Abstract
Extracellular vesicles (EVs) are naturally generated nanovesicles which potentially mediate the intercellular communication and inter-organ crosstalk. Recently, significant interest focuses on the potentials of EVs to serve as diagnostic and therapeutic agents, particularly, as the vehicles for drug delivery. Small RNAs, including small interfering RNA (siRNA) and microRNA (miRNA), provide a great therapeutic strategy for treating human diseases. However, it remains a challenge to deliver unconjugated small RNAs to the target tissue or cells. Delivery of small RNAs in an EV-encapsulating manner has a number of advantages, such as enhancing the concentration of small RNAs, improving the uptake of small RNAs by the recipient cells and potentially achieving a cell-specific delivery. In this chapter, we describe a protocol to prepare EVs, manipulate the EV-cargo small RNA molecules and the detailed experimental procedures for tracking and validating small RNA delivery in the lungs. This protocol allows researchers to transfer functional small RNA both in vitro and in vivo.
Keywords: microRNA, siRNA, microvesicle, exosomes, small RNA-based therapeutics
1. Introduction
Extracellular vesicles (EVs) are a group of heterogeneous, membrane surrounded structures released by all cells [1]. Despite that a consensus nomenclature has not been developed, currently, the term EVs refer to exosomes (EXOs), microvesicles (MVs) and apoptotic bodies (ABs), classified based on their sizes, components, and mechanisms of formation [1,2]. These evolutionally conserved vesicles carry out important functions in intercellular communication and inter-organ crosstalk, due to their capacity to transfer proteins, lipids and nucleic acids [1,2]. EVs also exert essential physiological and pathological effects on both recipient and parent cells. Emerging evidence in recent years suggests that EVs potentially serve as novel targets for the development of therapeutic and diagnostic reagents.
Small RNAs, particularly small interfering RNA (siRNA) and microRNA (miRNA), have great potentials to serve as a therapeutics of human diseases [3]. SiRNA is a chemically synthesized, short double-stranded RNA (dsRNA) molecules with 20–25 base-pairs in length [4]. In contrast, miRNAs are endogenously encoded small non-coding RNAs with about 22 nucleotides in length, which serve as crucial regulators in numerous biological processes such as proliferation and differentiation, development and cell death [5]. For years, a number of pharmaceutical companies have invested in the RNAi field and aim to bring siRNA drug candidates into clinical applications. For example, the Food and Drug Administration (FDA) has approved an RNAi-based therapy for the rare hereditary disease transthyretin-mediated amyloidosis in adult patients [6]. However, despite the scientific investigation and pharma’s investment, the delivery of small RNA remains a challenge. The major issues are stability and nonspecific uptake. Although chemical modification improves the stability, unconjugated free small RNAs still has poor permeability across cell membrane due to their charge and molecular weight, which leads to low delivery efficiency [3]. Delivery systems, such as polymer-based and lipid-based systems, significantly improve the stability of small RNAs and delivery efficiency by protecting them from degradation and enhancing the uptake by cells [7,8]. However, there are several concerns of using these delivery systems, including toxicity, nonspecific uptake and immunogenic effects [9,10].
Compared with man-made delivery systems, EVs provide many advantages as mentioned above, such as protecting EV-encapsulated miRNAs from enzymatic digestion and degradation, as well as providing improved efficiency of cell uptake [11]. In addition, EVs are naturally generated by most mammalian cells. MVs and EXOs are both released by healthy cells, and thus they are less toxic and less immunogenic compared to exogenous delivery vehicles [11,12]. It has been shown that EVs are able to cross the blood-brain barrier (BBB) and transmit biological signals from the circulation to the central nervous system [13]. The original components of EVs comprised of RNAs (such as tRNA, miRNA, lncRNA), lipids and proteins [1]. These components that are transported by EVs potentially serve as novel therapeutic agents [14]. Furthermore, engineered EVs were designed to target specific tissue/cell in vivo. For example, Alvarez Erviti and colleagues displayed neuron-specific RVG peptide on the surface of EXOs [15]. RVG-displayed EVs could deliver small RNA specifically into neurons, microglia, and oligodendrocytes in the mouse brain [15]. Taken together, these features make EV an ideal carrier for small nucleotide delivery.
