Abstract
Background:
Abiraterone and enzalutamide are high-cost oral therapies increasingly used to treat patients with advanced prostate cancer, carrying the potential for significant financial consequences to patients. We investigated coping and material measures of financial hardship of these therapies among patients with Medicare Part D.
Methods:
We performed a retrospective cohort study on a 20% sample of Medicare Part D enrollees who received abiraterone or enzalutamide between July 2013 and June 2015. We described the variability in adherence and out-of-pocket payments among hospital-referral regions in the first 6 months of therapy. We determined whether adherence and out-of-pocket payments were associated with patient factors and socioeconomic characteristics of where a patient was treated.
Results:
There were 4,153 patients who filled abiraterone or enzalutamide through Medicare Part D in 228 hospital-referral regions. Mean adherence was 75%. Median monthly out-of-pocket payment for abiraterone and enzalutamide was $706 (range $0 to $3,505). After multilevel, multivariable adjustment for patient and regional factors, adherence was lower in patients who were older (69% for 85+ vs. 76% for <70 years; p<0.01), and lower in those with low-income subsidies (69% with subsidy vs. 76% without subsidy; p<0.01). Hispanic ethnicity and living in an HRR with a higher proportion of Hispanic beneficiaries were both independently associated with higher out-of-pocket payments for abiraterone and enzalutamide.
Conclusions:
There was substantial variation in adherence and out-of-pocket payments among Medicare Part D beneficiaries prescribed abiraterone and enzalutamide. Sociodemographic patient and regional factors were associated with both adherence and out-of-pocket payments.
Keywords: medication adherence, out-of-pocket cost, prostate cancer, urologists, hospital referral region
Introduction
Abiraterone and enzalutamide are oral androgen inhibitors that have been approved for the treatment of men with advanced prostate cancer. Both have demonstrated an improvement in survival and quality of life and are generally taken continuously until disease progression. These therapies are increasingly used in earlier settings of advanced disease, with average treatment times of 2–3 years for patients with non-metastatic castration-resistant and metastatic castration-sensitive prostate cancer.1–3 With each passing year, they are also being prescribed more often and by a greater number of providers.4 Novel oral androgen inhibitors are specialty medications covered under Medicare Part D, all with high list prices and the potential for considerable out-of-pocket costs to patients.5
Patients prescribed high-cost therapies for their cancer often suffer from significant financial toxicity and may engage in coping behaviors such as rationing their medications or discontinuing their medication all together. While studies of adherence to treatments in prostate cancer are lacking, there are studies in other cancers demonstrating that high out-of-pocket costs can lead to lower adherence to therapy and ultimately worse cancer-related and overall outcomes.6–11 Patient characteristics such as age, race, and ethnicity have been associated with out-of-pocket expenses and adherence to therapy in other diseases with African American and Hispanic patients observed to have lower adherence and out-of-pocket payments compared to white patients.12–18 Furthermore, healthcare resources within a particular market, such as regional policies, access to nurse care managers, or access to financial counselors, may have significant effects on adherence to treatment and out-of-pocket responsibilities.19
We sought to describe adherence and out-of-pocket payments among Medicare beneficiaries treated across different health care markets, and the association between patient and regional sociodemographic variables and measures of financial hardship. Understanding the extent of variation in these measures of financial hardship and whether some patient groups and hospital-referral regions are disproportionately affected will allow healthcare systems and policy-makers to develop targeted strategies to improve adherence, reduce out-of-pocket payments, and ultimately improve patient outcomes and quality of life.
Methods
Data and Study Population
We performed a retrospective cohort study on a 20% sample of patients eligible for Medicare Part D who received their first fill for abiraterone or enzalutamide between July 2013 and June 2015, as well as survived and had sustained eligibility for at least six months following their first fill. Six months was chosen as an appropriate follow-up time since most patients receive more than 6 months of therapy. In the disease setting with the fewest and shortest responses (i.e. metastatic castration-resistant prostate cancer), patients are treated a median of 8–9 months.5 Patients with any type of Medicare Part D plan (stand-alone or Medicare Advantage), Medigap plans, and those with low-income subsidies were included to evaluate difference in outcomes by expected out-of-pocket payments among enrollees. We also restricted our cohort to patients who lived in a hospital-referral region where at least 5 patients received treatment, a typical cutoff used in other studies that investigated outcomes associated with hospital referral regions.20,21
Outcomes
Outcomes included two primary and two secondary dimensions of financial hardship12,22 measured during the first six months of therapy. Adherence was chosen as our primary coping measure and monthly out-of-pocket payment as the primary material measure. To further characterize financial consequences of these therapies, we also included proportion of days covered (PDC) and total 6-month out-of-pocket payment as secondary coping and material outcomes, respectively. PDC is a continuous variable calculated by summing the number of days of supply of prescriptions filled by the patient from initiation through 180 days post-initiation and dividing by the number of days in the period of interest (180 days). Adherence is a binary outcome defined as PDC ≥ 80%.12 Monthly out-of-pocket payment is a continuous variable calculated by totaling the “patient pay amount” during the first six months, divided by their days on treatment within the first six months, multiplied by 30 days. Finally, total out-of-pocket payment is a continuous variable that was the sum of all patient pay amounts over the first 180 days of treatment. Only those payments in Medicare Part D associated with abiraterone and enzalutamide were included. We expected adherence and PDC to demonstrate similar associative patterns since adherence is determined from PDC. In contrast, monthly and total 6-month out-of-pocket payment variables may differ slightly because of the Part D cost-sharing structure and based on a patient’s adherence to therapy. For example, since the first month of therapy is generally the most expensive until a patient reaches their catastrophic limit, a patient who discontinues therapy after one to two months may have a higher monthly out-of-pocket payment but a lower total 6-month payment compared to someone who remains on treatment for the full 6 months.
