both grade-specific and all-grade irAEs, we selected the latter. The Newcastle-Ottawa scale (NOS) criteria were applied to assess the quality of the included studies. Discrepancies were resolved by discussions among DW, CC, and YG.
Data Analyses
Review Manager (RevMan) Version 5.4 (Nordic Cochrane Center, Copenhagen, Denmark) was used to perform the statistical analyses. The log HRs of irAEs versus non-irAEs were obtained by calculating RevMan. If the HR of non-irAEs versus irAEs was provided instead of the opposite comparison, the HR and 95% CI of irAEs versus non-irAEs were calculated by determining the reciprocal of the original HR and 95% CI (6). Heterogeneity was assessed by applying the chi-square test and I2 statistic. A chi-square p<0.05 or an I2>50% was considered to indicate significant heterogeneity (7). The random-effects model was used in case of significant heterogeneity (8); if not, the fixed-effects model was used. Publication bias was evaluated using Begg’s test and Egger’s test. If there is a significant publication bias, we will further use the trim and fill method to evaluate.
Results
Records Selection and Characterization of the Identified Studies
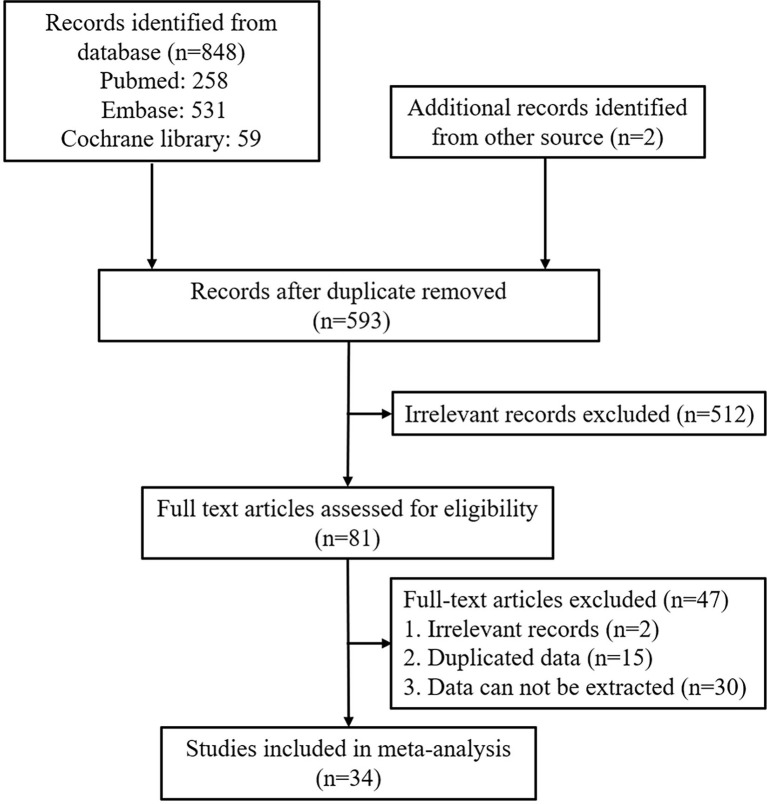
A total of 848 records were retrieved from PubMed, Embase, and Cochrane Library databases, and two were acquired from additional resources. The flowchart of the records selection is given in Figure 1 . After removing the duplicate records and excluding the irrelevant studies, 81 records were assessed for their eligibility. Finally, 34 records encompassing 8115 patients were chosen for this meta-analysis (9–42). Among the included studies, 31 had employed a retrospective cohort design, and 12 had adopted a landmark analysis. Twenty-one studies had reported the ORR of patients with or without irAEs, 23 had provided the HR of OS and 25 had provided the HR of PFS. Seventeen studies had reported organ-specific irAEs, and four had reported grade-specific irAEs. The characteristics of the included studies were listed in Table 1 and Supplementary Material 1 (Table S1) .
Figure 1.
Flowchart of the study selection process.
Table 1.