Here, we present protocols using a mouse model for 1) EVs isolation and preparation for small RNA delivery. In this protocol, we use MVs and EXOs as small RNA carrier and exclude ABs due to their size and heterogeneity. 2) Two methods for encapsulating small RNA in EVs, including modified calcium chloride-mediated transfection and electroporation [16]. We also describe the labeling of EVs using PKH26 dye. 3) Administration of small RNA-loaded EVs in vitro and in vivo [12]. For in vivo, two routes of EVs administration are described in the protocol, including intravenous injection (i.v.) and intratracheal inhalation (i.h.). In addition, we also provide experimental procedures for visualizing the EV biodistribution and validating the efficiency of small RNA delivery.
Subtitle 1:
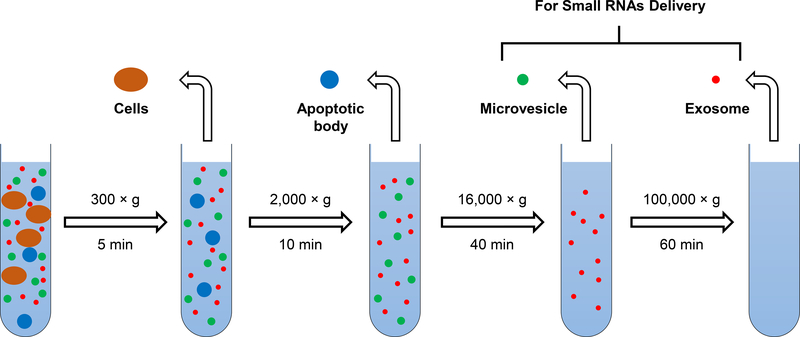
1). Prepare EVs which serve as vehicles used to deliver small RNAs (Fig. 1):
Fig. 1.
Schematic diagram: preparation of extracellular vesicles used for small RNAs delivery.
1.1. Materials
Sterile solutions and supplies, as well as aseptic technique, must always be applied in the following protocol. Results could be affected by microbial contamination both in vivo and in vitro.
1.1.1. Equipment
Ultracentrifuge (Beckman)
Benchtop Centrifuges (Eppendorf)
1.1.2. Reagents
Phosphate-buffered saline (PBS) (Gibco): pH 7.4; no Calcium, no Magnesium; store at room temperature.
Poly (ethylene glycol) average Mn 6,000 (Sigma-Aldrich)
1.1.3. Supplies
Centrifuge/ultracentrifuge tubes (Beckman)
20G × 1″ safelet catheter (EXEL): Sterile, Nonpyrogenic, and Nontoxic
2 ml and 15 ml collection tubes (Thermo Fisher)
Forane (isoflurane, USP): store at room temperature.
Thread (Thermo Fisher)
1-mL Syringe (BD)
1.2. Methods
1.2.1. Isolation of EVs from mouse BALF (see Note 1)
Isoflurane euthanasia: exposure the mouse to the isoflurane (5% or greater) until one minute after breathing stops (see Note 2).
Use scissors to expose the thoracic cage and neck. Dissect tissue from neck to expose trachea.
Proceed to open the diaphragm by cutting the rib cage to expose both the heart and lungs. Take caution not to pierce the heart or lungs.
Use forceps to slide a 1-inch long piece of thread underneath trachea.
Carefully insert the needle catheter into trachea and tie thread into a single knot around tubing in the trachea.
Slowly inject 1 ml cold PBS using 1 ml syringe (takes 15 – 20 sec): watch lungs inflating and do not overinflate.
Slowly collect BAL fluid (BALF) from lungs using 1 ml syringe (take 15 – 20 sec) and transfer the BALF to a pre-chilled tube on ice.
Repeat steps 6–7.
Centrifuge the BALF at 300 × g for 5 min.
Carefully collect BALF supernatant.
Centrifuge the BALF supernatant at 2,000 × g for 10 min.
Carefully collect AB pellets and BALF supernatant.
