Obstructive sleep apnea (OSA) is a common cause of fatigue and has cardiovascular consequences. OSA is common in people living with HIV (PLWH) but is infrequently recognized or diagnosed. Treatment of OSA can improve the quality of life of PLWH.
Keywords: HIV, PLWH, sleep, obstructive sleep apnea, fatigue
Abstract
Obstructive sleep apnea (OSA) is defined by repetitive collapse of the upper airway during sleep leading to transient hypoxemia and arousals from sleep. Surges in sympathetic activity, repeated oxygen desaturation, and sleep fragmentation can lead to cardiovascular (eg, myocardial infarction) and neurocognitive (eg, excessive daytime sleepiness) consequences. Emerging data suggest that OSA is common in people living with human immunodeficiency virus (PLWH) and that traditional risk factors for OSA, such as obesity, are not highly predictive of OSA in PLWH. Untreated OSA is associated with increased fatigue and levels of inflammation. Despite these data, most PLWH with OSA remain undiagnosed and untreated. Improved awareness of OSA among healthcare providers and greater use of OSA diagnostic approaches have the potential to substantially improve quality of life and outcomes in PLWH.
(See the Major Article by Alvi et al on pages 447–55.)
In 2013, Deeks and colleagues wrote that “the end of AIDS” had arrived, and that human immunodeficiency virus (HIV) infection was now a chronic disease [1]. While provocative, the authors noted that the availability of effective and generally well-tolerated antiretroviral therapy (ART) for people living with HIV (PLWH) had translated into dramatically improved survival and life expectancy. For those who have access to therapy and are adherent, AIDS-related illnesses are unlikely to substantially limit overall survival. The impact of greatly improved ART includes a remarkable change in the epidemiology of HIV in the United States where it is now estimated that >50% of PLWH are >50 years old [2]. However, treatment is not cure, and it does not fully restore immune health or eliminate other consequences of chronic HIV infection. Furthermore, chronic treatment may have long-term side effects that only become apparent over years of use. Thus, while the current research focus on the issues of HIV infection prevention and cure is appropriate and ongoing, an additional important area of investigation is improved understanding of the symptoms and comorbidities of PLWH. Specifically, understanding HIV-associated comorbidities, coinfections, and complications is a high-priority topic of research by the National Institutes of Health and Office of AIDS Research [3].
Among the most common and prominent symptoms reported by PLWH are those related to fatigue and difficulty sleeping. Nearly half of HIV-infected adults report poor sleep quality [4]. Fatigue is very commonly reported by PLWH even when HIV viral replication is suppressed and restoration of normal CD4 cell counts has been achieved [5, 6]. A host of other cardiovascular and metabolic complications such as coronary artery disease, diabetes mellitus, and nonalcoholic fatty liver disease are increasingly recognized as important complications of HIV infection and its treatment [7, 8]. We believe that one relatively unexplored comorbidity that may link these symptoms and complications in PLWH is obstructive sleep apnea (OSA). As illustrated in Table 1, many comorbidities experienced by PLWH are also negatively affected by OSA.
Table 1.
