Abstract
Multiple sclerosis (MS) is a neuroinflammatory disease in which unresolved and uncontrolled inflammation disrupts normal cellular homeostasis and leads to a pathological disease state. It has long been recognized that endogenously derived metabolic by-products of omega fatty acids, known as specialized pro-resolving lipid mediators (SPMs), are instrumental in resolving the pathologic inflammation. However, there is minimal data available on the functional status of SPMs in MS, despite the fact that MS presents a classical model of chronic inflammation. Studies to date indicate that dysfunction of the SPM biosynthetic pathway is responsible for their altered levels in patient-derived biofluids, which contributes to heightened inflammation and disease severity. Collectively, current findings suggest the contentious role of SPMs in MS due to variable outcomes in biological matrices across studies conducted so far, which could, in part, also be attributed to differences in population characteristics. It seems that SPMs have neuroprotective action on MS by exerting proresolving effects on brain microglia in its preclinical model; however, there are no reports demonstrating the direct effect of SPMs on oligodendrocytes or neurons. This reveals that “one size does not fit all” notion holds significance for MS in terms of the status of SPMs in other inflammatory conditions. The lack of clarity served as the impetus for this review, which is the first of its kind to summarize the relevant data regarding the role of SPMs in MS and the potential to target them for biomarker development and future alternative therapies for this disease. Understanding the mechanisms behind biological actions of SPMs as resolution mediators may prevent or even cure MS and other neurodegenerative pathologies.
Keywords: Multiple sclerosis, Autoimmune, Demyelination, Inflammation, Resolution, Specializedpro-resolving mediators, Therapeutics
Introduction
Multiple sclerosis (MS) is a chronic progressive inflammatory disease affecting the central nervous system (CNS) that disrupts the communication between the brain and the rest of the body [24]. The currently available epidemiologic data estimates that over 2.5 million people across the globe are affected by this crippling disease [13]. It predominantly affects young adults in their productive years of life between the ages of 20 and 40 years [67], causing a significant burden on the financial and emotional wellbeing, as well as the overall quality of life, of patients and their caregivers [66]. The sex distribution is increasingly showing more females are affected by the disease than males [99]. The prognosis of MS remains highly unpredictable and is largely determined by the clinical phenotype exhibited by each patient [65]. Thus, an active area of investigation is to identify novel methods that can advance current therapeutic options to treat MS or reverse the course of disease.
Depending on the location of the damage within the CNS, MS patients exhibit highly variable symptomatology, which is why it has been coined the “snow-flake disease”. There are more than 50 disease signs of chronic clinical deficits and a heterogeneous clinical presentation with diverse immunopathology that ranges from a mild to severe-disabling form [24, 34]. In 2013, the International Advisory Committee on Clinical Trials put forth recommendations to identify clinical subtypes of MS based on the four dominant clinical phenotypes, which include: clinically isolated syndrome (CIS), relapsing-remitting (RR), secondary progressive (SP), and primary progressive (PP) [65, 81]. These categories help guide treatment strategies for MS patients.
As of now, there exists a broad continuum of approaches to manage the disease, which include pharmacological treatment, physical therapy and rehabilitative procedures [64], and alternative or complementary medicine such as yoga, herbal medicine, physiotherapy, and acupuncture [89, 97, 153]. The commonly available immunomodulatory and anti-inflammatory drugs are partially effective in the early stage of the disease when they are capable of preventing the recurrence of new attacks and disability [86]. However, most of the drugs are expensive and are accompanied by substantial adverse effects. In addition, they fail to reverse the disease or delay its progression [117]. The present drug regimens are exclusively applicable for RRMS with sub-optimal tolerance, and there are no therapies for the progressive types of disease, which is why current research is largely aimed at developing novel approaches to combat non-motor symptoms as well as modifying the course of the disease [18, 117].
Given its convoluted etiology, the exact cause of MS is not known [51]. As such, MS is somewhat of a medical mystery. Some of the notable epidemiological studies strongly suggest a combination of environmental, genetic, epigenetic, and immunological components are involved in the causal pathways that trigger the disease [45, 96, 98]. There is enough literature to support the role of genetic milieu in disease predisposition through the presence of genetic variants in the form of polymorphisms [11, 41]. Furthermore, epigenetic factors have also been shown to influence disease relapse and progression [110]. However, the exact mechanism by which epigenetics can modulate disease risk is not known. The underlying cause of the disease process can be considered a vicious cycle that is characterized in the early stages by the increased presence of inflammatory cells that are generated by widespread activation of immune responses in the brain and periphery, followed in later stages by the loss of myelin with scar formation [93]. There is a plethora of genetic and pathological reports to suggest profound role of the immune system in its pathogenesis owing to which it is considered as an autoimmune demyelinating disease wherein immune system is evoked against self otherwise healthy bodily components, particularly myelin-associated proteins [56, 143].
MS is generally regarded as a T cell-mediated neuroinflammatory disease with the typical pathological feature being infiltration of major histocompatibility complex (MHC) class II restricted lymphocytic CD4+ T cells [76, 137]. Furthermore, there is significant evidence that supports the role of both T helper Th1 and Th17 subsets in its immunopathogenesis [44, 136]. The mediators of neuroinflammation and neuroimmune activation are brain-resident cells including microglial cells, ependymal cells, macrophages, astrocytes, and mast cells [102]. Inflammatory mediators target the CNS by facilitating infiltration of activated immune cells (autoreactive T cells, B cells, granulocytes, monocytes/macrophages) into the brain by disrupting the blood-brain barrier (BBB) whose integrity is modulated by these chemical mediators [24, 84, 144]. The most commonly targeted CNS components include myelin, white matter, neurons, axons, and blood vessels [104]. Over time, repeated attacks by the immune system leads to accumulation of disability due to irreversible damage, which initially starts as demyelination and axonal loss that is accompanied by neurodegeneration followed by an inability of built-in repair systems to effectively replace the lost myelin (i.e., remyelination) [15, 39]. This ultimately leads to chronic inflammation that is accompanied by the formation of inflammatory lesions, plaques, or scar tissue at the damaged site [63]. Recently, it was observed that the ongoing inflammation in MS induces an endothelial to mesenchymal transition (EndoMT) that promotes inflammation-induced dysfunction of brain endothelial cells (BECs), indicating EndoMT plays a role in the pathophysiological process of the disease [35]. Thus, MS pathogenesis is the result of pathogenic inflammation targeting the CNS, which can arise from multiple different sources.
By and large, about 80% of MS patients undergo temporary remission or a recovery phase in which they experience regression of symptoms after periodic episodes of relapse. This remission may be attributed to the limited resolution of the inflammatory response in peripheral and brain immune system. In turn, this leads to partial self-remyelination or redistribution of ion channels along demyelinated axonic regions [84, 92]. As the disease progresses, ineffective remyelination occurs due to repeated flare-ups, which turn into a more aggressive autoimmune attack that is characterized by permanent and irreversible brain damage [108]. The scientific knowledge available so far on MS comes from its imperfect and approximate, but widely used, preclinical murine model, experimental autoimmune encephalomyelitis (EAE) [112]. This model has been instrumental in providing critical insights into the basic understanding of the mechanisms driving development of disease.
Over time, as novel discoveries unravel new facets of the disease, the mechanistic understanding of MS as a disorder of the immune system has become progressively more complicated [143]. The traditional “outside-in” hypothesis involving an autoimmune response has been confronted by an alternative “inside-out” hypothesis, which suggests the disease is essentially a neurodegenerative disease that begins within the CNS and that this subsequently ignites the pathogenic inflammatory activity [143]. Through this review, we will focus on the latest discoveries that connect MS, inflammation, and the mechanism by which it is resolved through mediators, such as specialized pro-resolving lipid mediators (SPMs). This necessitates discussing the general idea of inflammation and the mechanism of its resolution, followed by a summary of the role of SPMs in MS. To the best of our knowledge, the present review is the first of its kind to provide an updated overview on the spectrum of SPMs in a preclinical animal model of MS (EAE) and patient-derived biological fluids. Particular emphasis is placed on the mechanistic approach of SPMs which will undoubtedly provide information on their application as a new therapy with the capability of modulating a catastrophic inflammatory state.
Inflammation and Resolution
The primary role of the immune system is to defend and protect the body from foreign attack by pathogenic invaders that gain entry through disruptions in the protective barriers in the form of skin cuts, trauma, burn injury or wound, or from pathogenic invasion in the form of infections or internal insults within the body [74]. This leads to the initiation of a physiological immune response, such as acute inflammation (angiophlogosis), that is mediated by a wide range of pro-inflammatory signals and mediators including immune cells, chemokines, cytokines, and classical lipid-derived mediators collectively known as eicosanoids (e.g., prostaglandins (PG), leukotrienes (LT), and thromboxanes (TX)) [38, 47, 87]. These initiate, mediate, and sustain the local or systemic inflammatory response by promoting changes that affect the permeability of the epithelium and endothelium, trafficking of leukocytes, chemotaxis, and cellular infiltration of neutrophils, polymorphonuclear neutrophils (PMN), and inflammatory macrophages. The effects of these changes are reflected through the cardinal signs of a protective response: pain, redness, heat, swelling, and loss of function [26]. If this occurs successfully, it eliminates foreign antigens from the body, clears the inflammatory exudate, promotes tissue healing and repair, and eventually restores cellular homeostasis. This process is characterized by the 5Rs, i.e., removal, relief, regeneration, restoration, and remission, and is the physiologic outcome of the recovery from an acute inflammatory response that mediates the end stage of inflammation, termed catabasis or resolution [119, 125, 128].
Despite daily encounters with inflammatory challenges, as human beings, we do not fully realize the importance of timely resolution of the acute inflammatory process. This is because it often goes unnoticed due to its inherent self-limiting abilities that prevent it from becoming a chronic state. The turning point in the transition from an inflammatory to a non-inflammatory state is the switch in the synthesis of lipid mediators; the synthesis of pro-inflammatory lipid mediators (PGs, LTs, and TXBs) shifts to the synthesis of pro-resolving endogenous mediators, or stop signals [78]. Interestingly, it is the initial stage (onset) of inflammation that programs its resolution or, more precisely, promotes its end-stage (termination) by provoking the synthesis of these pro-resolving endogenous mediators (resolution mediators). These are the master players that resolve the inflammation [126] and are synthesized locally depending on the initial stimuli that trigger their synthesis and release [27]. Ultimately, this activates the classical signs of the resolution process and controls the early cellular events that counter-regulate pro-inflammatory mediators and signals [122, 127].
A breakthrough in our understanding of the physiology of inflammatory resolution came more than 18 years ago from the research group led by Dr. Charles N. Serhan at Harvard Medical School. They first proposed the process of resolution and identified novel families of specific initiators known as specialized pro-resolving lipid mediators (SPMs) or immunoresolvents [119, 120, 132]. The ideal outcome of inflammation is resolution which is believed to play a critical role in health and disease. It is a highly active process coordinated by locally synthesized chemical mediators (i.e., SPMs) that control the magnitude and duration of inflammation by modulating innate and adaptive immune responses. These subsequently assist in reestablishing the normal physiological structures and conditions by regulating the recruitment and response of PMNs, monocytes, macrophages, and lymphocytes (B and T cells) [16, 19, 20, 37, 77, 122]. The activation of specific biochemical and cellular events that mark the resolution process includes reducing leukocyte trafficking, decreasing PMN and pro-inflammatory cytokine and chemokine recruitment, modulating reactive oxygen species (ROS) production, promoting the production of anti-inflammatory mediators, and recruiting macrophages for macrophage-assisted clearance of cellular debris, microbes, and apoptotic immune cells in inflammatory exudate via phagocytosis (efferocytosis) [14, 122]. Macrophage-mediated phagocytosis coincides with the biosynthesis of SPMs and together this governs the clearance of inflammatory infiltrates through the processing of apoptotic cell engulfment [55]. Altogether, these events decrease the time it takes to resolve the inflammation by activating innate resolution programs, which ultimately leads to tissue regeneration and healing to reinstate the homeostatic environment (Fig. 1).