Main characteristics of the included studies.
| Study | Design | ICIs types | Landmark analysis | irAEs types | irAEs grade | ORR (irAEs vs non-irAEs) | OS Hazard ratio (95%CI) | PFS Hazard ratio (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Osorioet al. (9) | Retrospective | P | no | Thyroid dysfunction | 1–3 | NA | 0.29 (0.09–0.94) | 0.58 (0.27–1.21) |
| Teraoka et al. (10) | Prospective | N | 6-week | Global | 1–2 | 33.3%/12.5% | NA | NA |
| Kim et al. (11) | Retrospective | N/P | no | Thyroid dysfunction | 1–2 | 31.6%/10.3% | 0.11 (0.01–0.92) | 0.38 (0.17–0.85) |
| Sato et al. (12) | Retrospective | N | no | Global | 1–4 | 63.6%/7.4% | NA | 0.10 (0.02–0.37) |
| Lisberg et al. (13) | Retrospective | P | no | Global | 1–4 | NA | 0.75 (0.58–0.96) | 0.75 (0.56–0.99) |
| Haratani et al. (14) | Retrospective | N | 6-week | Global Skin Endocrine |
1–4 | 52.3%/27.9% | 0.285 (0.102–0.675) 0.209 (0.049–0.618) 0.504 (0.027–2.629) |
0.542 (0.295–0.971) 0.476 (0.232–0.912) 0.237 (0.037–0.842) |
| Owen et al. (15) | Retrospective | NA | no | Global | 1–5 | NA | 0.364 (0.203–0.649)* | NA |
| Grangeon et al. (16) | Retrospective | NA | no | Global Pneumonitis Thyroiditis Colitis Hepatitis |
1–5 | 23.1%/5.7% | 0.29 (0.18–0.46) 1.42 (0.45–4.54) 0.46 (0.25–0.86) 0.24 (0.03–1.73) 0.97 (0.3–3.08) |
0.42 (0.32–0.57) 1.19 (0.52–2.70) 0.58 (0.39–0.85) 0.73 (0.35–1.50) 0.97 (0.45–2.08) |
| Ricciuti et al. (17) | Retrospective | N | no | Global Lung Gastrointestinal Endocrine Skin Hepatobiliary |
1–4 | 43.5%/10% | 0.38 (0.26–0.56) 0.46 (0.24–0.89) 0.5 (0.26–0.98) 0.45 (0.28–0.72) 0.8 (0.46–1.39) 0.94 (0.53–1.66) |
0.48 (0.34–0.67) 0.56 (0.33–0.96) 0.52 (0.3–0.9) 0.59 (0.4–0.89) 0.57 (0.35–0.95) 0.72 (0.41–1.24) |
| Cortellini et al. (18) | Retrospective | NA | 6-week | Global Endocrine Skin Gastrointestinal Hepatic |
1–4 | 46.5%/25.7% | 0.53 (0.41–0.69) 0.55 (0.37–0.83) 0.43 (0.27–0.70) 0.61 (0.38–0.98) 1.09 (0.48–2.45) |
0.57 (0.45–0.72) 0.63 (0.45–0.89) 0.46 (0.31–0.69) 0.68 (0.47–1.01) 1.47 (0.72–2.96) |
| Toi et al. (19) | Retrospective | N/P | no | Global | 1–4 | 51.5%/12.7% | 0.42 (0.24–0.71) | 0.45 (0.30–0.68) |
| Ahn et al. (20) | Retrospective | N/P | 6-week | Global Skin Endocrine Pneumonitis |
1–4 | 41.2%/26.7% | 0.484 (0.255–0.919) 0.420 (0.162–1.087) 0.255 (0.051–1.288) 4.117 (1.420–11.942) |
0.434 (0.256–0.735) 0.643 (0.350–1.180) 0.368 (0.132–1.028) 1.686 (0.618–4.597) |
| Berner et al. (21) | Prospective | N/P | no | Skin | NA | 60%/14.6% | 0.29 (0.12–0.71) | 0.22 (0.09–0.49) |
| Fukihara et al. (22) | Retrospective | N/P | 8-week | Pneumonitis | 1–5 | 42.9%/25.4% | NA | NA |