Strong Overlap in Comorbidities Experienced by People Living With Human Immunodeficiency Virus and Those Impacted by Obstructive Sleep Apnea
| Comorbidity | In PLWH | In OSA |
|---|---|---|
| Diabetes | 2–3 times the incidence of new-onset diabetes compared to non-HIV [29, 30] | Multiple experiments/models show decreased insulin sensitivity with decreased sleep or OSA (reviewed in [31]) |
| Cardiovascular disease | HIV associated with 50% higher risk of acute MI beyond traditional risk factors; ART may predispose [32] (reviewed in [33]) |
OSA a risk factor for hypertension and sudden cardiac death among others [34] (reviewed in [35]) |
| Stroke | 17% higher risk for ischemic stroke in men with HIV compared to non-HIV [36] (reviewed in [37]) | OSA is a potential modifiable risk factor [38]; treatment poststroke may aid recovery [39] (reviewed in [40]) |
| COPD | >40% have respiratory symptoms, 21% with airway obstruction, linked with ART use [41] (reviewed in [42]) | Coexistent COPD and OSA associated with increased mortality [43]; mortality attenuated by CPAP adherence [44] (reviewed in [45]) |
| Neurocognitive dysfunction | Problems persist into the modern treatment era [46] (reviewed in [47]) | OSA associated with brain morphology changes [48]; treatment with CPAP improves neurocognitive architecture [49] (reviewed in [50]) |
| Non-AIDS-defining cancers | Lung, liver, lymphoma, and melanoma, among others, higher in HIV compared to non-HIV [51] | OSA increasingly linked with higher incidence of cancers compared to non-OSA [52] |
| Liver disease | NAFLD is common in PLWH, even those without HBV or HCV [53, 54] | OSA independently associated with NAFLD and advanced liver histology; absence of OSA is protective of fibrosis [55] (reviewed in [56]) |
Abbreviations: ART, antiretroviral therapy; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MI, myocardial infarction; NAFLD, nonalcoholic fatty liver disease; OSA, obstructive sleep apnea; PLWH, people living with human immunodeficiency virus.
OSA is defined by repetitive collapse of the upper airway during sleep leading to transient hypoxemia and arousals from sleep. In the general population, OSA is associated with, broadly speaking, cardiometabolic and neurocognitive consequences. Surges in sympathetic activity that accompany arousal from sleep engender the release of adrenaline, cortisol, and other hormones, which, coupled with repeated oxygen desaturations, are hypothesized to lead to increased incidence of hypertension, stroke, and myocardial infarction. These hormones tend to antagonize the effects of insulin, potentially increasing the risk of diabetes in people with OSA. The fragmentation of sleep that defines OSA is thought to be the cause of neurocognitive symptoms such as excessive daytime sleepiness and lapses in attention that can have serious consequences, for example, increased automobile accidents [9]. Although mechanisms have not been elucidated, the degree and duration of OSA-associated hypoxemia are correlated with increased cancer risk.
Recent estimates suggest that roughly 10% of the US population has clinically important OSA (roughly 13% of middle-aged men and 6% of middle-aged women). This relatively high prevalence reflects both aging of the population and the obesity pandemic, 2 factors known to contribute to OSA risk [10]. In older populations, the prevalence may be even higher [11]. The most commonly prescribed treatment for OSA is continuous positive airway pressure (CPAP), which acts as a pneumatic splint to keep the upper airway open during sleep. Unfortunately, adherence to CPAP is far from ideal. Delayed diagnosis of OSA and erratic adherence to CPAP treatment may explain the lack of definitive data demonstrating improvement in mortality with the use of CPAP. Nonetheless, for adherent patients there are well-established benefits of CPAP in many patient-centric domains impacted by untreated OSA, such as fatigue, quality of life, and reduction in motor vehicle accidents [12–14].
WHAT IS KNOWN ABOUT OSA IN PLWH?
There has been limited study to date of OSA in PLWH (“HIV sleep apnea” yields only 42 citations in PubMed since 1995 [the last Clinical Infectious Diseases publication was in 2003], vs >1000 for “HIV fatigue”). Early case reports focused on adenotonsillar hypertrophy as a potential cause of OSA in HIV-infected persons [15]. Later, lipodystrophy and weight gain after ART were postulated as potential causative factors [16, 17, 18]. To our knowledge, there have been no studies examining the impact of OSA treatment in PLWH. More recent work in the last decade has included the following observations:
OSA appears to be common in PLWH. Studies of the prevalence of OSA in PLWH are imperfect, largely due to challenges of completing gold standard diagnostic testing (polysomnography) on large groups of PLWH. Recent emphasis on issues of chronic disease diagnosis and management in PLWH has generated some relevant data. Using a subset of subjects at one site of the Multicenter AIDS Cohort Study (MACS) who underwent polysomnography, Patil and colleagues found that 70% of HIV-infected subjects in their cohort had OSA [19]. Although the prevalence of OSA was similar in their control group, this finding suggests that a substantial fraction of men living with HIV may have undiagnosed OSA. (We know of no studies using polysomnography in women with HIV.) Goswami and colleagues used a questionnaire-based approach to try to estimate OSA prevalence in PLWH [20]. In a sample of mostly men (about 20% women), nearly a quarter endorsed witnessed apneic events, a fairly specific (but insensitive) marker for OSA. Taken together, these data suggest that there may be substantial numbers of PLWH with undiagnosed OSA. As prevalence estimates using administrative databases have been far lower (see below), these data also suggest the need for better estimates of the prevalence of OSA, ideally using objective testing in unselected populations.