Fig. 1.
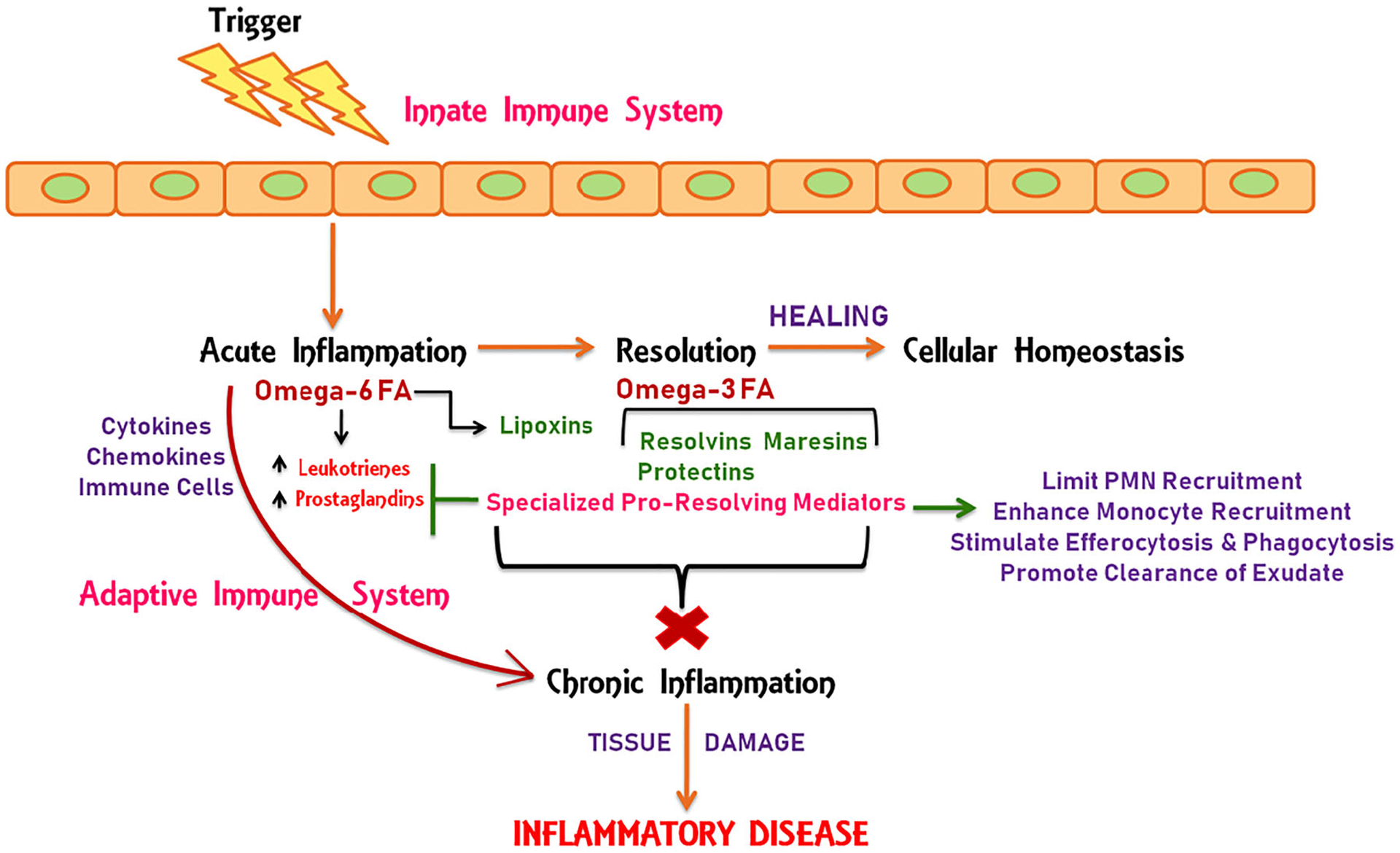
Cellular events in acute inflammation and resolution. The barrier break that occurs through different insults encountered by the body activates the immune system which in turn triggers acute inflammation. This process is regulated by the production of pro-inflammatory mediators from activated immune cells. The classical mediators include cytokines, chemokines, and omega-6 fatty acid-derived eicosanoids. Both innate and adaptive immune responses are involved in propagating ongoing inflammation in response to different inflammatory igniters. The inflammatory process is resolved by an active intrinsic mechanism regulated by pro-resolving lipid mediators (lipoxins, resolvins, maresins, and protectins) derived from omega-6 and omega-3 fatty acids, collectively termed as specialized pro-resolving mediators (SPMs). SPMs promote the end-stage of inflammation by counter-regulating pro-inflammatory signals and mediators through regulation of leukocyte trafficking, polymorphonuclear neutrophil (PMN) recruitment, and monocyte recruitment. They promote macrophage-assisted phagocytosis and efferocytosis, which leads to the clearance of inflammatory exudate and restoration of cellular homeostasis. Unresolved inflammation leads to nonstop chronic inflammation and tissue damage that are the underlying causes for several inflammatory diseases
SPMs are highly potent mediators that signal to dampen inflammation and are primarily generated by macrophages and neutrophils as a self-limiting response across different tissues [119]. As suggested by their name, these pro-resolution mediators are the metabolic by-products of two essential polyunsaturated fatty acids (PUFAs): linoleic acid (LA) omega-6 fatty acid (ω-6 FA) and alpha-linolenic acid (ALA) omega-3 fatty acid (ω-3 FA) (Fig. 2). ω-6 FA and ω-3 FA are synthesized through different pathways through a series of enzymatically driven reactions, catalyzed mainly by arachidonate 5-lipoxygenase (ALOX-5; 5-LOX), arachidonate 12-lipoxygenase (ALOX-12; 12-LOX), arachidonate 15-lipoxygenase (ALOX-15; 15-LOX), cyclooxygenase-2 (COX-2), cytochrome P450 (CYP), and epoxide hydrolases [68, 78, 127, 129]. The main intermediate precursors include arachidonic acid (AA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). The six primary SPM families include lipoxins (LXA4 and LXB4) derived from AA; resolvin E series (RvE1–3) derived from EPA; resolvin T series (RvT1–4) derived from DPA; resolvin D series (RvD1–6), protectins (NPD1/PD1 and PDX), and maresins (MaR1 and MaR2) derived from DHA [32, 123, 124, 132]. Simply put, SPMs can be thought of as the fire fighters that extinguish inflammation by activating innate resolution programs.
Fig. 2.
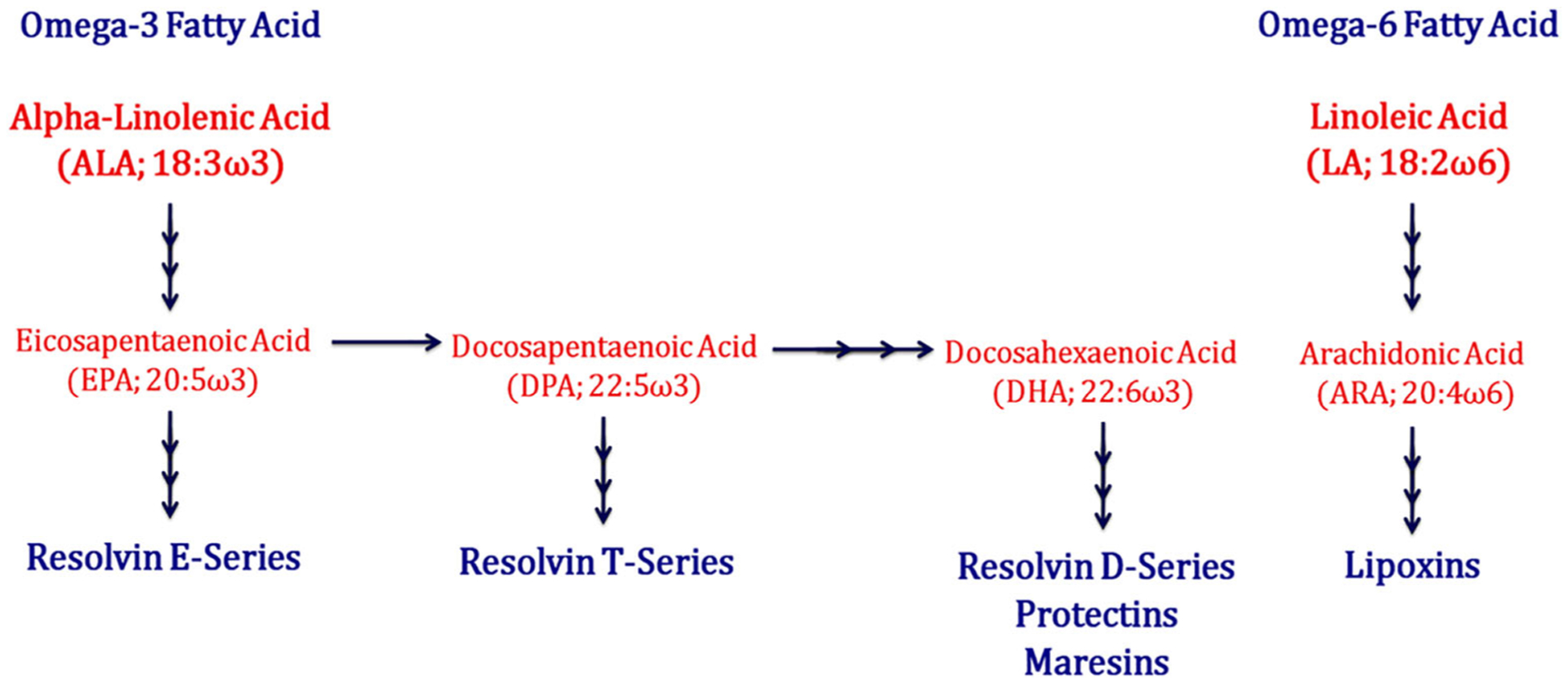
Schematic outline of the biosynthetic pathways that generate resolution mediators from different fatty acid-derived substrates
The metabolism of the different lipid mediators presents a complex interlinked system that is yet to be fully explored [20]. Some studies suggest there is co-existence of a comprehensive continuum of lipid mediators ranging from pro-inflammatory eicosanoids to pro-resolving SPMs at the different phases of inflammation, which would reflect a link between their biosynthesis and degradation and also suggest their interconversion between several interlinked pathways of inflammation machinery. For example, eicosanoids are dominantly present during the onset stage of inflammation (2–4 h), whereas their subsequent reduction along the process is marked by the dominance of SPMs, which reach maximal levels at the peak of acute inflammation (6–12 h), with some members being exclusively generated during the end-stage [20, 31, 123, 124]. This illustrates the significance of critically regulating their spatial and temporal production in order to maintain the essence of physiological inflammation and its subsequent dilution [132]. A recent report suggested that SPMs maintain the balance between pathogenic (Th1/Th17) T cells and tolerogenic regulatory T cells (Treg) by specifically acting on adaptive immune cells. Specifically, SPMs reduce cytokine production from activated CD8+ and CD4+ T cells, prevent differentiation of naïve CD4+ T cells into Th1 and Th17 by downregulating the transcription factors T-bet and Rorc, and enhance the de novo generation and function of Foxp3+ Treg cells [19]. It is worth highlighting the existence of such an intricate mechanism within the body that drives the balance between inflammation and resolution as the very same innate immune cells that propagate the acute inflammation by producing inflammatory mediators stop their synthesis and start generating bioactive SPMs locally via same metabolic enzymes involved in the synthesis of inflammatory mediators in a very stereoselective and coordinated manner [16, 132].
