Abstract
ATP-binding cassette transporter (ABC) A7 is a membrane protein that belongs to the large family of ABC transporters. It is 54% homologous in amino acid residue sequence to ABCA1 which mediates biogenesis of plasma high density lipoprotein (HDL) from cellular phospholipid and cholesterol with extracellular helical apolipoproteins such as apolipoprotein (apo) A-I. When transfected and expressed, ABCA7 also mediates generation of HDL-like particles but small and of less cholesterol content. However, endogenous ABCA7 is unlikely involved in HDL biogenesis and rather to regulate the host-defense system such as phagocytotic function of the cells. ABCA1 expression is regulated by cellular cholesterol levels, positively by the liver X receptor (LXR) in extrahepatic peripheral cells. However, it is modulated dually in the liver being relevant to transport of cholesterol for its catabolism; positively by LXR and negatively by sterol regulatory element binding protein (SREBP) or hepatic nuclear factor 4α (HNF4α). In contrast, ABCA7 expression was shown to be regulated negatively by the SREBP system so that decrease of cell cholesterol enhances ABCA7 function such as cellular phagocytotic reaction, suggesting that it links cholesterol metabolism to the host defense system. The interest is being build up in ABCA7 as its genomic diversity has been found related to a risk for late-onset Alzheimer’s diseases. More recent findings indicate that ABCA7 is involved in metabolism of amyloid β peptide including its phagocytotic clearance. Accordingly, modulation of ABCA7 activity by manipulating cholesterol metabolism may open a new path for management of Alzheimer’s disease.
Keywords: ABC transporter, Cholesterol, High density lipoprotein, Phagocytosis, Alzheimer’s disease
1. Introduction
Since human MDR1, a multi-drug transporter gene, was isolated as the first eukaryote ATP Binding Cassette (ABC) transporters in 1986, 48 genes in that category have been identified so far and half of them mediate translocation of lipids or lipid-like molecules (Ueda, 2011; Neumann et al., 2017). Functional ABC transporters contain two transmembrane domains (TMDs) (composed of six or eleven alpha-helices) and two nucleotide binding domains (NBDs), Walker motifs. They are classified based on the amino acid sequence similarity and domain organization. In “full transporters”, all the two TMDs and MBDs are expressed in a single polypeptide while “half transporters” are expressed with only one TMD and NBD. Among mammalian ABC transporter subfamilies, ABCA and ABCC are full transporters and ABCD and ABCG are half transporters. There are both full and half transporters in ABCB subfamily (Ueda, 2011; Neumann et al., 2017). Impairment of their functions became known to cause various disorders. Some of them play important roles in sterol homeostasis, such as ABCG5 and ABCG8 in sorting sterols in their excretion from intestinal and liver cells, so that defect of their function causes uncontrolled overabsorption of plant sterols (Berge et al., 2000). ABCA1 and ABCG1 are found crucial for generating high density lipoproteins (HDL) in its catabolic transport from the peripheral tissues to the liver (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Rust et al., 1999; Klucken et al., 2000). ABCA1 has especially been focused as a target of research on pathogenesis of atherosclerosis since it was identified as a causative molecule in genetic deficiency of HDL biogenesis, Tangier disease.
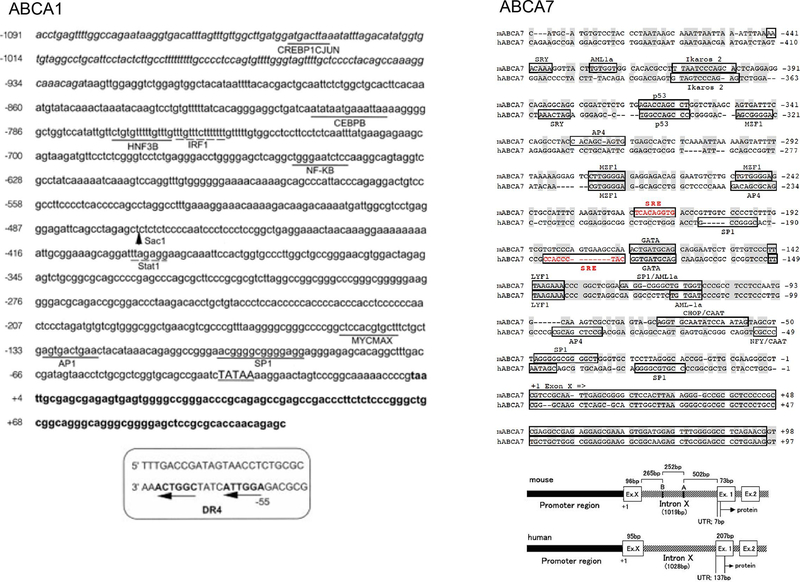
ABCA7 is one of the ABC transporters with two sets of TMD and two NBDs, and found highly homologous and structurally comparable to ABCA1, as ABCA7 and ABCA1 share 54% homogeneity in amino acid sequences (Kaminski et al., 2000a). On the other hand, its identity among species seems lower than ABCA1, e.g., human and mice chromosomes show 79% amino acid identity for ABCA7 compared to 95% identity for ABCA1 (Kaminski et al., 2000b), indicating its function more diverse in ABCA7 than ABCA1 (Abe-Dohmae et al., 2006b). ABCA7 was first identified as a “sterol-sensitive” ABC transporter, reportedly upregulated by low density lipoprotein (LDL) anddownregulated by HDL (Kaminski et al., 2000b) though sterol regulatory element (SRE) was not obviously conserved crossing species (Broccardo et al., 2001). A new exon was identified later and the functional SRE was found in the new promoter regionboth in human and mouse, confirmed by luciferase activity assay (Fig. 2) (Iwamoto et al., 2006).