-
Traditional OSA risk factors may not predict OSA in PLWH. Traditional risk factors for OSA include older age and increased weight. In the study mentioned above, PLWH who had OSA were younger and substantially leaner (as assessed by body mass index [BMI]) than the non-HIV-infected controls in this study—and therefore would have been predicted to have a lower prevalence of OSA [21]. Furthermore, while OSA is often thought of as a disease of obesity, only 12% of the HIV-infected OSA patients in the Patil et al study were obese. In cohorts of people with OSA from the general population (which generally exclude HIV-infected persons), most affected individuals are indeed overweight or obese. In contrast, in the subset of men in the MACS cohort described above (mean age, 49.9 years; mean BMI, 25.4 kg/m2), the prevalence of OSA was 70%.
The apparent high prevalence of OSA in the absence of traditional risk factors in PLWH suggests that other factors may be important in this population. In this same study, current or prior ART use was associated with an increased prevalence of OSA compared to those who had no prior exposure to ART (90% vs 57%). The underlying mechanisms by which HIV and or ART may contribute to OSA are unknown. Some older ART drugs/regimens were associated with lipodystrophy, a disorder that could increase airway narrowing/collapse at lower BMI levels. However, more contemporary treatment regimens are not linked to lipodystrophy, so other mechanisms must be considered. In addition to anatomy, nonanatomical factors such as upper airway muscle responsiveness and ventilatory control are important predisposing factors for OSA. Could neuropathic or myopathic dysfunction induced by HIV/ART in the upper airway promote collapse? The physiology of OSA in PLWH is being explored in the authors’ ongoing work [22], but more research is clearly needed.
Untreated OSA may be relevant for symptoms in PLWH. When examining predictors of fatigue in PLWH, Goswami and colleagues found expected covariates including opioid use, antidepressant use, depression, and sleep duration <6 hours. But the strongest predictor of fatigue was witnessed apnea [20]. Fatigue is one of the most commonly reported symptoms among PLWH, even in persons with suppressed HIV replication (undetectable HIV RNA). We note that although sleepiness and fatigue may be distinct symptoms with distinct underlying pathobiology, the 2 terms are often closely intertwined and/or used interchangeably by patients. To emphasize this point, in the MACS, 25% of PLWH complained of persistent fatigue, but there was also a similar proportion (26%) of patients who were considered excessively sleepy by questionnaire results. Thus, clinicians may need to consider disorders of excessive sleepiness (such as OSA) when evaluating patients who complain of fatigue.
Untreated OSA is likely to be associated with ongoing inflammation. In patients without HIV infection, OSA is associated with increased inflammation via a variety of pathways (see, eg, [23, 24]). In PLWH, the presence of moderate or severe OSA was associated with an increased likelihood of C-reactive protein elevations >3.0 mg/dL (odds ratio [OR], 6.9; 95% confidence interval [CI], 1.1–43.1; P = .04) [21], as well as higher levels of tumor necrosis factor α [25]. How OSA might be related to ongoing viral replication or diminished viral clearance is not known, but in PLWH with untreated OSA, greater than moderate sleep apnea was associated with HIV RNA levels >10000 copies/mL (OR, 7.1; 95% CI, 1.0–50.6; P = .05).
