Abstract
Patient: Male, 51-year-old
Final Diagnosis: COVID-19 • Klebsiella pneumoniae infection
Symptoms: Ascites • cough • fatigue • feve
Medication: —
Clinical Procedure: Antibiotics • oxygen therapy • paracentesis
Specialty: Gastroenterology and Hepatology • Medicine, General and Internal • Pulmonology
Objective:
Unusual clinical course
Background:
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), which manifests mainly as a respiratory condition, has become a global pandemic that causes coronavirus disease-2019 (COVID-19). Although the symptoms remain mild in most patients, the elderly and patients with previous comorbidities have higher rates of morbidity and mortality. Patients with liver cirrhosis, especially after decompensation, may be more susceptible to SARS-CoV-2 infection due to systemic immune dysfunction.
Case Report:
The patient was a 51-year-old man who was hypertensive, an ex-alcoholic abstinent for 6 months, and a smoker. He was diagnosed with alcoholic liver cirrhosis in July 2019, and was using norfloxacin at home for secondary prophylaxis of bacterial peritonitis. He was also using furosemide and spironolactone to control ascites and propranolol for primary prophylaxis of esophageal varices. The patient entered our hospital in July 2020 with cough, dyspnea, runny nose, diarrhea, and fever. During hospitalization, we confirmed infection by COVID-19 and secondary nosocomial pulmonary infection. Chest tomography compatible with ground-glass standard was performed. The patient developed the need for auxiliary oxygen but without invasive mechanical ventilation. The patient received dexamethasone 6 mg/day and broad-spectrum antibiotic therapy (he was started on cefepime but switched to meropenem). At the end of the 14-day isolation period, he was discharged with improved respiratory status.
Conclusions:
Despite high mortality rates in patients with advanced cirrhosis who become infected with COVID-19, we report a case with a favorable outcome. Success has been achieved with the use of medications in studies of broad-spectrum antibiotics and the rapid detection of complications caused by the virus. Further studies in SARS-CoV-2 patients with chronic liver disease are needed.
Keywords: COVID-19, Liver, Liver Cirrhosis, Liver Diseases, SARS Virus
Background
The coronavirus disease 2019 (COVID-19) pandemic has been caused by the severe acute respiratory syndrome-coronavirus (SARS-CoV-2). As of 18 November 2020, there had been 55 326 907 infected patients and 1 333 742 deaths recorded worldwide according to the World Health Organization (WHO) [1,2]. The most common symptoms of COVID-19 are fever, fatigue, and dry cough. Shortness of breath, pain, nasal congestion, sore throat, anosmia, and ageusia are less frequent but are present in several reports [1]. Although the symptoms remain mild in most patients, the elderly and patients with previous comorbidities have higher morbidity and mortality from infection [3]. Patients with liver cirrhosis, especially after decompensation, may be more susceptible to infection by SARS-CoV-2 due to systemic immune dysfunction [4]. Although a preliminary study reported that 2–11% of those infected with COVID-19 have previous liver disease [5], the true prevalence of infected cirrhotic patients remains unknown [6]. The purpose of this study was to report a case of a patient with liver cirrhosis who was infected with SARS-CoV-2 and to perform a literature review on COVID-19 patients with cirrhosis as well as liver involvement by the virus.