2. ABCA7 as a good artificial reference model for studying HDL biogenesis reaction mediated by ABCA1
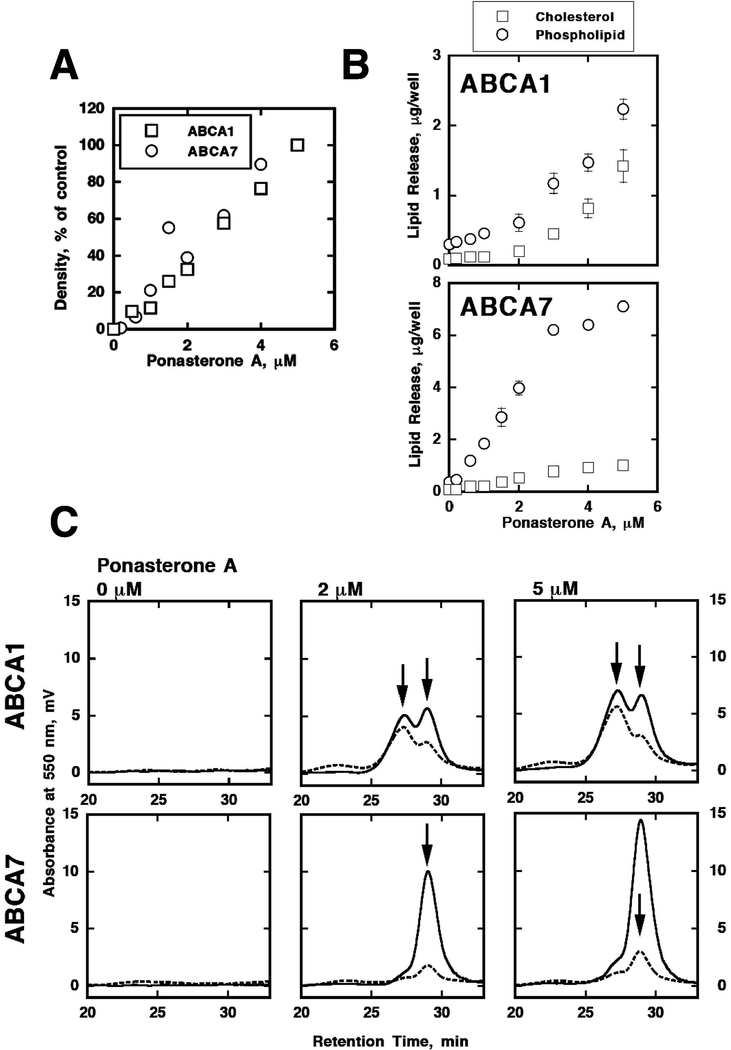
Knowing ABCA1 as a key molecule in HDL biogenesis and ABCA7 as a highly homologous molecule to ABCA1, ABCA7 was examined for its function in HDL biogenesis. When transfected and expressed, ABCA7 mediated production of HDL of cellular phospholipid with extracellular alpha-helical apolipoproteins, such as apolipoprotein (apo) A-I (Wang et al., 2003; Abe-Dohmae et al., 2004). The HDL particles thus generated contained much less cholesterol than those formed by the reaction mediated by similarly transfected ABCA1 (Wang et al., 2003; Abe-Dohmae et al., 2004). When the products were analyzed for their size, HDL produced by ABCA7 was smaller than those generated by ABCA1 (Abe-Dohmae et al., 2006a). Further analysis by HPLC was conducted by using the expression system induced by insect molting hormone for ABCA1 and ABCA7 (Fig. 1A) and accordingly lipid release (Fig. 1B). It revealed that the products by ABCA7 appeared as a single peak of small and cholesterol-poor particles, in contrast to the products by ABCA1 that consist of two peaks of small and cholesterol-poor particles and large and cholesterol-rich particles (Fig. 1C) (Hayashi et al., 2005). When the production increased by increasing ABCA1 or apolipoprotein, the cholesterol-rich large component increased predominantly (Fig. 1C) (Hayashi et al., 2005). These findings suggested that HDL particles are essentially assembled by helical apolipoprotein with membrane phospholipid and cholesterol is the secondary component to consist the particles. Analysis of phospholipid molecular species in the origin cells and in the HDL generated indicated that those with shorter and less unsaturated acyl chains are preferably used for assembly of HDL, and there seems no difference between small cholesterol-poor and large cholesterol-rich particles (Hotta et al., 2015). ABCA7 may preferably remove lysophosphatidylcholine among phospholipids (Tomioka et al., 2017). Rate of HDL production is linear to expression level of ABCA7 but it appears as an accelerating profile for the level of ABCA1 expression, potentially implicating a kind of cooperativity between the transporter molecules (Fig. 1) (Hayashi et al., 2005). ABCA7 mRNA generates spliced cDNA in addition to full-length cDNA, and only the latter is located on cell surface and mediates generation of HDL when transfected (Ikeda et al., 2003). Thus, ABCA7-mediated HDL biogenesis is a good tool to investigate and examine the mechanism for ABCA1-mediated “physiological” reaction as a reference model. Comparison of the HDL biogenesis reactions between the ABCA1- and ABCA7-transfected cells should be useful to study insight of molecular mechanism of ABCA1-mediated HDL biogenesis reaction. We attempted to generate hybrid proteins to investigate this reaction, which has been so far unsuccessful.