| Baldini et al. (23) | Retrospective | N | no | Global | 1–4 | 27.2%/16.5% | 0.69 (0.58–0.82)* | 0.69 (0.6–0.79)* |
| Cui et al. (24) | Retrospective | N/P/A/D | no | Pneumonitis | 1–4 | 61.9%/29.9% | NA | NA |
| Naqash et al. (25) | Retrospective | N | no | Global Thyroid Pneumonitis Hepatitis Colitis/diarrhea Skin |
NA | NA | 0.65 (0.52–0.8) 0.79 (0.53–1.19) 1.35 (0.89–2.02) 1.18 (0.63–1.97) 0.65 (0.35–1.21) 0.67 (0.41–1.07) |
0.69 (0.55–0.87) 0.98 (0.67–1.42) 1.36 (0.91–2.02) 0.75 (0.45–1.31) 0.65 (0.35–1.21) 0.57 (0.36–0.88) |
| Serrano et al. (26) | Retrospective | NA | no | Global | 1–3 | NA | 0.53 (0.31–0.93) | 0.44 (0.26–0.73) |
| Akamatsu et al. (27) | Retrospective | NA | no | Global | NA | 48.4%/10.7% | 0.45 (0.11–1.88) | 0.30 (0.10–0.85) |
| Pawel et al. (28) | Retrospective | A | no | Global | 1–4 | NA | 0.79 (0.60–1.05) | NA |
| Hosoya et al. (29) | Prospective | N | 2-week | Global Rash Diarrhea |
1–4 | 60.9%/12.8% | 0.92 (0.47–1.79) | 0.6 (0.36–0.99) 0.57 (0.33–0.99) 1.08 (0.46–2.5) |
| Boussageon et al. (30) | Retrospective | NA | 12-week | Global | 1–3 | NA | NA | 0.33 (0.14–0.76) |
| Sosa et al. (31) | Retrospective | NA | no | Global | NA | NA | 0.18 (0.06–0.53) | NA |
| Rizzi et al. (32) | Retrospective | NA | 6-week | Global | 3–4 | 47.9%/30.0% | NA | NA |
| Aso et al. (33) | Retrospective | N/P | 6-week | Skin | 1–3 | 72.0%/24.0% | 0.75 (0.4–1.43) | 0.58 (0.33–0.99) |
| Riudavets et al. (34) | Retrospective | NA | 2.4-month | Global Rash Endocrine |
1–5 | 48.6%/23.2% | 0.32 (0.22–0.46) 0.42 (0.23–0.75) 0.59 (0.34–1) |
0.43 (0.28–0.64) |
| Kubo et al. (35) | Retrospective | NA | no | Global | NA | NA | NA | 0.43 (0.24–0.76) |
| Lim et al. (36) | Retrospective | N | no | Global | 1–4 | 31.6%/11.3% | 0.5 (0.33–0.76) | NA |
| Noguchi et al. (37) | Retrospective | P | no | Global | 1–4 | NA | NA | 0.33 (0.17–0.65) |
| Sugano et al. (38) | Retrospective | N/P/A | no | ILD | NA | 62.5%/20.9% | NA | 0.39 (0.19–0.77) |
| Usui et al. (40) | Retrospective | N | 8-week | Skin rash | NA | 22.2%/20.8% | NA | 0.435 (0.105–1.196) |
| Ahmed et al. (39) | Retrospective | N/P | no | Thyroid | NA | NA | NA | 0.38 (0.2–0.71) |
| Bjørnhart et al. (41) | Retrospective | N/P | no | Global | 3–4 | NA | 0.47 (0.21–1.05) | 0.71 (0.39–1.27) |
| Ksienski et al. (42) | Retrospective | N/P | 6-week | Global | ≥3 | NA | 2.29 (1.05–4.98) | NA |
irAEs, immune-related adverse events; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; CI, confidence interval; NA, not available; N, nivolumab; P, pembrolizumab; A, atezolizumab; D, durvalumab; ILD, interstitial lung disease.
*The HR and 95% CI was calculated through taking reciprocal.