The fine regulation of the resolution process involves highly specific temporal and spatial action of resolution agents (SPMs), which exert their biological functions in the cellular milieu by acting as agonists or ligands at specific membrane-bound G protein-coupled receptors (GPCRs) [16]. Each SPM targets specific receptors with varying affinities and activates different signaling pathways. These receptors include formyl peptide receptor 2 (ALX/FPR2), G protein-coupled receptor 32/resolvin D1 receptor (GPR32/DRV1), G protein-coupled receptor 18/resolvin D2 receptor (GPR18/DRV2), and chemokine receptor-like 1/E-series resolvin receptor (CMKLR1/ChemR23/ERV) [16, 40]. Additional studies to identify the receptors for other classes of SPMs, such as protectins and maresins, are still ongoing. In particular, an in vivo study in mice showed maresin R1 (MaR1) blocked transient receptor potential V1 (TRPV1)-mediated signaling in neurons, suggesting this receptor may play a role in transmitting the action of SPMs [131]. Other potential receptors include G protein-coupled receptor 37 (GPR37) or parkin-associated endothelin-like receptor (Pael-R) for neuroprotectin D1 (NPD1) in macrophages [4], and leucine-rich repeat-containing G protein-coupled receptor 6 (LGR6) for MaR1 [17]. There are subtle differences in ligand-receptor combinations across different species, especially between mice and humans. A detailed description of SPM receptors, their respective agonists, and the biological actions they execute within the cellular milieu is summarized in Table 1.
Table 1.
Biological features of pro-resolving receptors and associated SPMs
| Receptor | Site of expression | SPM | Bioaction |
|---|---|---|---|
| ALX/FPR2 | Neutrophils, eosinophils, airway epithelium, monocytes, macrophages, T cells, synovial fibroblasts, intestinal epithelial cells, human natural killer cells, and innate lymphoid cells | RvD1 | Stimulate phagocytosis of apoptotic cells by macrophages |
| RvD3 | |||
| LXA4 | |||
| ATL | |||
| DRV1/GPR32 | Neutrophils, lymphocytes, macrophages, monocytes, and vascular tissues | RvD1 | Enhance macrophage-assisted phagocytosis of apoptotic cells |
| AT-RvD1 | Increase M2 macrophage polarization | ||
| RvD3 | |||
| RvD5 | |||
| LXA4 | |||
| ATL | |||
| DRV2/GPR18 | Neutrophils, monocytes, and macrophages | RvD2 | Promote non-phlogistic efferocytosis |
| Inhibit chemotaxis of neutrophils | |||
| Decrease pro-inflammatory cytokines | |||
| ERV1/ChemR32 | Brain, kidney, cardiovascular, gastrointestinal, and myeloid tissues. Natural killer cells, innate lymphoid cells, dendritic cells, epithelial cells, naïve and M1 macrophages | RvE1 | Promote phagocytosis of apoptotic PMNs by macrophages |
| RvE2 | Increase C-C chemokine receptor type 5 (CCR5) expression and reduce nuclear factor-kappa B (NF-kB) signaling | ||
| GPR37 | Macrophages | NPD1 | Promote phagocytosis of neutrophils by macrophages and resolve inflammatory pain |
| LRG6 | Phagocytes | MaR1 | Enhance phagocytosis, efferocytosis, and phosphorylation of several proteins including extracellular receptor kinase (ERK) and cAMP response element-binding protein (CREB) |
ALX/FPR2 formyl peptide receptor 2, DRV1/GPR32 G protein-coupled receptor 32/resolvin D1 receptor, DRV2/GPR18 G protein-coupled receptor 18/resolvin D2 receptor, ERV1/ChemR32 chemokine receptor-like 1/E-series resolvin receptor, GPR37 G protein-coupled receptor 37, LGR6 leucine-rich repeat containing G protein-coupled receptor 6, LXA4 lipoxin A4, ATL aspirin-triggered 15-epi-lipoxin A4, RvD1–5 resolvin D series, AT-RvD1 aspirin-triggered epimer resolvin D1, RvE1–2 resolvin E series, NPD1 neuroprotectin D1, MaR1 maresin 1, PMN polymorphonuclear neutrophils or polymorphonuclear leukocytes, CCR5 C-C chemokine receptor type 5, NF-kB nuclear factor kappa-light-chain-enhancer of activated B cells, ERK extracellular receptor kinase, cAMP cyclic adenosine monophosphate, CREB cAMP response element binding protein
Macrophages have a crucial role in driving inflammation and resolution [29]. M1-M2 phenotypic transition, enhanced monocyte recruitment, and phagocytosis by macrophages are the hallmarks of resolution process. However, SPM binding to specific receptor initiates downstream signaling pathways that regulate macrophage phenotypes while mediating resolution. RvE1/ChemR23 has been shown to activate pathway involving intense ribosomal protein S6 (rS6) phosphorylation [95]. Furthermore, there is major evidence for the role of Erv1/ChemR23 receptor in antiatherogenic macrophage signaling that results in anti-inflammation, reduction in oxidized low-density lipoprotein (oxLDL) uptake, and increase in phagocytosis [75]. This suggests that RvE1/ChemR23 signaling axis may have a potential therapeutic implications in atherosclerotic cardiovascular disease. Recently, Titos and colleagues have explored the therapeutic potential of RvD1 in inflamed visceral adipose tissues and macrophages from obese patients [139]. Incubation with RvD1showed significant reduction in the activation of interleukin 10 (IL-10) pathway by reducing phosphorylation of signal transducer and activator of transcription (STAT) proteins. RvD1 was shown to block STAT-1 and its associated inflammatory genes including C-X-C motif chemokine ligand 9 (CXCL9). At the same time, it also limited overactivation of STAT3. However, no effect was observed on IL-10-mediated anti-inflammation. Also, RvD1 increased expression of IL-10 target gene heme oxygenase-1 (HO-1) by positive regulation via p38 mitogen-activated protein kinase (MAPK) signaling pathway. This pathway is also involved in mediating the effect of RvD1 on interleukin 1 receptor antagonist (IL-1RA) which is a natural inhibitor of pro-inflammatory cytokine interleukin 1 beta (IL-1β). These findings clearly provide a broader picture of promising therapeutic targets in metabolic diseases.
Furthermore, inflammatory exudates from in vivo models have revealed that micro RNAs (miRNAs) are differentially regulated during inflammation and resolution [135]. Also, SPM actions via ligand-receptor interaction are associated with miRNAs and their target genes in resolution circuitry, thereby further modulating them [49, 79]. RvD1 receptor-dependent regulation is involved in temporal control of specific set of miRNAs such as miR-146b, miR-142-3p, miR-5p, miR-219, miR-21, miR-203, miR-208, and miR-302d [48, 107]. It has been reported that treatment with RvD1 (300 ng/mouse or 15 μg per kg) upregulates miR-21, miR-146b, and miR-219 and downregulates miR-208a in an in vivo mouse model of peritonitis. However, in vitro treatment with recombinant RvD1 receptors ALX/FPR2 or GPR32 in human macrophages significantly upregulated (P < 0.05) same set of miRNAs even at low concentrations (10 nM). It has been observed that RvD1-miRNAs control resolution process by counter regulating nuclear-factor kappa B (NF-κB) signaling and 5-LOX enzyme [107]. Overall, it increases anti-inflammatory cytokine IL-10, downregulates phosphor-IκB, and reduces leukotriene B4 (LTB4), NF-κB, and SMADs. Furthermore, miRNA expression in in vivo model of peritonitis has shown that RvD1 administration (10 ng per mouse) upregulates miR-208a and miR-219 in exudates obtained from ALX/FPR2 transgenic mice and overexpression of miR-208a in human macrophages increases IL-10. At the same time, RvD1 treatment in ALX/FPR2 knockout mice had no significant effect on reducing leukocyte infiltration and regulating miR-208a and IL-10 [72]. These findings point towards the selective and specific role of RvD1 in relation to receptors and miRNAs. Additionally, high-throughput sequencing has shown that protective actions of MaR1 also induce specific miRNAs in hepatocytes exposed to lipotoxic damage [111]. They mainly include miR-181a-1-3p, miR-129-2-3p, and miR-125b, involved in apoptosis and protein folding during endoplasmic reticulum (ER) stress. In another report by Fredman and colleagues, they investigated lipid mediators and miRNAs at resolution phase in the resolving inflammatory exudates and compared with delayed resolution system in in vivo peritonitis in murine model and human macrophages [49]. They have observed increase in pro-inflammatory mediators accompanied by decrease in SPMs in delayed resolution. There has been a substantial increase in miR-208 with reduced expression of miR-219-2 that has been be linked to the direct regulation of 5-LOX enzyme, which eventually results in decreasing leukotrienes while increasing SPM production. Altogether, these findings provide mechanistic basis for the immunoresolving actions of SPMs in disease models.
Regardless of the protective role of inflammation, any delay, impairment, or failure in its resolution can lead to excessive inflammation that ultimately transforms into a chronic pathological state known as histophlogosis [88]. Subsequently, this prolonged inflammatory process and insufficient resolution results in tissue damage, such as neurodegeneration, scar formation, and organ dysfunction due to abnormal remodeling and fibrosis. This serves as evidence to support the idea that uncontrolled and unresolved inflammation due to aberrant metabolism and altered levels of SPMs is the root cause and pathological hallmark for many inflammatory diseases, such as periodontitis, inflammatory bowel disease, obesity, sepsis, asthma, chronic obstructive pulmonary disease, cardiovascular disease, cancer, and neurodegenerative diseases [71, 77, 88, 122, 132, 148, 149]. So far, the major contributions to understanding the mechanisms of the resolution process and its associated mediators (SPMs) come from the pioneer in this field, Charles N Serhan and his co-workers [16, 19, 20, 121–124, 130, 133]. Given the central role of SPMs in resolving inflammation, their role in different autoimmune diseases is beginning to be investigated, including in rheumatoid arthritis, systemic lupus erythematosus (SLE), acute inflammatory demyelinating polyradiculoneuropathy (AIDP), autoimmune hepatitis, and type 1 diabetes mellitus [1]. Additionally, some reports strongly suggest dysregulated mechanisms of resolution serve as the underlying basis for neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and neuromyelitis optica [71, 80, 134, 148, 149]. A detailed overview of studies in which SPMs have been tested in different preclinical disease models is provided in Table 2.