Fig. 1.
Generation of HDL-like particles by ABCA1 and ABCA7 which are transfected in HEK293 cells in ecdysone-inducible forms (Hayashi et al., 2005). Their expression were linearly proportional to concentration of ponasterone, an ecdysone analogue, (A), and apoA-I mediated cell lipid release was exponentially increased by ABCA1 whereas linearly by ABCA7 (B). HPLC analysis of the HDL-like products showed two peaks for ABCA1-generated particles and a single peak for ABCA7-generated particles (C).
ABCA7 has thus been shown to be a good reference model for ABCA1 for studying HDL biogenesis reaction in the transfected cell system. However, lack of ABCA7 expression in mice did not show apparent influence on cell cholesterol release reactions (Kim et al., 2005; Meurs et al., 2012). Phenotype of ABCA7 null mice appeared with mild decrease in HDL and visceral adipose tissue but only in female (Kim et al., 2005) though they were unable to show evidence for ABCA7 to support apolipoprotein-related sterol transport or to relate to food intake regardless of gender (Kim et al., 2005). However, these findings were later confirmed and accompanied by gender-specific decrease in plasma adiponectin levels (Bhatia et al., 2017). Although some compensatory increase of expression was observed between ABCA1 and ABCA7, it is not clear how such finding is related to cholesterol/lipid metabolism (Iwamoto et al., 2006; Meurs et al., 2012). On the other hand, deletion of ABCA7 indicated its relation to T-cell proliferation/development (Meurs et al., 2012; Nowyhed et al., 2017). In fact, structural analysis of the promoter and genomic organization indicated its potential involvement in developmental specification of myelolymphoid cell lineages (Broccardo et al., 2001; Iwamoto et al., 2006).
3. ABCA7 links sterol metabolism to host defense system
ABCA7 expression has been found increased in the fibroblast of ABCA1-deficient mice but apoA-I-dependent cell cholesterol release is not compensated at all (Iwamoto et al., 2006). Thus, ABCA7 unlikely plays a significant role in HDL metabolism in vivo. It has been then rather noted that manipulation of endogenous ABCA7 expression in mouse cells is associated with phagocytosis activity of the cells rather than cell cholesterol release (Iwamoto et al., 2006; Jehle et al., 2006; Tanaka et al., 2010, 2011a).
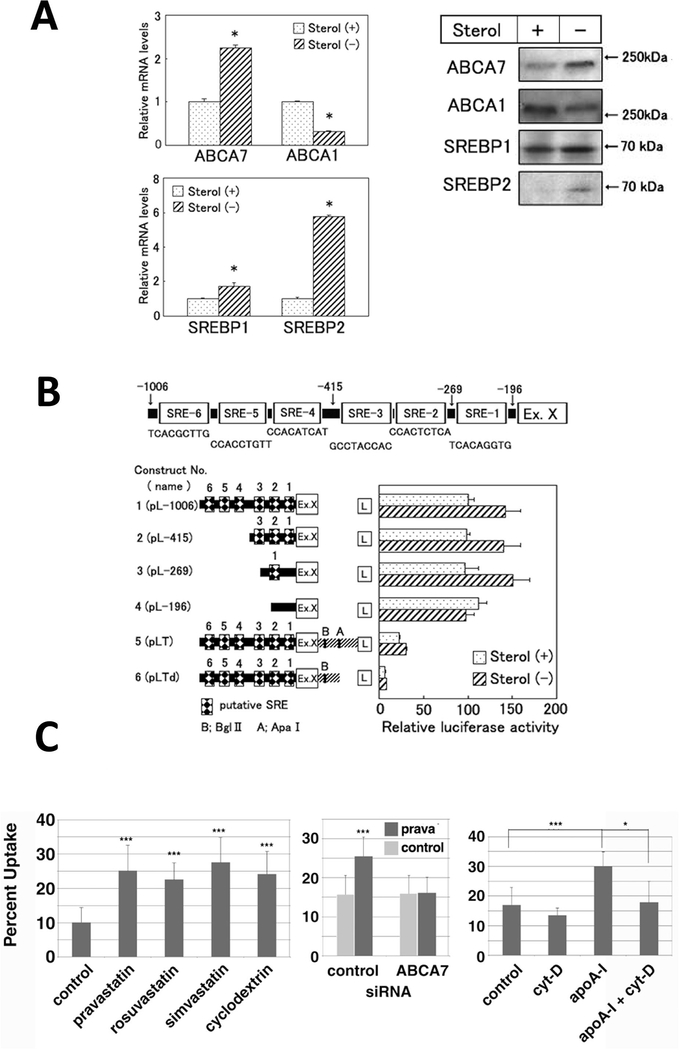
Expression of ABCA7 was down-regulated by increase of cellular cholesterol while ABCA1 was up-regulated, and the results were consistent by forced expression or down-regulation of SRE binding proteins (SREBPs) (Fig. 3A) (Iwamoto et al., 2006). The promoter of the ABCA7 gene includes the exon encoding 96 bp (mouse) and 95 bp (human) of the 5′-untranslated region and the transcription-starting site at 1122 bp (mouse) and 1260 bp (human) upstream of the initiation methionine codon. The 5′ upstream of this exon is the ABCA7 proximal promoter containing multiple binding sites of transcription factors for hematopoiesis, and SRE of 9 bp at 212 bp (mouse) and 179 bp (human) upstream of this exon (Fig. 2). While the apoA-I-mediated lipid release was not influenced by suppression of the endogenous ABCA7 with siRNA inmouse fibroblasts or by its increase in the ABCA1-deficient mouse cells, the phagocytic activity was in contrast altered in parallel to the ABCA7 expression in these cells (Iwamoto et al., 2006). When phagocytosis was induced, the messages increased for SREBP2, ABCA7 and other SREBP2-regulated proteins. The ABCA1 message rather decreased in this condition.
