Summary
Dormant bacterial spores are extraordinarily resistant to environmental insults and are vectors of various illnesses. However, spores cannot cause disease unless they germinate and become vegetative cells. The molecular details of initiation of germination are not understood, but proteins essential in early stages of germination, such as nutrient germinant receptors (GRs) and GerD, are located in the spore inner membrane. In this study, we examine how these germination proteins are organized in dormant Bacillus subtilis spores by expressing fluorescent protein fusions that were at least partially functional and observing spores by fluorescence microscopy. We show that GRs and GerD colocalize primarily to a single cluster in dormant spores, reminiscent of the organization of chemoreceptor signalling complexes in Escherichia coli. GRs require all their subunits as well as GerD for clustering, and also require diacylglycerol addition to GerD and GRs’ C protein subunits. However, different GRs cluster independently of each other, and GerD forms clusters in the absence of all the GRs. We predict that the clusters represent a functional germination unit or ‘germinosome’ in the spore inner membrane that is necessary for rapid and cooperative response to nutrients, as conditions known to block nutrient germination also disrupt the protein clusters.
Introduction
Spores of Bacillus and Clostridium species are metabolically dormant and resistant to environmental stresses, allowing them to survive for years (Nicholson et al., 2000; Setlow, 2006). However, when favourable conditions arise, as signalled by the presence of specific nutrients, spores lose their dormancy and resistance properties in the process of germination. In order to trigger germination, nutrients must traverse the coat, outer membrane, cortex and germ cell wall layers of the dormant spore to access nutrient germinant receptors (GRs) located in the spore’s inner membrane (Hudson et al., 2001; Paidhungat and Setlow, 2001; Setlow, 2003). Upon nutrient binding to specific GRs, monovalent cations and pyridine-2,6-dicarboxylic acid [dipicolinic acid (DPA)] are rapidly released from the spore core, leading to some water uptake into the core and some loss of spore resistance. DPA release then triggers degradation of the spore’s peptidoglycan cortex by cortex-lytic enzymes, resulting in core expansion, additional water uptake and further loss of spore resistance. Ultimately, the germinated spore escapes the spore coat and outgrows as its metabolism begins and it becomes a growing cell (Setlow, 2003).
In Bacillus subtilis, proteins important for germination include three GRs (GerA, GerB and GerK), and the GerD and SpoVA proteins. Each GR is encoded by a homologous tricistronic operon, whose products are two polytopic integral transmembrane proteins (proteins A and B) and a lipoprotein (protein C). The GerA GR is required for germination with L-alanine while the GerB and GerK GRs are both necessary for germination with a combination of L-asparagine, D-glucose, D-fructose and potassium (AGFK) (Setlow, 2003; Paredes-Sabja et al., 2010). GerD is a lipoprotein encoded by a monocistronic gene, and although its function is unknown its deletion greatly decreases rates of GR-dependent germination (Pelczar et al., 2007). However, spores that lack GerD germinate normally with non-nutrient germinants that bypass the GRs, suggesting that GerD is necessary for rapid transduction of some signal from the nutrient-GR complex (Pelczar et al., 2007). The spoVA operon likely encodes six polytopic integral transmembrane proteins SpoVAA-F, and SpoVA proteins may be involved in DPA release during spore germination (Vepachedu and Setlow, 2004).