Table 2.
Overview of experimental findings from studies on SPMs tested in different preclinical inflammatory disease models
| SPM type | Dosage | Route | Disease/in-vivo model | Outcome | Reference |
|---|---|---|---|---|---|
| Lipoxins | A. LXA4 5 μg per kg body weight | A. Intraperitoneal | A. Endometriosis surgically induced female C57BL/6J mice model | A. LXA4 treatment showed an effect on disease progression by attenuating pro-inflammatory and angiogenic mediators, matrix remodeling enzymes, estrogen metabolism, and signaling, as well as downstream proliferative pathways | A. [73] |
| B. ATL 1 μg per mouse | B. Intravenous | B. Murine melanoma model Male C57BL/6 mice | B. Treatment with ATL diminished the population of Ly6Chi monocytes in spleen, blood, and bone marrow, which decreased macrophage infiltration into the tumor and reduced the expression of the M2 marker on tumor-associated macrophages (TAMs) | B. [33] | |
| Resolvin D series | A. RvD1 5 μg per kg body weight | A. Intravenous | A. Acute inflammatory demyelinating polyradiculoneuropathy (AIDP)/Experimental autoimmune neuritis (EAN) model male Lewis rats | A. RvD1 promoted macrophage phagocytosis of apoptotic T cells in the peripheral nervous system (PNS). Increased anti-inflammatory macrophage counts in PNS, upregulated TGFβ−1 by macrophages and increased total Treg cell counts | A. [82] |
| B. RvD1 20 ng per mouse | B. Intraplantar injection | B. Inflammatory model (CFA-induced hindpaw inflammation) Male Wistar rats | B. RvD1 did not stimulate neutrophils or macrophages | B. [94] | |
| C. RvD1 or AT-RvD1 1, 10 or 100 ng per mouse | C. Intravenous | C. Asthma FVB male mice | C. Relative to RvD1, AT-RvD1 resisted metabolic inactivation by macrophages and significantly enhanced macrophage phagocytosis in in vitro and in vivo, a new pro-resolving mechanism for the clearance of allergen from the airways | C. [113] | |
| D. RvD1 0–1000 ng, 100, 500, and 1000 ng per mouse | D. Intraperitoneal | D. Collagen antibody-induced arthritis (CAIA) model Female DBA/1J mice | D. RvD1 did not affect murine RAW 264.7 macrophage viability. It reduced TNF-α, IL-1 β, IFN-γ, PGE 2, and receptor activator of nuclear factor κ B (RANK) and concurrently enhanced IL-10 | D. [6] | |
| Resolvin E series | RvE1 50 ng per mouse | Intraperitoneal | Osteolysis model C57BL/6 mice | RvE1 significantly reduced inflammatory bone resorption by downregulating osteoclast differentiation mediated by differential regulation of NFκB and PI3K-AKT pathways | [43] |
| Resolvin T series | RvT1–4500 ng per mouse | Intraperitoneal | Murine E coli infectious inflammation model | RvT treatment limited further neutrophil recruitment to sites of inflammation and also reduced systemic inflammation, as demonstrated by reduced platelet-leukocyte aggregates. RvT-treated mice showed a reduction in TxB2, PGD2, and PGE2 and significantly increased exudate macrophage efferocytosis | [30, 31] |
| Protectins | A. PDX 300 ng and 1000 ng per mouse | A. Intraperitoneal | A. Sepsis Male C57BL/6 mice | A. PDX promoted M2 polarization, enhanced phagocytotic activity of macrophage, and accelerated resolution of inflammation, finally leading to the increased survival rate of septic mice | A. [152] |
| B.NPD1 50 ng (50 μl) per wound | B. Intradermal injection | B. Diabetic mouse model type 2 diabetic db/db and non-diabetic db/+ female mice | B. NPD1 appeared to be a macrophage produced factor that accelerated diabetic wound healing and promoted macrophage pro-healing functions in diabetic wounds | B. [59] | |
| Maresins | A. MaR1 2 μg per ml (50 μl), 100 ng per mouse | A. Intraperitoneal | A. Postoperative neuroinflammation Adult male C57BL/6 mice | A. MaR1 exerted distinct anti-inflammatory and pro-resolving effects through regulation of macrophage infiltration, NF-κB signaling, and cytokine release after surgery | A. [154] |
| B. MaR1 and RvD2 100 ng each per mouse | B. Intraperitoneal | B. Atheroprogression C57Bl/6 mice | B. MaR1 and RvD2 induced a shift in macrophage profile towards a reparative phenotype, which stimulated collagen synthesis in smooth muscle cells | B. [145] |
LXA4 lipoxin A4, ATL aspirin-triggered 15-epi-lipoxin A4, RvD1–5 resolvin D series, AT-RvD1 aspirin-triggered epimer resolvin D1, RvE1–2 resolvin E series, RvT1–4 resolvin T series, PDX protectin DX, NPD1 neuroprotectin D1, MaR1 maresin 1, TAM tumor-associated macrophages, AIDP acute inflammatory demyelinating polyradiculoneuropathy, EAN experimental autoimmune neuritis, PNS peripheral nervous system, TGFβ-1 transforming growth factor beta 1, Treg T regulatory cells, CFA complete Freund’s adjuvant, CAIA collagen antibody-induced arthritis, TNF-α tumor necrosis factor alpha, IL-1 β interleukin 1 beta, IFN-γ interferon gamma, PGE2 prostaglandin E2, TxB2, thromboxane B2, PGD2 prostaglandin D2, RANK receptor activator of nuclear factor κ B, IL-10 interleukin 10, NFkB nuclear factor kappa-light-chain-enhancer of activated B cells, PI3K-AKT phosphatidylinositol 3-kinase and protein kinase B
MS is believed to have two levels of immune activation, peripheral and CNS, which result in neurological manifestations and govern overall disease progression [34, 92]. In particular, the CNS-specific mechanisms regulating inflammation and resolution have yet to be unraveled. The primary mechanism underlying its pathophysiological process is considered to be excessive inflammation that induces an array of inflammatory cascades within the body, leading to disease progression and rapid neurologic deterioration (Fig. 3). By and large, the role of SPMs in MS is in its infancy. Considering the necessity for disease-modifying drugs in the present era of MS treatment, it is critical to determine how SPMs, along with their receptors, can be exploited as potential targets for developing future biological markers and therapeutic agents for this non-traumatic inflammatory disease.
Fig. 3.
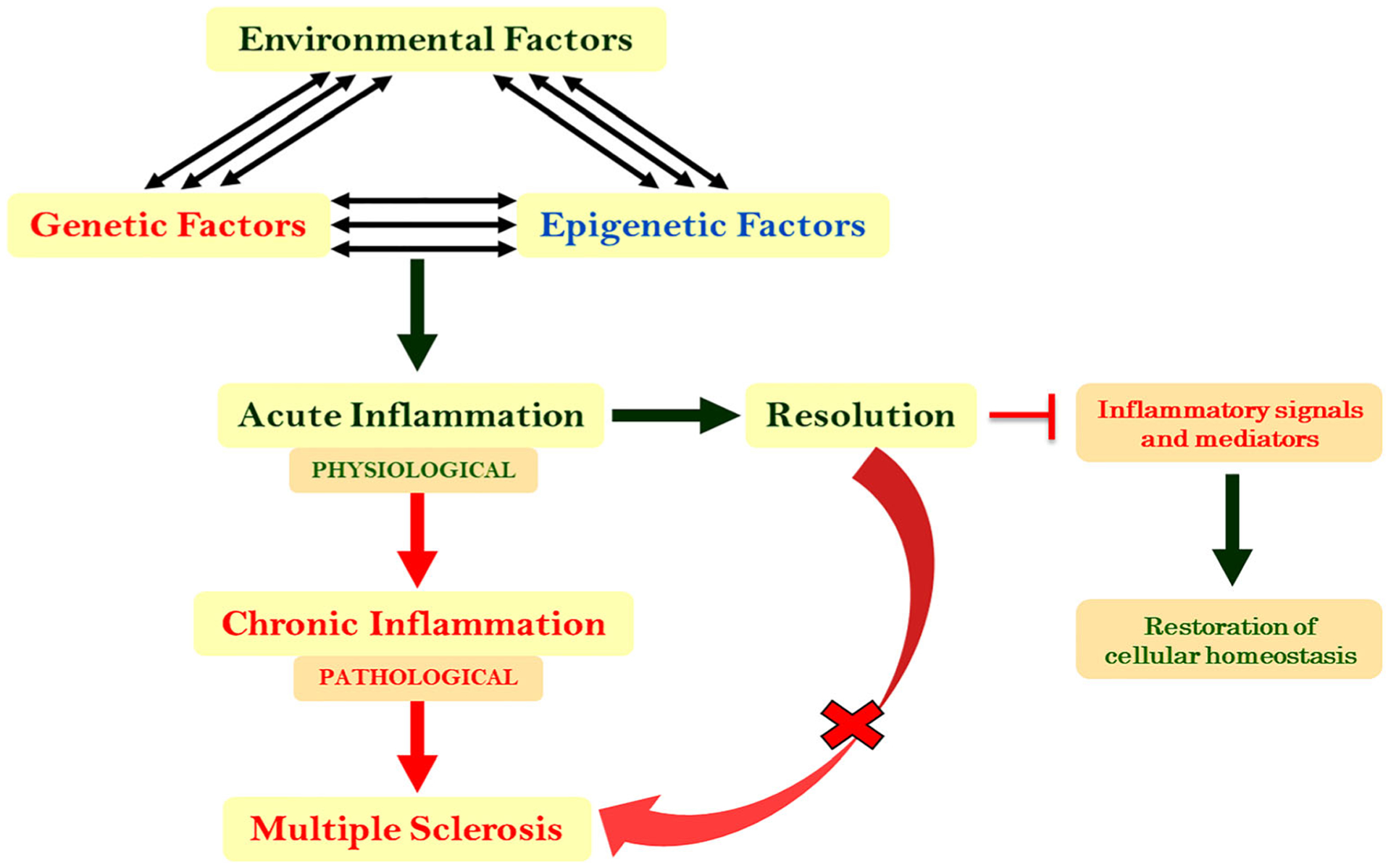
Uncontrolled and unresolved inflammation as the underlying causes of MS. The complex interactions between different triggers, including trauma, barrier break (skin cut, wound, and injury), stress, pathogenic attack, internal insults, and pro-inflammatory diet, elicit protective physiological inflammation. Ideally, inflammation is actively resolved by endogenous mediators to maintain cellular homeostasis; however, when there is failed resolution of the inflammation, this leads to a pathological state in which progressive tissue damage, including neurodegeneration, can occur and ultimately give rise to a pathological condition such as MS
Specialized Pro-Resolving Lipid Mediators in Multiple Sclerosis
MS has long been recognized as a severe inflammatory disease; however, there is still a lack of available treatment options that can effectively promote long-term recovery in patients after damage to the CNS [138, 141]. In addition, current therapeutic options have failed to adequately suppress the ongoing inflammation and stabilize the disease process, which exacerbates disease severity and progression. This could be due, in part, to the compartmentalization of the immune response mediated particularly by tissue-resident CD8+ T lymphocytes and B cells within the CNS [83]. As a result, many present investigations are focused on understanding the endogenous control points (i.e., metabolites and associated pathways) in hopes they will provide novel biomarker signatures for early disease detection and its timely management [23]. These strategies are expected to identify methods to curtail the unwarranted inflammation, speed up its resolution, and arrest disease progression. Despite the ground-breaking discovery of SPMs in the early twenty-first century [119] and their established role in the pathogenesis of a wide continuum of peripheral and brain inflammatory diseases [71, 88, 122, 134, 148, 149], it is surprising that their role in MS, a widely accepted chronic inflammatory disease, remains understudied and elusive. There is a lack of MS-based research in this direction, with only a few published reports so far [116]. Most of the findings of SPMs in MS have come from the metabolipidomic studies carried out on patient-derived samples. The observations made for other diseases have revealed an imbalance between eicosanoids and immunoresolvents as reflected by their lower levels in patient-derived samples [7, 21, 50, 57, 60, 101, 118]. Thus, the primary purpose of the present review is to provide a potentially new paradigm that illustrates the therapeutic potential of immunoresolvents in MS as alternative treatment option. Consequently, we have also provided the pros and cons of relevant studies that need to be taken into consideration while making any firm conclusions about the emerging role of SPMs in MS.