Fig. 3.
Negative regulation of ABCA7 expression and its function by cell cholesterol via SREBP. Expression of ABCA1 and ABCA7 are regulated in opposite directions by cell cholesterol in BALB/3T3 cells (A) and the responsible site for this regulation is SRE coded between positions −296 to −196 (SRE-1) (B) (Iwamoto et al., 2006). Accordingly, phagocytosis was upregulated by various statins in J774 cells (C) (Tanakaet al., 2011a).
Fig. 2.
Putative structures of the promoters of ABCA1 (Costet et al., 2000) and ABCA7 (Iwamoto et al., 2006).
Accordingly, phagocytosis was found enhanced by hydroxymethylglutaryl (HMG)-CoA reductase inhibitors, pravastatin, rosuvastatin and simvastatin as well as cyclodextrin in J774 macrophages (Fig. 3B), as cellular cholesterol was reduced and expressions of the cholesterol-related genes were modulated, including an increase of ABCA7 mRNA and decrease of ABCA1 mRNA (Tanaka et al., 2011a). Conversely, knock-down of ABCA7 expression by siRNA ablated enhancement of phagocytosis by statins. In vivo, pravastatin enhanced phagocytosis in wild-type mice, but not in ABCA7-knockout mice (Tanaka et al., 2011a). Phagocytotic activity to various targets, including foreign bodies, Gram-positive and -negative bacteria, and fungi, were upregulated by statins (Tanaka et al., 2011a). These findings provide a molecular basis for enhancement of the host-defense system by statins showing that one of their “pleiotropic” effects is in fact achieved through their reaction to a primary target.
ABCA1 is degraded by calpain after its internalization and helical apolipoproteins such as apoA-I retards this process and accordingly results in its increase (Arakawa and Yokoyama, 2002; Lu et al., 2008). ABCA7 was similarly shown stabilized by apoA-I and apoA-II against this degradation, and phagocytic activity was found enhanced by apoA-I and apoA-II more than twice at maximum in J774 and mouse peritoneal macrophages (Tanaka et al., 2010). Cell surface biotinylation experiments demonstrated that endogenous ABCA7 predominantly resides on the cell surface and the apolipoproteins increase the surface ABCA7. The increase of phagocytosis by apolipoproteins was retained in the J774 cells treated with ABCA1 siRNA and in the peritoneal macrophages from ABCA1-knockout mice, but was abolished in the J774 cells treated with ABCA7 siRNA and in the peritoneal macrophages from ABCA7-knockout mice (Tanaka et al., 2010). Phagocytosis was shown decreased in the cells in the peritoneal cavity of ABCA1-knockout mouse with low apoA-I level in the peritoneal fluid, compared to the wild type control. Thus, extracellular helical apolipoproteins augment ABCA7-associated phagocytosis by stabilizing ABCA7 (Tanaka et al., 2010).
Thus, expression of ABCA7 is regulated in association with cell cholesterol metabolism, but its physiological function may not be associated with regulation of cholesterol metabolism but rather related to host-defense system (Tanaka et al., 2011b; Abe-Dohmae and Yokoyama, 2012). It is still not clear how these functions are related to apparent involvement of ABCA7 in adipose tissue development in female mice (Kim et al., 2005; Bhatia et al., 2017)
4. ABCA7 and Alzheimer’s disease
ABCA7 became a focus of interest by scientific community when genome-wide association studies have identified this protein as a susceptibility locus for late-onset Alzheimer’s disease (Hollingworth et al., 2011; Naj et al., 2011; Lambert et al., 2013; Reitz et al., 2013). Single nucleotide polymorphisms associated with this disease are found in various domains of the gene including introns and exons including the one coding G1527A substitution. These findings have later been further investigated to find the association with more specific symptoms of the disease, memory decline and cognitive impairment (Carrasquillo et al., 2015), and with image findings of cortical and hippocampal atrophy (Carrasquillo et al., 2014; Ramirez et al., 2016). These findings were indicated to relate with loss of function of ABCA7 (Steinberg et al., 2015).