Case Report
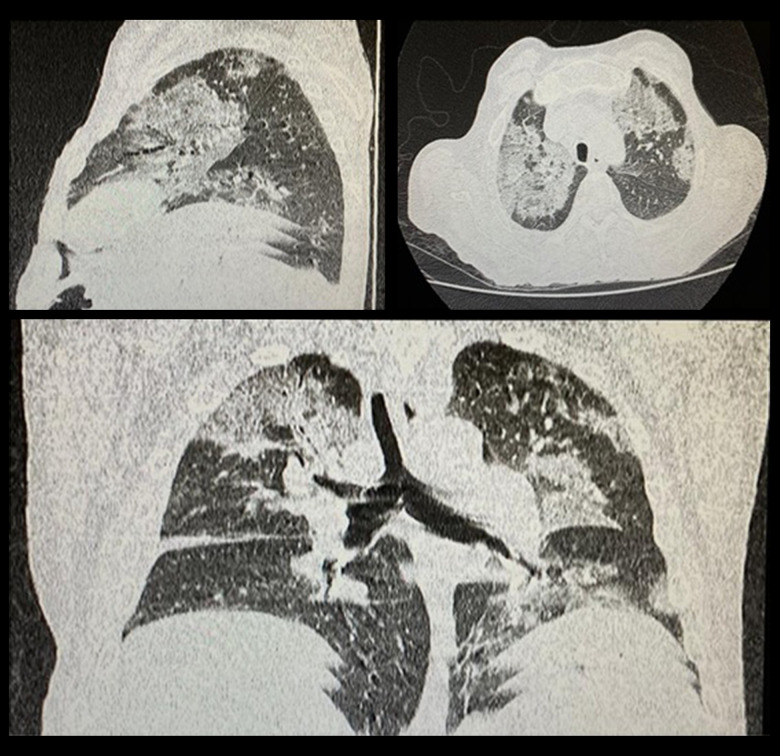
A 51-year-old man with liver cirrhosis was admitted to the hospital in July 2020 complaining of cough, dyspnea, runny nose, diarrhea, and fever (temperature 38.1ºC). The first flu-like symptoms (cough, fever, and runny nose) and the abdominal symptoms started 3 days before admission. He was hypertensive, ex-alcoholic (abstinent for 6 months), and a smoker. He was admitted to a tertiary hospital in September 2019 with an increase in abdominal volume. In the physical examination, he had signs of chronic liver disease, ascites, and grade I encephalopathy. He was then diagnosed with alcoholic liver cirrhosis, classified using the Child-Turcotte-Pugh (CTP) (with 10 points in class C) and with the Model for End-Stage Liver Disease Sodium (MELD-Na) (with a score of 11). Since this diagnosis, the patient had been following up with the hepatology team to control his chronic liver disease and its decompensations. During 2019, the patient started diuretic therapy with spironolactone and furosemide to control ascites. He received follow-up with upper digestive system endoscopies, elastic ligation, and beta-blocker therapy to control esophageal varices. He developed spontaneous bacterial peritonitis (SBP), which was successfully treated and then started secondary prophylaxis with norfloxacin. In the physical examination at admission, the patient was lightly lethargic, tachypneic, pale, and feverish, but was anicteric and acyanotic. Pulmonary auscultation indicated fine crackles in the bases of the lungs, no respiratory distress, and 90% oxygen saturation. The abdomen was globose and distended but not tense, with signs of collateral circulation and a small volume of ascites. At admission, laboratory testing identified anemia (Hb 9.2 g/dL – reference value [RV] >12 g/dL) and leukopenia (2900/mm3 – RV 4000–12 000/mm3) with lymphopenia (20.2% – RV 23–33%), thrombocytopenia (123 000/mm3 – RV 140 000–440 000/mm3), albuminemia (2.4 g/dL – RV 3.5–5 g/dL), increased transaminases: alanine aminotransferase (AST) (71 U/L – RV 17–59 U/L), and aspartate aminotransferase (ALT) (45 U/L – RV <50 U/L), and high levels of alkaline phosphatase (231 U/L – RV <126 U/L). In the laboratory control tests before admission, the patient already had anemia (Hb 10.7 g/dL) and decreased serum albumin (3.0 g/dL), but the other tests were within normal limits. Diagnostic paracentesis was performed to analyze ascitic fluid and it was negative for SPB. The patient was swabbed to test for SARS-CoV-2 two days after admission. A chest computed tomography scan was performed, which showed an increase in the number of lymph nodes in the upper and lower paratracheal mediastinal chains, as well as in the pre-vascular area. The lungs showed multiple ground-glass opacities that were predominantly perihilar, especially in the middle and upper third of the lung fields, and were associated with a thickening of the inter- and intralobular septa. They featured a mosaic pavement pattern and a slight laminar effusion on the right with an extension for the oblique fissure (Figure 1). The final diagnosis was severe acute respiratory syndrome-coronavirus-2. The oxygen desaturation improved with the use of 5L/min auxiliary O2 nasal catheter. After the SARS-CoV-2 confirmation, dexamethasone 6mg/day was introduced. At 3 days after admission, cefepime and azithromycin were also given due to suspected secondary bacterial pulmonary infection, and SBP prophylaxis was temporarily suspended. The flu-like and diarrhea symptoms improved after 72 h, but the fever and dyspnea remained. Oxygen flow also needed to be increased to 7 L/min and a secondary nosocomial infection was suspected.
Figure 1.
Clockwise, from left to right: sagittal, axial, and coronal section. They demonstrate multiple ground-glass opacities, predominantly perihilar, in the middle and upper third of the pulmonary fields, associated with thickening of the inter- and intralobular septa, which forma mosaic paving pattern.
To corroborate the results, we confirmed the presence of Klebsiella pneumoniae sensitive to carbapenems in the culture of tracheal aspirate; therefore, the antibiotic treatment was exchanged for meropenem due to worsening of the respiratory condition. Dexamethasone was maintained for 10 days. Two days after the antibiotic exchange and with the aid of respiratory physiotherapy, the patient began to improve. The patient was weaned off auxiliary oxygen and discharged from the hospital after 14 days with normalization of laboratory tests.