Centrifuge the BALF supernatant at 16,000 × g for 40 min.
Carefully collect MV pellets and BALF supernatant.
Centrifuge the BALF supernatant at 100,000 × g for 60 min.
Carefully collect Exo pellets.
Wash the EV pellets with cold-PBS using centrifugation.
Resuspend the washed EV pellets with cold-PBS.
Store at −80 °C.
1.2.2. Isolation of EVs from serum
Isoflurane euthanasia: exposure the mouse to the isoflurane (5% or greater) until one minute after breathing stops.
Collect the blood from the mouse heart.
Incubate the collected blood at RT for 30 min.
Centrifuge the sample for 20 min at 3,000 rpm (1500 × g).
Carefully transfer the supernatant (serum) to new tubes.
Centrifuge the supernatant at 16,000 × g for 40 min.
Carefully collect MV pellets and remaining supernatant.
Centrifuge the remaining supernatant at 100,000 × g for 60 min.
Carefully collect Exo pellets.
Wash the EV pellets with cold-PBS using centrifugation.
Resuspend the washed EV pellets with cold-PBS.
Store at −80 °C.
1.2.3. Alternative method of EV isolation
Add 5 % PEG to the collected BALF supernatant or serum.
Incubate the mixture at 4 °C overnight.
Centrifuge the mixture at 3,000 × g for 40 min
Resuspend the EV pellets with cold-PBS.
Store at −80 °C.
Subtitle 2:
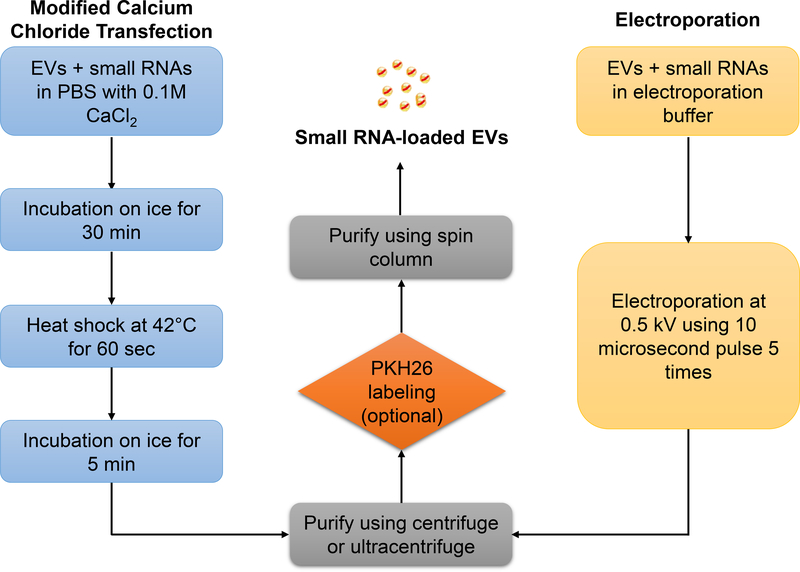
2). Manipulation of EV small RNA cargos (Fig. 2):
Fig. 2.
Flowchart illustrating two methods to introduce small RNA molecules into extracellular vesicles via modified calcium chloride transfection or electroporation.
2.1. Materials
Sterile solutions and supplies, as well as aseptic technique, must always be applied in the following protocol. Results could be affected by microbial contamination both in vivo and in vitro.
2.1.1. Equipment
Neon® Transfection System (Invitrogen)
Ultracentrifuge (Beckman)
Water bath (Fisher Scientific)
Benchtop Centrifuges (Eppendorf)
2.1.2. Reagents
Phosphate-buffered saline (PBS) (Gibco): pH 7.4; no Calcium, no Magnesium; store at room temperature.
Calcium chloride (CaCl2) (Sigma-Aldrich): a 10× stock solution (1 M CaCl2) can be prepared in ddH2O; store at room temperature.
Small interfering RNAs (siRNAs) and microRNA mimics/inhibitors: dissolve the small RNAs (20 µM) and prepare aliquots in RNase-free water (20 µM) to avoid freeze-thaw cycles. Store at −20 °C for up to six months. For extended storage, freeze at −80 °C.