Germinant receptors, and the GerD and SpoVA proteins all play important roles in the early stages of spore germination and are likely all located in the spore’s inner membrane (Hudson et al., 2001; Paidhungat and Setlow, 2001; Vepachedu and Setlow, 2005; Pelczar and Setlow, 2008). The GerB and GerK GRs act cooperatively, as do the GerA and GerB GRs, and the GerA and GerK GRs (Igarashi and Setlow, 2005; Atluri et al., 2006). GerD interaction with GRs is also likely, given that GerD seems to facilitate communication between the GRs and the SpoVA proteins. Such cooperative behaviour of proteins could certainly be mediated by direct or indirect protein–protein interactions. While such interactions have not been demonstrated, far Western blot and yeast two-hybrid experiments have confirmed interactions among the GerA subunits, and between SpoVA and GerA proteins (Vepachedu and Setlow, 2007). Also of note is that the components of the spore’s inner membrane and core are relatively immobile, and their mobility is not restored until late in spore germination (Cowan et al., 2003; 2004). Thus, to facilitate their interaction in the dormant spore, germination proteins might be pre-assembled into clusters or foci in the spore’s inner membrane, much like chemotaxis proteins in a variety of bacteria (Kentner and Sourjik, 2006). Such an organization would be beneficial in spores, given the need for: (i) rapid and cooperative responses to nutrient stimuli, (ii) the low abundance of GRs (likely averaging < 50 molecules/spore) compared with those of the GerD and SpoVA proteins (1000s of molecules/spore) (Paidhungat et al., 2001; Vepachedu and Setlow, 2005; Pelczar and Setlow, 2008), (iii) multiple interactions among germination proteins and (iv) the limited mobility of the spore’s inner membrane constituents. In this work, we have used fluorescence microscopy to localize functional fusions of fluorescent proteins to germination proteins in dormant B. subtilis spores, and show that GRs and GerD localize predominantly in single foci. We also show that the GR and GerD foci colocalize, suggesting that all these germination proteins are located at a specific site in the spore’s inner membrane.
Results
The B. subtilis GR proteins are located in the spore inner membrane (Hudson et al., 2001; Paidhungat and Setlow, 2001), although the GRs’ spatial organization within this membrane is unknown. We thus used fluorescence microscopy to determine the location of GR subunits fused to green fluorescent protein (GFP) or the mCherry derivative of red fluorescent protein in dormant B. subtilis spores. To facilitate the detection of the fluorescent proteins the fusion genes were inserted in a ΔcotE ΔgerE (PS4150) background, as PS4150 spores have greatly reduced autofluorescence compared with spores of the parental strain PS832 (Magge et al., 2009). PS4150 spores lack most of their coat layer, but are still able to germinate with nutrient germinants, albeit approximately twofold slower than wild-type spores (Ghosh et al., 2008). The fusion genes were placed at either the normal chromosomal loci or the non-essential amyE locus, and under the control of their native promoters.
Germination protein fusions facilitate germination
The fusion proteins studied were all at least partially functional because they facilitated germination in spores that lacked corresponding wild-type proteins (Fig. 1). Spores carrying the GerAA-mCherry fusion germinated twofold faster and twofold slower than PS4150 spores in L-alanine and AGFK, respectively, while spores carrying GerBA-GFP germinated at rates similar to PS4150 spores in AGFK but twofold slower than PS4150 spores with L-alanine (Fig. 1). These data suggest that GerAA-mCherry somehow enhances the GerA receptor’s response to L-alanine and depresses the GerB and GerK receptors’ response to AGFK. The observation that GerAA-mCherry inhibits AGFK germination and GerBA-GFP inhibits L-alanine germination further suggests that these two signalling pathways are intimately connected somehow. The rates of AGFK and L-alanine germination of spores carrying GerKA and GerKB fusions were twofold slower than with PS4150 spores (Fig. 1). Given that deletion of gerK typically does not adversely affect germination via the GerA receptor (Atluri et al., 2006), these data suggest that the germination defect in GerK fusion spores is not due to dysfunction of the GerK receptor itself, but instead to a general disruption of germination signalling. Spores carrying GerD-GFP germinate 4- to 5-fold slower than PS4150 spores with AGFK and L-alanine (Fig. 1), suggesting that the presence of the fluorescent protein significantly inhibits GerD’s function. However, the germination of spores carrying GerD-GFP was 4- to 5-fold faster than that of gerD spores (Pelczar et al., 2007, data not shown). Strains expressing all fusion proteins grew and sporulated normally, as expected, because neither GRs nor GerD are essential for growth or sporulation. In addition, a SpoVAE-GFP fusion made by single crossover insertion of spoVAE-gfp into the normal spoVAE locus (which separates the wild-type gene from its promoter) is also functional, because despite the loss of wild-type SpoVAE that is required for normal sporulation (Fort and Errington, 1985), cells containing this fusion protein sporulated normally and the resulting spores germinated normally.