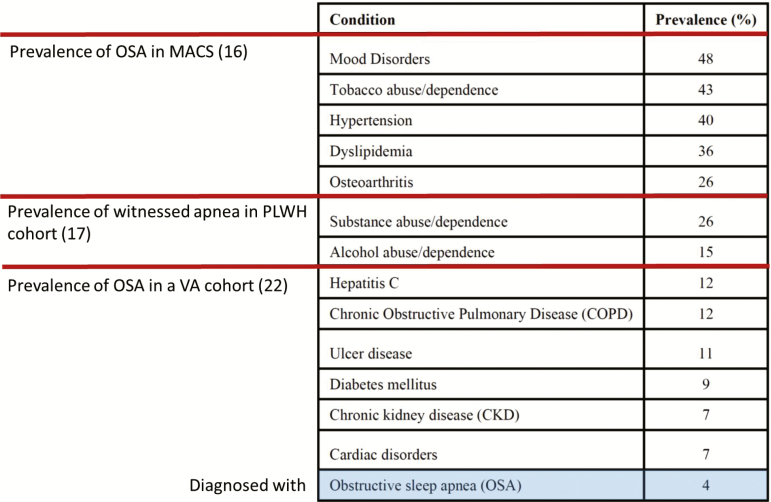
Few PLWH with OSA are diagnosed or treated. Few HIV providers have familiarity with or expertise in recognition and diagnosis of OSA. The lack of diagnostic consideration may be exacerbated by the fact that PLWH with OSA are younger and thinner than those in the general population. Moreover, common OSA symptoms such as fatigue/tiredness are often ascribed to HIV itself. As a consequence, few PLWH have been diagnosed with OSA. In multiple practice settings (including the Veterans Aging Cohort Study [26], an urban HIV clinic, and a university Center for AIDS Research clinic [27]), only about 4% of PLWH had been diagnosed with OSA. Although OSA is often underdiagnosed in general, in the same veterans cohort the rate of OSA diagnosis in HIV-uninfected persons was 12.4%. Thus, few patients or providers consider OSA in PLWH, although it may be important for many comorbidities (Figure 1).
Figure 1.
Prevalence of conditions in descending order for 1844 human immunodeficiency virus–infected patients receiving care at the University of Alabama Birmingham. However, based on other literature, obstructive sleep apnea is likely to be underdiagnosed. Reprinted with permission from Kim et al [27]. Abbreviations: MACS, Multicenter AIDS Cohort Study; OSA, obstructive sleep apnea; PLWH, people living with human immunodeficiency virus; VA, Veterans Affairs.
WHAT IS TO BE DONE?
Recently, there have been a variety of efforts to understand the role of sleep and sleep disorders in PLWH. More substantial resources have been made available by the National Institutes of Health (see, eg, RFA-HL 18-004–006, which allocate R01 and K12 resources potentially to sleep disorders in HIV). For ongoing research efforts, we encourage researchers to consider sleep and/or sleep apnea as a potential contributor to the some of the many comorbidities linked to HIV infection. As seen in Table 1, OSA impacts cardiovascular, metabolic, hepatic, and neurocognitive health—and emerging evidence appears to link OSA to the development and progression of cancer.
Most urgently, we suggest that clinicians who care for PLWH consider the diagnosis of OSA when evaluating patients with fatigue, tiredness, or excessive sleepiness. While the diagnosis and treatment of OSA in PLWH may produce modest improvements in control of comorbidities such as hypertension or diabetes, there may be very substantial improvements in fatigue/sleepiness and overall quality of life in PLWH whose OSA is appropriately treated. Although existing OSA screening tools sometimes focus on obesity and may be imperfect for PLWH, they represent a reasonable starting point and can be rapidly and easily completed during an office visit (see [28] for a review). In the absence of access to sleep medicine clinic diagnostics, portable or “home” sleep testing provides widespread availability to initial diagnostic testing for OSA (Figure 2). Following, diagnosis, CPAP remains the gold standard treatment for moderate to severe OSA. While adherence to CPAP has been relatively low in the past and remains an active area of research, advances in airway pressure delivery, interface (mask) design, and real-time feedback to users have all tended to improve adherence over time. Thus, at all levels beyond the initial suspicion of sleep apnea, diagnosis and therapy have improved and become more patient-friendly.