A defective resolution process can be an underlying predis-posing factor in some inflammatory conditions and underscores the beneficial health implications of omega-3 fatty acid supplementation to improve the clinical course of disease. This potential therapeutic nutritional intervention serves as the precursor for SPM biosynthesis [100]. Incidentally, studies have reported a decrease in polyunsaturated fatty acid (PUFA) metabolism in MS patients, and a large prospective study has established a critical role of ALA in MS, where an inverse relationship was observed between PUFA intake and MS risk [2, 9, 10, 28, 54, 90]. Similarly, a report by Holman and colleagues showed MS patients were deficient in PUFA and that these PUFAs were replaced by non-essential fatty acids in the body [58]. Early clinical trials on dietary interventions were based on the outcome of these epidemiological studies, and some trials in this direction even suggested beneficial effects of omega-3 supplementation in MS patients [5, 46, 106, 140, 151]. However, restoring omega-3 levels by supplementation in RRMS patients has not been successful in resolving neurological signs of the disease [46, 106, 109, 140]. Altogether, the outcome of studies based on the association of MS risk with PUFA intake in the form of fish and cod liver oil remains contradictory [3, 8, 25, 52, 62]. At the same time, few reports have provided evidence in support of the use of omega-3 PUFA supplementation in MS due to observations of better disease course in patients put on omega-3 supplements [61, 150]. Such observations strongly warrant continued investigation on the potential impact of n-3 PUFA supplementation on disease outcomes in MS patients.
The incongruous outcome from different studies raises the question as to why omega-3 supplementation may not be effective in combating MS, and indirectly suggests that MS patients harbor a defect in EPA, DPA, and DHA metabolism that has a direct impact on downstream SPM metabolites. This certainly highlights the significant contributions omega-3- and omega-6-derived SPM precursors have on disease pathogenesis as their deficiency and defective metabolism likely contributes toward impaired resolution and continued damage due to delayed healing/repair [142]. The protective role of SPMs found in various human disease models, due to their capability to diffuse inflammation and associated tissue damage, accentuates their prospective role as future therapeutic targets to treat MS pathogenesis [7, 30, 36, 42, 53, 85, 91, 115, 146, 147, 155].
The first study to describe a spectrum of lipid mediators (pro-inflammatory and pro-resolving) in MS comes from Pruss and colleagues, who used liquid chromatography-mass spectrometry (LC-MS/MS)-based lipidomics to quantify lipid mediators (AA and DHA derived) in the serum and cerebrospinal fluid (CSF) in 10 pairs of patients with either highly active or less active RRMS (categorized by clinical and paraclinical criteria) [105]. Significant differences were observed in the CSF, in which the authors observed an induction of RvD1 and PD1 in patients with highly active MS, with no increase in the levels of LXA4 observed. No difference was found in the levels of their corresponding substrate or precursor lipids, AA and DHA, across patient samples. Furthermore, there was no significant difference in the levels of mediators across serum samples derived from the patients. Overall, this study suggested that lipid mediators were differentially regulated and that their biosynthesis was dependent on the severity of the disease. Of note, this study had some inherent limitations, including a small sample size, the absence of healthy subjects as controls, and a narrow range of detected metabolites across subjects.
On the other hand, Poisson and colleagues employed an untargeted metabolomics approach using plasma samples from a B6 EAE mouse model of human MS to identify metabolic aberrations in chronic EAE [103]. With the help of different bioinformatics and pathway analysis tools, they observed significant alterations in the metabolic pathways of omega-3 and omega-6 PUFAs, including ALA and LA. They also found several metabolites of PUFAs, including RvD1, were downregulated in plasma samples from mice with EAE, which is in line with the findings reported for MS patients across different studies [2, 9, 10, 28, 54, 58, 90]. Based on these observations and evidence from literature, this group was the first to test the therapeutic potential of any SPM (RvD1) on disease progression in preclinical EAE model. Daily oral and intraperitoneal administration of RvD1 (100 ng per mouse) decreased disease progression to a greater extent by suppressing autoreactive T cells and inducing an M2 phenotype in monocytes/macrophages and resident brain microglial cells. Overall, RvD1 attenuated clinical symptoms in EAE and also significantly delayed disease progression by suppressing peripheral as well as central inflammation. This study provides the first experimental proof that the application of small endogenous mediator (i.e., RvD1) is an effective therapy in a mouse model of MS.
Findings from a recent study presented at the 35th Annual Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS, 2019) by Kooij and colleagues suggested impaired production of SPMs in MS patients [69]. They employed comprehensive metabolipidomics profiling using an LC-MS-MS platform to identify the spectrum of lipid mediator signatures in the CSF of patients across different clinical courses, including relapsing, remitting, and progressive modes of MS (RRMS, SPMS, and PPMS). CSF analysis revealed lower levels of LXB4 and RvD3 in different clinical courses of the disease (unpublished). Also, this study has validated data in epithelial cells isolated from post-mortem brain samples of patients, which further supports altered SPM biosynthesis in patients. It seems that this study is novel to put forth crucial evidence confirming the defective resolution pathways as a contributor to disease pathogenesis in MS. To provide further insights into the mechanistic aspects of SPMs in promoting inflammation resolution, the efficacy of SPM treatment was tested in an experimental preclinical mouse model of MS, which clearly showed that administering SPMs reduced the inflammatory profile and resulted in a better clinical course of disease, findings that have opened a new array of mechanistic insights into disease pathogenesis and therapeutic intervention.
Additionally, in yet another study by Kooij group, the peripheral blood of two different cohorts of patients (sample sizes of 20 and 18) recruited from two different hospitals in Italy was also analyzed for lipid mediators [70]. Using a targeted metabolipidomics strategy, human plasma samples were subjected to LC-MS-MS, which revealed the presence of typical profiles of lipid mediators in MS that were uniquely altered across separate forms and phases of the disease (relapsing, remitting, and progressive). Each distinct profile was associated with disease progression and the severity score of the patients. In particular, this work showed a significant reduction in EPA levels in MS patients compared to healthy controls, whereas AA and DHA levels were comparable between the two groups. Significantly higher levels of eicosanoids (PGE2, PGD2, and TXB2) and pro-resolving mediators (LXA4, LXB4, AT-LXA4, RvD1, RvD5), particularly PD1 and PDX, were present in MS patients than controls. However, EPA-derived RvE-series was not detected across subjects. Based on MS subtypes, the relapsing and progressive forms showed higher levels of PGE2 and PGD2, whereas the progressive form alone showed higher levels of LXA4, LXB4, and AT-LXA4 (also known as 15-epi-LXA4), RvD5, and PDX, and the relapsing form showed higher levels of RvD1 and PD1. The trend observed across two different cohorts was the same for all lipid mediators except for PDX, which has been predominantly increased in the relapsing form in one cohort (cohort 1) and in the progressive form in the other cohort (cohort 2).
Kooij and colleagues have also reported impaired expression of enzymes and their associated receptors involved in SPM biosynthesis in the peripheral blood of mononuclear cells (PBMCs) isolated from patients, which also correlated with disease stage [70]. However, one enzyme, 12-LOX, which is involved in maresin synthesis, was significantly higher in healthy subjects but reduced across all forms of the disease. Additionally, across all forms of MS, there was a reduction in relative gene expression of other enzymes (COX-2 and 5-LOX) and SPM-based target receptors that coincided with disease progression, with the exception of 15-LOX, which was constant. Interestingly, three receptors, including ALX/FPR2, GPR32/DRV1, and ChemR23/ERV, were further increased in the remitting form only, whereas GPR18/DRV2 was predominantly higher in the relapsing form. These findings indicate a role for these enzymes and receptors in resolving inflammation during remission. As far as the progressive phenotype of MS is concerned, SPMs such as LXA4, LXB4, RvD5, and PDX were found to be present in high levels and showed a direct association with disease severity. In contrast to this scenario, patients with the relapsing form of MS had relatively higher levels of RvD1 and PD1, which showed a surprising inverse correlation with clinical severity. Thus, the atypical trend observed for LXA4, LXB4, RvD1, and PD1 was selected for further validation by evaluating their ability to activate patient-derived monocytes and stimulate their production of cytokines under in vitro conditions. Overall, the SPM treatment reduced monocyte activation and cytokine production more strongly in cells from healthy subjects than those from patients, indicating a less responsive nature of patient-derived monocytes. Kooij and colleagues have also studied the actions of SPMs on inflammation-induced BBB dysfunction in MS using the human brain endothelial cell line hCMEC/D3 as a model system [70]. In this context, SPM treatment attenuated inflammation-induced BBB dysfunction and transendothelial migration of monocytes. There was also reduced expression of intercellular adhesion molecule 1 (ICAM-1, also known as CD54) and chemokine (C-C motif) ligand 2 (CCL2, more popularly known by the name of monocyte chemoattractant protein 1 (MCP1)) production in the treated brain endothelial cells. Kooij and colleagues concluded that the altered levels of SPMs observed across different forms of the disease are reflective of the specific use, unique method of degradation, and differential expression of their receptors [70]. Collectively, the studies carried out by Kooij and colleagues revealed defects in the resolution of peripheral inflammation, providing additional evidence that impaired SPM biosynthesis and dysfunctional resolution of inflammation as causal factors of the chronicity in MS [70].