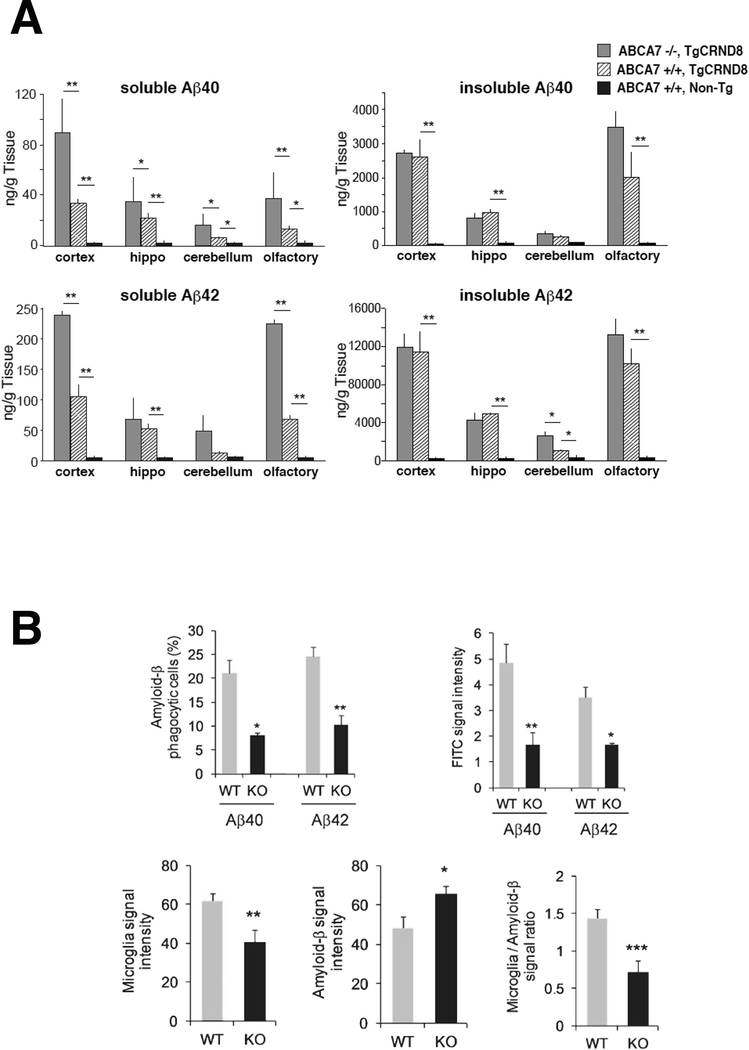
A role of ABCA7 in processing amyloid precursor protein was investigated in various cell lines and mouse brain (Fig. 4A) (Satoh et al., 2015). Suppression of endogenous ABCA7 increased β-secretase cleavage and elevated amyloid-β in several different cell lines. ABCA7 null mice showed an increased production of endogenous murine amyloid-β42 species. Crossing ABCA7-deficient mice to an amyloid precursor protein transgenic model (TgCRND8) resulted in significant increases in the soluble amyloid-β. While only modest changes in insoluble amyloid-β and amyloid plaque densities were observed once the amyloid pathology was well developed, amyloid-β deposition was enhanced in younger animals (Satoh et al., 2015). ABCA7-deficient mice crossed with another amyloidogenic mouse strain (hAPP swInd J20) also showed increase in amyloid-β accumulation, although their histological findings were different (Kim et al., 2013). In vitro studies indicated a more rapid endocytosis of amyloid precursor protein in ABCA7 knock-out cells being consistent with the increased amyloid-β production. The findings indicated a direct role of ABCA7 in amyloid processing and it may be associated with its primary biological function to regulate endocytic pathways (Satoh et al., 2015).
Fig. 4.
Accumulation amyloid β peptides in the mouse brain by deficiency of ABCA7. Increase in soluble amyloid β in the brain of the APP-transgenic mice by deficiency of ABCA7 (A) (Satoh et al., 2015). ABCA7-dependent phagocytosis is responsible for amyloid β clearance by microglia in mouse brain (B) (Fu et al., 2016).
Based on these findings, more direct focus were attempted to investigate production and clearance of amyloid-β. Suppression of ABCA7 function accelerated its production (Sakae et al., 2016; Aikawa et al., 2018) and decreased the clearance (Fig. 4B) (Fu et al., 2016). Microglia may be responsible for these alteration in metabolism of amyloid-β (Aikawa et al., 2019).
5. Potential link of sterol metabolism to ABCA7 gene expression
In spite of high homology of ABCA7 to ABCA1, one of the key players of cholesterol homeostasis, it is unclear how the ABCA7 gene expression is associated with sterol metabolism. Expression of ABCA1 gene is clearly regulated by cellular cholesterol (Fig. 2). In most of extrahepatic cells, it functions to release cell cholesterol as an essential part of catabolic pathway, so that the gene is upregulated by cell cholesterol level by using the liver X receptor system (LXR) (Costet et al., 2000; Santamarina-Fojo et al., 2000). In hepatocytes, however, HDL should not be overproduced in order to prevent back flow of cholesterol transported from the extrahepatic tissues to the liver. Its gene expression is therefore down-regulated by cell cholesterol via SREBP2 or hepatic nuclear factor (HNF) 4α transcriptional regulation (Tamehiro et al., 2007; Maejima et al., 2011; Ohoka et al., 2012). Involvement of protein kinase D and activator protein 2β has also been shown as cholesterol-unrelated regulation (Iwamoto et al., 2007, 2008; Iwamoto and Yokoyama, 2011), but regulation of ABCA1 gene expression is largely consistent with its roles in cholesterol metabolism/transport. In contrast, functional sterol-related transcription site in the ABCA7 promoter seems only that for SREBP2 (Fig. 2) (Iwamoto et al., 2006), which generally considered as down regulation site by cell cholesterol. Therefore, it is unlikely for this molecule to be involved in cholesterol catabolic pathway in any step. Nevertheless, it is clear that cell cholesterol does negatively regulate expression of ABCA7 and therefore its function of phagocytosis (Iwamoto et al., 2006). The reason is unknown yet why sterol metabolism is associated with such a function as related to host-defense and perhaps body immune system.