Adherence and both payment measures were quantified and illustrated across hospital-referral regions to demonstrate nation-wide variation.
Covariates
We then investigated the association between several patient- and regional-level variables and the measures of adherence and out-of-pocket payments. Patient-level variables we expected to affect adherence included age, race, socioeconomic status, and whether a beneficiary received low-income subsidies. The low-income subsidy variable indicates whether a patient receives extra help with their copayments and premiums. Beneficiaries “deemed” eligible for low-income subsidies are primarily patients with Medicaid or automatic assistance and have the least out-of-pocket payments.23–25 Patients who need to “apply” for low-income subsidies may only have partial subsidies, and may still have more difficulty paying for their medications or adhering to their medications compared to patients with no subsidies at all.25 Based on prior studies, we expected race and ethnicity to be associated with lower out-of-pocket payments.12,13,15
Varying local, state, and health system factors may also contribute to differences in adherence and out-of-pocket payments among patients. The characteristics of where a person is treated can capture differences related to policy (e.g. eligibility for low-income subsidies) and access to programs that address treatment adherence (e.g. nurse-directed education, reminder packaging), or reduce out-of-pocket payments through third-party mechanisms.19,26–29 Therefore, to understand more about the region or environment where a patient resides, we assigned patients to their hospital referral region (HRR, regional markets for tertiary medical care) based on the zip code of their residence. Regional variables included: 1) proportion of African American beneficiaries, 2) proportion of Hispanic beneficiaries, 3) proportion of Medicaid-eligible beneficiaries, and 4) the average health in the HRR measured using Hierarchical Condition Category scores.30 The proportion of beneficiaries in hospital system who are African American or Hispanic have been demonstrated to affect health outcomes in prior studies.31–33 We expected that living in an HRR with a greater proportion of African American, or Hispanic patients may have less resources available to lower out-of-pocket payments, similar to the literature that demonstrates regional differences in quality of care among hospitals with greater proportion of African American patients.31,33 Lastly, since medical oncology and urology offices may differ in their experience navigating these resources to address financial burdens and factors that may influence patient adherence,34–36 we included a regional-level variable that describes the proportion of abiraterone and enzalutamide prescriptions within an HRR that were being prescribed by urologists. Hospital referral region characteristics were determined using data from all Medicare beneficiaries within that HRR; characteristics of HRRs were analyzed as continuous variables in the models and displayed as quintiles in Table 1.
Table 1.
Patient Characteristics
| Frequency (%) n=4,153 |
||
|---|---|---|
| Patient Variables | ||
| Age (years) | ||
| <70 | (22.0) | |
| 70–74 | (20.4) | |
| 75–79 | (22.6) | |
| 80–84 | (18.5) | |
| 85+ | (16.5) | |
| Race | ||
| White | (77.8) | |
| Black | (15.3) | |
| Hispanic | (2.6) | |
| Unknown | (4.4) | |
| Socioeconomic Status Tertile | ||
| Low | (32.5) | |
| Middle | (32.3) | |
| High | (33.1) | |
| Missing | (2.1) | |
| Low-Income Subsidy | ||
| No | (80.7) | |
| Yes | (19.3) | |
| Hospital Referral Region Variables – Quintiles of Overall Beneficiary Characteristics | ||
| % African American (mean 6.5%, SD 7.6%) | ||
| 1 (<0.8% African American) | (7.9) | |
| 2 (0.8 − <2.3% African American) | (17.0) | |
| 3 (2.3 − <5.2% African American) | (21.2) | |
| 4 (5.2 − <11.3% African American) | (31.5) | |
| 5 (11.3%+ African American) | (22.5) | |
| % Hispanic (mean 4.7%, SD 8.8%) | ||
| 1 (<0.6% Hispanic) | (13.2) | |
| 2 (0.6 – <1.1% Hispanic) | (16.9) | |
| 3 (1.1 − <2.5% Hispanic) | (20.4) | |
| 4 (2.5 − <6.1% Hispanic) | (21.5) | |
| 5 (6.1%+ Hispanic) | (28.0) | |
