Abstract
In recent decades, dysregulation of proteases and atypical proteolysis have become increasingly recognized as important hallmarks of cancer, driving community-wide efforts to explore the proteolytic landscape of oncologic disease. With more than 100 proteases currently associated with different aspects of cancer development and progression, there is a clear impetus to harness their potential in the context of oncology. Advances in the protease field have yielded technologies enabling sensitive protease detection in various settings, paving the way towards diagnostic profiling of disease-related protease activity patterns. Methods including activity-based probes and substrates, antibodies, and various nanosystems that generate reporter signals, i.e., for PET or MRI, after interaction with the target protease have shown potential for clinical translation. Nevertheless, these technologies are costly, not easily multiplexed, and require advanced imaging technologies. While the current clinical applications of protease-responsive technologies in oncologic settings are still limited, emerging technologies and protease sensors are poised to enable comprehensive exploration of the tumor proteolytic landscape as a diagnostic and therapeutic frontier. This review aims to give an overview of the most relevant classes of proteases as indicators for tumor diagnosis, current approaches to detect and monitor their activity in vivo, and associated therapeutic applications.
Keywords: tumor microenvironment, protease activity, protease diagnostic and therapeutic modalities
1. Introduction
Dysregulated proteolysis, elevated protease expression, misfiring of protease signaling, or distorted protease-inhibitor equilibrium are frequently associated with developing or ongoing disease [1]. In healthy cells, proteases are instrumental for protein processing, metabolism, coagulation, tissue remodeling, homeostasis, programmed cell death and autophagy, antigen presentation, and immune response, among other physiological functions. Together, proteases represent one of the largest protein families, with a total of around 580 genetically encoded hydrolytic enzymes in humans [2], divided into the five major families of metallo, serine, cysteine, aspartic, and threonine proteases based on their catalytic mechanism [3,4]. While members of the same protease family often display a substantial degree of similarity in terms of structure and sequence homology, each individual protease has its own unique specificity fingerprint, activity patterns, expression profiles and localization [5]. Before the extensive developments in the fields of molecular and cell biology and the advent of the omics era in 1990s, proteases were essentially considered as protein-degrading enzymes instrumental for metabolic processes and maintenance of cell homeostasis [6]. Advances in the fields of molecular biology, chemical biology and proteomics have challenged this view, and proteases are now widely recognized as major players in diseases and considered important drug targets [7,8].
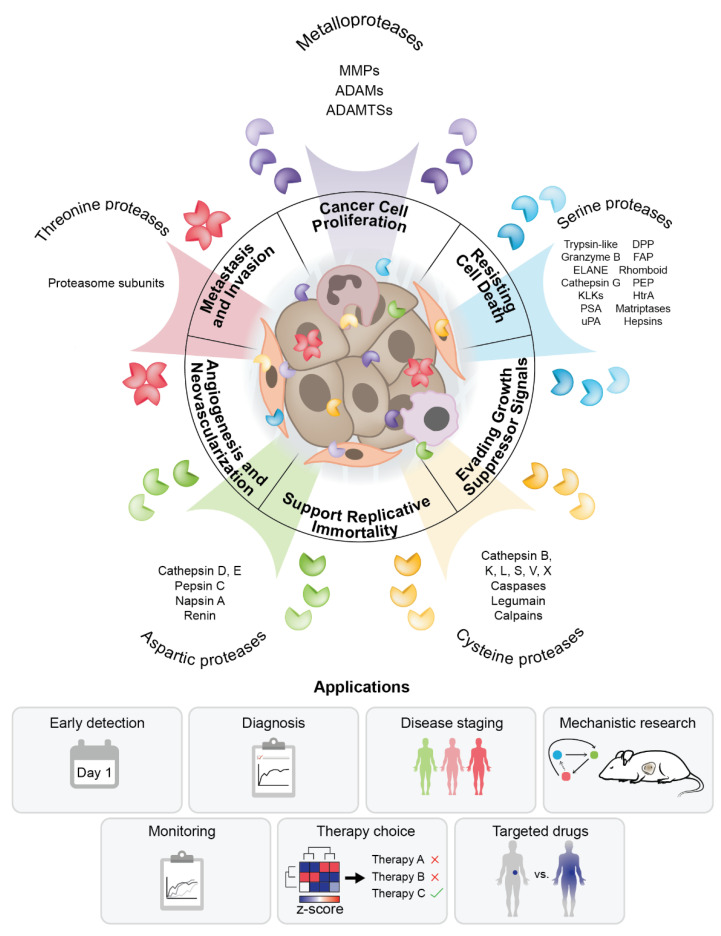
Past research provided solid evidence that proteases are heavily involved in the development and progression of cancer [9] and efforts to integrate them into cancer diagnosis and therapeutic management have grown [10]. In cancer, dysregulated proteases from different protease families are associated with a myriad of stages of development, extensive remodeling of the ECM (Extracellular matrix) [11,12,13], epithelial-to-mesenchymal transition [14], immune system evasion and hijacking [15], resistance to apoptosis signals [16], metastasis development [17,18,19], as well as tumor growth, invasion and metastasis spread [20,21,22]. Furthermore, proteases have roles in signaling pathways like MAPK (Mitogen-activated protein kinase), Akt (Protein kinase B) and TNFβ (Tumor necrosis factor β), among others, thereby exerting a substantial influence on cancer development and progression [23,24]. With the growing knowledge of protease molecular functions and characteristic activity patterns associated with cancer phenotypes, it has become clear that tumor-associated protease activity can be harnessed to develop diagnostic tools and biomarkers for early disease detection [10,25]. Moreover, the activity of disease proteases can be exploited for functional diagnostic imaging [26,27], translate into applications where proteases act as activators of prodrugs [28] or into protease-based drug delivery systems [29] to pave the way towards the next generation of clinical modalities as outlined in Figure 1.
Figure 1.
Knowledge of tumor proteolysis translates into diagnostic and therapeutic modalities. Proteases in the tumor microenvironment belong to different protease families and originate from tumor and other cells present in the tumor microenvironment. They exhibit different patterns of localization and activity that may overlap and generate an intricate proteolytic landscape with roles in mechanisms behind cancer hallmarks. Knowledge of the role of proteolysis in cancer can support diagnosis, staging, mechanistic studies, disease monitoring and therapy.
Here, we review the most important roles of each major catalytic type of protease in the development and progression of cancer. We describe the tools commonly used to detect and monitor their activity in preclinical settings and illustrate how this proteolytic landscape can be integrated into diagnostics and therapeutics. Attention is also given to the emerging synergies and interdisciplinary connections with the nanotechnology and nanomaterial field, where recent developments have shown potential to drive the evolution from bench-to-bedside.
2. Proteases in the Tumor Microenvironment
Representative members from each major protease family have been linked to selected cancer hallmarks (Figure 1) and elevated or dysregulated protease activity is mechanistically involved in sustaining cancer cell proliferation, resisting cell death, evading growth suppressor signals, supporting replicative immortality, inducing angiogenesis and neovascularization and metastasis development and invasion [21,30]. Accordingly, representative protease groups and their roles in context of the original six cancer hallmarks proposed by Hanahan and Weinberg are summarized in Table 1. Proteolytic cleavage is a relatively simple irreversible posttranslational modification that generates proteolytic fragments from proteins, thereby changing their structure and function. Understanding the complex contribution of proteases to cancer and leveraging this knowledge for clinical purposes is nevertheless a daunting task for several reasons. First, proteases from different families with different proteolytic activities are present in the tumor microenvironment. These can originate either from cancer cells or from other cells that are present in the tumor microenvironment (macrophages, neutrophils, stroma cells etc.) [31]. Second, the concentration of proteases can span several orders of magnitude, from those present in trace amounts to others with high expression levels or locally elevated concentration [32]. Third, proteases show different localization and activity patterns and can be found in multi-protein complexes or in complexes with their inhibitors that fine-tune their activity [9]. Finally, there is a substantial level of crosstalk between proteases from different families in the tumor microenvironment [17,19]. Together, all these factors contribute to an intricate and interconnected tumor proteolytic landscape. In the next sections, we summarize the current knowledge on the role of the five major classes of proteases in the context of the hallmarks of cancer.
Table 1.
Protease roles in the context of cancer hallmarks. Representative examples and selected roles of the most important proteases and protease groups are described with respect to cancer development and progression.
| Cancer Hallmark | Example Proteases and Protease Groups | Mechanistic Roles, Functions and Consequences | Selected References |
|---|---|---|---|
| Cancer cell proliferation | MMP2, 3, ADAM10, 17 Cathepsins Kallikreins |
ECM remodeling, signaling, processing of growth factors, sustain and boost proliferative signaling pathways | [33,34,35,36] [18,37] [38,39] |
| Resisting cell death | MMP7, ADAM10 Granzyme B HtrA Caspases Cathepsins B, L, S Cathepsin D |
Apoptosis signaling, apoptosis resistance, circumvent apoptosis triggers, autophagy recycling, immune system evasion | [22,40] [41,42] [43] [44,45] [18,37] [46] |
| Evading growth suppressor signals | Various MMPs and ADAMs ADAMTs Kalikreins, Cathepsins |
Cytokine and chemokine secretion, removal of cellular brakes, receptor signaling, disruption of p53 signaling | [22,36,40,47,48] [49,50,51] [39,52,53] [54,55] |
| Support replicative immortality | Multiple MMPs ADAM10, 17 Granyzme B Cathepsins B, L, S KLK4-7 |
Sustain growth signaling, release of biologically active fragments, immune system evasion, immune system hijacking | [33,56,57] [36,47,48] [41,42] [37,54,58,59] [60] |
| Angiogenesis and neovascularization | MMP1, 2, 9 Kallikreins PSA Cathepsins B, L, K, S Calpains |
Growth factor signaling, ECM remodeling, degradation of structural proteins, release of cytokines, receptor shedding | [61,62,63] [52,53] [64] [37,54,59,65] [66,67] |
| Metastasis and invasion | MMP1, 14 ADAM10, 17 Kalikreins Cathepsin G PSA FAP, DPPIV, PEP Cathepsins B, L, K, S Legumain Cathepsin D, E Calpains |
ECM remodeling, barrier degradation, cancer cell migration, receptor signaling, metabolic signaling, epithelial-to mesenchymal transition, release and modulation of signaling molecules, kinase signaling perturbation | [61,62,63] [36,47,48] [52,53] [68,69] [64] [70] [37,54,58,59] [71] [46,72] [73,74] |
2.1. Metalloproteases
Metalloproteases are a family of Zn2+ binding protease homologues with numerous cancer-related functions and represent the largest protease group in humans. They exhibit diverse roles, functions, and patterns of localization [33,34], and are instrumental for pericellular proteolysis with direct effects on ECM structure, function, and signaling [56]. The most important metalloproteases that are highly active in the tumor microenvironment are the matrix metalloproteases (MMPs), a disintegrin and metalloproteases (ADAMs), and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs). In a healthy state, metalloprotease activity is generally kept under tight regulation by TIMP (Tissue inhibitor of metalloproteinases, TIMP-1, -2, -3 and -4), which fine-tune the rate and extent of target protein proteolysis [75]. Any disruption of this equilibrium can unleash the proteases’ degradative power, with MMPs having particularly detrimental effects in the context of cancer [76].
Generally, MMPs target a broad range of ECM proteins, contributing to cancer development, progression, invasive growth and spread of cancer cells, and their elevated activity has so far been detected in almost all types of cancer [40]. Traditionally, MMPs are divided into groups of collagenases, gelatinases, stromelysins and matrilysins according to their effect on the ECM proteins [34,57,77]. MMP-1, -8 and -13 are strong collagenases that cleave triple helix collagens, while MMP-1 and -8 also cleave gelatin [78,79]. MMP-3, -10 and -11 belong to stromelysins, cleaving different ECM proteins like aggrecan and fibronectin, but do not cleave collagen. MMP-7 and MMP-26 are matrilysins that cleave gelatins, collagen and fibronectin, whereas MMP-2 and MMP-9 are gelatinases [34,57,77]. The proteolytic action of MMPs on these scaffolding proteins changes composition, structure and function of ECM [80]. Another important group of MMP substrates are cell adhesion molecules like syndecans or E-cadherin [81,82] and non-ECM substrates [83], and products of these cleavages are linked with disease development and progression. Interestingly, several MMPs (as well as serine proteases) can contribute to activation of other MMPs, amplifying their proteolytic activity in the ECM [84]. MMPs can also play a role in cell signaling and have pleotropic roles in cancer [85], such as the ability of MMP-7 to activate different kinase pathways [86]. In addition to soluble MMPs, there are six membrane-bound MMPs, affixed to the cell surface by a GPI-anchor. These include MMP-14, -15, -16, -17, -24 and -25, which have important roles in remodeling the ECM, processing growth factors, and shedding cell receptors, which drive cancer development and progression [61]. MMPs are also involved in the epithelial-to-mesenchymal transition, one of the most widely-recognized cancer hallmarks [62,63]. Furthermore, correlations discovered between MMPs and poor patient prognosis suggest that MMP levels could be used as a diagnostic or prognostic indicator [87,88,89,90,91].
ADAMs are transmembrane proteases with established roles in cell proliferation, adhesion and migration, and substantially contribute to the complexity of proteolysis in the tumor [35,36]. Whereas MMPs essentially target most of the structural components of the ECM, ADAMs mostly target the extracellular domains of transmembrane proteins (both type I and type II) and thus contribute substantially to the cleavage of cell adhesion molecules, shedding of cell surface receptors and maturation of cytokines and chemokines [36,47,48]. Currently, ADAM-10 and ADAM-17 present the strongest bodies of evidence for involvement in cancer. ADAM-10 is able to process and activate the EGFR (Epidermal growth factor receptor) ligands [92], cleave E-cadherin with implications in signaling [93], and cleave the CD44 adhesion molecule from the cell surface [94]. ADAM-10 expression was also shown to correlate with invasive growth of different cancers [95], establishing the protease as a potential therapeutic target. ADAM-17 is important for the release of the soluble TNFα (Tumor necrosis factor α) [96] and activation of IL-6/ERK signaling [97]. It can release the EGF receptor [98] and shed adhesion molecules such as CD44 [99]. While inhibition of ADAM-17 can reduce invasiveness of breast cancer [100], its activation as a consequence of chemotherapeutic drugs can contribute to resistance [101]. Different ADAMs, including ADAM-10 and ADAM-17, are also important for proteolytic processing of ligands and modulation of Notch signaling. This molecular mechanism requires ADAM-mediated cleavage events for its proper functioning and altered processing of Notch receptors and ligands has been linked to proliferation and differentiation as well as cancer cell death [102,103,104].
Another family of metalloproteases with roles in cancer are ADAMTSs, largely responsible for degrading structural ECM proteins [105]. They are generally divided into the group of hyalectanases, including ADAMTS-1, -4, -5, -8, -9, -15, and -20, which mostly target various (hyalectan) proteoglycans, ADAMTS-2, -3 and -14 which process the N-terminal propeptides of collagens, and the remaining group of ADAMTS-6, -7, -10, -12, -13 and -16, which have more specific functions [106,107,108]. Accumulating evidence suggests several ADAMTSs are involved in cancer development and progression and can promote tumor development [49,50,51], but their roles in proteoglycan proteolysis also have tumor suppressor functions [109] and their prognostic value was evaluated in the context of different cancers [110,111,112]. Since the involvement of different members of the metalloproteinase families has been reported in nearly all known forms of cancer, they are considered one of the most important target protease groups in cancer research.
2.2. Serine Proteases
Serine proteases comprise the second largest family of proteolytic enzymes, and execute functions ranging from metabolism and blood coagulation to homeostasis and immune response. They are mostly secreted, activated by limited proteolytic processing, and tightly regulated by their endogenous inhibitors, SERPINS [113]. Disturbance of this equilibrium can be a factor contributing to cancer development, progression, metastasis and invasion [114]. Trypsin and trypsin-like proteases (e.g., thrombin or tissue factor) are perhaps the most comprehensively characterized serine proteases. While assuming essential roles in metabolism, the coagulation cascade and blood pressure regulation, they have also been linked to cancer where hemostatic abnormalities are often observed [115]. The tumor-induced activation of the coagulation cascade can result in elevated levels of active coagulation proteases [116] with an impact on tumor growth and angiogenesis [117,118]. It is therefore unsurprising that elevated pro-coagulant activity was observed in cancer patient samples [119]. Currently, thrombin is considered a potential therapeutic target in lung cancer that could contribute to cancer progression [120]. In addition, tissue factor has been associated with aggressive behavior of malignancies and a substantial body of evidence suggests that coagulation proteases participate in activation of PARs (protease-activated receptors) to propagate tumor growth and invasiveness [121,122]. Notably, there is appreciable crosstalk between the coagulation proteases and the immune system and the activation of one can boost the activation of the other [123].