2.1.3. Supplies
1.5mL microtubes (Eppendorf)
Centrifuge/ultracentrifuge tubes (Beckman)
The Neon® Transfection System 100 µL Kit (Thermo Fisher Scientific)
2.2. Methods
2.2.1. Small RNA loading via electroportation
After pelleting EVs, resuspend the EVs using pre-cold E2 electrolytic buffer provided in Neon™ Transfection System 100 µL Kit (see Note 3).
To achieve a 110% reaction volume, add 5.5 µL 20 µM small RNA (totally 110 pmol) with 110 µg resuspend EVs (measured by EV protein) to 1.5 mL tube. Add E2 electrolytic buffer to make the final reaction volume to 110 µL.
Gently mix the tube by inverting 8–10 times and briefly centrifuge the tube.
Aspirate the EV/small RNA mixture into the 100 µL Neon™ Tip using Neon™ Pipette. Avoid air bubbles during pipetting.
Perform the electroporation at 0.5 kV using 10-microsecond pulse 5 times using the Neon™ Transfection System (see Note 4).
After electroporation, transfer 100 µL the small RNA loaded EVs into a new tube and proceed to wash steps below.
2.2.2. Small RNA loading via calcium-mediated transfection
1. After pelleting EVs, resuspend the EVs using pre-cold PBS (see Note 5).
2. Add 5 µL 20 µM small RNA (totally 100 pmol) with 100 µg resuspend EVs (measured by EV protein) to 1.5 mL tube.
3. Add pre-cold PBS to make the final reaction volume to 450 µL.
4. Add 50 µL 1 M CaCl2 to (the final concentration 0.1 M) (see Note 6).
5. Gently mix the tube by inverting 8–10 times and briefly centrifuge the tube.
6. Place the tube on ice for 30 minutes.
7. Place the bottom 2/3 of the tube into a 42°C water bath for 60 seconds (see Note 7).
8. Put the tubes back on the ice for 5 minutes.
6. After transfection, proceed to wash steps below.
2.2.3. Wash and purify small RNA-loaded EVs
To remove the unloaded small RNA molecules, add 10 mL pre-cold PBS to the electroporated mixture and transfer to Beckman™ centrifuge tubes.
Pellet the EVs: for MVs, centrifugation at 16,000 g for 40 minutes at 4 °C; for EXOs, ultracentrifugation at 100,000 g for 120 minutes at 4 °C.
Carefully remove the supernatant and resuspend the EVs in 90 µL PBS (see Note 8).
Subtitle 3:
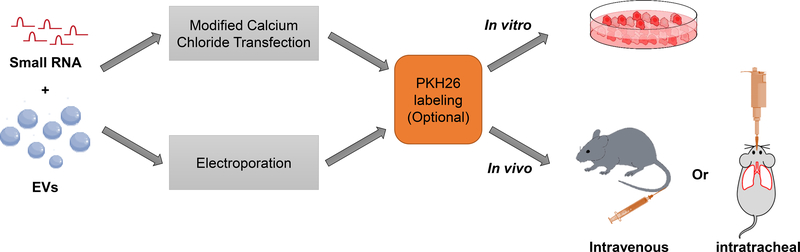
3). EV mediated delivery of small RNA molecules (Fig. 3):
Fig. 3.
Schematic diagram: experimental steps to deliver small RNA loaded-EVs in vivo and in vitro.
3.1. Materials
Sterile solutions and supplies, as well as aseptic technique, must always be applied in the following protocol. Results could be affected by microbial contamination both in vivo and in vitro.
3.1.1. Equipment
Tailveiner Restrainer for Mice (Braintree Scientific)
Benchtop Centrifuges (Eppendorf)
Cryostat (Leica)
Fluorescence microscope (Leica)
3.1.2. Reagents
Phosphate-buffered saline (PBS) (Gibco): pH 7.4; no Calcium, no Magnesium; store at room temperature.
PKH26 Red Fluorescent Cell Linker (Sigma-Aldrich): store at room temperature.