Discussion
This was the first case in our center of an infection from SARS-CoV-2 pneumonia in a patient with previous liver cirrhosis. The initial symptoms such as tachypnea, dyspnea, cough, and fever are common in patients hospitalized for COVID-19 [1]. The fact that the patient had a previous comorbidity represents a higher risk of morbidity and mortality [1,3]. However, in the largest study to date related to the characteristics of patients infected with COVID-19, the severity of the infection in patients with liver disease was not assessed [1].
When evaluating the patient’s laboratory exams, he presented anemia (Hb 9.2 g/dL), leukopenia (2.900/mm3) with lymphopenia (20.2%) and thrombocytopenia (123 000/mm3). In the study by Guan [1], when analyzing admission exams, lymphocytopenia was present in 83.2% of patients, thrombocytopenia in 36.2%, and leukopenia in 33.7%. However, in addition to the infectious factor, these changes are also associated with chronic liver disease [1,4]. In terms of liver biochemistry, our patient had albuminemia (2.4 g/dL), increased transaminases AST (71 U/L) and ALT (45 U/L), and high levels of alkaline phosphatase (231 U/L). Kunutsor et al [7] evaluated 10 540 patients with COVID-19 and found a possible link between the increase in laboratory serum markers of liver injury and severity of COVID-19. Such a correlation would justify regarding pre-existing liver disease as a severity factor, mainly due to the association with immunological injury [7]. Despite the relationship between previous comorbidities and increased morbidity and mortality due to COVID-19, our patient had a successful evolution.
Our patient was receiving oxygen through a nasal catheter. Thus, the treatment initiated for infection (dexamethasone 6 mg/day) was based on the RECOVERY study in which this drug reduced deaths by one-third in patients who received invasive mechanical ventilation and in one-fifth of patients who received auxiliary oxygen without invasive mechanical ventilation [8]. Regarding bacterial infection, in previous pandemics, it was concluded that the co-infections, secondary infections, or “superinfections” are common during viral pandemics [9]. The 1918 Spanish Flu pandemic saw around 50 million deaths ascribed to bacterial co-infections, and during the 2009 H1N1 Influenza pandemic up to 34% of all deaths were a result of bacterial co-infections [9]. While the precise mechanism for susceptibility to secondary infections is unclear, it is likely that virus-mediated immunosuppression of the host innate immune enables opportunistic bacteria to colonize the host [9].
Reports are inconclusive about whether COVID-19 causes direct liver damage. Musa [10] reviewed studies in which the incidence of liver damage (mainly due to high levels of ALT and AST) in COVID-19 patients ranged from 14.8% to 78%. A likely explanation for the high incidence rates is that SARS-CoV-2 binds to the angiotensin-converting enzyme 2 receptor (ACE2) to invade target cells [10,11]. A study showed that expression of ACE2 occurs in 59.7% of cholangiocytes, suggesting that SARS-CoV-2 can bind to these ACE2-positive cells and disrupt liver function [12].
Even with this observation of increased aminotransferase levels in severe COVID-19 cases, some other studies suggest that clinically significant liver dysfunction as a result of COVID-19 is uncommon and that there are alternative explanations for the causes of the damage [13]. Bangash et al [13], when analyzing liver function in patients with short- or long-term symptoms, found no evidence that the prolonged course of COVID-19 had caused a more serious liver function disorder [13,14]. Bangash et al [13] also pointed out that elevations in amino-transferases are not necessarily associated with liver damage and they suggested the hypothesis that COVID-19 induces myositis similar to that observed in infections by other respiratory viruses (eg, herpes, parvovirus, and adenovirus) [13,15]. Polakos et al [16] showed that specific viral CD8+ T cells, generated in response to a viral infection outside the liver, can trigger T cell-mediated hepatitis in the absence of viral antigens in the liver. During infection, the increases in interleukin (IL)-6, IL-10, IL-2, and gamma interferon, caused by typical lymphopenia, can worsen inflammatory responses in various organs, including the liver [17]. This cascade of cytokines and interleukins, generating an inflammatory insult in the body, explains the changes in liver biochemistry. It also shows the relationship between worse severity of COVID-19 and higher levels of liver injury markers [18]. Such arguments would characterize liver damage as collateral damage that is not directly caused by COVID-19. In support of this idea, anato-pathological analysis of the livers of patients who died of COVID-19 did not have viral inclusions in the hepatocytes [19,20].