| % Eligible for Medicaid (mean 13.7%, SD 6.2%) | ||
| 1 (<9.5% Medicaid-eligible) | (17.4) | |
| 2 (9.5 − <11.3% Medicaid-eligible) | (19.5) | |
| 3 (11.3 − <13.8% Medicaid-eligible) | (24.2) | |
| 4 (13.8 − <17.1% Medicaid-eligible) | (16.6) | |
| 5 (17.1%+ Medicaid-eligible) | (22.4) | |
| Average HCC score* (mean 1.0, SD 0.1) | ||
| 1 (<0.89) | (13.4) | |
| 2 (0.89 − <0.94) | (14.9) | |
| 3 (0.94 − <0.98) | (20.4) | |
| 4 (0.98 − <1.02) | (19.9) | |
| 5 (1.02+) | (31.4) | |
| % First prescription by Urology (mean 14.3, SD 14.4) | ||
| 1 (0%) | (18.8) | |
| 2 (>0 – <10.5%) | (27.1) | |
| 3 (10.5 − <16.7%) | (18.0) | |
| 4 (16.7 − <25.9%) | (21.7) | |
| 5 (25.9%+) | (14.5) | |
SD, standard deviation; HCC, hierarchical condition category
Hierarchical Condition Category score is an index for overall health of patients – higher score is poorer health
Statistical Analysis
We first described the characteristics of our cohort and the distribution of outcomes across the different hospital-referral regions in order to demonstrate the magnitude of variability. To further characterize this variation and determine whether patient and regional factors were associated with adherence and out-of-pocket payments, we conducted several regression analyses. We fit a multilevel mixed-effects logistic regression model for adherence and multilevel mixed-effects negative binomial regression models for both out-of-pocket payment measures. Payment models were further stratified by low-income subsidy status. Models were constructed at the patient level, and included covariates for patient age, race, socioeconomic status at the zip code level, low-income subsidy status (adherence only), and market-level variables described above. All models included HRR-level random effects. We then used the margins postestimation command in Stata to obtain adjusted adherence, monthly out-of-pocket payment, and total 6-month out-of-pocket payments from regression results. All statistical analyses were performed using Stata 15 (College Station, TX). This study was deemed exempt by the institutional IRB.
Sensitivity Analyses
While rationing behavior or discontinuing treatment after 1–2 months without a subsequent switch may be financially motivated, we considered other patient scenarios that may affect adherence to therapy, including drug intolerance or disease progression. Since drug intolerance or disease progression are commonly followed by a switch to the other oral therapy or a dose adjustment, we considered abiraterone and enzalutamide interchangeably, accounting for the potentially higher PDC a drug switch may cause by re-setting the fill date.37 Furthermore, since some patients intolerant to therapy may undergo dose adjustments, we determined PDC and adherence among patients who were maintained on full-dose therapy without dose reductions. We also considered whether a switch to chemotherapy for possible disease progression rather than the other oral agent may have affected adherence by identifying use of docetaxel or cabazitaxel within Medicare Part B claims in the latter half of the six-month study timeframe. Discontinuation of oral agents for disease progression is generally made after three months of therapy whereas discontinuation before three months could be due to financial reasons.
Results
Between July 1, 2013 and June 30, 2015, 4,153 patients received their first fill for abiraterone or enzalutamide in 228 HRRs. The Supplemental Figure details cohort selection. At least one urologist wrote a prescription for abiraterone or enzalutamide in 164 HRRs. There were 800 patients (19%) who received low-income subsidies – most patients with low-income subsidies were “deemed” eligible (88%) (e.g. through Medicaid), and 12% had to apply.
The majority of patients (n=3,289, 78%) continued treatment with abiraterone or enzalutamide for at least six months. There were 334 patients (8%) who discontinued treatment after one to two months. Figure 1 illustrates the mean unadjusted adherence, monthly out-of-pocket payment, and total out-of-pocket payment in the first six months of therapy by HRR. There was considerable variability across HRRs for all financial hardship outcomes, before adjusting for any covariates.
Figure 1.