Optimal cutting temperature compound (VWR): store at room temperature.
1 × trypsin/EDTA Solution (Gibco): store at −20°C. Freshly dilute to 0.2 × trypsin/EDTA with
PBS before use.
Paraformaldehyde solution 4% in PBS (Santa Cruz): store at 4° C. Aliquot and freeze at −20°C for long-term storage.
Forane (isoflurane, USP): store at room temperature.
3.1.3. Supplies
1.5mL microtubes (Eppendorf)
Exosome Spin Columns (Thermo Fisher Scientific)
Microscope Slides (Fisher Scientific)
Gauze pads (Thermo Fisher Scientific)
1-mL Syringe (BD)
3.2. Methods
3.2.1. EVs labeling using PKH26 fluorescent dye (see Note 9)
Dilute 1 µL PKH26 with 9 µL Diluent C in a 1.5 mL microtube. Mix gently by pipetting.
Add 10 µL diluted PKH26 to 90 µL EVs from the last step. Mix by pipetting and incubate the mixture for 10 minutes on ice.
During the incubation, rehydrate the spin column supplied by Exosome Spin Columns Kit (MW 3000) with 650 µL PBS at room temperature for 10 minutes.
Centrifuge the spin column in the tube at 750 g for 2 minutes at room temperature to remove excess PBS.
To remove the unincorporated PKH26 dye, transfer 100 µL EV labeling reaction into the rehydrated spin column. Centrifuge to elute the labeled EVs at 750 g for 2 minutes at 4 °C.
3.2.2. EV-mediated small RNAs delivery in vitro
1. Before performing the experiment, plate cells in a 60-mm cell culture dish with 5 mL of growth medium (EV-depleted). Keep the cells around 50 –70% confluent at the time of small RNAs delivery.
2. Directly add 100 µL small RNA-loaded, PKH26 labeled EVs prepared in Section II into the recipient cells (see Note 10). Incubate the cells at 37 ºC in a CO2 incubator for several hours until you can observe clear red fluorescence under a microscope. The medium may be changed after 2 to 6 hours.
3. To check the internalization of EVs, after incubation, treat the cells with 500 µL 0.2 × trypsin/EDTA buffer for 1 minute and then wash the cells extensively with 5 mL citric acid buffer to remove non-internalized EVs. The internalization of PKH26-labeled EVs can be viewed under a fluorescence microscope (see Note 11).
3. After 24 to 48 hours after incubation, collect the cells and proceed to RNA or protein purification. Small RNA delivery efficiency can be evaluated using Real-Time qRT-PCR or western blot analysis (see Note 12).
3.2.3. EV-mediated small RNAs delivery in vivo
Intravenously administration: place the mouse in the restraining device and soak the tail in warm water for 5 minutes to cause vasodilation of the tail vein. Locate one of the two lateral tail veins and slowly press the plunger to inject 5 µL/g (body weight of mouse) small RNA-loaded, PKH26 labeled EVs prepared in Section II into the vein. Then, remove the needle and slightly pressure the puncture site with a dry piece of gauze until the bleeding has stopped. Return the mouse to the cage.
Intratracheally administration: after the mouse is deeply anesthetized by inhalation anesthesia with 2% isoflurane (confirmed by the absence of reflexes on the footpad), the mouse is held by its teeth and 2.5 µL/g (body weight of mouse) of the small RNA-loaded EVs prepared in Section II is gradually released into the mouth and throat with the help of a micropipette. Adjust the rate of release so as to allow the mouse to inhale the solution without form bubbles. Hold the mouse in the hanging position for another couple of minutes till its breathing gradually returns to normal. Return the mouse to the cage.
To check the biodistribution of EVs, 2 hours after administration, fix the tissues from treated mice using 4% PFA. Freeze the fixed tissue in OCT in a suitable tissue mold. The tissue is typically cut into thin sections (5 –15 µm thick) in the cryostat at −20 °C and mounted onto microscope slides for the observation under a fluorescent microscope (see Note 13).