Immune response, or the lack thereof, is central in allowing cancer to develop, and several serine proteases are involved in immune response. Granzyme B is a serine protease from the natural killer cells and cytotoxic T lymphocytes [41,42]. It is important for the removal of tumor cells by the host immune system [124] and for different apoptosis mechanisms that contribute to the removal of cancer cells [125]. Generally, elimination of tumor cells is strongly dependent on granzyme B and this process can be enhanced by activating p53 to support tumor cell lysis [126]. Nevertheless, granzyme B can also contribute to cancer cell invasion by degrading ECM components [127]. Neutrophil elastase (ELANE), secreted into the tumor microenvironment primarily by immune cells like neutrophils and macrophages, has been shown to be upregulated in cancer [128]. ELANE can contribute to the remodeling of ECM [129], activation of toll-like receptors (e.g., TLR4) [130] and release of growth factors like TGFα (Transforming growth factor α), PDGF (Platelet-derived growth factor) and VEGF (Vascular endothelial growth factor) [131]. Another neutrophil protease involved in cancer is cathepsin G. It has been shown to be a mediator of MMP-9 activation and promotes TGFβ (Transforming growth factor β) signaling that is important for formation of bone lesions, cancer-induced osteolysis and metastasis [132,133]. Cathepsin G is also involved in perturbing E-cadherin-dependent cell adhesion, linking it to cancer cell migration and invasiveness [68,69]. Furin, a member of the subtilisin-like family has also been linked to cancer [134]. Its activity has been detected in various cancers and can promote the invasive phenotype, making it a potential therapeutic target [135].
Kallikreins, the largest subgroup of serine proteases, represent yet another class of serine proteases that appear to be implicated in cancer. Accounting for a total of 15 proteins in humans, KLK1–KLK15 are generally expressed within endocrine glands and organs and ultimately secreted in the extracellular space [38,136]. They are emerging as potential diagnostic targets in strategies for monitoring chemotherapeutic response [39] and there is evidence for their value as clinical biomarkers [137,138]. While the spectrum of functions of kallikreins is diverse, they appear to be essential in the development of prostate and skin cancers [52,139]. KLK4, 5, 6 and 7 are involved in TGFβ signaling as demonstrated by degradome analysis [60]. Kallikreins have important links between ECM proteolysis and infiltration of immune cells [53] and almost all kallikreins can cleave structural components of the ECM [140]. They are also involved in PAR and EGF receptor signaling [141]. PSA (Prostate-specific antigen), a protease very similar to the members of the kallikrein family, is typically expressed in prostate cells and routinely measured in clinics as a biomarker for diagnosis of prostate cancer [142]. PSA is key in ECM remodeling and signaling pathways associated with prostate cancer progression, metastasis and angiogenesis [64]. PSA is also capable of activating uPA (urokinase-type plasminogen activator) [143]. uPA is a trypsin-like protease that catalyzes the activation of uPAR (urokinase-type plasminogen activator receptor) and cleaves several components of ECM including fibronectin [144]. Currently, different components of the uPA/uPAR system are under consideration as prospective cancer biomarkers [145].
Moving away from trypsin-like serine proteases, DPPIV (Dipeptidyl peptidase IV), FAP (Fibroblast activation protein) and PEP (Prolyl endopeptidase) have emerged as potentially important players in cancer [70]. DPPIV seems to be closely linked to malignancies, though it has been suggested to have both cancer suppressing and promoting roles, indicating that further investigation is needed [146]. Notably, DPPIV serum levels have shown prognostic value in patients with colorectal [147] and gastric [148] cancer. FAP, which has strong gelatinase activity implicated in extensive remodeling of ECM, has been linked to elevated invasiveness and tumor progression [149,150]. Elevated PEP activity levels have also been found in various cancers [151] and tend to correlate with poor patient prognosis, as demonstrated in colorectal cancer [152].
Another group of serine proteases with possible links to cancer are the High-temperature requirement factors, HtrA1, 2, 3 and 4 [153]. From this group HtrA2 has emerged as the most promising target because of its role in apoptosis regulation [43]. There are also several membrane-anchored serine proteases, including matriptase and Hepsin [154]. Matriptase is considered a potential diagnostic marker [155] and has an important role in the serine protease—growth factor signaling axis [156]. Hepsin, a transmembrane protease, has received attention because of its overexpression in prostate [157] and gastric cancer [158], which correlated with poor patient prognosis.
Finally, there is the group of rhomboids [159]. These intramembrane proteases usually cleave transmembrane proteins to release their domains from the cell membrane. The most important of the rhomboids are RHBDL2 (Rhomboid-related protein 2), which can trigger the activation of the EGF receptor [160] and has been found to be highly expressed in breast cancer [161] and RHBDL4 (Rhomboid-related protein 4), which triggers non-canonical secretion of TGFα [162]. Rhomboids have been linked to angiogenesis [163], resistance to cell death, and proliferation of cancer cells [164]. Research interest in better understanding their functions has been increasing.
2.3. Cysteine Proteases
The cysteine proteases that appear to be most directly involved in cancer are cathepsins, caspases and calpains. Cysteine cathepsins are a family of 11 proteases with a papain-like fold and a catalytic Cys-His amino acid pair in their active site. While cathepsins B, C, H and X are exopeptidases, cathepsins F, K, L, O, S, V and W are potent endopeptidases [65]. Cysteine cathepsins are generally regarded as lysosomal proteases and their extra-lysosomal and extracellular activity has previously been linked to various aspects of cancer development and progression [37]. Activity of cysteine cathepsins is regulated by pH, localization, proteolytic degradation and by their protein inhibitors (cystatins and stefins). A substantial body of evidence links cathepsins B, C, H, K, L, S and X to various aspects of cancer, making them potentially useful biomarkers [58]. Their oncologic roles include remodeling the ECM via cleavage of structural proteins, altering the signaling pathways that govern cell growth, proliferation and cell death or helping to fuel the protease pool that drives chronic inflammation [54]. Cysteine cathepsins are mostly active in acidic conditions like the tumor microenvironment and can cleave many different proteins, ranging from components of the extracellular matrix like collagens, elastins, laminins, glycosaminoglycans and proteoglycans to various cell adhesion molecules [59]. These belong to groups of junction adhesion molecules (JAM) [165] and cell surface receptors (like the EGF receptor, plexins and neuropilins) [166]. Importantly, the extracellular proteolysis by cathepsins in the tumor microenvironment has been linked to cancer spread, altered cell adhesion, neovascularization, metastasis, and invasive growth [18,167,168] and evidence supports cathepsin secretion as one of the driving forces behind cancer progression [55]. Findings from mouse cancer models suggest that general degradation dominates over specific proteolysis in tumors [169]. Nevertheless, cathepsins can also cleave specific targets like chemokines and cytokines that are linked to inflammation [170]. While the elevated activity of cysteine cathepsins in cancer, especially in the extracellular space, is now widely recognized as a characteristic sign of the disease, their redundancy [171] and largely overlapping specificity features [172,173] make their direct translation into diagnostic and therapeutic modalities a challenge.
Caspases are a group of endopeptidases that cleave proteins specifically after aspartate. Generally, caspases are divided in groups of apoptotic and inflammatory caspases (caspase-1, -4, -5 and -12 in humans). Apoptotic caspases are further divided in initiation (caspase-2, -8, -9 and -10) and executioner caspases (caspase-3, -6 and -7) and are essential for facilitating programmed cell death [174]. An important hallmark of oncologic transformation is also the cancer cells’ resistance to programmed cell death, for instance by insensitivity towards apoptosis triggers [44,175]. Past studies have established strong links between levels of caspase expression and the sensitivity of cancer cells towards apoptosis. Moreover, caspase deregulation generally contributes to resistance towards therapeutic intervention [45]. Nevertheless, for several caspases, their exact cancer-related roles depend heavily on oncologic context and often involve functions that are not directly related to apoptosis [176,177,178]. This creates substantial difficulties for caspases as potential biomarkers, as demonstrated by caspase-3. While some studies found that the expression levels of caspase-3 correlated with poor prognosis for the disease outcome [179], others reported that the levels of caspase-3 in cancer samples were extremely low or even non-detectable [180], suggesting that further activity studies are needed to fully understand the role of caspases and to evaluate them as prospective disease biomarkers. Inflammatory caspases have also been linked to cancer, where especially caspase-1 and inflammasome activation can lead to inflammation-driven cancer development and progression [181,182]. Another notable cysteine protease that shares structural features with caspases is legumain. It is an asparaginyl endopeptidase highly expressed in breast, prostate, gastric and ovarian cancer [71]. Beside cancer cells, it is highly expressed by tumor-associated macrophages [183] and generally correlates with poor disease prognosis [184,185].
The third group of cysteine proteases are the 14 calpains, calcium-activated proteases with important roles in remodeling of ECM, regulation of apoptosis, and different cell signaling pathways [67]. They are involved in several aspects of tumor cell invasion, metastasis formation, and cancer spread [73]. Interestingly, there is evidence for calpains roles in cancer cell death and survival with important links to signaling pathways involved in cancer development and progression [74]. They are also implicated in activation of apoptosis and could be potentially interesting targets for chemotherapy-induced apoptosis of cancer cells [186,187]. Calpains were linked to poor outcomes in breast [188], pancreatic [189] and ovarian cancer [190], among others, and they are currently considered an important drug discovery frontier [66]. While the cysteine protease pool within the tumor microenvironment generates a complex proteolytic landscape, it is likely that even closely related disease phenotypes will have a characteristic protease activity fingerprint that could support precise disease diagnosis and staging.
2.4. Aspartic Proteases
In humans, the most important aspartic proteases with links to cancer are renin, cathepsins D and E, pepsin C, and napsin A. The family is characterized by two catalytic aspartic acid residues in the active site. Renin is the essential player in the RAS (Renin-angiotensin system), which regulates the blood pressure [191]. While elevated activity of RAS can result in hypertension, there is also evidence for involvement of the system in cancer development and progression by influencing tissue remodeling, inflammation, cancer cell proliferation and apoptosis [192]. Perturbations of the RAS system are linked to pathways that are deregulated in the pre-cancer stage and contribute to malignant transformation [193]. Furthermore, the RAS can impact immunosuppression in tumors [194] and activation or inhibition of the system has been associated with different cancers [195]. These findings present an opportunity to leverage knowledge of RAS to potentially improve cancer therapies [196].
Moving to aspartate cathepsins, cathepsin D is a protease residing in lysosomes under normal physiological conditions and is instrumental for protein degradation. It has been linked to multiple cancer related processes with experimental results showing its role in tumor progression, angiogenesis and apoptosis [46]. Increased expression and secretion of cathepsin D was observed in several cancers, including malignant melanoma, prostate, ovarian and breast cancer [197]. Elevated serum levels of cathepsin D were reported in breast cancer patients [198] and cathepsin D detected in tissues could have diagnostic value for ovarian cancer [199]. While serum levels of cathepsin D have been previously investigated as a biomarker with inconclusive results, its activity patterns could be potentially used to develop early diagnostics. Another promising candidate is cathepsin E, an intracellular protease expressed mostly by immune cells and in the gastrointestinal system, important for antigen processing/presentation, apoptosis, cytokine turnover and adipose tissue regulation [72,200]. Even as early as two decades ago, high levels of cathepsin E in pancreatic juice were suggested to be indicative of adenocarcinoma of the pancreas [201]. High levels of cathepsin E are characteristic for lesions found in pancreatic adenocarcinoma and could be a valuable biomarker for early detection of pancreatic cancer [202], which is still very challenging to diagnose at an early stage.
Pepsin C belongs to the group of common digestive enzymes of the gastrointestinal system, mostly present in the stomach from gastric mucosa cells. Pepsin C is essential for normal digestive processes, and significant changes in its expression levels were detected in breast, prostate and ovarian cancer [203,204]. Nevertheless, its diagnostic applications are currently very limited. Another aspartic protease similar to pepsin is napsin A, which is important for processing surfactant B in lungs [205] and is an established biomarker used in diagnosis of lung adenocarcinoma [206]. Despite the fact that aspartic proteases received less attention than other protease families in the context of cancer, new tools for monitoring their activity could be beneficial for their stratification as clinical biomarkers.
2.5. Threonine Proteases
The most important threonine proteases linked to several aspects of cancer development and progression are the proteasomes. These are multi-protease complexes composed of several subunits that efficiently and non-selectively degrade most of the cellular proteins marked for degradation by the ubiquitin-conjugation system [207]. Proteasomes have three different types of catalytic subunits with characteristic proteolytic activities. The b1 subunit has a caspase-like activity, the b2 subunit has a trypsin-like activity and the b5 subunit has a chymotrypsin-like activity as investigated with combinatorial peptide libraries [208]. While proteasomes are mostly responsible for nonspecific protein degradation, there is substantial evidence for their involvement in cancer. Proteasome inhibition is considered an important therapeutic strategy because of its central role in regulating cell homeostasis. Generally, this inhibition leads to cancer cell apoptosis by accumulation of pro-apoptotic proteins and disruption of the NF-κβ (Nuclear factor κΒ) pathway. There are currently several proteasome inhibitors at various stages of testing in clinical trials [209]. Inhibitors like bortezomib have been approved by the FDA for treatment of multiple myeloma [210]. With ongoing efforts to develop new compounds for targeting the proteasome in cancer, there is substantial interest in gaining a better understanding of how multiple proteasome activities could be exploited in clinical settings.
3. Monitoring Protease Activity in Cancer
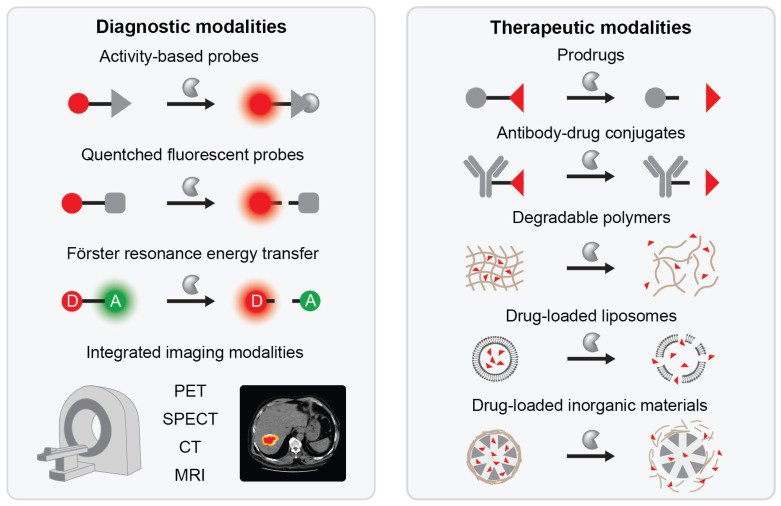
The proteolytic landscape of cancer consists of proteases representing different families, displaying different activity and patterns of localization, exhibiting varying degrees of substrate specificity, and acting upon considerably overlapping pools of natural substrates. All of this leads to nuanced pathological protease fingerprints, often characteristic for a specific disease phenotype. While this inherent complexity poses a formidable challenge for detection of proteases in the context of cancer, it also offers opportunities to harness and leverage specific pathologic protease activity as a basis for sensitive new tools for precision medicine [10] as outlined in Figure 2, left.
Figure 2.
Protease-responsive nanodevices and applications of protease-responsiveness. Protease-responsiveness can be centrally integrated into various diagnostic modalities that utilize optical, MRI, PET, SPECT or CT imaging for detection of protease activity (left). Protease-responsiveness can also serve as a trigger for activation of prodrugs and antibody-drug conjugates or on-site targeting of cancer drugs via polymeric nanoparticles, liposomes and inorganic nanoparticles (right).
3.1. Activity- and Substrate-Based Probes
Elevated protease activity can be successfully translated into activity-based probes and protease substrate reporters [211]. Both technologies aim to reveal the location and the amount of protease activity, accomplishing this via different strategies. Activity-based probes typically bear a warhead that specifically reacts with the active site of the protease and a reporter group that generates a signal upon interaction with the protease. This chemical biology approach has found broad application in visualization of cancer cells, tissues, and protease activity patterns. In 2006, Sieber et al. applied cocktails of active-site probes in combination with mass spectrometry for a comprehensive profiling of the metalloprotease family [212]. Fluorescent activity-based probes have been reported for all major protease families to date. Examples include metalloproteases [213,214,215], serine proteases [216] (e.g., neutrophil proteases [217] or inflammation-related serine proteases [218]), cysteine proteases (e.g., cathepsins [219,220], caspases [221,222] or legumain [223,224]), aspartic proteases [225], and recently also for multi-domain proteasomes [226,227]. Activity based probes have substantially improved our understanding of the role of proteases in cancer and the tumor microenvironment, as demonstrated by a cathepsin S activity-based probe applied to image tumor-associated macrophages [228]. Dual color activity-based probes were also developed to monitor the localization of cathepsin S activity to elucidate its cancer functions [229]. Activity-based probes can exactly pinpoint the protease location, but they have the disadvantage of inactivating the protease upon binding, which can not only cause unwanted perturbations in the experimental system, but also precludes the possibility of signal amplification. Another major challenge is to design selective probes that have good specificity towards the target enzyme without interference from other closely-related proteases [230].
Substrate probes exploit protease cleavage on the target site, usually to generate a fluorescent signal [231,232,233]. The majority of substrate probes are designed either as quenched substrates that fluoresce more intensely after cleavage or as FRET (Förster resonance energy transfer) probes in which cleavage causes a shift in the emission spectra that can be precisely quantified as a measure for protease activity. Both detection concepts have been successfully applied to cancer-related settings [234]. Selective substrates can be designed using substrate libraries like PS-SCL (positional scanning substrate combinatorial library) [235], CoSeSuL (counter selection substrate library) [236] or HyCoSuL (hybrid combinatorial substrate library) [237] and these strategies were successfully applied to caspases [222], cathepsins [238,239], neutrophil proteases [217] and kallikreins [240]. Recently, a HyCoSuL-based assay was used for screening protease activity in biopsies [241].