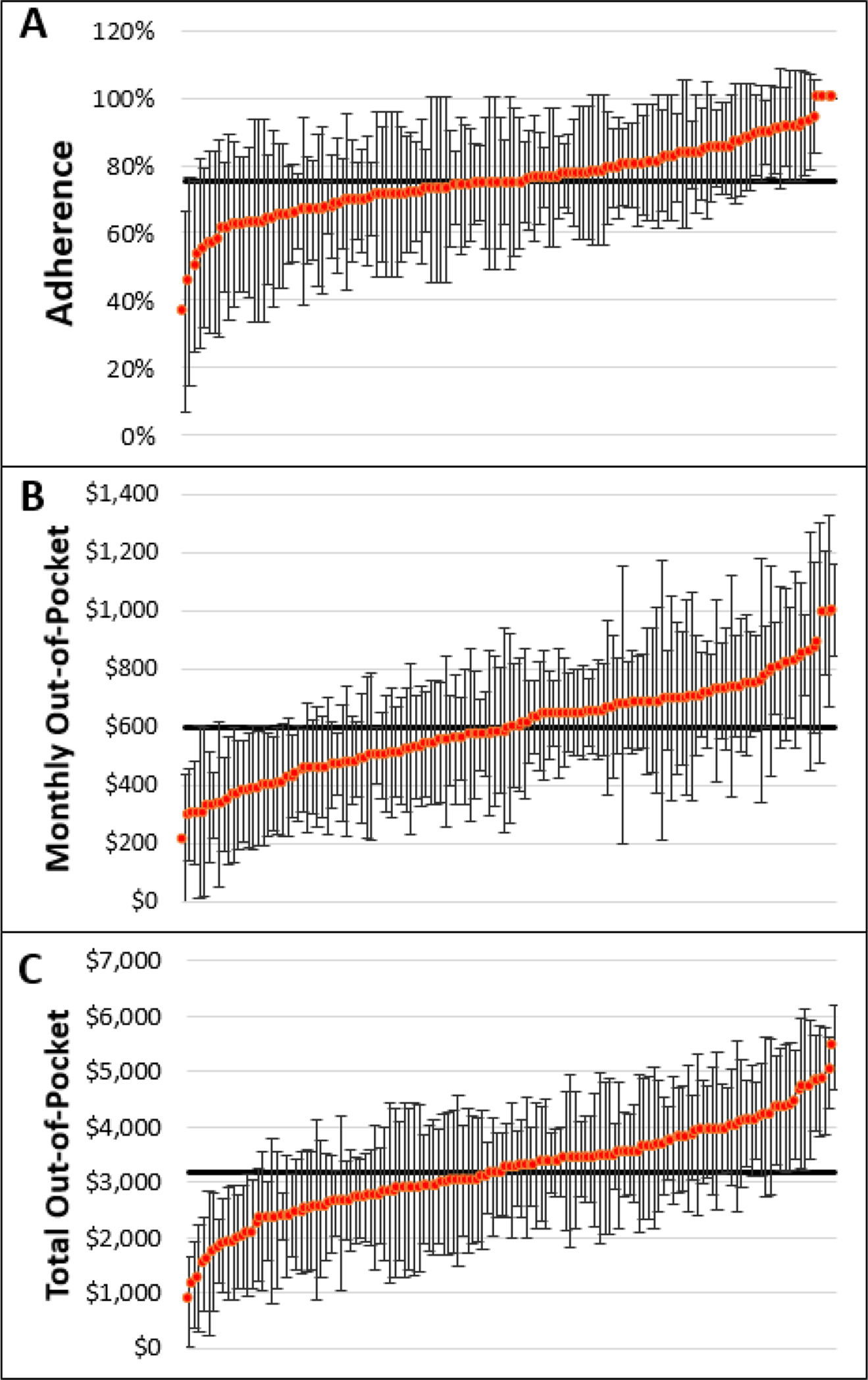
Unadjusted Mean Adherence and Out-of-Pocket Payments by Hospital Referral Region.
Unadjusted mean adherence and out-of-pocket payments by hospital referral region (HRR). Those HRRs with at least 11 patients receiving abiraterone or enzalutamide during the study timeframe (134 HRRs) were included and listed along the X-axis. Notably, fewer HRRs are included in this figure than are used in our analysis due to privacy limitations. The black horizontal line is the mean among the HRRs. The red dots represent the mean of the individual HRR. Panel A, Adherence (mean 75%), defined as proportion of days covered > 80%. Panel B, Monthly out-of-pocket payment (mean $601), defined as total amount patient paid out-of-pocket, divided by the number of days filled *30. Panel C, Total out-of-pocket payment (mean $3,176), defined as total out-of-pocket payment in the first 180 days of treatment.
Adherence
The overall mean adherence (having PDC of at least 80% over 6 months) was 75%. After adjusting for patient- and regional-level variables, patients who were 85 or older had a predicted adherence of 69% vs. 76% in patients less than 70 years old (p<0.01). Similarly, patients with low-income subsidies had a predicted adherence of 69% vs. 76% in patients without low-income subsidies (p<0.01). Living in an HRR with a higher proportion of Hispanic patients predicted for having lower adherence (p=0.01). (Table 2) Conversely, living in an HRR with a higher proportion of Medicaid-eligible patients trended toward an association with increased adherence (p=0.09). No association was found between living in an environment with a high proportion of urologists prescribing these therapies and adherence.
Table 2.