Collectively, the findings from the studies discussed in this review (Tables 3 and 4) provide meaningful insights into the fundamental understanding of inflammation resolution, as seen through the prism of MS. Despite the available armamentarium, there is still a lack of absolute diagnostic, imaging, and prognostic and therapeutic markers that differentiate the various MS forms and other diseases that mimic MS [12, 114]. This effort would indirectly entail the need for prognosticating the clinical progression of the disease course and its severity by carrying out multicenter collaborative research in this field. Notably, there is an ongoing clinical trial assessing the role of lipid mediators in maintaining the balance between effector and regulatory T cells in MS patients, and their ability to modulate mechanisms responsible for chronic inflammation. In addition, the study determined the ability to exploit lipid mediators in order to promote activation of anti-inflammatory and neuroprotective pathways using ex vivo, in vitro, and in vivo models [22]. It is clear that in MS, an imbalance exists between pro-inflammatory and pro-resolving lipid mediators. The studies discussed above show a similar trend with increased levels of classical pro-inflammatory lipid mediators across different clinical courses in MS patient-derived biological fluids relative to controls. This is in line with the underlying, uncontrolled, excessive inflammatory state of MS. However, variation in the profile of mediators across different studies, subjects, and biological fluids leaves the specific role of SPMs in MS unclear. This indirectly reflects the effects of population diversity and genetics in conferring specific disease attributes [156]. Thus, the question remains as to the role of the resolution process, as well as that of SPMs, in reducing BBB leakage vis a vis demyelination within the CNS and whether the inflammatory process can be alleviated by resolving the inflammation through stimulation of intrinsic SPM biosynthesis. Alleviating pathogenic inflammation could promote neuroprotection by allowing remyelination and repair of the damaged tissue, thereby reversing the disease process and preventing its further progression. To this end, understanding the endogenous mechanisms of resolution physiology could enable the development of potent immunoresolvent therapies that would downregulate the associated signal transduction processes that derail metabolism and promote autoimmunity, oxidative stress, neuroinflammation, demyelination, and neurodegeneration, all of which crossroad in the backdrop of MS.
Table 3.
Overview of studies conducted on SPMs and MS
| S. no. | Study description | Experimental material | Outcome | Reference |
|---|---|---|---|---|
| 1. | Differential metabolipidomic profiling of lipid-derived resolution agonists in the serum and CSF of patients with highly active and less active MS | Patient CSF and serum | RvD1 was significantly upregulated, and NPD1 was detected in the highly active group only. No change in LXA4 levels was observed in patients with highly active MS | [105] |
| 2. | Untargeted plasma metabolomics in preclinical EAE model | B6 EAE mouse model | RvD1 treatment in EAE reduced the inflammatory markers (Th1 and Th17) and promoted macrophage polarization to the M2 (anti-inflammatory) phenotype from M1. Altogether, there was a significant effect on disease pathology of EAE | [103] |
| B6 EAE plasma | ||||
| 3. | Metabolipidomic profiling of lipid mediator signatures in the CSF of RR, SP, PP patients and controls | Patient CSF and preclinical mouse model | LXB4 and RvD3 were reduced in various clinical disease stages while classical eicosanoids were found at high levels. SPM treatment in a mouse model reduced the inflammatory profile of both human innate (microglia) and adaptive (Th1 and Th17) immune cells under inflammatory conditions and improved clinical signs | [69] |
| 4. | SPMs are differentially altered in peripheral blood of MS patients | Patient plasma and PBMCs | Differentially altered levels of SPMs in the blood plasma of patients. Impaired expression of SPM biosynthetic enzymes and receptors in PBMCs. LXA4, LXB4, RvD1, and PD1 attenuate monocyte activation and inhibit BBB dysfunction and transendothelial migration | [70] |
SPM specialized pro-resolving lipid mediators, CSF cerebrospinal fluid, MS multiple sclerosis, RR relapsing remitting, SP secondary progressive, PP primary progressive, EAE experimental autoimmune encephalomyelitis, PBMC peripheral blood mononuclear cell, RvD1–5 resolvin D series, LXA4 lipoxin A4, LXB4 lipoxin B4, NPD1/PD1 neuroprotectin D1
Table 4.
Status of SPMs in MS patient-derived biological fluids across different studies
| S. no. | SPM | Biofluid | Outcome | Reference |
|---|---|---|---|---|
| 1. | RvD1 | CSF | Upregulated in highly active MS | [105] |
| 2 | NPD1 | CSF | Detected only in highly active MS | [105] |
| 3. | LXA4 | CSF | No change in patients with highly active MS | [105] |
| 4. | LXB4 | CSF | Reduced in various clinical disease stages | [69] |
| 5. | RvD3 | CSF | Reduced in various clinical disease stages | [69] |
| 6. | LXA4 | Plasma | A higher but non-significant increase in progressive MS patients | [70] |
| LXB4 | ||||
| AT-LXA4 | ||||
| 7. | RvD1 | Plasma | Increased in relapsing MS patients | [70] |
| NPD1 | ||||
| 8. | RvD5 | Plasma | Slightly increased in relapsing MS but significantly increased in progressive MS | [70] |
| PDX |
CSF cerebrospinal fluid, MS multiple sclerosis, RvD1–5 resolvin D series, LXA4 lipoxin A4, LXB4 lipoxin B4, AT-LXA4 aspirin-triggered 15-epi-lipoxin A4, NPD1 neuroprotectin D1, PDX protectin DX
Conclusions
MS is currently an incurable, non-traumatic neurological disease with serious emotional and financial implications. Inflammation is considered a major player in its pathogenesis as failed resolution of inflammation is the unifying contributor to pathological conditions in several inflammatory diseases. Although there are diverse regimens of pharmacological medications and rehabilitative approaches that are available for the management of this disease, at present, there are no effective strategies available to mitigate the dysregulated/disordered immune system from attacking different components of the nervous system. The drugs that are available for treatment are associated with substantial adverse effects which further complicate the management of this debilitating disease. Thus, existing therapies may only delay disease progression to some extent, but a curative or reparative approach to reverse the damage caused to myelin remains elusive. Considering the necessity for novel drugs that can impact the longevity, severity, and progression of disease, this review describes the mechanistic role of SPMs in MS, taking into consideration the strengths and limitations of the studies that have been conducted to date.
SPM treatment is still under investigation in preclinical mouse models of MS (EAE) and, considering its prospects as an alternative treatment option for this highly convoluted nervous system disease, the studies summarized in this review indicate the need for continued investigations aimed at developing innovative treatment options for MS that are based on innate mechanism of resolution and natural lipid-based mediators that can control the magnitude and duration of inflammation. Based on the outcomes from clinical and preclinical disease systems, we propose a hypothetical model of the therapeutic relevance of these immunoresolvents in MS, and provide key evidence t ha t supports intensifying pharmacotherapeutic research that is focused on the use of resolution mediators. Data from cellular and preclinical mouse model suggest that SPMs have neuroprotective action on MS by exerting proresolving effects on brain microglia in EAE; however, there are no reports demonstrating the direct effect of SPMs on oligodendrocytes or neurons in MS or its preclinical model. It can be concluded that SPMs promote an anti-inflammatory phenotype of macrophages/microglia, thus, in turn, induce T regulatory cells and suppress Th17 cells leading to resolve peripheral and brain immune response and restrain tissue damage. So far, the results are in early stage and just the beginning of new research into resolution in MS, and therefore, this review highlights the need for translational research exclusively aimed at improving quality of life, increasing life expectancy, and decreasing the level of disability in MS patients. As such, there is an urgent requirement for in-depth, long-term, and well-designed studies aimed at exploring the effectiveness of SPMs as a potential future therapy for MS.
We herein recommend the technical considerations to be looked into prior to drawing any conclusive decisions from the studies carried out in this direction (refer to the last paragraph of section on SPMs in MS). This makes it obligatory to conduct prospective longitudinal studies for establishing the correlation between disease progression and SPM levels across patient-derived biological fluids, and determining if the spectrum varies between different types of biological specimens (serum and CSF) in the same patient groups. The role played by class-specific degradation of lipid mediators cannot be ruled out and needs to be considered while designing such studies. The progression of MS and its clinical outcome can be delayed and improved by accelerating the endogenous mechanism of inflammation resolution; however, we still do not have a detailed understanding of the immunopathogenesis involving defective SPM metabolism, the mechanism of SPM action, and inflammation resolution, which leaves open the door for future research studies in this area. The approach of targeting intrinsic inflammatory and resolution pathways can open up a new era of diagnostics and immunoresolvent therapies against the conventional anti-inflammatory, immunosuppressive treatments with unwanted side effects.
In this review, we have provided in-depth information of many of the studies of SPMs in MS in order to identify the gap between preclinical and clinical research that can undoubtedly be bridged, and inform the research community of new potential directions for translational MS research. We present a comprehensive outlook on the unexplored role of SPMs as a promising option for diagnostic and therapeutic intervention in MS. Given the fact that there is limited information regarding the role of SPMs in MS across the different studies described herein, it would be premature and unwise to make any conclusive statements regarding the potential to target these factors for therapeutic purposes. This only highlights the need for continued investigations that will lead to testing the potential of SPMs as pharmacotherapeutic entrants in clinical trials. Progress in this area will only serve to translate findings from the bench to the bedside and help alleviate the distress faced by MS patients. Hopefully, it will eventually open up the cash pockets needed to do the translational and clinical trials on SPMs for neurodegenerative diseases.
Acknowledgments
Dr. Insha Zahoor is highly indebted to her family for being extraordinarily supportive through every odd of life and handling every worst situation when she was not available for them. It takes many sacrifices at the family level to pursue a career in research, especially for a caregiver. I dedicate this piece of writing to my little brother Imran Zahoor (Imu) who is suffering from seizure disorder with mental retardation and a plethora of psychiatric ailments. In serving as a caregiver for him, I have realized the impact that effective therapeutic research interventions can make on the life of a patient and his caregivers.
The authors thank Prof. Charles N. Serhan, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts, USA, for his valuable suggestions while reviewing this manuscript.
Funding Information This work was supported by the National Multiple Sclerosis Society (US) Research Grant (RG-1807-31964), the US National Institutes of Health Grant (R01 NS112727, AI144004), and Henry Ford Hospital Internal Grant (A10270) to SG. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethics Approval This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent No informed consent was required to prepare the manuscript.