Association Between the Occurrence of irAEs and ORR, OS, and PFS
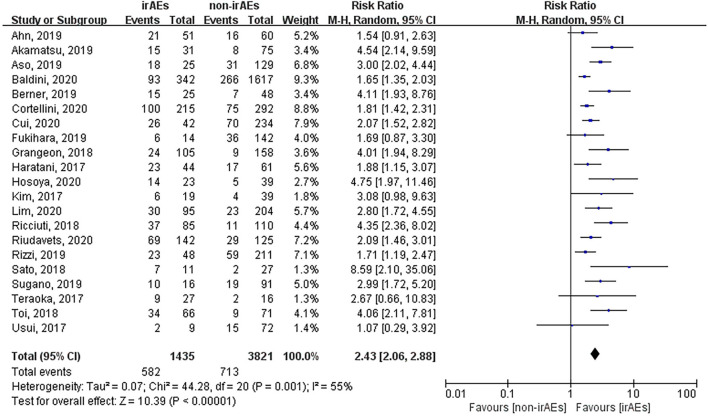
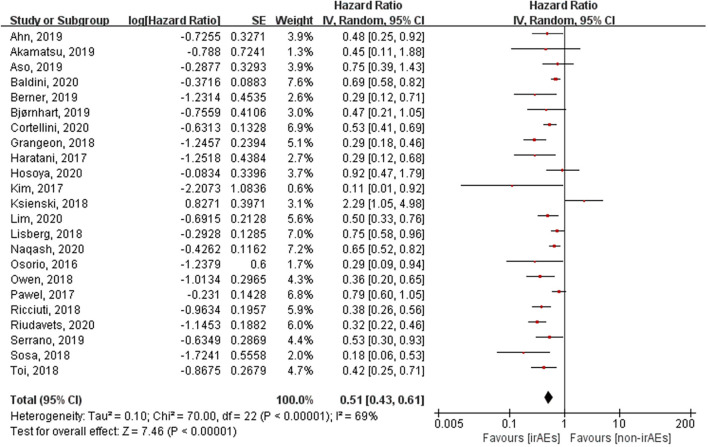
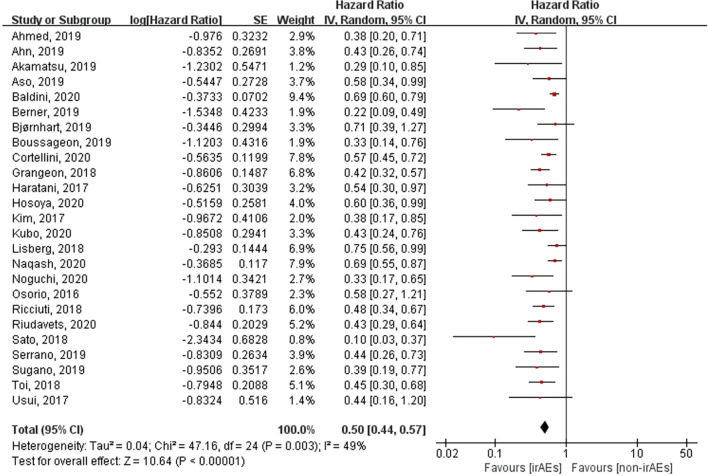
A total of 21 studies involving 5,256 patients had reported the ORR of patients with or without irAEs. The pooled RR for ORR was 2.43 (95% CI [2.06–2.88], p < 0.00001), which means that patients experiencing irAEs respond better to ICIs than those who do not ( Figure 2 ). A random-effect model was utilized because of significant heterogeneity (I2 = 55%, p = 0.001). The occurrence of irAEs also predicted improved OS and PFS in lung cancer patients undergoing immunotherapy. The pooled HR OS was 0.51 for OS (95% CI [0.43–0.61], p < 0.00001) and 0.50 for PFS (95% CI [0.44–0.57], p < 0.00001). However, significant heterogeneities were observed for OS (I2 = 69%, p < 0.00001) and PFS (I2 = 49%, p = 0.003) ( Figures 3 , 4 ).
Figure 2.
Forest plot of the association between the occurrence of irAEs and ORR. ORR, objective response rate; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Figure 3.
Forest plot of the association between the occurrence of irAEs and OS. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Figure 4.