Predicted Margins of Financial Hardship Measures after Multi-level, Multivariable Adjustment
| All Patients | No Low-Income Subsidies | Low-Income Subsidies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adherence (N=4,153) |
Monthly Out-of-Pocket (N=3,353) |
Total Out-of-Pocket (N=3,353) |
Monthly Out-of-Pocket (N=800) |
Total Out-of-Pocket (N=800) |
||||||
| Margin, p-value | Margin, p-value | Margin, p-value | Margin, p-value | Margin, p-value | ||||||
| Age (years) | ||||||||||
| <70 | Ref | Ref | Ref | Ref | Ref | |||||
| 70–74 | 0.69 | 0.36 | 0.13 | 0.88 | 0.74 | |||||
| 75–79 | 0.71 | 0.69 | 0.98 | 0.53 | 0.53 | |||||
| 80–84 | 0.15 | 0.03 | <0.01 | 0.02 | 0.03 | |||||
| 85+ | <0.01 | 0.05 | <0.01 | 0.10 | 0.11 | |||||
| Race | ||||||||||
| White | Ref | Ref | Ref | Ref | Ref | |||||
| African American | 0.51 | <0.01 | <0.01 | <0.01 | <0.01 | |||||
| Hispanic | 0.60 | 0.07 | <0.01 | <0.01 | <0.01 | |||||
| Missing | 0.35 | 0.73 | 0.15 | <0.01 | <0.01 | |||||
| Socioeconomic Status Tertile | ||||||||||
| Low | Ref | Ref | Ref | Ref | Ref | |||||
| Medium | 0.42 | 0.40 | 0.79 | 0.80 | 0.58 | |||||
| High | 0.91 | 0.35 | 0.15 | 0.62 | 0.64 | |||||
| Missing | 0.88 | 0.65 | 0.95 | 0.11 | 0.07 | |||||
| Low-Income Subsidy | ||||||||||
| No | Ref | - | - | - | - | |||||
| Yes | <0.01 | - | - | - | - | |||||
| % African American | 0.42 | 0.59 | 0.20 | 0.42 | 0.35 | |||||
| 1 (0.3%) | ||||||||||
| 2 (1.7%) | ||||||||||
| 3 (3.5%) | ||||||||||
| 4 (7.4%) | ||||||||||
| 5 (18.7%) | ||||||||||
| % Hispanic | 0.01 | <0.01 | 0.10 | 0.59 | 0.98 | |||||
| 1 (0.4%) | ||||||||||
| 2 (0.8%) | ||||||||||
| 3 (1.7%) | ||||||||||
| 4 (3.8%) | ||||||||||
| 5 (10.8%) | ||||||||||
| % Eligible for Medicaid | 0.09 | 0.12 | 0.09 | 0.32 | 0.43 | |||||
| 1 (8.0%) | ||||||||||
| 2 (10.5%) | ||||||||||
| 3 (12.7%) | ||||||||||
| 4 (14.9%) | ||||||||||
| 5 (20.4%) | ||||||||||
| Average HCC Score | 0.12 | 0.19 | 0.02 | 0.98 | 0.99 | |||||
| 1 (0.86) | ||||||||||
| 2 (0.91) | ||||||||||
| 3 (0.95) | ||||||||||
| 4 (0.99) | ||||||||||
| 5 (1.05) | ||||||||||
| % Prescriptions By Urologist | 0.99 | 0.23 | 0.53 | 0.28 | 0.17 | |||||
| 1 (0.0%) | ||||||||||
| 2 (7.7%) | ||||||||||
| 3 (14.3%) | ||||||||||
| 4 (21.6%) | ||||||||||
| 5 (40.0%) | ||||||||||
HCC, hierarchical condition category.
Outcome measures (adherence, monthly out-of-pocket payment, total out-of-pocket payment) were modeled over the first six months of treatment. Payment models (i.e. monthly out-of-pocket and total out-of-pocket) were modeled separately by low-income subsidy status. Models were constructed at the patient level, and included covariates for patient age, race, socioeconomic status at the zip code level, low-income subsidy status (adherence measure only), and geographic variables that characterize the different hospital-referral regions where patients reside. Characteristics of hospital-referral regions were based on all HRRs, except % urologists which was based on HRRs where at least one urologist wrote a prescription. HRR characteristics were modeled as continuous variables, but predictive margins were displayed for the median of each quintile. Significant findings (p<0.05) are bolded.
Out-Of-Pocket Payment – No Low-Income Subsidies
Among patients without low-income subsidies (n=3,353), median monthly out-of-pocket payment was $706, ranging between $0 and $3,505. After adjustment for other patient- and regional-level variables, African American patients had a lower predicted monthly out-of-pocket payment of $625 (p<0.01), and Hispanic patients trended toward a higher predicted monthly payment of $1,102 (p=0.07) versus white patients ($747). (Table 2) Furthermore, living in an HRR with a higher proportion of Hispanic patients was associated with a higher monthly out-of-pocket payment, after controlling for patient-level variables (p<0.01).
Median total out-of-pocket payment over the first six months of treatment was $4,498 (range $0 to $8,398). African American patients had a predicted total out-of-pocket payment of $3,289 (p<0.01), and Hispanic patients $5,380 (p<0.01), versus $3,964 for white patients. Patients who lived in HRRs where beneficiaries had higher Hierarchical Condition Category scores (i.e. more illnesses) had lower total out-of-pocket payments (p=0.02). (Table 2) No association was found between living in an environment with a high proportion of urologists prescribing these therapies and out-of-pocket payments.