References
- 1.Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A (2020) Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol 58:82–91 [DOI] [PubMed] [Google Scholar]
- 2.Aupperle RL, Denney DR, Lynch SG, Carlson SE, Sullivan DK (2008) Omega-3 fatty acids and multiple sclerosis: relationship to depression. J Behav Med 31:127–135 [DOI] [PubMed] [Google Scholar]
- 3.Baarnhielm M, Olsson T, Alfredsson L (2014) Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult Scler 20:726–732 [DOI] [PubMed] [Google Scholar]
- 4.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR (2018) GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 128:3568–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates D, Fawcett PR, Shaw DA, Weightman D (1978) Polyunsaturated fatty acids in treatment of acute remitting multiple sclerosis. Br Med J 2:1390–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benabdoun HA, Kulbay M, Rondon EP, Vallieres F, Shi Q, Fernandes J, Fahmi H, Benderdour M (2019) In vitro and in vivo assessment of the proresolutive and antiresorptive actions of resolvin D1: relevance to arthritis. Arthritis Res Ther 21:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB (2011) Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 187:1957–1969 [DOI] [PubMed] [Google Scholar]
- 8.Berr C, Puel J, Clanet M, Ruidavets JB, Mas JL, Alperovitch A (1989) Risk factors in multiple sclerosis: a population-based case-control study in Hautes-Pyrenees, France. Acta Neurol Scand 80: 46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornevik K, Chitnis T, Ascherio A, Munger KL (2017) Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult Scler 23:1830–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornevik K, Myhr KM, Beiske A, Bjerve KS, Holmoy T, Hovdal H, Midgard R, Riise T, Wergeland S, Torkildsen O (2019) Alpha-linolenic acid is associated with MRI activity in a prospective cohort of multiple sclerosis patients. Mult Scler 25:987–993 [DOI] [PubMed] [Google Scholar]
- 11.Briggs FBS, Yu JC, Davis MF, Jiangyang J, Fu S, Parrotta E, Gunzler DD, Ontaneda D (2019) Multiple sclerosis risk factors contribute to onset heterogeneity. Mult Scler Relat Dis 28:11–16 [DOI] [PubMed] [Google Scholar]
- 12.Brinar VV, Habek M (2010) Rare infections mimicking MS. Clin Neurol Neurosurg 112:625–628 [DOI] [PubMed] [Google Scholar]
- 13.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ (2014) Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83:1022–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley CD, Gilroy DW, Serhan CN (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chari DM (2007) Remyelination in multiple sclerosis. Int Rev Neurobiol 79:589–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang N, Serhan CN (2017) Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Asp Med 58:114–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN (2019) Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest 129:5294–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiurchiu V (2014) Novel targets in multiple sclerosis: to oxidative stress and beyond. Curr Top Med Chem 14:2590–2599 [DOI] [PubMed] [Google Scholar]
- 19.Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN (2016) Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med 8:353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiurchiu V, Leuti A, Maccarrone M (2018) Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN (2012) Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol 189:2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov (2018) Specialized pro-resolving lipid mediators in the resolution of multiple sclerosis US National Library of Medicine Identifier NCT03492606; https://clinicaltrials.gov/ct2/home. Accessed Nov 2019
- 23.Comi G (2008) Clinically isolated syndrome: the rationale for early treatment. Nat Clin Pract Neurol 4:234–235 [DOI] [PubMed] [Google Scholar]
- 24.Compston A, Coles A (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- 25.Cortese M, Riise T, Bjornevik K, Holmoy T, Kampman MT, Magalhaes S, Pugliatti M, Wolfson C, Myhr KM (2015) Timing of use of cod liver oil, a vitamin D source, and multiple sclerosis risk: the EnvIMS study. Mult Scler 21:1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotran RVK, Collins T (2009) Robbins pathologic basis of disease. Saunders, Philadelphia [Google Scholar]
- 27.Crean D, Godson C (2015) Specialised lipid mediators and their targets. Semin Immunol 27:169–176 [DOI] [PubMed] [Google Scholar]
- 28.Cunnane SC, Ho SY, Dore-Duffy P, Ells KR, Horrobin DF (1989) Essential fatty acid and lipid profiles in plasma and erythrocytes in patients with multiple sclerosis. Am J Clin Nutr 50:801–806 [DOI] [PubMed] [Google Scholar]
- 29.Dalli J, Serhan CN (2017) Pro-resolving mediators in regulating and conferring macrophage function. Front Immunol 8:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalli J, Colas RA, Serhan CN (2013) Novel n-3 immunoresolvents: structures and actions. Sci Rep 3:1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalli J, Chiang N, Serhan CN (2015) Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med 21:1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN (2015) Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. FASEB J 29:2120–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de-Brito NM, da-Costa HC, Simoes RL, Barja-Fidalgo C (2019) Lipoxin-induced phenotypic changes in CD115(+)LY6C(hi) monocytes TAM precursors inhibits tumor development. Front Oncol 9:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15:545–558 [DOI] [PubMed] [Google Scholar]
- 35.Derada Troletti C, Fontijn RD, Gowing E, Charabati M, van Het Hof B, Didouh I, van der Pol SMA, Geerts D, Prat A, van Horssen J, Kooij G, de Vries HE (2019) Inflammation-induced endothelial to mesenchymal transition promotes brain endothelial cell dysfunction and occurs during multiple sclerosis pathophysiology. Cell Death Dis 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dmitrieva N, Suess G, Shirley R (2014) Resolvins RvD1 and 17(R)-RvD1 alleviate signs of inflammation in a rat model of endometriosis. Fertil Steril 102:1191–1196 [DOI] [PubMed] [Google Scholar]
- 37.Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, Sime PJ (2018) Key roles for lipid mediators in the adaptive immune response. J Clin Invest 128:2724–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy D, Rouilly V, Libri V, Hasan M, Beitz B, David M, Urrutia A, Bisiaux A, Labrie ST, Dubois A, Boneca IG, Delval C, Thomas S, Rogge L, Schmolz M, Quintana-Murci L, Albert ML, Milieu Interieur C (2014) Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity 40:436–450 [DOI] [PubMed] [Google Scholar]
- 39.Dutta R, Trapp BD (2011) Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 93:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvall MG, Levy BD (2016) DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol 785:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyment DA, Ebers GC, Sadovnick AD (2004) Genetics of multiple sclerosis. Lancet Neurol 3:104–110 [DOI] [PubMed] [Google Scholar]
- 42.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD (2013) Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 6:256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Kholy K, Freire M, Chen T, Van Dyke TE (2018) Resolvin E1 promotes bone preservation under inflammatory conditions. Front Immunol 9:1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-behi M, Rostami A, Ciric B (2010) Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol 5:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etemadifar M, Ghourchian S, Sabeti F, Akbari M, Etemadifar F, Salari M (2019) The higher prevalence of multiple sclerosis among Iranian Georgians; new clues to the role of genetic factors. Rev Neurol 176:113–117 [DOI] [PubMed] [Google Scholar]
- 46.Farinotti M, Vacchi L, Simi S, Di Pietrantonj C, Brait L, Filippini G (2012) Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev 12:CD004192. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fredman G, Serhan CN (2011) Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J 437:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fredman G, Li Y, Dalli J, Chiang N, Serhan CN (2012) Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci Rep 2: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I (2016) An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun 7:12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaby A (2013) Multiple sclerosis. Glob Adv Health Med 2:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghadirian P, Jain M, Ducic S, Shatenstein B, Morisset R (1998) Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol 27:845–852 [DOI] [PubMed] [Google Scholar]
- 53.Gong J, Liu H, Wu J, Qi H, Wu ZY, Shu HQ, Li HB, Chen L, Wang YX, Li B, Tang M, Ji YD, Yuan SY, Yao SL, Shang Y (2015) Maresin 1 prevents lipopolysaccharide-induced neutrophil survival and accelerates resolution of acute lung injury. Shock 44: 371–380 [DOI] [PubMed] [Google Scholar]
- 54.Gul S, Smith AD, Thompson RH, Wright HP, Zilkha KJ (1970) Fatty acid composition of phospholipids from platelets and erythrocytes in multiple sclerosis. J Neurol Neurosurg Psychiatry 33: 506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han CZ, Ravichandran KS (2011) Metabolic connections during apoptotic cell engulfment. Cell 147:1442–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauser SL, Oksenberg JR (2006) The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron 52:61–76 [DOI] [PubMed] [Google Scholar]
- 57.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M (2011) Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holman RT, Johnson SB, Kokmen E (1989) Deficiencies of polyunsaturated fatty acids and replacement by nonessential fatty acids in plasma lipids in multiple sclerosis. Proc Natl Acad Sci U S A 86:4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong S, Tian H, Lu Y, Laborde JM, Muhale FA, Wang Q, Alapure BV, Serhan CN, Bazan NG (2014) Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am J Physiol Cell Physiol 307:C1058–C1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ (2015) Resolvin D1 reduces emphysema and chronic inflammation. Am J Pathol 185:3189–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jelinek GA, Hadgkiss EJ, Weiland TJ, Pereira NG, Marck CH, van der Meer DM (2013) Association of fish consumption and omega 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int J Neurosci 123:792–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kampman MT, Wilsgaard T, Mellgren SI (2007) Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic circle. J Neurol 254:471–477 [DOI] [PubMed] [Google Scholar]
- 63.Karussis D (2014) The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun 48–49:134–142 [DOI] [PubMed] [Google Scholar]
- 64.Kesselring J, Beer S (2005) Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol 4:643–652 [DOI] [PubMed] [Google Scholar]
- 65.Klineova S, Lublin FD (2018) Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med 8:a028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J, Group MS, European Multiple Sclerosis P (2017) New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 23: 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch-Henriksen N, Sorensen PS (2010) The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 9:520–532 [DOI] [PubMed] [Google Scholar]
- 68.Kohli P, Levy BD (2009) Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol 158:960–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kooij G, Chiurchiù V, Norris P, Olsson T, Iacobeus E, Teunissen C, Eggen B, Engelhardt B, de Vries H, Serhan C (2019) Specialized pro-resolving lipid mediator production in the cerebrospinal fluid is impaired in multiple sclerosis: implications for its pathogenesis and therapy ECTRIMS Online Library 279540
- 70.Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, Ruggieri S, Libreros S, van der Pol SMA, van Het Hof B, Schell Y, Guerrera G, Buttari F, Mercuri NB, Centonze D, Gasperini C, Battistini L, de Vries HE, Serhan CN, Chiurchiu V (2019) Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, Marino G, Calabrese V, Vedele F, Ghiglieri V, Picconi B, Di Lazzaro G, Schirinzi T, Sancesario G, Casadei N, Riess O, Bernardini S, Pisani A, Calabresi P, Viscomi MT, Serhan CN, Chiurchiu V, D’Amelio M, Mercuri NB (2019) Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun 10: 3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol 180: 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar R, Clerc AC, Gori I, Russell R, Pellegrini C, Govender L, Wyss JC, Golshayan D, Canny GO (2014) Lipoxin A(4) prevents the progression of de novo and established endometriosis in a mouse model by attenuating prostaglandin E(2) production and estrogen signaling. PLoS One 9:e89742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar V, Abbas AK, Fausto N, Aster JC (2014) Robbins and Cotran pathologic basis of disease, professional edition e-book. Elsevier Health Sciences [Google Scholar]
- 75.Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, Forteza MJ, Jiang X, Andonova T, Walker ME, Dalli J, Arnardottir H, Gistera A, Thul S, Wheelock CE, Paulsson-Berne G, Ketelhuth DFJ, Hansson GK, Back M (2018) ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation 138:1693–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lassmann H (2013) Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci 333:1–4 [DOI] [PubMed] [Google Scholar]
- 77.Leuti A, Maccarrone M, Chiurchiu V (2019) Proresolving lipid mediators: endogenous modulators of oxidative stress. Oxid Med Cell Longev 2019:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2:612–619 [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN (2013) Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity 39:885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu G, Fiala M, Mizwicki MT, Sayre J, Magpantay L, Siani A, Mahanian M, Chattopadhyay M, La Cava A, Wiedau-Pazos M (2012) Neuronal phagocytosis by inflammatory macrophages in ALS spinal cord: inhibition of inflammation by resolvin D1. Am J Neurodegener Dis 1:60–74 [PMC free article] [PubMed] [Google Scholar]