Forest plot of the association between the occurrence of irAEs and PFS. PFS, progression-free survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Subgroup Analysis
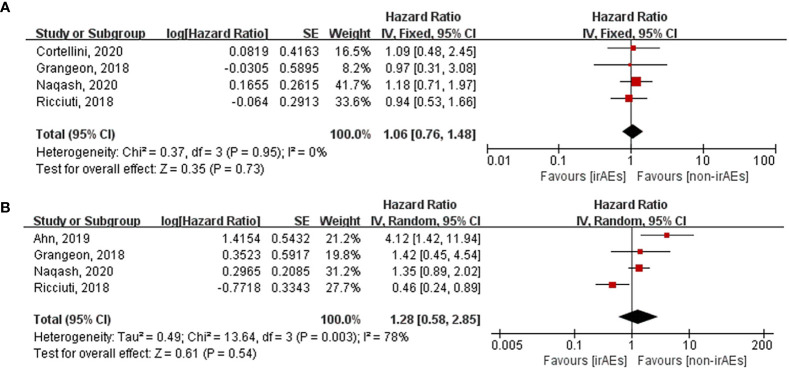
Various organ systems could be affected by irAEs. Zhou et al. had found that the association between the different irAE types and patient outcomes was inconsistent by conducting a meta-analysis that covered all cancer types (43). However, this issue remains unclear in case of lung cancer. Hence, we performed subgroup analysis according to irAE types and grades exclusively in lung cancer patients. After pooling the HRs of OS according to the irAE types, the occurrence of dermatological (HR: 0.53, 95% CI [0.42–0.65], p < 0.00001), endocrine (HR: 0.55, 95% CI [0.45–0.67], p < 0.00001), and gastrointestinal (HR: 0.58, 95% CI [0.42–0.80], p = 0.0009) irAEs was found to be significantly correlated with longer OS of lung cancer patients treated with ICIs ( Figure 5 ). Nevertheless, no significant association was seen between OS and the occurrence of hepatobiliary (HR: 1.06, 95% CI [0.76–1.48], p = 0.73) and pulmonary (HR: 1.28, 95% CI [0.58–2.85], p = 0.54) irAEs ( Figure 6 ). The association between irAE type and the PFS of patients was identical to that of OS ( Supplementary Material 1: Figures S1, S2 ). Furthermore, we conducted a subgroup analysis based on the irAE grades. It was inferred that high irAE grades (≥ 3) were not significantly associated with OS and PFS (OS: HR:0.83, 95% CI [0.49–1.43], p = 0.51; PFS: HR: 0.88, 95% CI [0.67–1.16], p = 0.37) ( Supplementary Material 1: Figure S3 ). Owing to data limitations, the association between low irAE grades (≤ 2) and patient outcomes could not be analyzed. In addition, we performed subgroup analysis based on the drug types. The pooled HR of OS was 0.57 (95%CI [0.45–0.72], p < 0.00001) for nivolumab monotherapy and 0.56 (95%CI [0.24-1.31], p = 0.18) for pembrolizumab monotherapy ( Supplementary Material 1: Figure S4 ).
Figure 5.
Forest plot of the association between the occurrences of different irAEs types and OS. (A) dermatological irAEs; (B) endocrine irAEs; (C) gastrointestinal irAEs. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Figure 6.
Forest plot of the association between the occurrences of different irAEs types and OS. (A) hepatobiliary irAEs; (B) pulmonary irAEs. OS, overall survival; irAEs, immune-related adverse events; non-irAEs, non-immune-related adverse events.
Sensitivity Analysis and Publication Bias
Sensitivity analyses were conducted to assess the stability of our results. The results of OS and PFS remained consistent even upon deleting any included study or changing the random-effect model into the fixed-effect model ( Supplementary Material 1: Figures S5, S6 ). The funnel plots assessing the publication bias of OS and PFS are presented in Supplementary Material 1 (Figures S7, S8) . Regarding OS analysis, Begg’s test (p = 0.369) revealed no publication bias, however, Egger’s test (p = 0.043) presented with the opposite result. We further adopted the trim and fill method to evaluate the publication bias. The result revealed that no study was trimmed or filled and that the pooled result of OS was unchanged, which indicated that our results were stable. Regarding PFS analysis, Begg’s test (p = 0.016) and Egger’s test (p < 0.001) suggested the existence of significant publication bias. The result of the trim and fill method was similar to that of OS.
Discussion
Although the underlying pathophysiology has not been clearly elucidated until date, accumulating evidence suggest that the occurrence of irAEs can predict the outcomes of patients receiving immunotherapy. In our meta-analysis focusing on lung cancer, the results demonstrated that the occurrence of irAEs was significantly associated with ORR, OS, and PFS of patients treated with ICIs. In addition, patients who experienced dermatological, endocrine, and gastrointestinal irAEs had longer OS and PFS than those who did not. Nevertheless, pulmonary and hepatobiliary irAEs and high-grade (≥3) irAEs were not correlated with OS and PFS.