An alternative to these combinatorial approaches to design protease probes is to convert a pharmacologically optimized inhibitor to a substrate by replacing the warhead with a protease-cleavable peptide. This has been successfully demonstrated for luminescent probes for caspase-1 [242]. In general, substrate probes have the advantage that they do not inactivate the target protease and thus do not immediately influence on-site protease activity. The protease can thus cleave further substrate molecules, leading to signal amplification, an effect that is counteracted by diffusion away from the target site. One approach to address this problem has been to improve the reporter on-site retention after protease cleavage [243].
3.2. Integrating In Vivo Imaging Modalities
Synergies with the field of nanomaterials have shown great potential for the development of various protease-sensitive nanomaterials suitable for optical imaging modalities [244]. For example, QDs (quantum dots) consisting of ZnS or CdSe can be used as fluorescent reporters and these nanomaterials have a broad spectrum of application for in vivo imaging studies, including imaging of protease activity [245]. Like fluorescent molecules, QDs exhibit FRET effects, and several different FRET-based QD systems have been prepared and utilized as protease sensors for multiplexed tracking of protease activity, such as monitoring trypsin and chymotrypsin [246]. QDs exploit the FRET effect between the quantum dot and a suitable fluorescent dye bound by a protease-cleavable peptide, resulting in fluorescence emission spectrum change upon protease cleavage. This concept was used to design protease reporters for MMPs [247,248], caspase-1, collagenase, chymotrypsin and thrombin [249], uPAR [250], caspase-3 [251] and kallikrein [252]. Recently, the concept was also extended for monitoring MMP-2/-9 activity in the tumor microenvironment [253]. While these concepts can be employed for sensitive monitoring of clinically relevant proteases, they are very difficult to translate in modalities that would be applicable to clinical settings, mostly due to high costs and toxicity of materials for QD preparation. Fortunately, there are also non-toxic materials like silicon that can be used to make QDs [254], but even comparatively biocompatible QDs cannot not overcome the limitations of optical imaging.
Various classes of probes described in the previous sections have substantially advanced understanding of cancer mechanisms and can be applied in a broad range of contexts, from labeling cell lysates, intact cells to ex vivo, and in vivo imaging of small animals [255]. Nevertheless, a broad translation of fluorescent probes into clinical modalities has not yet occurred, despite the fact that NIR (near-infrared) fluorescent protease reporters have shown great potential for more than a decade [256]. Image-guided surgery with NIR fluorescent probes is an emerging application that seems especially well-poised for clinical translation. For example, a cathepsin-sensitive poly(L-glutamic acid)-based quenched fluorescent probe, Prosense® 680, which is commercially available from Perkin Elmer, was successfully used for detection of tumor margins in image guided surgery [257]. In a recent report, another cathepsin-sensitive quenched fluorescence activity-based probe designed for intravenous application was used to visualize surgical margins of the tumor and thus increase the probability of its complete removal [258]. Besides image-guided surgery, the NIR reporters have potential to translate into diagnostic assays as demonstrated by FAP and PREP [259]. A NIR FRET-based probe LUM015 sensitive for cysteine cathepsins has been tested in a mouse-human phase I co-clinical trial, offering the first promising human data for this technique [260].
Unfortunately, the use of fluorescent probes for deep tissue imaging is inherently precluded by the limitations of fluorescence as a readout. Nevertheless, the concepts developed for selective protease detection with fluorescent reporters are transferrable to other detection modalities [261]. Here, PET (positron emission tomography), SPECT (single-photon emission computed tomography), CT (computed tomography) and MRI (magnetic resonance imaging) have proven to be valuable technologies for noninvasively tracing protease activity. In PET and SPECT, a typical protease tracer consists of a radioactive isotope like 11C and 18F for PET or 123I and 131I for SPECT and CT. These tracers require not only excellent selectivity towards the target protease, but also good clearance properties to avoid background interference [262]. The half-lives of isotopes used in PET or SPECT tracers are usually short, posing a unique barrier to commonplace application of these modalities for protease imaging. Nevertheless, the technology was successfully demonstrated for imaging metalloproteases [263,264,265], cysteine cathepsins [266,267,268] and caspases [269,270] in preclinical settings. In CT imaging modalities, X-rays are used to create cross-sectional images with high resolution and this technique is widely used for noninvasive clinical imaging [271]. Contrast agents are an integral part of any CT imaging, but development of protease-sensitive molecules for CT applications has proven challenging. Recently, cancer imaging studies for CT imaging of multiple cysteine cathepsins were performed using a protease-targeted iodinated probe [272] or activity probes based on gold nanoparticles [273].
Another technology applicable to in vivo imaging of proteases is MRI, which detects certain nuclei or protons and is sensitive to interactions with their surrounding molecular context. The influence of molecular context on relaxation times offers a basis for proteolytic activity to be coupled to detectable changes in contrast, producing an informative readout. Paramagnetic Gd3+ compounds are conventionally used as contrast agents, and MRI probes have been developed to visualize protease activity. For example, a Gd-DOTA (Tetraazacyclododecane tetraacetic acid) probe was conjugated with a peptide cleavable by MMP-2, leading to solubility changes and allowing MMP-2 activity to be imaged in tumors [274]. Also, a Gd-based caspase drug was used to monitor caspase activity after drug-induced apoptosis [275]. Examples also extend into MRI modalities employing specialized contrast agents, including 19F MRI, in which Gd acts as a quencher [276], and Overhauser-enhanced MRI, which essentially combines MRI with electron paramagnetic resonance to reveal the cleavage of nitroxide-labeled macromolecules [277]. MRI protease-sensitive imaging is clearly a subject rich with possibilities and potential for further expansion, though it remains to be seen which modalities and contrast agents will ultimately be most translatable. Synergies with nanotechnology are one promising area, best typified by a cleavage-responsive nanosensor based on Gd complexes bound to magnetic nanoparticles, revealing MMP-2 activity in a rodent tumor model [278]. Protease-cleavable PLG-based MRI probes (poly-L-glutamate) have also been used in a rodent tumor model, mapping cysteine cathepsin expression [279]. A smart, self-assembling contrast agent developed for furin was also used to detect the protease in cell cancer models [280].
Additional advantages have been realized by combining multiple detection modalities simultaneously, resulting in probes that achieve better resolution and more sensitive detection of the target proteases. This is perhaps best exemplified by dual modality probes designed for several members of the MMP family. For example, NIR/PET probes sensitive for MMP-2, -9 and -13 were used for detection of tumors in a mouse cancer model [281], a FRET/SPECT probe designed for MMP-2 and was used for in vivo imaging of metastatic lymph nodes [282] and a FRET/MRI probe was developed for MMP-2 and bimodal imaging of gastric tumors [283]. There are several other examples of probes with protease-cleavable peptides enabling fluorescent and MRI imaging of proteases [284]. While there is still a dearth of technologies that could be applied for routine, cost-efficient imaging of protease activity in deep tissues, there have also been recent developments in the field of biosensors for acoustic enzyme detection [285].
3.3. New Developments and Trends
The methodologies described above can produce high-resolution data of spatiotemporally-resolved protease activity, but the technologies are technically demanding and cost-intensive, often precluding their routine use. Clearly, the continued development of alternative protease detection technologies is needed. Antibodies, one example of an alternative to activity-based probes and other contrast agents, can achieve excellent specificity towards the target protease or even the components of an activity-based probe used for labeling the target protease [286]. Besides antibodies, DARPins (Designed Ankyrin Repeat Proteins) are a useful alternative because of their superior stability and equal or better affinity towards the target [287]. Recently, a highly selective fluorescently-labeled DARPin for cathepsin B was used for in vivo imaging of the protease in cell and animal models of breast cancer [288]. DARPins could include other reporters and thus expand the spectrum of imaging applications.
A concept that has recently shown great potential for translation in clinical settings and is building on the disease-related activity of proteases are the synthetic biomarkers. While classic activity-based tools usually generate a direct readout for one target protease or a very narrow group of closely-related proteases, synthetic biomarkers have the capacity to be applied in multiplexed setups [289]. These nanosensors employ protease-cleavable tagged peptides conjugated to nanoparticles that are cleaved by different disease proteases at the target site (e.g., in tumors), liberating reporters that can be detected in urine by mass spectrometry. Kwong and co-workers demonstrated that detection of urinary biomarkers can outperform the standard CEA (carcinoembryonic antigen) detection in plasma for early diagnosis of colorectal cancer [289]. Also, magnetically actuated protease sensors (MAPS) have been developed. In this assay, magnetic nanoparticles and peptide substrates with fluorescent reporters and biotin affinity tags were co-encapsulated in thermosensitive liposomes, enabling the targeted application of an alternating magnetic field to initiate selective cargo release. Protease activity was then measured by cleaved reporter peptides excreted in the urine, reflecting MMP tumor profiles in colorectal cancer [290]. Knowledge of disease-related protease activity can be integrated into the design of nanosensor libraries that measure protease activity and these ABNs (activity-based nanosensors) have great potential to reveal disease-associated protease activity, as demonstrated by detection of MMP-9, a protease commonly upregulated in human cancers [291]. The concept of synthetic biomarkers is gaining momentum in cancer diagnostic applications and MMP-responsive nanosensors based on AuNC (Ultra small gold nanoclusters) have shown promising results in colorectal mouse cancer models. The AuNC can be renally excreted after cleavage and enable catalytically amplified readouts [292]. Importantly, similar protease nanosensors designed in a bottom-up approach for prostate cancer detection could outperform the PSA prostate cancer marker [293] and a multiplexed substrate panel for lung cancer proteases has demonstrated excellent specificity and sensitivity for disease detection [294]. These encouraging results suggest that the development of future protease activity sensors will be based on synergies with the fields of nanotechnology and nanomedicine. With the development of synthetic biomarkers, it is also essential to develop frameworks to analyze this type of data to extract the maximal amount of meaningful information and aid the application of such diagnostic platforms [295].
Finally, promising results are coming from synergies with microfluidics to enable rapid, multiplexed detection of activity patterns of various proteases with minimum sample requirements. Chen et al. [296] developed a protease assay that uses a picoliter-scale droplet microfluidic platform, utilizing a mixture of inhibitors and FRET substrates and PrAMA (Proteolytic Activity Matrix Analysis) analysis to calculate protease activities [297], enabling simultaneous monitoring of multiple proteases. To further boost the performance and decrease the material requirements, a lab-on-a-chip assay was developed for simultaneous monitoring of multiple MMPs and ADAMs in a breast cancer cell line [298]. While integration of protease-responsiveness into lab-on-a-chip modalities is still limited, such multiplexed and highly parallel platforms could potentially expedite the diagnostic profiling of cancer-related proteases.
4. Leveraging Protease Activity in Drug Delivery for Cancer Therapy
One of the key challenges in cancer treatment with potent drugs is to ensure that their influence is focused on the tumor while minimizing off target effects. Drug delivery paradigms have been designed for selective activation of therapeutic agents based on physical and chemical cues from the tumor microenvironment that differ from elsewhere in the body, such as pH or redox potential [299]. Pathologic activity of proteases represents another biochemical feature of the tumor microenvironment that can be leveraged for this purpose. A growing number of protease-sensitive nanosystems and nanomaterials utilize disease-associated protease activity for their activation, selective targeting, or on-site release of drug payloads [300,301]. These strategies (Figure 2, right) serve to enhance specificity, improve targeting, decrease off-target effects, and increase the therapeutic index of a drug, thus enhancing its efficacy. In this section, therapeutic modalities that centrally integrate protease responsiveness are reviewed.
4.1. Prodrugs
The first group are the protease-activated prodrugs that use protease cleavage to release or activate the drug on-site. They integrate knowledge of disease-specific proteases to incorporate a specific cleavable sequence that ensures release dependent on the activity of a specific target protease. Leucine-doxorubicine prodrug [302] offers perhaps the simplest example to demonstrate the utility of the concept, and showed promising results in tumor models. In this case, a short peptide attached to the drug needs to be cleaved by the target protease for the drug to be activated. While this prodrug is rather unspecific, there are other examples that activate in response to more specific protease cleavage. These include the legumain-sensitive Ala-Ala-Asn linker [303], cathepsin B sensitive linker Val-Cit [304], and several other prodrugs requiring different MMPs, kallikreins, cathepsins, and coagulation proteases, among others [305].
4.2. Antibody Drugs
Antibody-drug conjugates constitute another group of therapeutics that rely on proteolysis in the tumor. In this case, the antibody recognizes a cancer cell-specific molecular feature (e.g., cell surface receptor) and is eventually internalized into the target cell [306,307]. The conjugate is subsequently processed by lysosomal proteases and released from the lysosome, destroying the target cells. There are currently two antibody-drug conjugates activated by cleavage of the Val-Cit linker on the market, ADCERTIS [308] and Polivy [309], both of which are approved for cancer treatment. Presently, there is considerable interest in leveraging this concept to develop cleavable linkers for other proteases to improve the serum stability and on-site release of drugs. While the use of antibodies is beneficial for achieving targeting and specificity, antibodies can also have unwanted effects and further development is needed to improve selectivity toward targeted cells [310]. Protease cleavage-activation can be also integrated into therapeutic antibodies to help overcome this challenge. This concept was recently incorporated in Ab prodrugs (ProbodiesTM) by CytomX Therapeutics [311]. Their probody targets the EGF receptor highly expressed on the surface of cancer cells, while incorporating a protease-cleavable peptide into cetuximab to boost selectivity. This peptide needs to be cleaved by legumain, uPA, or matriptase to activate the probody and has shown promising preclinical results [312]. Of note, application of ProbodiesTM to preclinical imaging is generally simple and could be applied in multiplexed settings, thus potentially aiding the evaluation of different proteases as prospective cancer biomarkers.
4.3. Polymers
Responsiveness to disease-related proteases can also be incorporated into polymer architectures, including the biodegradable and biocompatible polymer-based nanoparticles that have been investigated over the last decade as drug delivery vehicles [313]. Targeting of polymeric nanoparticles to tumors has been thought to rely on the EPR (Enhanced permeability and retention) effect, exploiting the leaky vasculature and poor lymphatic drainage [314], however the practical relevance of this effect in determining the fate of injected nanomaterials has been increasingly called into question [315]. Only a tiny fraction of an injected dose of nanomaterial reaches tumors, with one recent metaanalysis suggesting a median delivery efficiency of 0.7% of the initial dose [316]. This underscores the importance of strategies for site-specific release or activation in tumors for improving selectivity.
Polymers were integrated into various therapeutics to date [317]. One of the most widely used polymers in nanoscale therapeutic architectures for cancer is PEG (Poly(ethylene glycol)) [318], favored mostly due to its solubility and biodegradability, though the occurrence of anti-PEG antibodies in patients is an emerging issue that may eventually cause PEG to be supplanted by similar alternatives [319]. For an example of a PEG-based protease-responsive nanotherapeutic, PEG-functionalized QDs clusters sensitive to MMP-2 were developed to enable multistage penetration into tumor tissue [320], with their initial 100 nm size promoting accumulation in the tumor, followed by cleavage into 10 nm particles that could more readily diffuse in the tumor. Similarly, utilizing cancer-associated MMP-2 activity, a PEG 2000-paclitaxel conjugate showed improved tumor targeting characteristics over standard paclitaxel [321]. Another interesting example includes SELPs (Silk-elastin-like protein polymers). Since several MMPs exhibit strong elastase activity, these polymers could serve as specific MMP-responsive delivery agents for tumor targeted drug delivery [322]. Also poly(L-glutamic acid) conjugated with paclitaxel enabled a cathepsin B-dependent drug release, showing promising results in preclinical ovarian cancer models [323]. Similar conjugates were developed for thrombin [324] and trypsin [325]. A further polymer successfully used for preparation of polymer-drug conjugates with protease-responsiveness is HPMA (N-(2-hydroxypropyl)methacrylamide copolymer) [326]. In this case, the conjugate incorporated a PSA-cleavable peptide to release thapsigargin, a natural cytotoxin, and the system showed promising results in a prostate cancer model. Like the polymer drug delivery vehicles, the serum protein albumin has been successfully used as a macromolecular carrier in the preparation of albumin-drug conjugates. Several conjugates with cancer therapeutics and cleavable protease linkers were developed for cathepsin B [327] and PSA [328] among others. These efforts corroborate protease-sensitive macromolecular drug conjugates as a viable strategy for improved on-site drug targeting.
4.4. Liposomes
Liposomes are yet another type of delivery system with oncologic applications. Their advantages include a size range suitable for accumulation in tumors and surface chemistry readily adaptable to various functionalities [329]. In a common approach, the drug (e.g., cytotoxic compound) is encapsulated in liposomes functionalized with protease-sensitive ligands. These protease-responsive liposomes have been integrated with cancer gene therapy to boost the efficiency of cancer cell transduction [330]. One such design relied on a PEGylated MMP-cleavable peptide serving as a steric hindrance to regulate liposome cell entry. Highly-targeted liposomes can be also designed by combining the advantages of multifunctional liposomes and protease-sensitive polymers to generate polymer-caged liposomes [331]. Here, a graft copolymer of poly(acrylic acid) containing a peptide with a cleavage site for the cancer protease uPA was used to crosslink liposomes. These liposomes showed excellent stability and efficient cargo release upon uPA cleavage, making them a promising cancer targeting modality. Recently, another version of liposomes using PEGylated MMP-cleavable lipopeptide was reportedly used to improve the cytotoxicity of anticancer drugs [332]. Liposomes can be also coated with cell-penetrating TAT (Transactivator of transcription) peptides that are modified with protease cleavage sequences, as demonstrated by a legumain-activated liposome for tumor targeting [333]. In this case, the tumor-expressed legumain cleavage ensures improved efficiency in the delivery of doxorubicin to tumors. A further strategy to generate protease-responsive liposomes is the destabilization of liposome integrity with ”uncorking” that is achieved by integrating MMP-9-sensitive triple helical lipopeptides that result in cargo release upon cleavage [334]. Additionally, a newly emerging concept in the liposome field is the use of proteases not directly as activators, but rather as targeting moieties. For example, a liposome drug delivery system was reported where the liposomes were coated with a selective cathepsin B inhibitor [335]. While cathepsin B resides in lysosomes under normal physiological conditions, it is highly expressed on the surface of cancer cells and can be used for tumor targeting.