Out-Of-Pocket Payment – Low-Income Subsidies
Among patients with low-income subsidies (n=800), median monthly out-of-pocket payment was $1 and ranged from $0 to $2,635. After adjusting for patient- and regional-level variables, African American patients and Hispanic patients with low-income subsidies had lower predicted monthly out-of-pocket payments than white patients ($27 and $13, respectively versus $61, p<0.01).
Median total out-of-pocket payments over 6 months for those with low-income subsidies was $6 (range $0 to $6,193). After adjusting for patient- and regional-level variables, predicted total out-of-pocket payments for African American and Hispanic patients were lower than among white patients ($112 and $59, respectively versus $271, p<0.01). (Table 2) No HRR characteristics were associated with out-of-pocket payments among patients with low-income subsidies.
Sensitivity Analyses
A drug switch (i.e. abiraterone to enzalutamide or enzalutamide to abiraterone) was observed in 464 (11%) of patients during the first six months of treatment. To ensure dose adjustments did not affect adherence, we calculated adherence among those who were maintained on full-dose therapy and found it similar to the total: 76% versus 75%. After excluding patients who discontinued therapy after 1–2 fills (n=334, 8%), adherence went up to 81%, demonstrating adherence was affected by patients who discontinued therapy quickly. To determine whether a switch to chemotherapy for disease progression would have impacted our results, we evaluated whether the non-adherent patients who were also eligible for Medicare Part B received docetaxel or cabazitaxel during months 4–6. A switch to chemotherapy during the first three months may have been likely for financial reasons since out-of-pocket payments are lower for intravenous chemo than these oral therapies. We found approximately 1% of total patients switched to docetaxel or cabazitaxel during months 4–6 and therefore, did not expect this small number of patients to have affected our results.
Discussion
We found substantial variation in adherence and out-of-pocket payments among patients with Medicare Part D prescribed abiraterone and enzalutamide for advanced prostate cancer. We also demonstrated that patient age, race, ethnicity, and the sociodemographic characteristics of the HRR where a patient lives are associated with varying adherence and out-of-pocket payments. Beneficiaries who were older or received low-income subsidies had lower adherence. Furthermore, living in a region with a greater proportion of Hispanic beneficiaries was associated with lower adherence, potentially reflecting unmeasured structural, policy, or differences in resources. Out-of-pocket payments varied substantially by whether patients had low-income subsidies or not. African American or Hispanic beneficiaries who received low-income subsidies had lower monthly and total out-of-pocket payments than white beneficiaries in the same group. In contrast, Hispanic beneficiaries without low-income subsidies had higher total out-of-pocket payments than white patients without low-income subsidies and living in an HRR with a greater proportion of Hispanic beneficiaries was associated with higher out-of-pocket payments.
There are several potential explanations for why some patient and regional factors predicted for varying adherence. Being adherent to a medication requires regular physician visits, which involves transportation and potentially time off work for the patient or caregiver. Thus, lack of transportation to the doctor’s office or the pharmacy may explain why older patients and those with low-income subsidies may have lower adherence to therapy. Furthermore, environmental factors such as living in a region that has a higher proportion of African American or Hispanic patients may reflect market level variables that can capture structural issues, policy differences, and access to different programs.19,26 Prior studies have demonstrated lower adherence and specifically lower cost-related adherence in African American and Hispanic patients in younger patients, with a narrowing of the disparity among patients with Medicare.38 That our adjusted analysis did not demonstrate an impact of race or ethnicity on adherence could be because our cohort included patients with Medicare where disparities were narrower. Alternatively, the adjustment for regional-level variables could reflect the fact that prior racial and ethnic disparities in adherence may be explained somewhat by regional characteristics. Interestingly, despite having lower adherence, living in an HRR with a higher proportion of Hispanic beneficiaires was associated with higher monthly out-of-pocket payments among those without low-income subsidies. The proportion of beneficiaries in hospital system who are African American or Hispanic have been demonstrated to affect health outcomes in prior studies.31–33 Markets with more Hispanic patients may have differing levels of resources and policy-driven interventions to address financial hardship than those with fewer Hispanic patients. There may also be something unique about the clinic infrastructure in HRRs with greater proportions of Hispanic patients that we are not able to capture in this data.