- 81.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo B, Han F, Xu K, Wang J, Liu Z, Shen Z, Li J, Liu Y, Jiang M, Zhang ZY, Zhang Z (2016) Resolvin D1 programs inflammation resolution by increasing TGF-beta expression induced by dying cell clearance in experimental autoimmune neuritis. J Neurosci 36: 9590–9603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Machado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J, Lassmann H (2018) The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 141:2066–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14:183–193 [DOI] [PubMed] [Google Scholar]
- 85.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB (2013) Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J Immunol 191:4288–4298 [DOI] [PubMed] [Google Scholar]
- 86.Miller JR (2004) The importance of early diagnosis of multiple sclerosis. J Manag Care Pharm 10:S4–S11 [PubMed] [Google Scholar]
- 87.Nathan C (2002) Points of control in inflammation. Nature 420: 846–852 [DOI] [PubMed] [Google Scholar]
- 88.Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140: 871–882 [DOI] [PubMed] [Google Scholar]
- 89.Nayak S, Matheis RJ, Schoenberger NE, Shiflett SC (2003) Use of unconventional therapies by individuals with multiple sclerosis. Clin Rehabil 17:181–191 [DOI] [PubMed] [Google Scholar]
- 90.Nightingale S, Woo E, Smith AD, French JM, Gale MM, Sinclair HM, Bates D, Shaw DA (1990) Red blood cell and adipose tissue fatty acids in mild inactive multiple sclerosis. Acta Neurol Scand 82:43–50 [DOI] [PubMed] [Google Scholar]
- 91.Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M (2016) Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI insight 1:e85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952 [DOI] [PubMed] [Google Scholar]
- 93.Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122:1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oehler B, Mohammadi M, Perpina Viciano C, Hackel D, Hoffmann C, Brack A, Rittner HL (2017) Peripheral interaction of Resolvin D1 and E1 with opioid receptor antagonists for antinociception in inflammatory pain in rats. Front Mol Neurosci 10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN (2010) Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285:3451–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oksenberg JR, Baranzini SE (2010) Multiple sclerosis genetics–is the glass half full, or half empty? Nat Rev Neurol 6:429–437 [DOI] [PubMed] [Google Scholar]
- 97.Olsen SA (2009) A review of complementary and alternative medicine (CAM) by people with multiple sclerosis. Occup Ther Int 16: 57–70 [DOI] [PubMed] [Google Scholar]
- 98.Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13:25–36 [DOI] [PubMed] [Google Scholar]
- 99.Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, Ebers GC, Canadian Collaborative Study G (2006) Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 5:932–936 [DOI] [PubMed] [Google Scholar]
- 100.Penesová A, Dean Z, Kollár B, Havranová A, Imrich R, Vlček M, Rádiková Ž (2018) Nutritional intervention as an essential part of multiple sclerosis treatment? Physiol Res 67:521–533 [DOI] [PubMed] [Google Scholar]
- 101.Perretti M, Norling LV (2017) Actions of SPM in regulating host responses in arthritis. Mol Asp Med 58:57–64 [DOI] [PubMed] [Google Scholar]
- 102.Pittock SJ, Lucchinetti CF (2007) The pathology of MS: new insights and potential clinical applications. Neurologist 13:45–56 [DOI] [PubMed] [Google Scholar]
- 103.Poisson LM, Suhail H, Singh J, Datta I, Denic A, Labuzek K, Hoda MN, Shankar A, Kumar A, Cerghet M, Elias S, Mohney RP, Rodriguez M, Rattan R, Mangalam AK, Giri S (2015) Untargeted plasma metabolomics identifies endogenous metabolite with drug-like properties in chronic animal model of multiple sclerosis. J Biol Chem 290:30697–30712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Popescu BF, Pirko I, Lucchinetti CF (2013) Pathology of multiple sclerosis: where do we stand? Continuum 19:901–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K, Schwab JM (2013) Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One 8:e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, Pacheco-Moises F, Torres-Sanchez ED, Sorto-Gomez TE, Cruz-Ramos JA, Orozco-Avina G, Celis de la Rosa AJ (2013) Efficacy of fish oil on serum of TNF alpha, IL-1 beta, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med Cell Longev 2013:709493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN (2011) MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J 25:544–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple sclerosis. N Engl J Med 378:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riccio P, Rossano R, Larocca M, Trotta V, Mennella I, Vitaglione P, Ettorre M, Graverini A, De Santis A, Di Monte E, Coniglio MG (2016) Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: a pilot study. Exp Biol Med 241:620–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rito Y, Torre-Villalvazo I, Flores J, Rivas V, Corona T (2018) Epigenetics in multiple sclerosis: molecular mechanisms and dietary intervention. Cent Nerv Syst Agents Med Chem 18:8–15 [DOI] [PubMed] [Google Scholar]
- 111.Rius B, Duran-Guell M, Flores-Costa R, Lopez-Vicario C, Lopategi A, Alcaraz-Quiles J, Casulleras M, Lozano JJ, Titos E, Claria J (2017) The specialized proresolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia-induced endoplasmic reticulum stress. FASEB J 31:5384–5398 [DOI] [PubMed] [Google Scholar]
- 112.Robinson AP, Harp CT, Noronha A, Miller SD (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol 122:173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD (2012) Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol 189:1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rolak LA, Fleming JO (2007) The differential diagnosis of multiple sclerosis. Neurologist 13:57–72 [DOI] [PubMed] [Google Scholar]
- 115.Rossi S, Di Filippo C, Gesualdo C, Potenza N, Russo A, Trotta MC, Zippo MV, Maisto R, Ferraraccio F, Simonelli F, D’Amico M (2015) Protection from endotoxic uveitis by intravitreal Resolvin D1: involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediat Inflamm 2015: 149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruiz F, Vigne S, Pot C (2019) Resolution of inflammation during multiple sclerosis. Semin Immunopathol 41:711–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saidha S, Eckstein C, Calabresi PA (2012) New and emerging disease modifying therapies for multiple sclerosis. Ann N Y Acad Sci 1247:117–137 [DOI] [PubMed] [Google Scholar]
- 118.Schwanke RC, Marcon R, Bento AF, Calixto JB (2016) EPA- and DHA-derived resolvins’ actions in inflammatory bowel disease. Eur J Pharmacol 785:156–164 [DOI] [PubMed] [Google Scholar]
- 119.Serhan CN (2004) A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol 122:305–321 [DOI] [PubMed] [Google Scholar]
- 120.Serhan CN (2007) Resolution phase of inflammation: novel endogenous anti-inflammatoryand proresolving lipid mediators and pathways. Annu Rev Immunol 25:101–137 [DOI] [PubMed] [Google Scholar]
- 121.Serhan CN (2011) The resolution of inflammation: the devil in the flask and in the details. FASEB J 25:1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Serhan CN (2017) Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Asp Med 58:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serhan CN (2017) Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J 31:1273–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serhan CN, Chiang N (2004) Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entree for resoleomics. Rheum Dis Clin N Am 30:69–95 [DOI] [PubMed] [Google Scholar]
- 126.Serhan CN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197 [DOI] [PubMed] [Google Scholar]
- 127.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K (2000) Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and trans-cellular processing. J Exp Med 192:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serhan CN, Arita M, Hong S, Gotlinger K (2004) Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 39: 1125–1132 [DOI] [PubMed] [Google Scholar]
- 129.Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N (2011) Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem 11:629–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA (2012) Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26:1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serhan CN, Chiang N, Dalli J, Levy BD (2014) Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 7: a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N (2015) Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 1851:397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shang P, Zhang Y, Ma D, Hao Y, Wang X, Xin M, Zhang Y, Zhu M, Feng J (2019) Inflammation resolution and specialized pro-resolving lipid mediators in CNS diseases. Expert Opin Ther Targets 23:967–986 [DOI] [PubMed] [Google Scholar]
- 135.Sheedy FJ, O’Neill LA (2008) Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis 67(Suppl 3):iii50–iii55 [DOI] [PubMed] [Google Scholar]
- 136.Sonobe Y, Jin S, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A (2007) Chronological changes of CD4(+) and CD8(+) T cell subsets in the experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. Tohoku J Exp Med 213: 329–339 [DOI] [PubMed] [Google Scholar]
- 137.Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747 [DOI] [PubMed] [Google Scholar]
- 138.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391:1622–1636 [DOI] [PubMed] [Google Scholar]
- 139.Titos E, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Lopategi A, Dalli J, Lozano JJ, Arroyo V, Delgado S, Serhan CN, Claria J (2016) Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J Immunol 197:3360–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Torkildsen O, Wergeland S, Bakke S, Beiske AG, Bjerve KS, Hovdal H, Midgard R, Lilleas F, Pedersen T, Bjornara B, Dalene F, Kleveland G, Schepel J, Olsen IC, Myhr KM (2012) Omega-3 fatty acid treatment in multiple sclerosis (OFAMS study): a randomized, double-blind, placebo-controlled trial. Arch Neurol 69:1044–1051 [DOI] [PubMed] [Google Scholar]
- 141.Torkildsen O, Myhr KM, Bo L (2016) Disease-modifying treatments for multiple sclerosis - a review of approved medications. Eur J Neurol 23(Suppl 1):18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tourki B, Halade G (2017) Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J 31: 4226–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trapp BD, Nave KA (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269 [DOI] [PubMed] [Google Scholar]
- 144.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285 [DOI] [PubMed] [Google Scholar]
- 145.Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O (2016) Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res 119:1030–1038 [DOI] [PubMed] [Google Scholar]
- 146.Wang L, Yuan R, Yao C, Wu Q, Christelle M, Xie W, Zhang X, Sun W, Wang H, Yao S (2014) Effects of resolvin D1 on inflammatory responses and oxidative stress of lipopolysaccharide-induced acute lung injury in mice. Chin Med J 127:803–809 [PubMed] [Google Scholar]
- 147.Wang Q, Zheng X, Cheng Y, Zhang YL, Wen HX, Tao Z, Li H, Hao Y, Gao Y, Yang LM, Smith FG, Huang CJ, Jin SW (2014) Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol 192:3765–3777 [DOI] [PubMed] [Google Scholar]
- 148.Wang X, Zhu M, Hjorth E, Cortes-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, Fitzgerald JM, Serhan CN, Granholm AC, Schultzberg M (2015) Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 11(40–50):e41–e42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X, Jiao W, Lin M, Lu C, Liu C, Wang Y, Ma D, Wang X, Yin P, Feng J, Zhu J, Zhu M (2019) Resolution of inflammation in neuromyelitis optica spectrum disorders. Mult Scler Relat Dis 27: 34–41 [DOI] [PubMed] [Google Scholar]
- 150.Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, Venkatraman J, Meksawan K, Deinehert S, Pendergast D, Awad AB, Ramanathan M, Munschauer F, Rudick R (2005) Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids 73:397–404 [DOI] [PubMed] [Google Scholar]
- 151.Wergeland S, Torkildsen O, Bo L, Myhr KM (2012) Polyunsaturated fatty acids in multiple sclerosis therapy. Acta Neurol Scand Suppl:70–75 [DOI] [PubMed] [Google Scholar]
- 152.Xia H, Chen L, Liu H, Sun Z, Yang W, Yang Y, Cui S, Li S, Wang Y, Song L, Abdelgawad AF, Shang Y, Yao S (2017) Protectin DX increases survival in a mouse model of sepsis by ameliorating inflammation and modulating macrophage phenotype. Sci Rep 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yadav V, Shinto L, Bourdette D (2010) Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol 6:381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang T, Xu G, Newton PT, Chagin AS, Mkrtchian S, Carlstrom M, Zhang XM, Harris RA, Cooter M, Berger M, Maddipati KR, Akassoglou K, Terrando N (2019) Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth 122:350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yaxin W, Shanglong Y, Huaqing S, Hong L, Shiying Y, Xiangdong C, Ruidong L, Xiaoying W, Lina G, Yan W (2014) Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J Surg Res 188: 213–221 [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y, Zhou Y, van der Mei IAF, Simpson S, Ponsonby AL, Lucas RM, Tettey P, Charlesworth J, Kostner K, Taylor BV, Ausimmune/AusLong Investigators G (2019) Lipid-related genetic polymorphisms significantly modulate the association between lipids and disability progression in multiple sclerosis. J Neurol Neurosurg Psychiatry 90:636–641 [DOI] [PubMed] [Google Scholar]