Several biomarkers have been investigated to predict the efficacy of ICIs prior to the treatment. Clinical practice suggests that not all predictors are effective. PD-L1 is the commonly adopted defective biomarker in clinical practice. Its expression in tumor cells, especially when ≥50%, was found to be significantly associated with the outcomes of lung cancer patients (44). Nevertheless, patients with negative PD-L1 expression also benefit from immunotherapy (45). Another acknowledged biomarker is the tumor mutational burden (TMB), whose predictive value is comparable to that of PD-L1 (46). Both tumor tissue and blood TMB have been shown to be associated with superior survival in lung cancer patients (47–50). However, technical limitations and the absence of standardization hampered its clinical application. Our results indicate that irAEs could be predictors of clinical outcomes in lung cancer patients after the initiation of treatment. These events are routinely witnessed within a few weeks of the initial administration, which helps clinicians in the early prediction of ICI efficacy. Most irAEs related to ipilimumab develop within 8–12 weeks of initiating the medication, with skin toxicity occurring at around 2–3 weeks, gastrointestinal and hepatic toxicity at 6–7 weeks, and endocrine toxicity at 9 weeks (51). The onset time of irAEs differs between anti-CTLA-4 and anti-PD-1 inhibitors. In patients treated with nivolumab, skin irAEs occur at about 5 weeks, followed by gastrointestinal irAEs at 7 weeks, hepatic irAEs at 8 weeks, pulmonary irAEs at 9 weeks, and endocrine irAEs at 10 weeks (51).
As mentioned previously, not all irAE occurrences are related to the efficacy of immunotherapy. The overall incidence of immune-related pneumonitis (IRP) was 4% (52, 53), with grade ≥3 accounting for 16.7%–42.9% of the cases (24, 25). A meta-analysis found that IRP was the major cause of death due to irAEs (54). Immune-related hepatitis (IRH) is usually asymptomatic, with elevated serum transaminase or bilirubin levels. Most patients developing IRH tended to receive corticosteroid treatment (55). For patients with grade ≥3 irAEs, which are usually life-threatening, ICIs should be permanently discontinued and treatment with immunosuppressive drugs such as glucocorticoids should be commenced. An increased risk of mortality and treatment with corticosteroids may together counteract the efficacy of ICIs.
While Zhou et al. had conducted a meta-analysis which mainly covered melanoma and non-small cell lung cancer to investigate the association between irAEs and the efficacy of ICIs (43), there are some key differences between our work and theirs. First, irAE profiles tend to vary according to the cancer type (56). For example, gastrointestinal irAEs occur more frequently in melanoma than in lung cancer, while the opposite is true for pneumonia. Therefore, the results obtained for other cancers may not be applicable for lung cancer. Although the cancer types studied by us are not as extensive as those investigated by Zhou et al., our results are more representative of the actual scenario in the specific field of lung cancer. Second, we pooled the RRs of ORR, which is lacking in Zhou et al.’s work. ORR is an important indicator of the response to immunotherapy, and our results demonstrated that patients experiencing irAEs respond better to ICIs than those who do not.
However, there are some limitations in our research. First, most of the included studies were retrospective, which might affect the quality of the evidences analyzed. Second, heterogeneities were significant in the analysis of ORR, OS, and PFS. After conducting a subgroup analysis based on the types of irAEs, the heterogeneities were significantly reduced. Third, Begg’s test and Egger’s test suggested the existence of significant publication bias. We further adopted the trim and fill method, which indicated that our results were stable. The last, but not the least, owing to the availability of only limited data, we were unable to analyze the association between low grade irAEs and patient outcomes. Hence, more cohort studies need to be performed in the future.