4.5. Inorganic Nanomaterials
Finally, systems based on inorganic nanomaterials that incorporate responsiveness to proteases for therapeutic effects have also seen recent advancements. Frequently, this includes various silica and iron oxide nanoparticles that can be integrated with protease-responsive elements to improve cancer targeting. Such approaches have already found application in the field of protease therapeutics and have already been extensively reviewed elsewhere [244,336,337,338]. As a representative example, in one study, mesoporous silica nanoparticles were packed with doxorubicin and coated with gelatin to prevent drug leakage. After accumulating in tumors, elevated activity of MMP-2 broke down the gelatin layer, releasing the drug cargo [339]. Nanomaterials may offer additional advantages if they can be developed into theranostic agents, which simultaneously integrate dual modalities as diagnostic reporters and targeted drug delivery systems [244]. One such example is the use of iron oxide nanoparticles prepared by conjugating ferumoxytol to an MMP-activatable peptide linked with azademethylcolchicine [340]. This theranostic system efficiently induced apoptosis of cancer cells in a breast cancer model, and also enabled precise monitoring of its biodistribution via T2 contrast. Although current work tends to emphasize ultrasmall iron oxide nanocrystals that act as T1 contrast agents [341], this example is broadly illustrative of the role that protease responsiveness can play in integrating multiple modalities into a single theranostic agent.
5. Conclusions and Outlook
Exploration of the protease landscape associated with cancer has enabled the emergence of promising clinical technologies exploiting pathologic patterns of proteolytic activity for diagnosis and therapy (Table 2). Engineering toolsets for incorporating protease responsiveness are more readily available than ever, with the potential to be integrated into nanosystems that empower the next generation of protease-based diagnostic and therapeutic modalities [342]. Protease research has already greatly benefited from interdisciplinary connections of molecular biology and biomedicine with nanotechnology, polymer chemistry, and microfluidics. These efforts strive toward a shared ideal of technologies that are sensitive, robust, and able to measure protease activity in real time, yet simultaneously cost-efficient, easily multiplexed, and practical to use at the point-of-care. We are convinced that the field of oncology will advance alongside the development of the next generation of protease sensors, protease-responsive nanomaterials, and lab-on-a-chip applications. While several of these technologies have already shown promising preclinical results for cancer diagnosis, disease staging, therapy stratification, and evaluation of therapeutic response, future developments are poised to unleash the full potential of proteases for oncologic applications.
Table 2.
Protease-responsive nanodevices for diagnostic and therapeutic purposes, application notes (describing advantages and shortcomings of the indicated modalities) and selected examples of use.
| Protease-Responsive Nanodevices | Application Notes (+ Advantages, − Shortcomings) |
Selected Examples and References |
|---|---|---|
| Activity-based probes | + Sensitive detection of proteases in situ in cells and animal models + Excellent selectivity for target proteases − No signal amplification |
Metalloproteases [213,214,215], serine proteases [216] including neutrophil proteases [217] and inflammation-related serine proteases [218], cysteine cathepsins [219,220], caspases [221,222] and legumain [223,224], aspartic proteases [225], and proteasome [226,227] |
| Protease-cleavable fluorescent substrate probes | + Signal amplification + Selectivity can be improved by designs that incorporate unnatural amino acids − Background fluorescence and signal diffusion |
Profiling of caspases [222], cathepsins [238,239], neutrophil proteases [217] and kallikreins [240] |
| QDs | + Sensitivity of integrated FRET + Versatile platform − Toxicity of nanoparticles |
Imaging and detection MMPs [247,248], caspase-1, collagenase, chymotrypsin and thrombin [249], uPAR [250], caspase-3 [251] and kallikrein [252] |
| PET and SPECT probes | + High sensitivity and resolution − Short half-life of reagents because of radioactive isotopes − Costly detection modalities |
Protease-responsive contrast agents for metalloproteases [263,264,265], cysteine cathepsins [266,267,268] and caspases [269,270] |
| MRI probes | + High sensitivity and resolution for soft tissues − Expensive detection modality |
MMP-2 in tumors [274], caspase activity after drug-induced apoptosis [275], caspase-3 [276], digestive elastases [277], cysteine cathepsins [279] and furin [280] |
| CT probes | + Resolution for in-depth tissue imaging − Lack of suitable protease-sensitive probes |
Protease-targeted iodinated probe [272] and protease activity probes with gold nanoparticles [273] were for cysteine cathepsins |
| Dual modality probes | + Improved spatiotemporal resolution and sensitivity − Expensive and do not overcome the problems of original modalities |
NIR/PET probes sensitive for MMP-2, MMP-9, and MMP-13 [281], FRET/SPECT probe for MMP-2 [282] and a FRET/MRI probe for MMP-2 [283] |
| DARPins | + Selectivity for target protease + Can be integrated with other modalities − Intensive development and selection process |
Imaging of cathepsin B in breast cancer [288] |
| Synthetic biomarkers | + Sensitive in situ detection of protease activity + High multiplexing capabilities − Not best-suited for on-site monitoring (i.e., imaging) |
Colorectal cancer biomarker detection in plasma [289], magnetically actuated protease sensors (MAPS) for measuring MMP tumor profiles in colorectal cancer [290], activity-based nanosensors (ABNs) for MMP-9 [291], Ultra small gold nanoclusters (AuNC) as MMP-responsive nanosensors [292], prostate cancer nanosensors [293] and lung cancer nanosensors [294] |
| Prodrugs with protease-cleavable linkers | + Protease-dependent on-site activation − Off-site drug release due to unspecific protease cleavage |
Legumain-sensitive Ala-Ala-Asn linker [303], cathepsin B-sensitive linker Val-Cit [304] and prodrugs that require metalloproteases, kallikreins, cathepsins or coagulation proteases [305] |
| Ab-drug conjugates | + Improved on-site targeting of the drug − Problems with selective cancer cell recognition |
ADCERTIS [308] and Polivy [309] |
| Probody | + Protease-dependent on-site activation of the Ab − Non-selective peptide linker cleavage |
EGFR targeting probody activated by legumain, uPA or matriptase cleavage from CytomX Therapeutics [311] |
| Polymers | + Favorable biologic properties of polymers + Accumulation on target site due to EPR effect + Selective on-site release of polymer-bound drug by the target protease − Prolonged retention and off-site accumulation − Non-selective protease degradation of the polymers − Anti-polymer antibodies arise in patients |
PEG-functionalized MMP-2-sensitive QDs clusters [320], MMP-2-sensitive PEG 2000-paclitaxel conjugate [321], SELPs (Silk-elastin-like protein polymers) polymers as MMP-responsive delivery agents [322], cathepsin B sensitive poly(L-glutamic acid)-paclitaxel conjugate [323] as well thrombin-sensitive [324] and trypsin-sensitive [325] conjugates, a PSA-sensitive HPMA-thapsigargin conjugate [326] |
| Liposomes | + Small size and favorable biological properties + Can be integrated with various other modalities − Accelerated clearance from cardiovascular system − Potential for allergic reactions |
PEGylated MMP-sensitive lyposome [330], polymer-caged uPA-sensitive liposomes [331], PEGylated liposome with a MMP-cleavable lipopeptide [332], TAT peptide bearing legumain-activated liposome [333], a MMP-9-sensitive ‘uncorking’ liposome [334], a cathepsin B targeting liposome [335] |
| Inorganic materials | + Biocompatible materials with various shapes and sizes + Can be integrated into theranostic agents − Nanoparticle toxicity or off-site accumulation |
MMP-responsive silica [339] and iron oxide nanoparticles [340] for targeted drug release |
Author Contributions
S.S. and M.V. conceptualized the paper. M.V., D.R. and S.M. wrote and M.G.C. and S.S. revised the paper. D.R., S.M. and M.G.C. prepared the figures. All authors edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Branco Weiss Fellowship-Society in Science (title: “Cancer-fighting magnetic biobots: Harnessing the power of synthetic biology and magnetism”).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turk B., Turk D., Turk V. Protease signalling: The cutting edge. EMBO J. 2012;31:1630–1643. doi: 10.1038/emboj.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond J.S. Proteases: History, discovery, and roles in health and disease. J. Biol. Chem. 2019;294:1643–1651. doi: 10.1074/jbc.TM118.004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawlings N.D., Barrett A.J., Thomas P.D., Huang X., Bateman A., Finn R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46:D624–D632. doi: 10.1093/nar/gkx1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlings N.D., Waller M., Barrett A.J., Bateman A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2014;42:D503–D509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawlings N.D. Peptidase specificity from the substrate cleavage collection in the MEROPS database and a tool to measure cleavage site conservation. Biochimie. 2016;122:5–30. doi: 10.1016/j.biochi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath H. Proteolytic enzymes, past and future. Proc. Natl. Acad. Sci. USA. 1999;96:10962–10963. doi: 10.1073/pnas.96.20.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drag M., Salvesen G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craik C.S., Page M.J., Madison E.L. Proteases as therapeutics. Biochem. J. 2011;435:1–16. doi: 10.1042/BJ20100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eatemadi A., Aiyelabegan H.T., Negahdari B., Mazlomi M.A., Daraee H., Daraee N., Eatemadi R., Sadroddiny E. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed. Pharmacother. 2017;86:221–231. doi: 10.1016/j.biopha.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Dudani J.S., Warren A.D., Bhatia S.N. Harnessing Protease Activity to Improve Cancer Care. Annu. Rev. Cancer Biol. 2018;2:353–376. doi: 10.1146/annurev-cancerbio-030617-050549. [DOI] [Google Scholar]
- 11.Mohan V., Das A., Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 2020;62:192–200. doi: 10.1016/j.semcancer.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75–76:1–11. doi: 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler J., Abisoye-Ogunniyan A., Metcalf K.J., Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitschke J., Burk U.C., Reinheckel T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev. 2019;38:431–444. doi: 10.1007/s10555-019-09808-2. [DOI] [PubMed] [Google Scholar]
- 15.Muenst S., Laubli H., Soysal S.D., Zippelius A., Tzankov A., Hoeller S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern. Med. 2016;279:541–562. doi: 10.1111/joim.12470. [DOI] [PubMed] [Google Scholar]
- 16.Olsson M., Zhivotovsky B. Caspases and cancer. Cell Death Differ. 2011;18:1441–1449. doi: 10.1038/cdd.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevenich L., Joyce J.A. Pericellular proteolysis in cancer. Genes Dev. 2014;28:2331–2347. doi: 10.1101/gad.250647.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandya P., Orgaz J.L., Sanz-Moreno V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017;11:5–27. doi: 10.1002/1878-0261.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Kessenbrock K., Wang C.Y., Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;44–46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Otin C., Hunter T. The regulatory crosstalk between kinases and proteases in cancer. Nat. Rev. Cancer. 2010;10:278–292. doi: 10.1038/nrc2823. [DOI] [PubMed] [Google Scholar]
- 24.Mason S.D., Joyce J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vizovisek M., Vidmar R., Drag M., Fonovic M., Salvesen G.S., Turk B. Protease Specificity: Towards In Vivo Imaging Applications and Biomarker Discovery. Trends Biochem. Sci. 2018;43:829–844. doi: 10.1016/j.tibs.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Edgington L.E., Verdoes M., Bogyo M. Functional imaging of proteases: Recent advances in the design and application of substrate-based and activity-based probes. Curr. Opin. Chem. Biol. 2011;15:798–805. doi: 10.1016/j.cbpa.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Withana N.P., Garland M., Verdoes M., Ofori L.O., Segal E., Bogyo M. Labeling of active proteases in fresh-frozen tissues by topical application of quenched activity-based probes. Nat. Protoc. 2016;11:184–191. doi: 10.1038/nprot.2016.004. [DOI] [PubMed] [Google Scholar]
- 28.Poreba M. Protease-activated prodrugs: Strategies, challenges, and future directions. FEBS J. 2020;287:1936–1969. doi: 10.1111/febs.15227. [DOI] [PubMed] [Google Scholar]
- 29.Vandooren J., Opdenakker G., Loadman P.M., Edwards D.R. Proteases in cancer drug delivery. Adv. Drug Deliv. Rev. 2016;97:144–155. doi: 10.1016/j.addr.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D., Coussens L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig T. Local proteolytic activity in tumor cell invasion and metastasis. Bioessays. 2005;27:1181–1191. doi: 10.1002/bies.20306. [DOI] [PubMed] [Google Scholar]
- 33.Alaseem A., Alhazzani K., Dondapati P., Alobid S., Bishayee A., Rathinavelu A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019;56:100–115. doi: 10.1016/j.semcancer.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Klein T., Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards D.R., Handsley M.M., Pennington C.J. The ADAM metalloproteinases. Mol. Asp. Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullooly M., McGowan P.M., Crown J., Duffy M.J. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol. Ther. 2016;17:870–880. doi: 10.1080/15384047.2016.1177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson O.C., Joyce J.A. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat. Rev. Cancer. 2015;15:712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 38.Filippou P.S., Karagiannis G.S., Musrap N., Diamandis E.P. Kallikrein-related peptidases (KLKs) and the hallmarks of cancer. Crit. Rev. Clin. Lab. Sci. 2016;53:277–291. doi: 10.3109/10408363.2016.1154643. [DOI] [PubMed] [Google Scholar]
- 39.Kontos C.K., Scorilas A. Kallikrein-related peptidases (KLKs): A gene family of novel cancer biomarkers. Clin. Chem. Lab. Med. 2012;50:1877–1891. doi: 10.1515/cclm-2012-0247. [DOI] [PubMed] [Google Scholar]
- 40.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rousalova I., Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation (review) Int. J. Oncol. 2010;37:1361–1378. doi: 10.3892/ijo_00000788. [DOI] [PubMed] [Google Scholar]
- 42.Cullen S.P., Brunet M., Martin S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 43.Zurawa-Janicka D., Skorko-Glonek J., Lipinska B. HtrA proteins as targets in therapy of cancer and other diseases. Expert Opin. Ther. Targets. 2010;14:665–679. doi: 10.1517/14728222.2010.487867. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad R.M., Muqbil I., Lowe L., Yedjou C., Hsu H.Y., Lin L.T., Siegelin M.D., Fimognari C., Kumar N.B., Dou Q.P., et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015;35:S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boice A., Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118688. doi: 10.1016/j.bbamcr.2020.118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berchem G., Glondu M., Gleizes M., Brouillet J.P., Vignon F., Garcia M., Liaudet-Coopman E. Cathepsin-D affects multiple tumor progression steps in vivo: Proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5955. doi: 10.1038/sj.onc.1205745. [DOI] [PubMed] [Google Scholar]
- 47.Weber S., Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- 48.Huovila A.P., Turner A.J., Pelto-Huikko M., Karkkainen I., Ortiz R.M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Rocks N., Paulissen G., Quesada-Calvo F., Munaut C., Gonzalez M.L., Gueders M., Hacha J., Gilles C., Foidart J.M., Noel A., et al. ADAMTS-1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivo. Cancer Res. 2008;68:9541–9550. doi: 10.1158/0008-5472.CAN-08-0548. [DOI] [PubMed] [Google Scholar]
- 50.Demircan K., Gunduz E., Gunduz M., Beder L.B., Hirohata S., Nagatsuka H., Cengiz B., Cilek M.Z., Yamanaka N., Shimizu K., et al. Increased mRNA expression of ADAMTS metalloproteinases in metastatic foci of head and neck cancer. Head Neck. 2009;31:793–801. doi: 10.1002/hed.21045. [DOI] [PubMed] [Google Scholar]