Mice are euthanized via isoflurane inhalation as described in Section I 24 to 48 hours after treatment. To determine the efficiency of small RNA delivery, collect the tissues/organs from the treated mouse and proceed to RNA or protein purification. Efficiency can be evaluated using Real-Time qRT-PCR or western blot analysis (see Note 14).
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R33 AI121644, R01 GM111313, R01 GM127596, Wing Tat Lee award (all to YJ); by NIH grant K99HL141685 (to DZ).
4 Notes
EVs here include MVs and EXOs. In this protocol, both MVs and EXOs can be used as carriers for small RNAs delivery.
In our experiments, no difference was observed among mice species, such as C57BL/6 and BALB/c.
The volume of E2 electrolytic buffer must be optimized to achieve the EVs protein concentration range from 5 to 10 mg/mL.
The optimized parameters may be varied among different electroporation devices.
The volume of PBS must be optimized to achieve the EVs protein concentration range from 5 to 10 mg/mL.
Depending on the origin of EVs, the mixture may appear cloudy after adding CaCl2. It does not affect the small RNA transfection.
Small RNA loading efficiency can be improved by longer incubation at 42°C, which also causes EVs degradation. This step might be optimized for the best performance of EV-mediated small RNA delivery.
Depending on the experiment, the volume of PBS for resuspension can be optimized.
This is an optional step. Proceed to the EVs delivery if the tracking of EVs is not necessary.
Depending on the origin of EVs, the dose of EVs can be optimized.
For long term storage, the cells can be fixed using 4% formalin. Do not use menthol or ethanol for the fixation, which can wash PKH26 dye away.
For the control group, small RNA control loaded EVs is recommended. Other groups, such as original EVs or PBS could also be used as a negative control.
Do not use the paraffin-embedded tissue section if you want to perform immunofluorescence. Procedure for deparaffinization can eliminate PKH26 fluorescence signal.
For the control group, small RNA control loaded EVs is recommended. Other groups, such as original EVs or PBS could also be used as a negative control.
References
- 1.van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19 (4):213–228 [DOI] [PubMed] [Google Scholar]
- 2.Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200 (4):373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam JK, Chow MY, Zhang Y, Leung SW (2015) siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol Ther Nucleic Acids 4:e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, Mehmandoost N, Moazzen F, Mazraeh A, Marmari V, Ebrahimi M, Rashno MM, Abadi SJ, Gharagouzlo E (2017) Molecular Mechanisms and Biological Functions of siRNA. Int J Biomed Sci 13 (2):48–57 [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang D, Li Y, Liu S, Wang YC, Guo F, Zhai Q, Jiang J, Ying H (2017) microRNA and thyroid hormone signaling in cardiac and skeletal muscle. Cell Biosci 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullard A (2018) FDA approves landmark RNAi drug. Nat Rev Drug Discov 17 (9):613. [DOI] [PubMed] [Google Scholar]
- 7.Dilnawaz F (2017) Polymeric Biomaterial and Lipid Based Nanoparticles for Oral Drug Delivery. Curr Med Chem 24 (22):2423–2438 [DOI] [PubMed] [Google Scholar]
- 8.Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol Rev 68 (3):701–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv H, Zhang S, Wang B, Cui S, Yan J (2006) Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release 114 (1):100–109 [DOI] [PubMed] [Google Scholar]
- 10.Wu SY, McMillan NA (2009) Lipidic systems for in vivo siRNA delivery. AAPS J 11 (4):639–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Zhang D, Rai A, Jin Y (2017) The Obstacles to Current Extracellular Vesicle-Mediated Drug Delivery Research. J Pharm Pharm 4 (2):156–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y (2018) Exosome-Mediated Small RNA Delivery: A Novel Therapeutic Approach for Inflammatory Lung Responses. Mol Ther 26 (9):2119–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto J, Stewart T, Banks WA, Zhang J (2017) The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr Pharm Des 23 (40):6206–6214 [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, Cao P (2017) Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front Pharmacol 8:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29 (4):341–345 [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y (2017) Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 312 (1):L110–L121 [DOI] [PMC free article] [PubMed] [Google Scholar]