In conclusion, our meta-analysis has demonstrated that the occurrence of irAEs, especially dermatological, endocrine, and gastrointestinal irAEs, could predict the enhanced efficacy of immunotherapy in lung cancer patients treated with ICIs.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Author Contributions
Conceptualization, supervision: FZ and YS. Data extraction: DW and CC. Writing – original draft preparation: DW. Methodology, software: YG and WL. Writing – reviewing and editing: PZ, HL, and TL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81970034 and 81570078).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.631949/full#supplementary-material
References
- 1. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (Clinical Res ed) (2018) 363:k4226. 10.1136/bmj.k4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6:38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 5. Ouwerkerk W, van den Berg M, van der Niet S, Limpens J, Luiten RM, et al. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res (2019) 29(5):453–64. 10.1097/CMR.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed) (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin trials (2007) 28:105–14. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 9. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol (2017) 28:583–9. 10.1093/annonc/mdw640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non–Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thoracic Oncol (2017) 12:1798–805. 10.1016/j.jtho.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 11. Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology (2018) 7:e1375642. 10.1080/2162402X.2017.1375642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung cancer (Amsterdam. Netherlands) (2018) 115:71–4. 10.1016/j.lungcan.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 13. Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Hardy A, et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res (2018) 6:288–94. 10.1158/2326-6066.CIR-17-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol (2018) 4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin Lung Cancer (2018) 19:e893–900. 10.1016/j.cllc.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non–small-cell Lung Cancer. Clin Lung Cancer (2019) 20:201–7. 10.1016/j.cllc.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 17. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol (2019) 145:479–85. 10.1007/s00432-018-2805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune-related Adverse Events of Pembrolizumab in a Large Real-world Cohort of Patients With NSCLC With a PD-L1 Expression ≥ 50% and Their Relationship With Clinical Outcomes. Clin Lung Cancer (2020) 21(6):498–508. 10.1016/j.cllc.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 19. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated with Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:376–83. 10.1001/jamaoncol.2018.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol (2019) 145:1613–23. 10.1007/s00432-019-02899-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:1043–7. 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20:442–450.e4. 10.1016/j.cllc.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 23. Baldini E, Lunghi A, Cortesi E, Turci D, Signorelli D, Stati V, et al. Immune-related adverse events correlate with clinical outcomes in NSCLC patients treated with nivolumab: The Italian NSCLC expanded access program. Lung Cancer (2020) 140:59–64. 10.1016/j.lungcan.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 24. Cui P, Huang D, Wu Z, Tao H, Zhang S, Ma J, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol (2020) 12:1–10. 10.1177/1758835920922033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother CII (2020) 69:1177–87. 10.1007/s00262-020-02536-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saavedra Serrano C, Barquín García A, Corral de la Fuente E, Serrano Domingo JJ, Albarrán Artahona V, Martin Huertas R, et al. Association of immune-related adverse events (irAEs) and immunotherapy (IT) benefit in advanced non-small cell lung cancer (NSCLC). Ann Oncol (2019) 30:ii57–8. 10.1093/annonc/mdz063.053 [DOI] [Google Scholar]
- 27. Akamatsu H, Murakami E, Oyanagi J, Shibaki R, Kaki T, Takase E, et al. Immune-Related Adverse Events by Immune Checkpoint Inhibitors Significantly Predict Durable Efficacy Even in Responders with Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25:e679–83. 10.1634/theoncologist.2019-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Von Pawel J, Syrigos K, Mazieres J, Cortinovis D, Dziadziuszko R, Gandara DR, et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: Analyses from the phase III study OAK. Ann Oncol (2017) 28:v469. 10.1093/annonc/mdx380.017 [DOI] [Google Scholar]
- 29. Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, et al. Association Between Early Immune-related Adverse Events and Clinical Outcomes in Patients With Non–Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin Lung Cancer (2020) 21:e315–28. 10.1016/j.cllc.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 30. Boussageon M, Ortiz-Cuaran S, Chabaud S, Pérol D, Avrillon V, Mastroianni B, et al. P1.01-116 Early Immune-Related Adverse Events Under PD-1/PD-L1 Inhibitors Predict Better Progression-Free Survival in NSCLC. J Thoracic Oncol (2019) 14:S407. 10.1016/j.jtho.2019.08.831 [DOI] [Google Scholar]