In light of the lack of epidemiological data on COVID-19 in the population with chronic liver disease, international voluntary records of patients with COVID-19 who have underlying liver disease (with or without cirrhosis) or transplantation was initiated. In a July 14, 2020 report, the SECURE-Cirrhosis and the COVID-HEP databases recorded 508 cases of patients with liver cirrhosis who developed COVID-19. Of these, 445 (88%) were hospitalized, 141 (27%) were admitted to the Intensive Care Unit (ICU), 95 (19%) needed mechanical ventilation, and 158 (31%) died [21,22]. Our patient had a favorable evolution, despite studies showing high mortality in patients with advanced cirrhosis. Only 46% of hospitalized patients with CTP-C cirrhosis survived, and this proportion dropped to 21% in those admitted to an ICU and further still to 10% in those receiving invasive ventilation [23].
Using these virtual databases, a large study was carried out that evaluated 745 patients with chronic liver disease (CLD) from 32 countries: 668 (90%) were hospitalized, 177 (24%) were admitted to the ICU, 132 (18%) received invasive mechanical ventilation, 32 (4%) started renal replacement therapy, and 150 (20%) died. When comparing this mortality, including 27/359 (8%) CLD patients without cirrhosis and 123/386 (32%) of patients with cirrhosis, with the CTP scores, a directly proportional increase was noted: CTP-A 33 (19%), CTP-B 44 (35%), and CTP-C 46 (51%). The risk factors significantly associated with death were age (OR 1.03 per year; 95%CI 1.01–1.04; p<0.001), heart disease (OR 1.76; 95%CI 1.16–2.66; p=0.008), White ethnicity (OR 2.52; 95%CI 1.73–3.68; p<0.001), and baseline serum creatinine (OR 1.19 per mg/dL; 95%CI 1.04–1.38; p=0.014). The presence of cirrhosis versus CLD without cirrhosis was significant in univariable analysis (OR 1.98; 95%CI 1.52–2.59). The predominant cause of death was COVID-19 lung injury, with only 19% of mortality in the patients with cirrhosis accounted for by liver-related complications [23].
For the population with cirrhosis, the COVID-19 pandemic is challenging. A careful balance must be struck between protecting these patients from exposure to the virus and instituting treatment to prevent progression of liver disease and treat the cirrhosis complications (relief paracentesis, elastic ligation of esophageal varices, outpatients with liver disease, PBE treatment, and liver transplantation). This strategy may ultimately be the best option for protecting patients from harm after SARS-CoV-2 infection [24].
Conclusions
COVID-19 is a new disease whose greatest morbidity and mortality rates have been identified in patients with comorbidities, including patients with cirrhosis. With cirrhotic immuno-suppression, it is extremely important to pay attention to the complications of coronavirus infection such as desaturation and the risk of secondary infection. If secondary bacterial infection is detected, as in the present case, broad-spectrum antibiotic therapy should be instituted. However, further studies in the population with chronic liver disease are necessary for health teams to understand the challenges of this new disease, mainly in terms of pathophysiology and therapy. The results based on large virtual databases are providing useful epidemiologic information.
Footnotes
Conflict of Interest
None.
References:
- 1.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization 2020. Nov 18, https://covid19.who.int/
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–30. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenblatt R, Verna EC. COVID 19: Management of decompensated cirrhosis and liver transplant recipients. Clin Liver Dis (Hoboken) 2020;15(5):200–3. doi: 10.1002/cld.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunutsor SK, Laukkanen JA. Markers of liver injury and clinical outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect. 2020;28(1):159–98. doi: 10.1016/j.jinf.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manohar P, Loh B, Nachimuthu R, et al. Secondary bacterial infections in patients with viral pneumonia. Front Med (Lausanne) 2020;7:420. doi: 10.3389/fmed.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21(1):3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M, Kleine-Weber H, Krüger N, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-1 coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. 2020:929042. [Google Scholar]
- 12.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bio. Rxiv. 2020:931766. [Google Scholar]
- 13.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–30. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20(4):425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–59. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polakos NK, Cornejo JC, Murray DA, et al. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169–78. doi: 10.2353/ajpath.2006.050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–30. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–22. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao XH, Li TY, He ZC, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:411–17. doi: 10.3760/cma.j.cn112151-20200312-00193. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 21.Surveillance epidemiology of coronavirus (COVID-19) under research exclusion. SECURE-Cirrhosis. 2020. https://covidcirrhosis.web.unc.edu/
- 22.COVID-Hep COVID-Hep.net: Coronavirus (COVID-19) in liver disease reporting registry. 2020. https://www.covid-hep.net/
- 23.Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.09.024. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: An international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–16. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]