Importantly, out-of-pocket payments for beneficiaries with low-income subsidies were not impacted by the same socioeconomic variables as was seen for beneficiaries without subsidies. Some of the differences in out-of-pocket payments among those with low-income subsidies may reflect differences in Medicaid eligibility. Most patients who are deemed eligible for low-income subsidies have Medicaid and are fully subsidized, whereas many of those who apply and are only partially subsidized are still expected to pay coinsurance for medications.25 There is a higher proportion of African American and Hispanic beneficiaries among Medicare beneficiaries who are Medicaid-eligible (33%) than among all patients with Medicare (18%).39
One limitation to this investigation was that we are only able to capture those prescriptions that were filled through Medicare Part D. Low-income patients who are eligible for Part D, but took advantage of free drug assistance programs would not be included in this analysis – only 20% patients in our cohort had low-income subsidies, compared to 29% of patients in Medicare overall. This difference may reflect the proportion of patients with Medicare Part D who sought out free-drug assistance from manufacturers.40 However, even though differential use of free-drug programs would affect our overall cohort number, it would not necessarily affect the trends in predictors of financial hardship measures among those observed in the data. Furthermore, to maximize the number of included beneficiaries and ensure our cohort was representative of all patients with Medicare Part D, we did not restrict our cohort to those with only traditional Medicare and included all of those patients with Medicare Part D, including those with Medicare Part C (e.g. Medicare Advantage). For this reason, we were only able to evaluate the use of docetaxel and cabazitaxel for potential disease progression as a sensitivity analysis in a subset of our cohort who were eligible for traditional Medicare Part B. Assuming a similar rate of use among beneficiaries in whom we did not have chemotherapy information, the number affected was still negligible and thus not expected to affect our results. Finally, while we accounted for some scenarios unrelated to financial toxicity that may impact adherence, such as disease progression and drug intolerance, there were some unmeasured variables that could have been associated with adherence. For example, we were not able to measure patient and provider beliefs and attitudes toward treatments. We also did not evaluate social factors such as employment, childcare, and family dynamics that could potentially impact adherence and may be considered indirectly related to financial toxicity.41 Nevertheless, prior work has demonstrated that these factors have less effect on adherence among patients with Medicare than among a younger cancer population.38
Conclusion
We demonstrated significant variation in adherence and out-of-pocket payments among Medicare Part D beneficiaries prescribed abiraterone and enzalutamide throughout hospital-referral regions. Measures of financial hardship such as coping behaviors (adherence) and direct material measures of financial toxicity (out-of-pocket payments) will become increasingly salient to those patients with advanced prostate cancer in the coming years as the use of abiraterone and enzalutamide continues to expand dramatically and as additional newly approved oral therapies (apalutamide, darolutamide, olaparib, rucaparib) are adopted. Thus, the stakes for understanding and further mitigating financial hardships experienced by this growing population of patients deserves urgent attention. Understanding the effects of patient and market-level variables on measures of adherence and out-of-pocket payments for patients with advanced prostate cancer and trying to minimize financial consequences of treatment will allow more patients to access and benefit from these and other important treatments.
Supplementary Material
Supplementary Figure. Diagram of Cohort Selection
MBSF, Medicare Master Beneficiary Summary File
Acknowledgement of Research Support:
This work was supported by funding from AHRQ (R01 HS 025707). Further funding is as follows: TS - National Cancer Institute (R01 CA222885-01); MC Prostate Cancer Foundation; SD Commonwealth Fund, the Leukemia & Lymphoma Society, and Arnold Ventures for related work.
Footnotes
Disclosures: The authors have no competing interests to disclose. Dr. Dusetzina is a member of the Institute for Clinical and Economic Review (ICER) Midwest Comparative Effectiveness Public Advisory Council, the West Health Council for Informed Drug Spending, and served on the National Academy of Sciences, Engineering, and Medicine Committee “Ensuring Patient Access to Affordable Drug Therapies.”
References:
- 1.Davis ID, Martin AJ, Stockler MR, et al. : Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 381:121–131, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Fizazi K, Saad F, et al. : Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 378:2465–2474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377:352–360, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Caram MEV, Kaufman SR, Modi PK, et al. : Adoption of Abiraterone and Enzalutamide by Urologists. Urology 131:176–183, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusetzina SB, Keating NL: Mind the Gap: Why Closing the Doughnut Hole Is Insufficient for Increasing Medicare Beneficiary Access to Oral Chemotherapy. J Clin Oncol 34:375–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight TG, Deal AM, Dusetzina SB, et al. : Financial Toxicity in Adults With Cancer: Adverse Outcomes and Noncompliance. J Oncol Pract:JOP 1800120, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Jagsi R, Pottow JA, Griffith KA, et al. : Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol 32:1269–76, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bestvina CM, Zullig LL, Rushing C, et al. : Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract 10:162–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zafar SY, Peppercorn JM, Schrag D, et al. : The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 