- 51.Filou S., Korpetinou A., Kyriakopoulou D., Bounias D., Stavropoulos M., Ravazoula P., Papachristou D.J., Theocharis A.D., Vynios D.H. ADAMTS expression in colorectal cancer. PLoS ONE. 2015;10:e0121209. doi: 10.1371/journal.pone.0121209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pampalakis G., Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim. Biophys. Acta. 2007;1776:22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Sotiropoulou G., Pampalakis G. Kallikrein-related peptidases: Bridges between immune functions and extracellular matrix degradation. Biol. Chem. 2010;391:321–331. doi: 10.1515/bc.2010.036. [DOI] [PubMed] [Google Scholar]
- 54.Kramer L., Turk D., Turk B. The Future of Cysteine Cathepsins in Disease Management. Trends Pharmacol. Sci. 2017;38:873–898. doi: 10.1016/j.tips.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Vidak E., Javorsek U., Vizovisek M., Turk B. Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment. Cells. 2019;8:264. doi: 10.3390/cells8030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jablonska-Trypuc A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 57.Fanjul-Fernandez M., Folgueras A.R., Cabrera S., Lopez-Otin C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Fonovic M., Turk B. Cysteine cathepsins and their potential in clinical therapy and biomarker discovery. Proteom. Clin. Appl. 2014;8:416–426. doi: 10.1002/prca.201300085. [DOI] [PubMed] [Google Scholar]
- 59.Vizovisek M., Fonovic M., Turk B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix Biol. 2019;75–76:141–159. doi: 10.1016/j.matbio.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 60.Shahinian H., Loessner D., Biniossek M.L., Kizhakkedathu J.N., Clements J.A., Magdolen V., Schilling O. Secretome and degradome profiling shows that Kallikrein-related peptidases 4, 5, 6, and 7 induce TGFbeta-1 signaling in ovarian cancer cells. Mol. Oncol. 2014;8:68–82. doi: 10.1016/j.molonc.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turunen S.P., Tatti-Bugaeva O., Lehti K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1974–1988. doi: 10.1016/j.bbamcr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Radisky E.S., Radisky D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland Biol. Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintero-Fabian S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argaez V., Lara-Riegos J., Ramirez-Camacho M.A., Alvarez-Sanchez M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moradi A., Srinivasan S., Clements J., Batra J. Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019;38:333–346. doi: 10.1007/s10555-019-09815-3. [DOI] [PubMed] [Google Scholar]
- 65.Turk V., Stoka V., Vasiljeva O., Renko M., Sun T., Turk B., Turk D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta. 2012;1824:68–88. doi: 10.1016/j.bbapap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ono Y., Saido T.C., Sorimachi H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016;15:854–876. doi: 10.1038/nrd.2016.212. [DOI] [PubMed] [Google Scholar]
- 67.Storr S.J., Carragher N.O., Frame M.C., Parr T., Martin S.G. The calpain system and cancer. Nat. Rev. Cancer. 2011;11:364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- 68.Yui S., Osawa Y., Ichisugi T., Morimoto-Kamata R. Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism. Mediat. Inflamm. 2014;2014:971409. doi: 10.1155/2014/971409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morimoto-Kamata R., Yui S. Insulin-like growth factor-1 signaling is responsible for cathepsin G-induced aggregation of breast cancer MCF-7 cells. Cancer Sci. 2017;108:1574–1583. doi: 10.1111/cas.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu D.M., Yao T.W., Chowdhury S., Nadvi N.A., Osborne B., Church W.B., McCaughan G.W., Gorrell M.D. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010;277:1126–1144. doi: 10.1111/j.1742-4658.2009.07526.x. [DOI] [PubMed] [Google Scholar]
- 71.Dall E., Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. doi: 10.1016/j.biochi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 72.Zaidi N., Hermann C., Herrmann T., Kalbacher H. Emerging functional roles of cathepsin E. Biochem. Biophys. Res. Commun. 2008;377:327–330. doi: 10.1016/j.bbrc.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 73.Chen B., Tang J., Guo Y.S., Li Y., Chen Z.N., Jiang J.L. Calpains are required for invasive and metastatic potentials of human HCC cells. Cell Biol. Int. 2013;37:643–652. doi: 10.1002/cbin.10062. [DOI] [PubMed] [Google Scholar]
- 74.Chen J., Wu Y., Zhang L., Fang X., Hu X. Evidence for calpains in cancer metastasis. J. Cell Physiol. 2019;234:8233–8240. doi: 10.1002/jcp.27649. [DOI] [PubMed] [Google Scholar]
- 75.Jackson H.W., Defamie V., Waterhouse P., Khokha R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer. 2017;17:38–53. doi: 10.1038/nrc.2016.115. [DOI] [PubMed] [Google Scholar]
- 76.Brew K., Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. BBA–Mol. Cell Res. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 78.Fields G.B. Interstitial Collagen Catabolism. J. Biol. Chem. 2013;288:8785–8793. doi: 10.1074/jbc.R113.451211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houghton A.M., Mouded M., Shapiro S.D. Consequences of Elastolysis. Extracell. Matrix Degrad. 2011:217–249. doi: 10.1007/978-3-642-16861-1_9. [DOI] [Google Scholar]
- 80.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 81.Manon-Jensen T., Multhaupt H.A., Couchman J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280:2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- 82.Symowicz J., Adley B.P., Gleason K.J., Johnson J.J., Ghosh S., Fishman D.A., Hudson L.G., Stack M.S. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007;67:2030–2039. doi: 10.1158/0008-5472.CAN-06-2808. [DOI] [PubMed] [Google Scholar]
- 83.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 84.Saunders W.B., Bayless K.J., Davis G.E. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J. Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 85.Overall C.M., Dean R.A. Degradomics: Systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- 86.Tan X., Egami H., Abe M., Nozawa F., Hirota M., Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. J. Clin. Pathol. 2005;58:1242–1248. doi: 10.1136/jcp.2004.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasala K., Turpeenniemi-Hujanen T. Serum tissue inhibitor of metalloproteinase-2 (TIMP-2) and matrix metalloproteinase-2 in complex with the inhibitor (MMP-2:TIMP-2) as prognostic markers in bladder cancer. Clin. Biochem. 2007;40:640–644. doi: 10.1016/j.clinbiochem.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Szarvas T., Jager T., Becker M., Tschirdewahn S., Niedworok C., Kovalszky I., Rubben H., Ergun S., vom Dorp F. Validation of circulating MMP-7 level as an independent prognostic marker of poor survival in urinary bladder cancer. Pathol. Oncol. Res. 2011;17:325–332. doi: 10.1007/s12253-010-9320-4. [DOI] [PubMed] [Google Scholar]
- 89.Kasurinen A., Tervahartiala T., Laitinen A., Kokkola A., Sorsa T., Bockelman C., Haglund C. High serum MMP-14 predicts worse survival in gastric cancer. PLoS ONE. 2018;13:e0208800. doi: 10.1371/journal.pone.0208800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laitinen A., Hagstrom J., Mustonen H., Kokkola A., Tervahartiala T., Sorsa T., Bockelman C., Haglund C. Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol. 2018;40:1010428318799266. doi: 10.1177/1010428318799266. [DOI] [PubMed] [Google Scholar]
- 91.Bockelman C., Beilmann-Lehtonen I., Kaprio T., Koskensalo S., Tervahartiala T., Mustonen H., Stenman U.H., Sorsa T., Haglund C. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer. 2018;18:679. doi: 10.1186/s12885-018-4589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahin U., Weskamp G., Kelly K., Zhou H.M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solanas G., Cortina C., Sevillano M., Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat. Cell Biol. 2011;13:1100–1107. doi: 10.1038/ncb2298. [DOI] [PubMed] [Google Scholar]
- 94.Murai T., Miyauchi T., Yanagida T., Sako Y. Epidermal growth factor-regulated activation of Rac GTPase enhances CD44 cleavage by metalloproteinase disintegrin ADAM10. Biochem. J. 2006;395:65–71. doi: 10.1042/BJ20050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith T.M., Jr., Tharakan A., Martin R.K. Targeting ADAM10 in Cancer and Autoimmunity. Front. Immunol. 2020;11:499. doi: 10.3389/fimmu.2020.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scheller J., Chalaris A., Garbers C., Rose-John S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Saad M.I., Alhayyani S., McLeod L., Yu L., Alanazi M., Deswaerte V., Tang K., Jarde T., Smith J.A., Prodanovic Z., et al. ADAM17 selectively activates the IL-6 trans-signaling/ERK MAPK axis in KRAS-addicted lung cancer. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohtsu H., Dempsey P.J., Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 99.Parra L.M., Hartmann M., Schubach S., Li Y., Herrlich P., Herrlich A. Distinct Intracellular Domain Substrate Modifications Selectively Regulate Ectodomain Cleavage of NRG1 or CD44. Mol. Cell Biol. 2015;35:3381–3395. doi: 10.1128/MCB.00500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caiazza F., McGowan P.M., Mullooly M., Murray A., Synnott N., O’Donovan N., Flanagan L., Tape C.J., Murphy G., Crown J., et al. Targeting ADAM-17 with an inhibitory monoclonal antibody has antitumour effects in triple-negative breast cancer cells. Br. J. Cancer. 2015;112:1895–1903. doi: 10.1038/bjc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kyula J.N., Van Schaeybroeck S., Doherty J., Fenning C.S., Longley D.B., Johnston P.G. Chemotherapy-induced activation of ADAM-17: A novel mechanism of drug resistance in colorectal cancer. Clin. Cancer Res. 2010;16:3378–3389. doi: 10.1158/1078-0432.CCR-10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zolkiewska A. ADAM proteases: Ligand processing and modulation of the Notch pathway. Cell Mol. Life Sci. 2008;65:2056–2068. doi: 10.1007/s00018-008-7586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bray S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016;17:722–735. doi: 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- 104.Kopan R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cal S., Lopez-Otin C. ADAMTS proteases and cancer. Matrix Biol. 2015;44–46:77–85. doi: 10.1016/j.matbio.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 106.Wagstaff L., Kelwick R., Decock J., Edwards D.R. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front. Biosci. 2011;16:1861–1872. doi: 10.2741/3827. [DOI] [PubMed] [Google Scholar]
- 107.Stanton H., Melrose J., Little C.B., Fosang A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta. 2011;1812:1616–1629. doi: 10.1016/j.bbadis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 108.Kelwick R., Desanlis I., Wheeler G.N., Edwards D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Binder M.J., McCoombe S., Williams E.D., McCulloch D.R., Ward A.C. The extracellular matrix in cancer progression: Role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett. 2017;385:55–64. doi: 10.1016/j.canlet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Lima M.A., Dos Santos L., Turri J.A., Nonogaki S., Buim M., Lima J.F., de Jesus Viana Pinheiro J., Bueno de Toledo Osorio C.A., Soares F.A., Freitas V.M. Prognostic Value of ADAMTS Proteases and Their Substrates in Epithelial Ovarian Cancer. Pathobiology. 2016;83:316–326. doi: 10.1159/000446244. [DOI] [PubMed] [Google Scholar]
- 111.Porter S., Span P.N., Sweep F.C., Tjan-Heijnen V.C., Pennington C.J., Pedersen T.X., Johnsen M., Lund L.R., Romer J., Edwards D.R. ADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinoma. Int. J. Cancer. 2006;118:1241–1247. doi: 10.1002/ijc.21476. [DOI] [PubMed] [Google Scholar]
- 112.Liang L., Zhu J.H., Chen G., Qin X.G., Chen J.Q. Prognostic Values for the mRNA Expression of the ADAMTS Family of Genes in Gastric Cancer. J. Oncol. 2020;2020:9431560. doi: 10.1155/2020/9431560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Law R.H., Zhang Q., McGowan S., Buckle A.M., Silverman G.A., Wong W., Rosado C.J., Langendorf C.G., Pike R.N., Bird P.I., et al. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lucas A., Yaron J.R., Zhang L., Macaulay C., McFadden G. Serpins: Development for Therapeutic Applications. Methods Mol. Biol. 2018;1826:255–265. doi: 10.1007/978-1-4939-8645-3_17. [DOI] [PubMed] [Google Scholar]
- 115.Unruh D., Horbinski C. Beyond thrombosis: The impact of tissue factor signaling in cancer. J. Hematol. Oncol. 2020;13:93. doi: 10.1186/s13045-020-00932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Jiang P., Capkova K., Xue D., Ye L., Sinha S.C., Mackman N., Janda K.D., Liu C. Tissue factor-activated coagulation cascade in the tumor microenvironment is critical for tumor progression and an effective target for therapy. Cancer Res. 2011;71:6492–6502. doi: 10.1158/0008-5472.CAN-11-1145. [DOI] [PubMed] [Google Scholar]
- 117.Rickles F.R., Patierno S., Fernandez P.M. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58S. [DOI] [PubMed] [Google Scholar]
- 118.Wojtukiewicz M.Z., Hempel D., Sierko E., Tucker S.C., Honn K.V. Thrombin-unique coagulation system protein with multifaceted impacts on cancer and metastasis. Cancer Metastasis Rev. 2016;35:213–233. doi: 10.1007/s10555-016-9626-0. [DOI] [PubMed] [Google Scholar]
- 119.Debaugnies F., Azerad M.A., Noubouossie D., Rozen L., Hemker H.C., Corazza F., Efira A., Demulder A. Evaluation of the procoagulant activity in the plasma of cancer patients using a thrombin generation assay. Thromb. Res. 2010;126:531–535. doi: 10.1016/j.thromres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Zhao B., Wu M., Hu Z., Ma Y., Qi W., Zhang Y., Li Y., Yu M., Wang H., Mo W. Thrombin is a therapeutic target for non-small-cell lung cancer to inhibit vasculogenic mimicry formation. Signal. Transduct. Target. Ther. 2020;5:117. doi: 10.1038/s41392-020-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaffner F., Versteeg H.H., Schillert A., Yokota N., Petersen L.C., Mueller B.M., Ruf W. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010;116:6106–6113. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palumbo J.S., Talmage K.E., Massari J.V., La Jeunesse C.M., Flick M.J., Kombrinck K.W., Hu Z., Barney K.A., Degen J.L. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Langer F., Bokemeyer C. Crosstalk between cancer and haemostasis. Implications for cancer biology and cancer-associated thrombosis with focus on tissue factor. Hamostaseologie. 2012;32:95–104. doi: 10.5482/ha-1160. [DOI] [PubMed] [Google Scholar]
- 124.Boivin W.A., Cooper D.M., Hiebert P.R., Granville D.J. Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab. Investig. 2009;89:1195–1220. doi: 10.1038/labinvest.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chowdhury D., Lieberman J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008;26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chollat-Namy M., Ben Safta-Saadoun T., Haferssas D., Meurice G., Chouaib S., Thiery J. The pharmalogical reactivation of p53 function improves breast tumor cell lysis by granzyme B and NK cells through induction of autophagy. Cell Death Dis. 2019;10:695. doi: 10.1038/s41419-019-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D’Eliseo D., Pisu P., Romano C., Tubaro A., De Nunzio C., Morrone S., Santoni A., Stoppacciaro A., Velotti F. Granzyme B is expressed in urothelial carcinoma and promotes cancer cell invasion. Int. J. Cancer. 2010;127:1283–1294. doi: 10.1002/ijc.25135. [DOI] [PubMed] [Google Scholar]
- 128.Lerman I., Hammes S.R. Neutrophil elastase in the tumor microenvironment. Steroids. 2018;133:96–101. doi: 10.1016/j.steroids.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kristensen J.H., Karsdal M.A., Sand J.M., Willumsen N., Diefenbach C., Svensson B., Hagglund P., Oersnes-Leeming D.J. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm. Med. 2015;15:53. doi: 10.1186/s12890-015-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Julier Z., Martino M.M., de Titta A., Jeanbart L., Hubbell J.A. The TLR4 agonist fibronectin extra domain A is cryptic, exposed by elastase-2; use in a fibrin matrix cancer vaccine. Sci. Rep. 2015;5:8569. doi: 10.1038/srep08569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wada Y., Yoshida K., Tsutani Y., Shigematsu H., Oeda M., Sanada Y., Suzuki T., Mizuiri H., Hamai Y., Tanabe K., et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol. Rep. 2007;17:161–167. [PubMed] [Google Scholar]
- 132.Wilson T.J., Nannuru K.C., Singh R.K. Cathepsin G-mediated activation of pro-matrix metalloproteinase 9 at the tumor-bone interface promotes transforming growth factor-beta signaling and bone destruction. Mol. Cancer Res. 2009;7:1224–1233. doi: 10.1158/1541-7786.MCR-09-0028. [DOI] [PubMed] [Google Scholar]