Figure 2.
Example of portable sleep monitor, which can be applied and worn in the patient’s own home.
In the >3 decades since the first cases of AIDS were recognized, enormous progress in the diagnosis and treatment of HIV infection has been achieved. If we are indeed at a time when we can think about “the end of AIDS,” we should recognize that these accomplishments are the fruit of extraordinary efforts of patient/public engagement in the research process. The time has come to devote similar energy and passion to the many comorbidities impacting quality of life in the millions who live with HIV worldwide, including OSA. While there is still much to learn about sleep and sleep apnea in PLWH, diagnostic and treatment devices that could make a big difference among PLWH are widely available in the developed world, and it is time for HIV providers to wake up to the idea of OSA in PLWH.
Note
Potential conflicts of interest. R. L. O. has received consulting fees from Novartis, and honoraria and travel fee reimbursements from ResMed and Itamar Medical. C. B. H. is an employee of ViiV Healthcare. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson AS, Hall HI, Hu X, Lansky A, Holtgrave DR, Mermin J. Trends in diagnoses of HIV infection in the United States, 2002–2011. JAMA 2014; 312:432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ba D, Babadi B, Purdon PL, Brown EN. Robust spectrotemporal decomposition by iteratively reweighted least squares. Proc Natl Acad Sci U S A 2014; 111:E5336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allavena C, Guimard T, Billaud E, et al. ; COREVIH-Pays de la Loire Troubles du Sommeil Study Group . Prevalence and risk factors of sleep disturbance in a large HIV-infected adult population. AIDS Behav 2016; 20:339–44. [DOI] [PubMed] [Google Scholar]
- 5. Fredericksen RJ, Edwards TC, Merlin JS, et al. Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care 2015; 27:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Payne BA, Hateley CL, Ong EL, et al. HIV-associated fatigue in the era of highly active antiretroviral therapy: novel biological mechanisms?HIV Med 2013; 14:247–51. [DOI] [PubMed] [Google Scholar]
- 7. Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses 2013; 29:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pullinger CR, Aouizerat BE, Gay C, et al. Metabolic abnormalities and coronary heart disease risk in human immunodeficiency virus-infected adults. Metab Syndr Relat Disord 2010; 8:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383:736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177;1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehra R, Stone KL, Blackwell T, et al. ; Osteoporotic Fractures in Men Study . Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayas NT, FitzGerald JM, Fleetham JA, et al. Cost-effectiveness of continuous positive airway pressure therapy for moderate to severe obstructive sleep apnea/hypopnea. Arch Intern Med 2006; 166:977–84. [DOI] [PubMed] [Google Scholar]
- 13. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 1997; 6:199–204. [DOI] [PubMed] [Google Scholar]
- 14. Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–71. [DOI] [PubMed] [Google Scholar]
- 15. Epstein LJ, Strollo PJ Jr, Donegan RB, Delmar J, Hendrix C, Westbrook PR. Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep 1995; 18:368–76. [DOI] [PubMed] [Google Scholar]
- 16. Schulz R, Lohmeyer J, Seeger W. Obstructive sleep apnea due to HIV-associated lipodystrophy. Clin Infect Dis 2003; 37:1398–9. [DOI] [PubMed] [Google Scholar]
- 17. Dorey-Stein Z, Amorosa VK, Kostman JR, Lo Re V 3rd, Shannon RP. Severe weight gain, lipodystrophy, dyslipidemia, and obstructive sleep apnea in a human immunodeficiency virus-infected patient following highly active antiretroviral therapy. J Cardiometab Syndr 2008; 3:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo Re V 3rd, Schutte-Rodin S, Kostman JR. Obstructive sleep apnoea among HIV patients. Int J STD AIDS 2006; 17:614–20. [DOI] [PubMed] [Google Scholar]
- 19. Patil SP, Brown TT, Jacobson LP, et al. Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and -uninfected men. PLoS One 2014; 9:e99258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goswami U, Baker JV, Wang Q, Khalil W, Kunisaki KM. Sleep apnea symptoms as a predictor of fatigue in an urban HIV clinic. AIDS Patient Care STDS 2015; 29:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown TT, Patil SP, Jacobson LP, et al. Anthropometry in the prediction of sleep disordered breathing in HIV-positive and HIV-negative men. Antivir Ther 2010; 15:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Center for AIDS Research (CFAR). Creative and Novel Ideas in HIV Research (CNIHR). Available at http://cnihr.org/awardees/2016-awardees/.