ABCA7 now is considered to play a crucial role in development of Alzheimer’s disease. More seemingly, impairment of ABCA7 function is associated to increase in generation and decrease in processing of amyloid-β, and the later reaction seems related to cellular phagocytosis activity of ABCA7 (Fu et al., 2016). It is therefore possible to enhance this function of ABCA7 by manipulation of cell cholesterol metabolism, as inhibition of HMG-CoA reductase has been shown to enhance cell phagocytosis activity (Tanaka et al., 2011a). These findings would provide the molecular background for the previous clinical findings that statins may prevent development of Alzheimer’s disease (Zissimopoulos et al., 2017; Chu et al., 2018). It is interesting to note that the effect of statins was found more apparent in females in some ethnic groups (Zissimopoulos et al., 2017), which would remind us that phenotypes by ABCA7 deletion were only seen in female mice (Kim et al., 2005; Bhatia et al., 2017).
6. Closing remarks
Function of ABCA7 is still puzzling. It is seemingly involved in lipid metabolism as its deficiency in mice appears as moderate decrease in plasma HDL and adipogenesis in female only, while expression of ABCA7 is regulated by cell cholesterol level. ABCA7 is associated with cellular phagocytotic activity, which is upregulated by decrease in cell cholesterol. This function may be a background for association of ABCA7 variants with a risk for Alzheimer’s disease, related to clearance of Amyloid β peptide.
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series–a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org./wiki/ABCA7.
Funding
This work was supported by the MEXT-Supported Program for Strategic Founding of Research in Private Universities (S1201007) and by Grants-in-aid from MEXT Japan (24614018, 15H02903, 19K11805).
Abbreviations:
- ABC
ATP binding cassette transporter
- TMD
transmembrane domain
- NBD
nucleotide binding domain
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- SRE
sterol regulatory element
- apo
apolipoprotein
- SREBP
SRE binding protein
- HMG
hydroxymethylglutaryl
- LXR
liver X receptor
- HNF
hepatic nuclear factor
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abe-Dohmae S, Ikeda Y, Matsuo M, Hayashi M, Okuhira K, Ueda K, Yokoyama S, 2004. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 279, 604–611. [DOI] [PubMed] [Google Scholar]
- Abe-Dohmae S, Kato KH, Kumon Y, Hu W, Ishigami H, Iwamoto N, Okazaki M, Wu CA, Tsujita M, Ueda K, Yokoyama S, 2006a. Serum amyloid A generates high density lipoprotein with cellular lipid in an ABCA1- or ABCA7-dependent manner. J. Lipid Res. 47, 1542–1550. [DOI] [PubMed] [Google Scholar]
- Abe-Dohmae S, Ueda K, Yokoyama S, 2006b. ABCA7, a molecule with unknown function. FEBS Lett. 580, 1178–1182. [DOI] [PubMed] [Google Scholar]
- Abe-Dohmae S, Yokoyama S, 2012. ABCA7: a potential mediator between cholesterol homeostasis and the host defense system. Clinical Lipidology 7, 677–687. [Google Scholar]
- Aikawa T, Holm ML, Kanekiyo T, 2018. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa T, Ren Y, Yamazaki Y, Tachibana M, Johnson MR, Anderson CT, Martens YA, Holm ML, Asmann YW, Saito T, Saido TC, Fitzgerald ML, Bu G, Kanekiyo T, 2019. ABCA7 haplodeficiency disturbs microglial immune responses in the mouse brain. Proc. Natl. Acad. Sci. USA 116, 23790–23796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa R, Yokoyama S, 2002. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 277, 22426–22429. [DOI] [PubMed] [Google Scholar]
- Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH, 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Fu Y, Hsiao JT, Halliday GM, Kim WS, 2017. Deletion of Alzheimer’s Disease Risk Gene ABCA7 Alters White Adipose Tissue Development and Leptin Levels. J. Alzheimers Dis. Rep. 1, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G, 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22, 347–351. [DOI] [PubMed] [Google Scholar]
- Broccardo C, Osorio J, Luciani MF, Schriml LM, Prades C, Shulenin S, Arnould I, Naudin L, Lafargue C, Rosier M, Jordan B, Mattei MG, Dean M, Denèfle P, Chimini G, 2001. Comparative analysis of the promoter structure and genomic organization of the human and mouse ABCA7 gene encoding a novel ABCA transporter. Cytogenet. Cell Genet. 92, 264–270. [DOI] [PubMed] [Google Scholar]
- Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J Jr., Hayden MR, 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22, 336–345. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, Crook JE, Pedraza O, Thomas CS, Pankratz VS, Allen M, Nguyen T, Malphrus KG, Ma L, Bisceglio GD, Roberts RO, Lucas JA, Smith GE, Ivnik RJ, Machulda MM, Graff-Radford NR, Petersen RC, Younkin SG, Ertekin-Taner N, 2015. Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging 36, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo MM, Khan Q, Murray ME, Krishnan S, Aakre J, Pankratz VS, Nguyen T, Ma L, Bisceglio G, Petersen RC, Younkin SG, Dickson DW, Boeve BF, Graff-Radford NR, Ertekin-Taner N, 2014. Late-onset Alzheimer disease genetic variants in posterior cortical atrophy and posterior AD. Neurology 82, 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, Yang WC, Chen YW, Wu CK, Veronese N, Carvalho AF, Fernandes BS, Herrmann N, Lin PY, 2018. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 8, 5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costet P, Luo Y, Wang N, Tall AR, 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245. [DOI] [PubMed] [Google Scholar]