- 133.Wilson T.J., Nannuru K.C., Futakuchi M., Singh R.K. Cathepsin G-mediated enhanced TGF-beta signaling promotes angiogenesis via upregulation of VEGF and MCP-1. Cancer Lett. 2010;288:162–169. doi: 10.1016/j.canlet.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 135.Jaaks P., Bernasconi M. The proprotein convertase furin in tumour progression. Int. J. Cancer. 2017;141:654–663. doi: 10.1002/ijc.30714. [DOI] [PubMed] [Google Scholar]
- 136.Loessner D., Goettig P., Preis S., Felber J., Bronger H., Clements J.A., Dorn J., Magdolen V. Kallikrein-related peptidases represent attractive therapeutic targets for ovarian cancer. Expert Opin. Ther. Targets. 2018;22:745–763. doi: 10.1080/14728222.2018.1512587. [DOI] [PubMed] [Google Scholar]
- 137.Schmitt M., Magdolen V., Yang F., Kiechle M., Bayani J., Yousef G.M., Scorilas A., Diamandis E.P., Dorn J. Emerging clinical importance of the cancer biomarkers kallikrein-related peptidases (KLK) in female and male reproductive organ malignancies. Radiol. Oncol. 2013;47:319–329. doi: 10.2478/raon-2013-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tailor P.D., Kodeboyina S.K., Bai S., Patel N., Sharma S., Ratnani A., Copland J.A., She J.X., Sharma A. Diagnostic and prognostic biomarker potential of kallikrein family genes in different cancer types. Oncotarget. 2018;9:17876–17888. doi: 10.18632/oncotarget.24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Avgeris M., Scorilas A. Kallikrein-related peptidases (KLKs) as emerging therapeutic targets: Focus on prostate cancer and skin pathologies. Expert Opin. Ther. Targets. 2016;20:801–818. doi: 10.1517/14728222.2016.1147560. [DOI] [PubMed] [Google Scholar]
- 140.Lawrence M.G., Lai J., Clements J.A. Kallikreins on steroids: Structure, function, and hormonal regulation of prostate-specific antigen and the extended kallikrein locus. Endocr. Rev. 2010;31:407–446. doi: 10.1210/er.2009-0034. [DOI] [PubMed] [Google Scholar]
- 141.Michel N., Heuze-Vourc’h N., Lavergne E., Parent C., Jourdan M.L., Vallet A., Iochmann S., Musso O., Reverdiau P., Courty Y. Growth and survival of lung cancer cells: Regulation by kallikrein-related peptidase 6 via activation of proteinase-activated receptor 2 and the epidermal growth factor receptor. Biol. Chem. 2014;395:1015–1025. doi: 10.1515/hsz-2014-0124. [DOI] [PubMed] [Google Scholar]
- 142.Saini S. PSA and beyond: Alternative prostate cancer biomarkers. Cell Oncol. 2016;39:97–106. doi: 10.1007/s13402-016-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Webber M.M., Waghray A. Urokinase-mediated extracellular matrix degradation by human prostatic carcinoma cells and its inhibition by retinoic acid. Clin. Cancer Res. 1995;1:755–761. [PubMed] [Google Scholar]
- 144.Gondi C.S., Kandhukuri N., Dinh D.H., Gujrati M., Rao J.S. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int. J. Oncol. 2007;31:19–27. doi: 10.3892/ijo.31.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McMahon B.J., Kwaan H.C. Components of the Plasminogen-Plasmin System as Biologic Markers for Cancer. Adv. Exp. Med. Biol. 2015;867:145–156. doi: 10.1007/978-94-017-7215-0_10. [DOI] [PubMed] [Google Scholar]
- 146.Beckenkamp A., Davies S., Willig J.B., Buffon A. DPPIV/CD26: A tumor suppressor or a marker of malignancy? Tumour Biol. 2016;37:7059–7073. doi: 10.1007/s13277-016-5005-2. [DOI] [PubMed] [Google Scholar]
- 147.De Chiara L., Rodriguez-Pineiro A.M., Cordero O.J., Vazquez-Tunas L., Ayude D., Rodriguez-Berrocal F.J., de la Cadena M.P. Postoperative serum levels of sCD26 for surveillance in colorectal cancer patients. PLoS ONE. 2014;9:e107470. doi: 10.1371/journal.pone.0107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boccardi V., Marano L., Rossetti R.R., Rizzo M.R., di Martino N., Paolisso G. Serum CD26 levels in patients with gastric cancer: A novel potential diagnostic marker. BMC Cancer. 2015;15:703. doi: 10.1186/s12885-015-1757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee H.O., Mullins S.R., Franco-Barraza J., Valianou M., Cukierman E., Cheng J.D. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu R., Li H., Liu L., Yu J., Ren X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol. Ther. 2012;13:123–129. doi: 10.4161/cbt.13.3.18696. [DOI] [PubMed] [Google Scholar]
- 151.Larrinaga G., Perez I., Blanco L., Lopez J.I., Andres L., Etxezarraga C., Santaolalla F., Zabala A., Varona A., Irazusta J. Increased prolyl endopeptidase activity in human neoplasia. Regul. Pept. 2010;163:102–106. doi: 10.1016/j.regpep.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 152.Larrinaga G., Perez I., Blanco L., Sanz B., Errarte P., Beitia M., Etxezarraga M.C., Loizate A., Gil J., Irazusta J., et al. Prolyl endopeptidase activity is correlated with colorectal cancer prognosis. Int. J. Med. Sci. 2014;11:199–208. doi: 10.7150/ijms.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Skorko-Glonek J., Zurawa-Janicka D., Koper T., Jarzab M., Figaj D., Glaza P., Lipinska B. HtrA protease family as therapeutic targets. Curr. Pharm. Des. 2013;19:977–1009. doi: 10.2174/1381612811319060003. [DOI] [PubMed] [Google Scholar]
- 154.Choi S.Y., Bertram S., Glowacka I., Park Y.W., Pohlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol. Med. 2009;15:303–312. doi: 10.1016/j.molmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 155.Uhland K. Matriptase and its putative role in cancer. Cell Mol. Life Sci. 2006;63:2968–2978. doi: 10.1007/s00018-006-6298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ustach C.V., Huang W., Conley-LaComb M.K., Lin C.Y., Che M., Abrams J., Kim H.R. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res. 2010;70:9631–9640. doi: 10.1158/0008-5472.CAN-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Magee J.A., Araki T., Patil S., Ehrig T., True L., Humphrey P.A., Catalona W.J., Watson M.A., Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61:5692–5696. [PubMed] [Google Scholar]
- 158.Zhang M., Zhao J., Tang W., Wang Y., Peng P., Li L., Song S., Wu H., Li C., Yang C., et al. High Hepsin expression predicts poor prognosis in Gastric Cancer. Sci. Rep. 2016;6:36902. doi: 10.1038/srep36902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Urban S., Dickey S.W. The rhomboid protease family: A decade of progress on function and mechanism. Genome Biol. 2011;12:231. doi: 10.1186/gb-2011-12-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adrain C., Strisovsky K., Zettl M., Hu L., Lemberg M.K., Freeman M. Mammalian EGF receptor activation by the rhomboid protease RHBDL2. EMBO Rep. 2011;12:421–427. doi: 10.1038/embor.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abba M.C., Lacunza E., Nunez M.I., Colussi A., Isla-Larrain M., Segal-Eiras A., Croce M.V., Aldaz C.M. Rhomboid domain containing 2 (RHBDD2): A novel cancer-related gene over-expressed in breast cancer. Biochim. Biophys. Acta. 2009;1792:988–997. doi: 10.1016/j.bbadis.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wunderle L., Knopf J.D., Kuhnle N., Morle A., Hehn B., Adrain C., Strisovsky K., Freeman M., Lemberg M.K. Rhomboid intramembrane protease RHBDL4 triggers ER-export and non-canonical secretion of membrane-anchored TGFalpha. Sci. Rep. 2016;6:27342. doi: 10.1038/srep27342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Noy P.J., Swain R.K., Khan K., Lodhia P., Bicknell R. Sprouting angiogenesis is regulated by shedding of the C-type lectin family 14, member A (CLEC14A) ectodomain, catalyzed by rhomboid-like 2 protein (RHBDL2) FASEB J. 2016;30:2311–2323. doi: 10.1096/fj.201500122R. [DOI] [PubMed] [Google Scholar]
- 164.Cheng T.L., Lai C.H., Jiang S.J., Hung J.H., Liu S.K., Chang B.I., Shi G.Y., Wu H.L. RHBDL2 is a critical membrane protease for anoikis resistance in human malignant epithelial cells. Sci. World J. 2014;2014:902987. doi: 10.1155/2014/902987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sevenich L., Bowman R.L., Mason S.D., Quail D.F., Rapaport F., Elie B.T., Brogi E., Brastianos P.K., Hahn W.C., Holsinger L.J., et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014;16:876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sobotic B., Vizovisek M., Vidmar R., Van Damme P., Gocheva V., Joyce J.A., Gevaert K., Turk V., Turk B., Fonovic M. Proteomic Identification of Cysteine Cathepsin Substrates Shed from the Surface of Cancer Cells. Mol. Cell Proteomics. 2015;14:2213–2228. doi: 10.1074/mcp.M114.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Small D.M., Burden R.E., Jaworski J., Hegarty S.M., Spence S., Burrows J.F., McFarlane C., Kissenpfennig A., McCarthy H.O., Johnston J.A., et al. Cathepsin S from both tumor and tumor-associated cells promote cancer growth and neovascularization. Int. J. Cancer. 2013;133:2102–2112. doi: 10.1002/ijc.28238. [DOI] [PubMed] [Google Scholar]
- 168.Vasiljeva O., Papazoglou A., Kruger A., Brodoefel H., Korovin M., Deussing J., Augustin N., Nielsen B.S., Almholt K., Bogyo M., et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 169.Prudova A., Gocheva V., Auf dem Keller U., Eckhard U., Olson O.C., Akkari L., Butler G.S., Fortelny N., Lange P.F., Mark J.C., et al. TAILS N-Terminomics and Proteomics Show Protein Degradation Dominates over Proteolytic Processing by Cathepsins in Pancreatic Tumors. Cell Rep. 2016;16:1762–1773. doi: 10.1016/j.celrep.2016.06.086. [DOI] [PubMed] [Google Scholar]
- 170.Repnik U., Starr A.E., Overall C.M., Turk B. Cysteine Cathepsins Activate ELR Chemokines and Inactivate Non-ELR Chemokines. J. Biol. Chem. 2015;290:13800–13811. doi: 10.1074/jbc.M115.638395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nagler D.K., Menard R. Family C1 cysteine proteases: Biological diversity or redundancy? Biol. Chem. 2003;384:837–843. doi: 10.1515/BC.2003.094. [DOI] [PubMed] [Google Scholar]
- 172.Vizovisek M., Vidmar R., Van Quickelberghe E., Impens F., Andjelkovic U., Sobotic B., Stoka V., Gevaert K., Turk B., Fonovic M. Fast profiling of protease specificity reveals similar substrate specificities for cathepsins K, L and S. Proteomics. 2015;15:2479–2490. doi: 10.1002/pmic.201400460. [DOI] [PubMed] [Google Scholar]
- 173.Vidmar R., Vizovisek M., Turk D., Turk B., Fonovic M. Protease cleavage site fingerprinting by label-free in-gel degradomics reveals pH-dependent specificity switch of legumain. EMBO J. 2017;36:2455–2465. doi: 10.15252/embj.201796750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ramirez M.L.G., Salvesen G.S. A primer on caspase mechanisms. Semin. Cell Dev. Biol. 2018;82:79–85. doi: 10.1016/j.semcdb.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Indran I.R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Biophys. Acta. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 176.Parsons M.J., McCormick L., Janke L., Howard A., Bouchier-Hayes L., Green D.R. Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell Death Differ. 2013;20:1174–1182. doi: 10.1038/cdd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang Q., Li F., Liu X., Li W., Shi W., Liu F.F., O’Sullivan B., He Z., Peng Y., Tan A.C., et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Strilic B., Yang L., Albarran-Juarez J., Wachsmuth L., Han K., Muller U.C., Pasparakis M., Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 179.Pu X., Storr S.J., Zhang Y., Rakha E.A., Green A.R., Ellis I.O., Martin S.G. Caspase-3 and caspase-8 expression in breast cancer: Caspase-3 is associated with survival. Apoptosis. 2017;22:357–368. doi: 10.1007/s10495-016-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Devarajan E., Sahin A.A., Chen J.S., Krishnamurthy R.R., Aggarwal N., Brun A.M., Sapino A., Zhang F., Sharma D., Yang X.H., et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- 181.Hu B., Elinav E., Huber S., Booth C.J., Strowig T., Jin C., Eisenbarth S.C., Flavell R.A. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl. Acad. Sci. USA. 2010;107:21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kolb R., Liu G.H., Janowski A.M., Sutterwala F.S., Zhang W. Inflammasomes in cancer: A double-edged sword. Protein Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lin Y., Wei C., Liu Y., Qiu Y., Liu C., Guo F. Selective ablation of tumor-associated macrophages suppresses metastasis and angiogenesis. Cancer Sci. 2013;104:1217–1225. doi: 10.1111/cas.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang L., Chen S., Zhang M., Li N., Chen Y., Su W., Liu Y., Lu D., Li S., Yang Y., et al. Legumain: A biomarker for diagnosis and prognosis of human ovarian cancer. J. Cell Biochem. 2012;113:2679–2686. doi: 10.1002/jcb.24143. [DOI] [PubMed] [Google Scholar]
- 185.Ohno Y., Nakashima J., Izumi M., Ohori M., Hashimoto T., Tachibana M. Association of legumain expression pattern with prostate cancer invasiveness and aggressiveness. World J. Urol. 2013;31:359–364. doi: 10.1007/s00345-012-0977-z. [DOI] [PubMed] [Google Scholar]
- 186.Del Bello B., Moretti D., Gamberucci A., Maellaro E. Cross-talk between calpain and caspase-3/-7 in cisplatin-induced apoptosis of melanoma cells: A major role of calpain inhibition in cell death protection and p53 status. Oncogene. 2007;26:2717–2726. doi: 10.1038/sj.onc.1210079. [DOI] [PubMed] [Google Scholar]
- 187.Leloup L., Wells A. Calpains as potential anti-cancer targets. Expert Opin. Ther. Targets. 2011;15:309–323. doi: 10.1517/14728222.2011.553611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Storr S.J., Thompson N., Pu X., Zhang Y., Martin S.G. Calpain in Breast Cancer: Role in Disease Progression and Treatment Response. Pathobiology. 2015;82:133–141. doi: 10.1159/000430464. [DOI] [PubMed] [Google Scholar]
- 189.Yu L.M., Zhu Y.S., Xu C.Z., Zhou L.L., Xue Z.X., Cai Z.Z. High calpain-1 expression predicts a poor clinical outcome and contributes to tumor progression in pancreatic cancer patients. Clin. Transl. Oncol. 2019;21:924–932. doi: 10.1007/s12094-018-02006-6. [DOI] [PubMed] [Google Scholar]
- 190.Zhang S., Deen S., Storr S.J., Chondrou P.S., Nicholls H., Yao A., Rungsakaolert P., Martin S.G. Calpain system protein expression and activity in ovarian cancer. J. Cancer Res. Clin. Oncol. 2019;145:345–361. doi: 10.1007/s00432-018-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Crowley S.D., Coffman T.M. Recent advances involving the renin-angiotensin system. Exp. Cell Res. 2012;318:1049–1056. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.George A.J., Thomas W.G., Hannan R.D. The renin-angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 193.Wegman-Ostrosky T., Soto-Reyes E., Vidal-Millan S., Sanchez-Corona J. The renin-angiotensin system meets the hallmarks of cancer. J. Renin Angiotensin Aldosterone Syst. 2015;16:227–233. doi: 10.1177/1470320313496858. [DOI] [PubMed] [Google Scholar]
- 194.Nakamura K., Yaguchi T., Ohmura G., Kobayashi A., Kawamura N., Iwata T., Kiniwa Y., Okuyama R., Kawakami Y. Involvement of local renin-angiotensin system in immunosuppression of tumor microenvironment. Cancer Sci. 2018;109:54–64. doi: 10.1111/cas.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Afsar B., Afsar R.E., Ertuglu L.A., Kuwabara M., Ortiz A., Covic A., Kanbay M. Renin-angiotensin system and cancer: Epidemiology, cell signaling, genetics and epigenetics. Clin. Transl. Oncol. 2020 doi: 10.1007/s12094-020-02488-3. [DOI] [PubMed] [Google Scholar]
- 196.Pinter M., Jain R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pranjol M.Z., Gutowski N., Hannemann M., Whatmore J. The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules. 2015;5:3260–3279. doi: 10.3390/biom5043260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brouillet J.P., Dufour F., Lemamy G., Garcia M., Schlup N., Grenier J., Mani J.C., Rochefort H. Increased cathepsin D level in the serum of patients with metastatic breast carcinoma detected with a specific pro-cathepsin D immunoassay. Cancer. 1997;79:2132–2136. doi: 10.1002/(SICI)1097-0142(19970601)79:11<2132::AID-CNCR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 199.Chai Y., Wu W., Zhou C., Zhou J. The potential prognostic value of cathepsin D protein in serous ovarian cancer. Arch. Gynecol. Obstet. 2012;286:465–471. doi: 10.1007/s00404-012-2318-2. [DOI] [PubMed] [Google Scholar]
- 200.Zaidi N., Kalbacher H. Cathepsin E: A mini review. Biochem. Biophys. Res. Commun. 2008;367:517–522. doi: 10.1016/j.bbrc.2007.12.163. [DOI] [PubMed] [Google Scholar]
- 201.Uno K., Azuma T., Nakajima M., Yasuda K., Hayakumo T., Mukai H., Sakai T., Kawai K. Clinical significance of cathepsin E in pancreatic juice in the diagnosis of pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2000;15:1333–1338. [PubMed] [Google Scholar]
- 202.Cruz-Monserrate Z., Abd-Elgaliel W.R., Grote T., Deng D., Ji B., Arumugam T., Wang H., Tung C.H., Logsdon C.D. Detection of pancreatic cancer tumours and precursor lesions by cathepsin E activity in mouse models. Gut. 2012;61:1315–1322. doi: 10.1136/gutjnl-2011-300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sereg-Bahar M., Jerin A., Hocevar-Boltezar I. Higher levels of total pepsin and bile acids in the saliva as a possible risk factor for early laryngeal cancer. Radiol. Oncol. 2015;49:59–64. doi: 10.2478/raon-2014-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shen S., Jiang J., Yuan Y. Pepsinogen C expression, regulation and its relationship with cancer. Cancer Cell Int. 2017;17:57. doi: 10.1186/s12935-017-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brasch F., Ochs M., Kahne T., Guttentag S., Schauer-Vukasinovic V., Derrick M., Johnen G., Kapp N., Muller K.M., Richter J., et al. Involvement of napsin A in the C- and N-terminal processing of surfactant protein B in type-II pneumocytes of the human lung. J. Biol. Chem. 2003;278:49006–49014. doi: 10.1074/jbc.M306844200. [DOI] [PubMed] [Google Scholar]