- 23. Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol 2017; 595:2423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akinnusi M, Jaoude P, Kufel T, El-Solh AA. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath 2013; 17:1009–16. [DOI] [PubMed] [Google Scholar]
- 25. Brigham EP, Patil SP, Jacobson LP, et al. Association between systemic inflammation and obstructive sleep apnea in men with or at risk for HIV infection. Antivir Ther 2014; 19:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunisaki KM, Akgün KM, Fiellin DA, et al. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med 2015; 16:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DJ, Westfall AO, Chamot E, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr 2012; 61:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller JN, Berger AM. Screening and assessment for obstructive sleep apnea in primary care. Sleep Med Rev 2016; 29:41–51. [DOI] [PubMed] [Google Scholar]
- 29. Capeau J, Bouteloup V, Katlama C, et al. ; ANRS CO8 APROCO-COPILOTE Cohort Study Group . Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012; 26:303–14. [DOI] [PubMed] [Google Scholar]
- 30. Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr HIV/AIDS Rep 2016; 13:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest 2017; 152:1070–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nou E, Lo J, Hadigan C, Grinspoon SK. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol 2016; 4:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005; 352:1206–14. [DOI] [PubMed] [Google Scholar]
- 35. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009; 373:82–93. [DOI] [PubMed] [Google Scholar]
- 36. Sico JJ, Chang CC, So-Armah K, et al. ; Veterans Aging Cohort Study . HIV status and the risk of ischemic stroke among men. Neurology 2015; 84:1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One 2017; 12:e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–53. [DOI] [PubMed] [Google Scholar]
- 39. Lyons OD, Ryan CM. Sleep apnea and stroke. Can J Cardiol 2015; 31:918–27. [DOI] [PubMed] [Google Scholar]
- 40. Kim Y, Koo YS, Lee HY, Lee SY. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PLoS One 2016; 11:e0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med 2010; 182:790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Presti RM, Flores SC, Palmer BE, et al. Mechanisms underlying HIV-associated noninfectious lung disease. Chest 2017; 152:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182:325–31. [DOI] [PubMed] [Google Scholar]
- 44. Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med 2013; 9:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Owens RL, Macrea MM, Teodorescu M. The overlaps of asthma or COPD with OSA: a focused review. Respirology 2017; 22:1073–83. [DOI] [PubMed] [Google Scholar]
- 46. Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eggers C, Arendt G, Hahn K, et al. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 2017; 264:1715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med 2003; 4:451–4. [DOI] [PubMed] [Google Scholar]
- 49. Rosenzweig I, Glasser M, Crum WR, et al. Changes in neurocognitive architecture in patients with obstructive sleep apnea treated with continuous positive airway pressure. EBioMed 2016; 7:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bucks RS, Olaithe M, Rosenzweig I, Morrell MJ. Reviewing the relationship between OSA and cognition: where do we go from here?Respirology 2017; 22:1253–61. [DOI] [PubMed] [Google Scholar]
- 51. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 52. Owens RL, Gold KA, Gozal D, et al. Sleep and breathing ... and cancer?Cancer Prev Res (Phila) 2016; 9:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr 2009; 50:464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017; 31:1621–32. [DOI] [PubMed] [Google Scholar]
- 55. Corey KE, Misdraji J, Gelrud L, et al. Obstructive sleep apnea is associated with nonalcoholic steatohepatitis and advanced liver histology. Dig Dis Sci 2015; 60:2523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath 2018. Jan 15. doi: 10.1007/s11325-018-1625-7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]