18:381–90, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias AJ, Hansen RN, Zeliadt SB, et al. : The Association Between Out-of-Pocket Costs and Adherence to Adjuvant Endocrine Therapy Among Newly Diagnosed Breast Cancer Patients. Am J Clin Oncol 41:708–715, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaisaeng N, Harpe SE, Carroll NV: Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm 20:669–75, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farias AJ, Du XL: Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer. J Clin Oncol 35:86–95, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Z, St Clair P, Goldman DP, et al. : Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One 14:e0212117, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MJ, Shaykevich S, Cawthon C, et al. : Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med 7:470–5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber BS, Cho YI, Arozullah AM, et al. : Racial differences in medication adherence: A cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother 8:136–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaskin DJ, Briesacher BA, Limcangco R, et al. : Exploring racial and ethnic disparities in prescription drug spending and use among Medicare beneficiaries. Am J Geriatr Pharmacother 4:96–111, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gellad WF, Haas JS, Safran DG: Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med 22:1572–8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu KT, Borders TF: Racial and ethnic disparities in the financial burden of prescription drugs among older Americans. J Health Hum Serv Adm 30:28–49, 2007 [PubMed] [Google Scholar]
- 19.Camacho FT, Tan X, Alcala HE, et al. : Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in medicare breast cancer survivors. Medicine (Baltimore) 96:e7147, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faris NR, Smeltzer MP, Lu F, et al. : Evolution in the Surgical Care of Patients With Non-Small Cell Lung Cancer in the Mid-South Quality of Surgical Resection Cohort. Semin Thorac Cardiovasc Surg 29:91–101, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeltzer MP, Faris NR, Ray MA, et al. : Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 4:80–87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altice CK, Banegas MP, Tucker-Seeley RD, et al. : Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst 109, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Centers for Medicare and Medicaid Services Guidance to States on the Low-Income Subsidy, in Services TCfMaM (ed), 2009
- 24.HI 03001.005 Medicare Part D Extra Help (Low-Income Subsidy or LIS), in Administration SS (ed). Program Operations Manual System, 2019 [Google Scholar]
- 25.Chou YT, Farley JF, Stinchcombe TE, et al. : The Association Between Medicare Low-income Subsidy and Anticancer Treatment Uptake in Advanced Lung Cancer. J Natl Cancer Inst, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White C, Taychakhoonavudh S, Parikh R, et al. : Roles of prices, poverty, and health in Medicare and private spending in Texas . Am J Manag Care 21:e303–11, 2015 [PubMed] [Google Scholar]
- 27.Waalen J, Bruning AL, Peters MJ, et al. : A telephone-based intervention for increasing the use of osteoporosis medication: a randomized controlled trial. Am J Manag Care 15:e60–70, 2009 [PubMed] [Google Scholar]
- 28.Dupclay L, Eaddy M, Jackson J, et al. : Real-world impact of reminder packaging on antihypertensive treatment adherence and persistence. Patient Prefer Adherence 6:499–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmer A, Hardeman W, Hughes D, et al. : An explanatory randomised controlled trial of a nurse-led, consultation-based intervention to support patients with adherence to taking glucose lowering medication for type 2 diabetes. BMC Fam Pract 13:30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope GC, Kautter J, JIngber MJ, et al. : Evaluation of the CMS-HCC Risk Adjustment Model, in Centers for Medicare and Medicaid Services’ Office of Research D, and Information (ed). Research Triangle Park, NC, 2011 [Google Scholar]
- 31.Lucas FL, Stukel TA, Morris AM, et al. : Race and surgical mortality in the United States. Ann Surg 243:281–6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henning-Smith CE, Hernandez AM, Hardeman RR, et al. : Rural Counties With Majority Black Or Indigenous Populations Suffer The Highest Rates Of Premature Death In The US. Health Aff (Millwood) 38:2019–2026, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Dimick J, Ruhter J, Sarrazin MV, et al. : Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions. Health Aff (Millwood) 32:1046–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankaran V, Leahy T, Steelquist J, et al. : Pilot Feasibility Study of an Oncology Financial Navigation Program. J Oncol Pract 14:e122–e129, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Shankaran V, Linden H, Steelquist J, et al. : Development of a financial literacy course for patients with newly diagnosed cancer. Am J Manag Care 23:S58–S64, 2017 [PubMed] [Google Scholar]
- 36.Yezefski T, Steelquist J, Watabayashi K, et al. : Impact of trained oncology financial navigators on patient out-of-pocket spending. Am J Manag Care 24:S74–S79, 2018 [PubMed] [Google Scholar]
- 37.Leslie RS: Using Arrays to Calculate Medication Utilization. Presented at the SAS Global Forum 2007, Orlando, FL, April 16–19, 2007, 2007 [Google Scholar]
- 38.Lee M, Salloum RG: Racial and ethnic disparities in cost-related medication non-adherence among cancer survivors. J Cancer Surviv 10:534–44, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Scher HI, Morris MJ, Stadler WM, et al. : Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402–18, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cubanski J, Damico A, Neuman T: Medicare Part D in 2018: The Latest on Enrollment, Premiums, and Cost Sharing, 2018
- 41.Gallups SF, Connolly MC, Bender CM, et al. : Predictors of Adherence and Treatment Delays among African American Women Recommended to Receive Breast Cancer Chemotherapy. Womens Health Issues 28:553–558, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Diagram of Cohort Selection
MBSF, Medicare Master Beneficiary Summary File