- 206.Stoll L.M., Johnson M.W., Gabrielson E., Askin F., Clark D.P., Li Q.K. The utility of napsin-A in the identification of primary and metastatic lung adenocarcinoma among cytologically poorly differentiated carcinomas. Cancer Cytopathol. 2010;118:441–449. doi: 10.1002/cncy.20108. [DOI] [PubMed] [Google Scholar]
- 207.Collins G.A., Goldberg A.L. The Logic of the 26S Proteasome. Cell. 2017;169:792–806. doi: 10.1016/j.cell.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rut W., Drag M. Human 20S proteasome activity towards fluorogenic peptides of various chain lengths. Biol. Chem. 2016;397:921–926. doi: 10.1515/hsz-2016-0176. [DOI] [PubMed] [Google Scholar]
- 209.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen D., Frezza M., Schmitt S., Kanwar J., Dou Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sanman L.E., Bogyo M. Activity-based profiling of proteases. Annu. Rev. Biochem. 2014;83:249–273. doi: 10.1146/annurev-biochem-060713-035352. [DOI] [PubMed] [Google Scholar]
- 212.Sieber S.A., Niessen S., Hoover H.S., Cravatt B.F. Proteomic profiling of metalloprotease activities with cocktails of active-site probes. Nat. Chem. Biol. 2006;2:274–281. doi: 10.1038/nchembio781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Saghatelian A., Jessani N., Joseph A., Humphrey M., Cravatt B.F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl. Acad. Sci. USA. 2004;101:10000–10005. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amara N., Tholen M., Bogyo M. Chemical Tools for Selective Activity Profiling of Endogenously Expressed MMP-14 in Multicellular Models. ACS Chem. Biol. 2018;13:2645–2654. doi: 10.1021/acschembio.8b00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Scherer R.L., McIntyre J.O., Matrisian L.M. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- 216.Pan Z., Jeffery D.A., Chehade K., Beltman J., Clark J.M., Grothaus P., Bogyo M., Baruch A. Development of activity-based probes for trypsin-family serine proteases. Bioorg. Med. Chem. Lett. 2006;16:2882–2885. doi: 10.1016/j.bmcl.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 217.Kasperkiewicz P., Altman Y., D’Angelo M., Salvesen G.S., Drag M. Toolbox of Fluorescent Probes for Parallel Imaging Reveals Uneven Location of Serine Proteases in Neutrophils. J. Am. Chem. Soc. 2017;139:10115–10125. doi: 10.1021/jacs.7b04394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Edgington-Mitchell L.E., Barlow N., Aurelio L., Samha A., Szabo M., Graham B., Bunnett N. Fluorescent diphenylphosphonate-based probes for detection of serine protease activity during inflammation. Bioorg. Med. Chem. Lett. 2017;27:254–260. doi: 10.1016/j.bmcl.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Blum G., von Degenfeld G., Merchant M.J., Blau H.M., Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 220.Edgington-Mitchell L.E., Bogyo M., Verdoes M. Live Cell Imaging and Profiling of Cysteine Cathepsin Activity Using a Quenched Activity-Based Probe. Methods Mol. Biol. 2017;1491:145–159. doi: 10.1007/978-1-4939-6439-0_11. [DOI] [PubMed] [Google Scholar]
- 221.Edgington L.E., Berger A.B., Blum G., Albrow V.E., Paulick M.G., Lineberry N., Bogyo M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 2009;15:967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Poreba M., Groborz K., Navarro M., Snipas S.J., Drag M., Salvesen G.S. Caspase selective reagents for diagnosing apoptotic mechanisms. Cell Death Differ. 2019;26:229–244. doi: 10.1038/s41418-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Edgington L.E., Verdoes M., Ortega A., Withana N.P., Lee J., Syed S., Bachmann M.H., Blum G., Bogyo M. Functional imaging of legumain in cancer using a new quenched activity-based probe. J. Am. Chem. Soc. 2013;135:174–182. doi: 10.1021/ja307083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sexton K.B., Witte M.D., Blum G., Bogyo M. Design of cell-permeable, fluorescent activity-based probes for the lysosomal cysteine protease asparaginyl endopeptidase (AEP)/legumain. Bioorg. Med. Chem. Lett. 2007;17:649–653. doi: 10.1016/j.bmcl.2006.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chattopadhaya S., Chan E.W.S., Yao S.Q. An affinity-based probe for the proteomic profiling of aspartic proteases. Tetrahedron Lett. 2005;46:4053–4056. doi: 10.1016/j.tetlet.2005.04.015. [DOI] [Google Scholar]
- 226.de Bruin G., Xin B.T., Kraus M., van der Stelt M., van der Marel G.A., Kisselev A.F., Driessen C., Florea B.I., Overkleeft H.S. A Set of Activity-Based Probes to Visualize Human (Immuno)proteasome Activities. Angew. Chem. Int. Ed. 2016;55:4199–4203. doi: 10.1002/anie.201509092. [DOI] [PubMed] [Google Scholar]
- 227.Hewings D.S., Flygare J.A., Wertz I.E., Bogyo M. Activity-based probes for the multicatalytic proteasome. FEBS J. 2017;284:1540–1554. doi: 10.1111/febs.14016. [DOI] [PubMed] [Google Scholar]
- 228.Verdoes M., Edgington L.E., Scheeren F.A., Leyva M., Blum G., Weiskopf K., Bachmann M.H., Ellman J.A., Bogyo M. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem. Biol. 2012;19:619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Oresic Bender K., Ofori L., van der Linden W.A., Mock E.D., Datta G.K., Chowdhury S., Li H., Segal E., Sanchez Lopez M., Ellman J.A., et al. Design of a highly selective quenched activity-based probe and its application in dual color imaging studies of cathepsin S activity localization. J. Am. Chem. Soc. 2015;137:4771–4777. doi: 10.1021/jacs.5b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kasperkiewicz P., Poreba M., Groborz K., Drag M. Emerging challenges in the design of selective substrates, inhibitors and activity-based probes for indistinguishable proteases. FEBS J. 2017;284:1518–1539. doi: 10.1111/febs.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mahmood U., Weissleder R. Near-infrared optical imaging of proteases in cancer. Mol. Cancer Ther. 2003;2:489–496. [PubMed] [Google Scholar]
- 232.Weissleder R., Tung C.H., Mahmood U., Bogdanov A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 233.Yhee J.Y., Kim S.A., Koo H., Son S., Ryu J.H., Youn I.C., Choi K., Kwon I.C., Kim K. Optical imaging of cancer-related proteases using near-infrared fluorescence matrix metalloproteinase-sensitive and cathepsin B-sensitive probes. Theranostics. 2012;2:179–189. doi: 10.7150/thno.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hu H.Y., Gehrig S., Reither G., Subramanian D., Mall M.A., Plettenburg O., Schultz C. FRET-based and other fluorescent proteinase probes. Biotechnol. J. 2014;9:266–281. doi: 10.1002/biot.201300201. [DOI] [PubMed] [Google Scholar]
- 235.Poreba M., Szalek A., Kasperkiewicz P., Drag M. Positional scanning substrate combinatorial library (PS-SCL) approach to define caspase substrate specificity. Methods Mol. Biol. 2014;1133:41–59. doi: 10.1007/978-1-4939-0357-3_2. [DOI] [PubMed] [Google Scholar]
- 236.Poreba M., Solberg R., Rut W., Lunde N.N., Kasperkiewicz P., Snipas S.J., Mihelic M., Turk D., Turk B., Salvesen G.S., et al. Counter Selection Substrate Library Strategy for Developing Specific Protease Substrates and Probes. Cell Chem. Biol. 2016;23:1023–1035. doi: 10.1016/j.chembiol.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Poreba M., Salvesen G.S., Drag M. Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity. Nat. Protoc. 2017;12:2189–2214. doi: 10.1038/nprot.2017.091. [DOI] [PubMed] [Google Scholar]
- 238.Poreba M., Rut W., Vizovisek M., Groborz K., Kasperkiewicz P., Finlay D., Vuori K., Turk D., Turk B., Salvesen G.S., et al. Selective imaging of cathepsin L in breast cancer by fluorescent activity-based probes. Chem. Sci. 2018;9:2113–2129. doi: 10.1039/C7SC04303A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Poreba M., Groborz K., Vizovisek M., Maruggi M., Turk D., Turk B., Powis G., Drag M., Salvesen G.S. Fluorescent probes towards selective cathepsin B detection and visualization in cancer cells and patient samples. Chem. Sci. 2019;10:8461–8477. doi: 10.1039/C9SC00997C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gruba N., Bielecka E., Wysocka M., Wojtysiak A., Brzezinska-Bodal M., Sychowska K., Kalinska M., Magoch M., Pecak A., Falkowski K., et al. Development of Chemical Tools to Monitor Human Kallikrein 13 (KLK13) Activity. Int. J. Mol. Sci. 2019;20:1557. doi: 10.3390/ijms20071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tholen M., Yim J.J., Groborz K., Yoo E., Martin B.A., van den Berg N.S., Drag M., Bogyo M. Design of Optical-Imaging Probes by Screening of Diverse Substrate Libraries Directly in Disease-Tissue Extracts. Angew. Chem. Int. Ed. 2020;59:19143–19152. doi: 10.1002/anie.202006719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kindermann M., Roschitzki-Voser H., Caglic D., Repnik U., Miniejew C., Mittl P.R., Kosec G., Grutter M.G., Turk B., Wendt K.U. Selective and sensitive monitoring of caspase-1 activity by a novel bioluminescent activity-based probe. Chem. Biol. 2010;17:999–1007. doi: 10.1016/j.chembiol.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 243.Hu H.Y., Vats D., Vizovisek M., Kramer L., Germanier C., Wendt K.U., Rudin M., Turk B., Plettenburg O., Schultz C. In vivo imaging of mouse tumors by a lipidated cathepsin S substrate. Angew. Chem. Int. Ed. 2014;53:7669–7673. doi: 10.1002/anie.201310979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Smith B.R., Gambhir S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017;117:901–986. doi: 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- 245.Larson D.R., Zipfel W.R., Williams R.M., Clark S.W., Bruchez M.P., Wise F.W., Webb W.W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 246.Algar W.R., Malanoski A.P., Susumu K., Stewart M.H., Hildebrandt N., Medintz I.L. Multiplexed tracking of protease activity using a single color of quantum dot vector and a time-gated Forster resonance energy transfer relay. Anal. Chem. 2012;84:10136–10146. doi: 10.1021/ac3028068. [DOI] [PubMed] [Google Scholar]
- 247.Shi L., De Paoli V., Rosenzweig N., Rosenzweig Z. Synthesis and application of quantum dots FRET-based protease sensors. J. Am. Chem. Soc. 2006;128:10378–10379. doi: 10.1021/ja063509o. [DOI] [PubMed] [Google Scholar]
- 248.Li X., Deng D., Xue J., Qu L., Achilefu S., Gu Y. Quantum dots based molecular beacons for in vitro and in vivo detection of MMP-2 on tumor. Biosens. Bioelectron. 2014;61:512–518. doi: 10.1016/j.bios.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 249.Medintz I.L., Clapp A.R., Brunel F.M., Tiefenbrunn T., Uyeda H.T., Chang E.L., Deschamps J.R., Dawson P.E., Mattoussi H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006;5:581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 250.Lowe S.B., Dick J.A., Cohen B.E., Stevens M.M. Multiplex sensing of protease and kinase enzyme activity via orthogonal coupling of quantum dot-peptide conjugates. ACS Nano. 2012;6:851–857. doi: 10.1021/nn204361s. [DOI] [PubMed] [Google Scholar]
- 251.Boeneman K., Mei B.C., Dennis A.M., Bao G., Deschamps J.R., Mattoussi H., Medintz I.L. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc. 2009;131:3828–3829. doi: 10.1021/ja809721j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Breger J.C., Sapsford K.E., Ganek J., Susumu K., Stewart M.H., Medintz I.L. Detecting kallikrein proteolytic activity with peptide-quantum dot nanosensors. ACS Appl. Mater. Interfaces. 2014;6:11529–11535. doi: 10.1021/am502135h. [DOI] [PubMed] [Google Scholar]
- 253.Jeong S., Song J., Lee W., Ryu Y.M., Jung Y., Kim S.Y., Kim K., Hong S.C., Myung S.J., Kim S. Cancer-Microenvironment-Sensitive Activatable Quantum Dot Probe in the Second Near-Infrared Window. Nano Lett. 2017;17:1378–1386. doi: 10.1021/acs.nanolett.6b04261. [DOI] [PubMed] [Google Scholar]
- 254.Cheng X., McVey B.F.P., Robinson A.B., Longatte G., O’Mara P.B., Tan V.T.G., Thordarson P., Tilley R.D., Gaus K., Justin Gooding J. Protease sensing using nontoxic silicon quantum dots. J. Biomed. Opt. 2017;22:087002. doi: 10.1117/1.JBO.22.8.087002. [DOI] [PubMed] [Google Scholar]
- 255.Edgington L.E., Bogyo M. In vivo imaging and biochemical characterization of protease function using fluorescent activity-based probes. Curr. Protoc. Chem. Biol. 2013;5:25–44. doi: 10.1002/9780470559277.ch120235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hilderbrand S.A., Weissleder R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 257.Blau R., Epshtein Y., Pisarevsky E., Tiram G., Israeli Dangoor S., Yeini E., Krivitsky A., Eldar-Boock A., Ben-Shushan D., Gibori H., et al. Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics. 2018;8:3437–3460. doi: 10.7150/thno.23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Suurs F.V., Qiu S.Q., Yim J.J., Schroder C.P., Timmer-Bosscha H., Bensen E.S., Santini J.T., Jr., de Vries E.G.E., Bogyo M., van Dam G.M. Fluorescent image-guided surgery in breast cancer by intravenous application of a quenched fluorescence activity-based probe for cysteine cathepsins in a syngeneic mouse model. EJNMMI Res. 2020;10:111. doi: 10.1186/s13550-020-00688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bainbridge T.W., Dunshee D.R., Kljavin N.M., Skelton N.J., Sonoda J., Ernst J.A. Selective Homogeneous Assay for Circulating Endopeptidase Fibroblast Activation Protein (FAP) Sci. Rep. 2017;7:12524. doi: 10.1038/s41598-017-12900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Whitley M.J., Cardona D.M., Lazarides A.L., Spasojevic I., Ferrer J.M., Cahill J., Lee C.L., Snuderl M., Blazer D.G., 3rd, Hwang E.S., et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 2016;8:320ra324. doi: 10.1126/scitranslmed.aad0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yang Y., Hong H., Zhang Y., Cai W. Molecular Imaging of Proteases in Cancer. Cancer Growth Metastasis. 2009;2:13–27. doi: 10.4137/CGM.S2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rempel B.P., Price E.W., Phenix C.P. Molecular Imaging of Hydrolytic Enzymes Using PET and SPECT. Mol. Imaging. 2017;16:1536012117717852. doi: 10.1177/1536012117717852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.auf dem Keller U., Bellac C.L., Li Y., Lou Y., Lange P.F., Ting R., Harwig C., Kappelhoff R., Dedhar S., Adam M.J., et al. Novel matrix metalloproteinase inhibitor [18F]marimastat-aryltrifluoroborate as a probe for in vivo positron emission tomography imaging in cancer. Cancer Res. 2010;70:7562–7569. doi: 10.1158/0008-5472.CAN-10-1584. [DOI] [PubMed] [Google Scholar]
- 264.Matusiak N., van Waarde A., Bischoff R., Oltenfreiter R., van de Wiele C., Dierckx R.A., Elsinga P.H. Probes for non-invasive matrix metalloproteinase-targeted imaging with PET and SPECT. Curr. Pharm. Des. 2013;19:4647–4672. doi: 10.2174/1381612811319250011. [DOI] [PubMed] [Google Scholar]
- 265.Kasten B.B., Jiang K., Cole D., Jani A., Udayakumar N., Gillespie G.Y., Lu G., Dai T., Rosenthal E.L., Markert J.M., et al. Targeting MMP-14 for dual PET and fluorescence imaging of glioma in preclinical models. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1412–1426. doi: 10.1007/s00259-019-04607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Withana N.P., Ma X., McGuire H.M., Verdoes M., van der Linden W.A., Ofori L.O., Zhang R., Li H., Sanman L.E., Wei K., et al. Non-invasive Imaging of Idiopathic Pulmonary Fibrosis Using Cathepsin Protease Probes. Sci. Rep. 2016;6:19755. doi: 10.1038/srep19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Laube M., Frizler M., Wodtke R., Neuber C., Belter B., Kniess T., Bachmann M., Gutschow M., Pietzsch J., Loser R. Synthesis and preliminary radiopharmacological characterisation of an (11) C-labelled azadipeptide nitrile as potential PET tracer for imaging of cysteine cathepsins. J. Label. Comp. Radiopharm. 2019;62:448–459. doi: 10.1002/jlcr.3729. [DOI] [PubMed] [Google Scholar]