- Fu Y, Hsiao JH, Paxinos G, Halliday GM, Kim WS, 2016. ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J. Alzheimers Dis. 54, 569–584. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Abe-Dohmae S, Okazaki M, Ueda K, Yokoyama S, 2005. Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J. Lipid Res. 46, 1703–1711. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Abraham R, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Jones N, Stretton A, Thomas C, Richards A, Ivanov D, Widdowson C, Chapman J, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Beaumont H, Warden D, Wilcock G, Love S, Kehoe PG, Hooper NM, Vardy ER, Hardy J, Mead S, Fox NC, Rossor M, Collinge J, Maier W, Jessen F, Rüther E, Schürmann B, Heun R, Kölsch H, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Gallacher J, Hüll M, Rujescu D, Giegling I, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Pankratz VS, Sando SB, Aasly JO, Barcikowska M, Wszolek ZK, Dickson DW, Graff-Radford NR, Petersen RC, et al. , 2011. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Abe-Dohmae S, Taguchi R, Yokoyama S, 2015. Preferential incorporation of shorter and less unsaturated acyl phospholipids into high density lipoprotein-like particles in the ABCA1- and ABCA7-mediated biogenesis with apoA-I. Chem. Phys. Lipids 187, 1–9. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Abe-Dohmae S, Munehira Y, Aoki R, Kawamoto S, Furuya A, Shitara K, Amachi T, Kioka N, Matsuo M, Yokoyama S, Ueda K, 2003. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem. Biophys. Res. Commun. 311, 313–318. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Abe-Dohmae S, Ayaori M, Tanaka N, Kusuhara M, Ohsuzu F, Yokoyama S, 2007. ATP-binding cassette transporter A1 gene transcription is downregulated by activator protein 2alpha. Doxazosin inhibits activator protein 2alpha and increases high-density lipoprotein biogenesis independent of alpha1-adrenoceptor blockade. Circ. Res. 101, 156–165. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Abe-Dohmae S, Lu R, Yokoyama S, 2008. Involvement of protein kinase D in phosphorylation and increase of DNA binding of activator protein 2 alpha to downregulate ATP-binding cassette transporter A1. Arterioscler. Thromb. Vasc. Biol. 28, 2282–2287. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Abe-Dohmae S, Sato R, Yokoyama S, 2006. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J. Lipid Res. 47, 1915–1927. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Yokoyama S, 2011. Protein kinase D regulates the adiponectin gene expression through phosphorylation of AP-2: a common pathway to the ABCA1 gene regulation. Atherosclerosis 216, 90–96. [DOI] [PubMed] [Google Scholar]
- Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N, Greenberg S, Dale BM, Qin C, Henson PM, Tall AR, 2006. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J. Cell Biol. 174, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski WE, Orsó E, Diederich W, Klucken J, Drobnik W, Schmitz G, 2000a. Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7). Biochem. Biophys. Res. Commun. 273, 532–538. [DOI] [PubMed] [Google Scholar]
- Kaminski WE, Piehler A, Schmitz G, 2000b. Genomic organization of the human cholesterol-responsive ABC transporter ABCA7: tandem linkage with the minor histocompatibility antigen HA-1 gene. Biochem. Biophys. Res. Commun. 278, 782–789. [DOI] [PubMed] [Google Scholar]
- Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N, Moore KJ, Freeman MW, 2005. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J. Biol. Chem. 280, 3989–3995. [DOI] [PubMed] [Google Scholar]
- Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T, Garner B, 2013. Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer’s disease. J. Neurosci. 33, 4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Büchler C, Orsó E, Kaminski WE, Porsch-Ozcürümez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, Allikmets R, Schmitz G, 2000. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci U S A 97, 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, et al. , 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45, 1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Arakawa R, Ito-Osumi C, Iwamoto N, Yokoyama S, 2008. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler. Thromb. Vasc. Biol. 28, 1820–1824. [DOI] [PubMed] [Google Scholar]
- Maejima T, Sugano T, Yamazaki H, Yoshinaka Y, Doi T, Tanabe S, Nishimaki-Mogami T, 2011. Pitavastatin increases ABCA1 expression by dual mechanisms: SREBP2-driven transcriptional activation and PPARα-dependent protein stabilization but without activating LXR in rat hepatoma McARH7777 cells. J. Pharmacol. Sci. 116, 107–115. [DOI] [PubMed] [Google Scholar]