- 31. Melián Sosa M, Puchades C, Mendez JA, Torres A, Rodrigo C, Gimeno G, et al. Immune-related adverse events (irAEs) and survival (OS) in metastatic non-small cell lung cancer (mNSCLC) patients (pts) treated with immunotherapy. Ann Oncol (2018) 29:viii501. 10.1093/annonc/mdy292.014 [DOI] [Google Scholar]
- 32. Rizzi M, Rizzo M, Minatta N, Recondo G, Naveira M, Lupinacci L, et al. P1.04-82 Toxicity as a Clinical Marker for Efficacy of Immunotherapy in NSCLC: A Multicentric Experience from Argentina. J Thoracic Oncol (2019) 14:S474–5. 10.1016/j.jtho.2019.08.985 [DOI] [Google Scholar]
- 33. Aso M, Toi Y, Sugisaka J, Aiba T, Kawana S, Saito R, et al. Association Between Skin Reaction and Clinical Benefit in Patients Treated with Anti-Programmed Cell Death 1 Monotherapy for Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25:e536–44. 10.1634/theoncologist.2019-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riudavets M, Mosquera J, Garcia-Campelo R, Serra J, Anguera G, Gallardo P, et al. Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents. Front Oncol (2020) 10:1677. 10.3389/fonc.2020.01677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kubo S, Kobayashi N, Somekawa K, Hirata M, Kamimaki C, Aiko H, et al. Identification of biomarkers for non-small-cell lung cancer patients treated with an immune checkpoint inhibitor. Anticancer Res (2020) 40:3889–96. 10.21873/anticanres.14379 [DOI] [PubMed] [Google Scholar]
- 36. Lim SM, Kim SW, Cho BC, Kang JH, Ahn MJ, Kim DW, et al. Real World Experience of Nivolumab in Non-Small Cell Lung Cancer in Korea. Cancer Res Treat Off J Korean Cancer Assoc (2020) 52(4):1112–9. 10.4143/crt.2020.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noguchi S, Suminaga K, Kaki T, Kawachi H, Fukao A, Terashita S, et al. Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer. Lung Cancer: Targets Ther (2020) 11:53–7. 10.2147/LCTT.S254146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thoracic Cancer (2020) 11:1052–60. 10.1111/1759-7714.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmed Y, Lee J, Calvert P. EP1.01-47 Thyroid Related Adverse Events Predict Survival in NSCLC Patients Receiving Anti-PD-1/ PD-L1 Therapy. J Thoracic Oncol (2019) 14:S930. 10.1016/j.jtho.2019.08.2020 [DOI] [Google Scholar]
- 40. Usui Y, Udagawa H, Kirita K, Umemura S, Matsumoto S, Yoh K, et al. Association between immune-related adverse events and efficacy of nivolumab in advanced non-small cell lung cancer. Ann Oncol (2017) 28:ix86. 10.1093/annonc/mdx697.053 [DOI] [Google Scholar]
- 41. Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol (Stockholm Sweden) (2019) 58:953–61. 10.1080/0284186X.2019.1615636 [DOI] [PubMed] [Google Scholar]
- 42. Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z, et al. Efficacy of Nivolumab and Pembrolizumab in Patients With Advanced Non–Small-Cell Lung Cancer Needing Treatment Interruption Because of Adverse Events: A Retrospective Multicenter Analysis. Clin Lung Cancer (2019) 20:e97–e106. 10.1016/j.cllc.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 43. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med (2020) 18:87. 10.1186/s12916-020-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res (2019) 8:413–28. 10.21037/tlcr.2019.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu X, Guo CY, Tou FF, Wen XM, Kuang YK, Zhu Q, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: A systematic review and meta-analysis. Int J Cancer (2020) 147:116–27. 10.1002/ijc.32744 [DOI] [PubMed] [Google Scholar]
- 46. Lu S, Stein JE, Rimm DL, Wang DW, Bell JM, Johnson DB, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol (2019) 5:1195–204. 10.1001/jamaoncol.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol (2019) 5:696–702. 10.1001/jamaoncol.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell (2018) 33:853–861.e4. 10.1016/j.ccell.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med (2018) 24:1441–8. 10.1038/s41591-018-0134-3 [DOI] [PubMed] [Google Scholar]
- 50. Velcheti V, Kim ES, Mekhail T, Dakhil C, Stella PJ, Shen X, et al. Prospective clinical evaluation of blood-based tumor mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC): Interim B-F1RST results. J Clin Oncol (2018) 36:12001–1. 10.1200/JCO.2018.36.15_suppl.12001 [DOI] [Google Scholar]
- 51. Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol (2018) 10:1758835918764628. 10.1177/1758835918764628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest (2017) 152:271–81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 53. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol (2016) 2:1607–16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 54. Sun X, Roudi R, Dai T, Chen S, Fan B, Li H, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer (2019) 19:558. 10.1186/s12885-019-5701-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Romanski NA, Holmstroem RB, Ellebaek E, Svane IM. Characterization of risk factors and efficacy of medical management of immune-related hepatotoxicity in real-world patients with metastatic melanoma treated with immune checkpoint inhibitors. Eur J Cancer (Oxford Engl 1990) (2020) 130:211–8. 10.1016/j.ejca.2020.02.041 [DOI] [PubMed] [Google Scholar]
- 56. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28:2377–85. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.