- 268.Rodnick M.E., Shao X., Kozloff K.M., Scott P.J., Kilbourn M.R. Carbon-11 labeled cathepsin K inhibitors: Syntheses and preliminary in vivo evaluation. Nucl. Med. Biol. 2014;41:384–389. doi: 10.1016/j.nucmedbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Garcia-Arguello S.F., Lopez-Lorenzo B., Cornelissen B., Smith G. Development of [(18)F]ICMT-11 for Imaging Caspase-3/7 Activity during Therapy-Induced Apoptosis. Cancers. 2020;12:2191. doi: 10.3390/cancers12082191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dubash S.R., Merchant S., Heinzmann K., Mauri F., Lavdas I., Inglese M., Kozlowski K., Rama N., Masrour N., Steel J.F., et al. Clinical translation of [(18)F]ICMT-11 for measuring chemotherapy-induced caspase 3/7 activation in breast and lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2285–2299. doi: 10.1007/s00259-018-4098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lusic H., Grinstaff M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013;113:1641–1666. doi: 10.1021/cr200358s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gaikwad H.K., Tsvirkun D., Ben-Nun Y., Merquiol E., Popovtzer R., Blum G. Molecular Imaging of Cancer Using X-ray Computed Tomography with Protease Targeted Iodinated Activity-Based Probes. Nano Lett. 2018;18:1582–1591. doi: 10.1021/acs.nanolett.7b03813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Tsvirkun D., Ben-Nun Y., Merquiol E., Zlotver I., Meir K., Weiss-Sadan T., Matok I., Popovtzer R., Blum G. CT Imaging of Enzymatic Activity in Cancer Using Covalent Probes Reveal a Size-Dependent Pattern. J. Am. Chem. Soc. 2018;140:12010–12020. doi: 10.1021/jacs.8b05817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jastrzebska B., Lebel R., Therriault H., McIntyre J.O., Escher E., Guerin B., Paquette B., Neugebauer W.A., Lepage M. New enzyme-activated solubility-switchable contrast agent for magnetic resonance imaging: From synthesis to in vivo imaging. J. Med. Chem. 2009;52:1576–1581. doi: 10.1021/jm801411h. [DOI] [PubMed] [Google Scholar]
- 275.Ye D., Shuhendler A.J., Pandit P., Brewer K.D., Tee S.S., Cui L., Tikhomirov G., Rutt B., Rao J. Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis. Chem. Sci. 2014;4:3845–3852. doi: 10.1039/C4SC01392A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mizukami S., Takikawa R., Sugihara F., Hori Y., Tochio H., Walchli M., Shirakawa M., Kikuchi K. Paramagnetic relaxation-based 19f MRI probe to detect protease activity. J. Am. Chem. Soc. 2008;130:794–795. doi: 10.1021/ja077058z. [DOI] [PubMed] [Google Scholar]
- 277.Koonjoo N., Parzy E., Massot P., Lepetit-Coiffe M., Marque S.R., Franconi J.M., Thiaudiere E., Mellet P. In vivo Overhauser-enhanced MRI of proteolytic activity. Contrast Media Mol. Imaging. 2014;9:363–371. doi: 10.1002/cmmi.1586. [DOI] [PubMed] [Google Scholar]
- 278.Choi J.S., Kim S., Yoo D., Shin T.H., Kim H., Gomes M.D., Kim S.H., Pines A., Cheon J. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 2017;16:537–542. doi: 10.1038/nmat4846. [DOI] [PubMed] [Google Scholar]
- 279.Haris M., Singh A., Mohammed I., Ittyerah R., Nath K., Nanga R.P., Debrosse C., Kogan F., Cai K., Poptani H., et al. In vivo magnetic resonance imaging of tumor protease activity. Sci. Rep. 2014;4:6081. doi: 10.1038/srep06081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cao C.Y., Shen Y.Y., Wang J.D., Li L., Liang G.L. Controlled intracellular self-assembly of gadolinium nanoparticles as smart molecular MR contrast agents. Sci. Rep. 2013;3:1024. doi: 10.1038/srep01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lee S., Kang S.W., Ryu J.H., Na J.H., Lee D.E., Han S.J., Kang C.M., Choe Y.S., Lee K.C., Leary J.F., et al. Tumor-homing glycol chitosan-based optical/PET dual imaging nanoprobe for cancer diagnosis. Bioconjug. Chem. 2014;25:601–610. doi: 10.1021/bc500020g. [DOI] [PubMed] [Google Scholar]
- 282.Yin L., Sun H., Zhao M., Wang A., Qiu S., Gao Y., Ding J., Ji S.J., Shi H., Gao M. Rational Design and Synthesis of a Metalloproteinase-Activatable Probe for Dual-Modality Imaging of Metastatic Lymph Nodes In Vivo. J. Org. Chem. 2019;84:6126–6133. doi: 10.1021/acs.joc.9b00331. [DOI] [PubMed] [Google Scholar]
- 283.Shi H., Sun Y., Yan R., Liu S., Zhu L., Liu S., Feng Y., Wang P., He J., Zhou Z., et al. Magnetic Semiconductor Gd-Doping CuS Nanoparticles as Activatable Nanoprobes for Bimodal Imaging and Targeted Photothermal Therapy of Gastric Tumors. Nano Lett. 2019;19:937–947. doi: 10.1021/acs.nanolett.8b04179. [DOI] [PubMed] [Google Scholar]
- 284.Olson E.S., Jiang T., Aguilera T.A., Nguyen Q.T., Ellies L.G., Scadeng M., Tsien R.Y. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. USA. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lakshmanan A., Jin Z., Nety S.P., Sawyer D.P., Lee-Gosselin A., Malounda D., Swift M.B., Maresca D., Shapiro M.G. Acoustic biosensors for ultrasound imaging of enzyme activity. Nat. Chem. Biol. 2020;16:988–996. doi: 10.1038/s41589-020-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Walker E., Gopalakrishnan R., Bogyo M., Basilion J.P. Microscopic detection of quenched activity-based optical imaging probes using an antibody detection system: Localizing protease activity. Mol. Imaging Biol. 2014;16:608–618. doi: 10.1007/s11307-014-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pluckthun A. Designed ankyrin repeat proteins (DARPins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015;55:489–511. doi: 10.1146/annurev-pharmtox-010611-134654. [DOI] [PubMed] [Google Scholar]
- 288.Kramer L., Renko M., Zavrsnik J., Turk D., Seeger M.A., Vasiljeva O., Grutter M.G., Turk V., Turk B. Non-invasive in vivo imaging of tumour-associated cathepsin B by a highly selective inhibitory DARPin. Theranostics. 2017;7:2806–2821. doi: 10.7150/thno.19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kwong G.A., von Maltzahn G., Murugappan G., Abudayyeh O., Mo S., Papayannopoulos I.A., Sverdlov D.Y., Liu S.B., Warren A.D., Popov Y., et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 2013;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Schuerle S., Dudani J.S., Christiansen M.G., Anikeeva P., Bhatia S.N. Magnetically Actuated Protease Sensors for in Vivo Tumor Profiling. Nano Lett. 2016;16:6303–6310. doi: 10.1021/acs.nanolett.6b02670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kwon E.J., Dudani J.S., Bhatia S.N. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat. Biomed. Eng. 2017;1 doi: 10.1038/s41551-017-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Loynachan C.N., Soleimany A.P., Dudani J.S., Lin Y., Najer A., Bekdemir A., Chen Q., Bhatia S.N., Stevens M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019;14:883–890. doi: 10.1038/s41565-019-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Dudani J.S., Ibrahim M., Kirkpatrick J., Warren A.D., Bhatia S.N. Classification of prostate cancer using a protease activity nanosensor library. Proc. Natl. Acad. Sci. USA. 2018;115:8954–8959. doi: 10.1073/pnas.1805337115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kirkpatrick J.D., Warren A.D., Soleimany A.P., Westcott P.M.K., Voog J.C., Martin-Alonso C., Fleming H.E., Tammela T., Jacks T., Bhatia S.N. Urinary detection of lung cancer in mice via noninvasive pulmonary protease profiling. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aaw0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kwong G.A., Dudani J.S., Carrodeguas E., Mazumdar E.V., Zekavat S.M., Bhatia S.N. Mathematical framework for activity-based cancer biomarkers. Proc. Natl. Acad. Sci. USA. 2015;112:12627–12632. doi: 10.1073/pnas.1506925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chen C.H., Miller M.A., Sarkar A., Beste M.T., Isaacson K.B., Lauffenburger D.A., Griffith L.G., Han J. Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J. Am. Chem. Soc. 2013;135:1645–1648. doi: 10.1021/ja307866z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Miller M.A., Barkal L., Jeng K., Herrlich A., Moss M., Griffith L.G., Lauffenburger D.A. Proteolytic Activity Matrix Analysis (PrAMA) for simultaneous determination of multiple protease activities. Integr. Biol. 2011;3:422–438. doi: 10.1039/C0IB00083C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ng E.X., Miller M.A., Jing T., Lauffenburger D.A., Chen C.H. Low-volume multiplexed proteolytic activity assay and inhibitor analysis through a pico-injector array. Lab Chip. 2015;15:1153–1159. doi: 10.1039/C4LC01162G. [DOI] [PubMed] [Google Scholar]
- 299.Lu Y., Sun W., Gu Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J. Control. Release. 2014;194:1–19. doi: 10.1016/j.jconrel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Anderson C.F., Cui H. Protease-Sensitive Nanomaterials for Cancer Therapeutics and Imaging. Ind. Eng. Chem. Res. 2017;56:5761–5777. doi: 10.1021/acs.iecr.7b00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hu Q., Katti P.S., Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273–12286. doi: 10.1039/C4NR04249B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Breistol K., Hendriks H.R., Fodstad O. Superior therapeutic efficacy of N-L-leucyl-doxorubicin versus doxorubicin in human melanoma xenografts correlates with higher tumour concentrations of free drug. Eur. J. Cancer. 1999;35:1143–1149. doi: 10.1016/S0959-8049(99)00074-X. [DOI] [PubMed] [Google Scholar]
- 303.Bajjuri K.M., Liu Y., Liu C., Sinha S.C. The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity. ChemMedChem. 2011;6:54–59. doi: 10.1002/cmdc.201000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhong Y.J., Shao L.H., Li Y. Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy (Review) Int. J. Oncol. 2013;42:373–383. doi: 10.3892/ijo.2012.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Choi K.Y., Swierczewska M., Lee S., Chen X. Protease-activated drug development. Theranostics. 2012;2:156–178. doi: 10.7150/thno.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sievers E.L., Senter P.D. Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 307.Chari R.V., Miller M.L., Widdison W.C. Antibody-drug conjugates: An emerging concept in cancer therapy. Angew. Chem. Int. Ed. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 308.Senter P.D., Sievers E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 309.Deeks E.D. Polatuzumab Vedotin: First Global Approval. Drugs. 2019;79:1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Beck A., Wurch T., Bailly C., Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 311.Desnoyers L.R., Vasiljeva O., Richardson J.H., Yang A., Menendez E.E., Liang T.W., Wong C., Bessette P.H., Kamath K., Moore S.J., et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 2013;5:207ra144. doi: 10.1126/scitranslmed.3006682. [DOI] [PubMed] [Google Scholar]
- 312.Wong K.R., Menendez E., Craik C.S., Kavanaugh W.M., Vasiljeva O. In vivo imaging of protease activity by Probody therapeutic activation. Biochimie. 2016;122:62–67. doi: 10.1016/j.biochi.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hu J., Zhang G., Liu S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012;41:5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 314.Maeda H., Bharate G.Y., Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 315.Salvioni L., Rizzuto M.A., Bertolini J.A., Pandolfi L., Colombo M., Prosperi D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers. 2019;11:1855. doi: 10.3390/cancers11121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 317.Duncan R. Polymer therapeutics as nanomedicines: New perspectives. Curr. Opin. Biotechnol. 2011;22:492–501. doi: 10.1016/j.copbio.2011.05.507. [DOI] [PubMed] [Google Scholar]
- 318.Knop K., Hoogenboom R., Fischer D., Schubert U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 319.Zhang P., Sun F., Liu S., Jiang S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release. 2016;244:184–193. doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wong C., Stylianopoulos T., Cui J., Martin J., Chauhan V.P., Jiang W., Popovic Z., Jain R.K., Bawendi M.G., Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhu L., Wang T., Perche F., Taigind A., Torchilin V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gustafson J.A., Price R.A., Frandsen J., Henak C.R., Cappello J., Ghandehari H. Synthesis and characterization of a matrix-metalloproteinase responsive silk-elastinlike protein polymer. Biomacromolecules. 2013;14:618–625. doi: 10.1021/bm3013692. [DOI] [PubMed] [Google Scholar]
- 323.Li C., Yu D.F., Newman R.A., Cabral F., Stephens L.C., Hunter N., Milas L., Wallace S. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58:2404–2409. [PubMed] [Google Scholar]
- 324.Gabriel D., Busso N., So A., van den Bergh H., Gurny R., Lange N. Thrombin-sensitive photodynamic agents: A novel strategy for selective synovectomy in rheumatoid arthritis. J. Control. Release. 2009;138:225–234. doi: 10.1016/j.jconrel.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 325.Gabriel D., Campo M.A., Gurny R., Lange N. Tailoring protease-sensitive photodynamic agents to specific disease-associated enzymes. Bioconjug. Chem. 2007;18:1070–1077. doi: 10.1021/bc060321l. [DOI] [PubMed] [Google Scholar]
- 326.Chandran S.S., Nan A., Rosen D.M., Ghandehari H., Denmeade S.R. A prostate-specific antigen activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol. Cancer Ther. 2007;6:2928–2937. doi: 10.1158/1535-7163.MCT-07-0392. [DOI] [PubMed] [Google Scholar]
- 327.Schmid B., Chung D.E., Warnecke A., Fichtner I., Kratz F. Albumin-binding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: Synthesis and antitumor efficacy. Bioconjug. Chem. 2007;18:702–716. doi: 10.1021/bc0602735. [DOI] [PubMed] [Google Scholar]
- 328.Graeser R., Chung D.E., Esser N., Moor S., Schachtele C., Unger C., Kratz F. Synthesis and biological evaluation of an albumin-binding prodrug of doxorubicin that is cleaved by prostate-specific antigen (PSA) in a PSA-positive orthotopic prostate carcinoma model (LNCaP) Int. J. Cancer. 2008;122:1145–1154. doi: 10.1002/ijc.23050. [DOI] [PubMed] [Google Scholar]
- 329.Ramishetti S., Huang L. Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy. Ther. Deliv. 2012;3:1429–1445. doi: 10.4155/tde.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wan Y., Han J., Fan G., Zhang Z., Gong T., Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34:3020–3030. doi: 10.1016/j.biomaterials.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 331.Basel M.T., Shrestha T.B., Troyer D.L., Bossmann S.H. Protease-sensitive, polymer-caged liposomes: A method for making highly targeted liposomes using triggered release. ACS Nano. 2011;5:2162–2175. doi: 10.1021/nn103362n. [DOI] [PubMed] [Google Scholar]
- 332.Ringgaard L., Melander F., Eliasen R., Henriksen J.R., Jolck R.I., Engel T.B., Bak M., Fliedner F.P., Kristensen K., Elema D.R., et al. Tumor repolarization by an advanced liposomal drug delivery system provides a potent new approach for chemo-immunotherapy. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liu Z., Xiong M., Gong J., Zhang Y., Bai N., Luo Y., Li L., Wei Y., Liu Y., Tan X., et al. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat. Commun. 2014;5:4280. doi: 10.1038/ncomms5280. [DOI] [PubMed] [Google Scholar]
- 334.Banerjee J., Hanson A.J., Gadam B., Elegbede A.I., Tobwala S., Ganguly B., Wagh A.V., Muhonen W.W., Law B., Shabb J.B., et al. Release of liposomal contents by cell-secreted matrix metalloproteinase-9. Bioconjug. Chem. 2009;20:1332–1339. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Mikhaylov G., Klimpel D., Schaschke N., Mikac U., Vizovisek M., Fonovic M., Turk V., Turk B., Vasiljeva O. Selective targeting of tumor and stromal cells by a nanocarrier system displaying lipidated cathepsin B inhibitor. Angew. Chem. Int. Ed. 2014;53:10077–10081. doi: 10.1002/anie.201402305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mumtaz T., Qindeel M., Asim Ur R., Tarhini M., Ahmed N., Elaissari A. Exploiting proteases for cancer theranostic through molecular imaging and drug delivery. Int. J. Pharm. 2020;587:119712. doi: 10.1016/j.ijpharm.2020.119712. [DOI] [PubMed] [Google Scholar]
- 337.de la Rica R., Aili D., Stevens M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 338.Liu D., Yang F., Xiong F., Gu N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics. 2016;6:1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zou Z., He X., He D., Wang K., Qing Z., Yang X., Wen L., Xiong J., Li L., Cai L. Programmed packaging of mesoporous silica nanocarriers for matrix metalloprotease 2-triggered tumor targeting and release. Biomaterials. 2015;58:35–45. doi: 10.1016/j.biomaterials.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 340.Ansari C., Tikhomirov G.A., Hong S.H., Falconer R.A., Loadman P.M., Gill J.H., Castaneda R., Hazard F.K., Tong L., Lenkov O.D., et al. Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small. 2014;10:417, 566–575. doi: 10.1002/smll.201301456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cao Y., Mao Z., He Y., Kuang Y., Liu M., Zhou Y., Zhang Y., Pei R. Extremely Small Iron Oxide Nanoparticle-Encapsulated Nanogels as a Glutathione-Responsive T1 Contrast Agent for Tumor-Targeted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces. 2020;12:26973–26981. doi: 10.1021/acsami.0c07288. [DOI] [PubMed] [Google Scholar]
- 342.Oliveira-Silva R., Sousa-Jeronimo M., Botequim D., Silva N.J.O., Paulo P.M.R., Prazeres D.M.F. Monitoring Proteolytic Activity in Real Time: A New World of Opportunities for Biosensors. Trends Biochem. Sci. 2020;45:604–618. doi: 10.1016/j.tibs.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]