- Meurs I, Calpe-Berdiel L, Habets KL, Zhao Y, Korporaal SJ, Mommaas AM, Josselin E, Hildebrand RB, Ye D, Out R, Kuiper J, Van Berkel TJ, Chimini G, Van Eck M, 2012. Effects of deletion of macrophage ABCA7 on lipid metabolism and the development of atherosclerosis in the presence and absence of ABCA1. PLoS ONE 7, e30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, et al. , 2011. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 43, 436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Rose-Sperling D, Hellmich UA, 2017. Diverse relations between ABC transporters and lipids: An overview. Biochim. Biophys. Acta, Biomembr. 1859, 605–618. [DOI] [PubMed] [Google Scholar]
- Nowyhed HN, Chandra S, Kiosses W, Marcovecchio P, Andary F, Zhao M, Fitzgerald ML, Kronenberg M, Hedrick CC, 2017. ATP Binding CassetteTransporter ABCA7 Regulates NKT Cell Development and Function by Controlling CD1d Expression and Lipid Raft Content. Sci. Rep. 7, 40273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N, Okuhira K, Cui H, Wu W, Sato R, Naito M, Nishimaki-Mogami T, 2012. HNF4α increases liver-specific human ATP-binding cassette transporter A1 expression and cholesterol efflux to apolipoprotein A-I in response to cholesterol depletion. Arterioscler. Thromb. Vasc. Biol. 32, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Ramirez LM, Goukasian N, Porat S, Hwang KS, Eastman JA, Hurtz S, Wang B, Vang N, Sears R, Klein E, Coppola G, Apostolova LG, 2016. Common variants in ABCA7 and MS4A6A are associated with cortical and hippocampal atrophy. Neurobiol. Aging 39, 82–89. [DOI] [PubMed] [Google Scholar]
- Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, Valladares O, Lin CF, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue M, Baldwin CT, Green RC, Barnes LL, Cantwell LB, Fallin MD, Go RC, Griffith P, Obisesan TO, Manly JJ, Lunetta KL, Kamboh MI, Lopez OL, Bennett DA, Hendrie H, Hall KS, Goate AM, Byrd GS, Kukull WA, Foroud TM, Haines JL, Farrer LA, Pericak-Vance MA, Schellenberg GD, Mayeux R, 2013. Variants in the ATP-binding cassette transporter (ABCA7), apolipoproteinE ∊4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309, 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, Assmann G, 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22, 352–355. [DOI] [PubMed] [Google Scholar]
- Sakae N, Liu CC, Shinohara M, Frisch-Daiello J, Ma L, Yamazaki Y, Tachibana M, Younkin L, Kurti A, Carrasquillo MM, Zou F, Sevlever D, Bisceglio G, Gan M, Fol R, Knight P, Wang M, Han X, Fryer JD, Fitzgerald ML, Ohyagi Y, Younkin SG, Bu G, Kanekiyo T, 2016. ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer’s Neuronal Pathology. J. Neurosci. 36, 3848–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng JF, Osorio J, Remaley A, Yang XP, Haudenschild C, Prades C, Chimini G, Blackmon E, Francois T, Duverger N, Rubin EM, Rosier M, Denèfle P, Fredrickson DS, Brewer HB Jr., 2000. Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci U S A 97, 7987–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Abe-Dohmae S, Yokoyama S, St George-Hyslop P, Fraser PE, 2015. ATP-binding cassette transporter A7 (ABCA7) loss of function alters Alzheimer amyloid processing. J. Biol. Chem. 290, 24152–24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Stefansson H, Jonsson T, Johannsdottir H, Ingason A, Helgason H, Sulem P, Magnusson OT, Gudjonsson SA, Unnsteinsdottir U, Kong A, Helisalmi S, Soininen H, Lah JJ, Aarsland D, Fladby T, Ulstein ID, Djurovic S, Sando SB, White LR, Knudsen GP, Westlye LT, Selbæk G, Giegling I, Hampel H, Hiltunen M, Levey AI, Andreassen OA, Rujescu D, Jonsson PV, Bjornsson S, Snaedal J, Stefansson K, 2015. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat. Genet. 47, 445–447. [DOI] [PubMed] [Google Scholar]
- Tamehiro N, Shigemoto-Mogami Y, Kakeya T, Okuhira K, Suzuki K, Sato R, Nagao T, Nishimaki-Mogami T, 2007. Sterol regulatory element-binding protein and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282, 21090–21099. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S, 2010. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J. Lipid Res. 51, 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S, 2011a. HMG-CoA reductase inhibitors enhance phagocytosis by upregulating ATP-binding cassette transporter A7. Atherosclerosis 217, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Abe-Dohmae S, Iwamoto N, Yokoyama S, 2011b. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J Atheroscler Thromb 18, 274–281. [DOI] [PubMed] [Google Scholar]
- Tomioka M, Toda Y, Mañucat NB, Akatsu H, Fukumoto M, Kono N, Arai H, Kioka N, Ueda K, 2017. Lysophosphatidylcholine export by human ABCA7. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids 1862, 658–665. [DOI] [PubMed] [Google Scholar]
- Ueda K, 2011. ABC proteins protect the human body and maintain optimal health. Biosci. Biotechnol. Biochem. 75, 401–409. [DOI] [PubMed] [Google Scholar]
- Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR, 2003. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 278, 42906–42912. [DOI] [PubMed] [Google Scholar]
- Zissimopoulos JM, Barthold D, Brinton RD, Joyce G, 2017. Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurol 74, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]