Fig. 1.
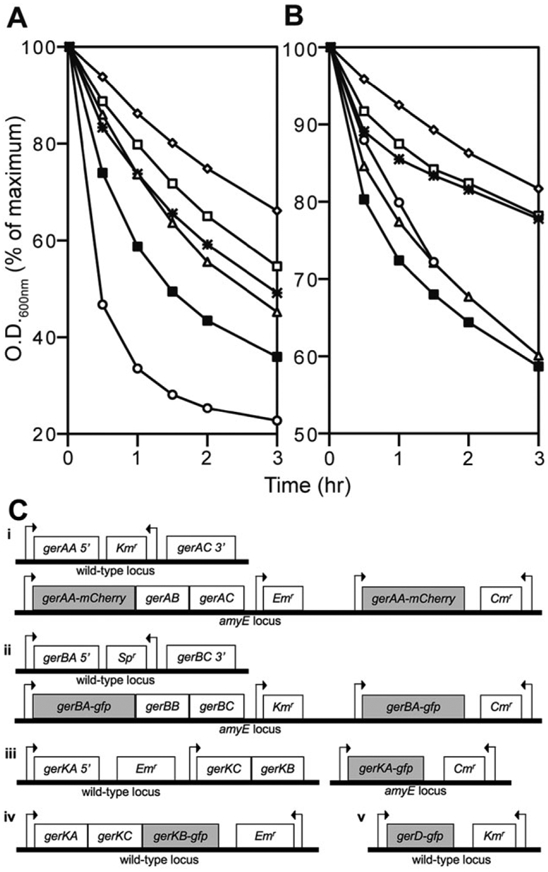
Germination protein fusions are at least partially functional. Spores were germinated with (A) AGFK and (B) L-alanine and the decrease in the OD600 was monitored. The genotype of the strains used in germination assays is shown in (C), arrows indicate the relative position of promoters as well as the direction of transcription. The symbols for the strains used to generate spores are: ■, PS4150; ○, GerAA-mCherry (KGB174 – Ci); △, GerBA-GFP (KGB202 – Cii); ⁕, GerKA-GFP (KGB203 – Ciii); □, GerKB-GFP (KGB08 – Civ); and ◇, GerD-GFP (KGB52 – Cv). Strains KGB174 and KGB202 contain two copies of their respective fusion genes, and KGB203, KGB08 and KGB52 contain one copy of their respective fusion genes expressed under the control of their native promoters, shown in (C). All these strains lack the full-length wild-type gene as shown in (C). There was no germination when spores were incubated without AGFK or L-alanine. Results similar to those with GerKB-GFP spores were also obtained with spores carrying GerKB-mCherry (KGB34).
Germination protein fusions localize to the inner membrane
At least two GRs and GerD have been previously localized to the spore inner membrane (Hudson et al., 2001; Paidhungat and Setlow, 2001; Pelczar and Setlow, 2008). To confirm that the GerAA-mCherry fusion was located in the spore inner membrane, we made lysates from decoated spores treated with lysozyme, and then fractionated the lysates by centrifugation at 100 000 g. The pellet fraction (P100) from this treatment, which contains inner membrane vesicles, was then subjected to Western blot analysis with polyclonal antibodies to GerAA (Fig. 2). A signal at 54 kDa representing wild-type GerAA was detected in the inner membrane fraction of spores of strain PS4150 (lane 1) that contains only the wild-type copy of gerAA, and a signal at 82 kDA representing GerAA-mCherry was detected in the inner membrane fraction of spores of strain KGB138 (lane 5) that contains only the gerAA-mCherry fusion gene. Neither signal was observed in the inner membranes from spores of strain KGB136 (lane 4), which lacks both wild-type and fusion genes, and both signals were observed in the inner membrane from spores of strains KGB114 (lane 2) and KGB129 (lane 3), which contain both gerAA and gerAA-mCherry. It is unclear why the ratio of the GerAA/GerAA-mCherry signal from spores of strain KGB129 (lane 3) is lower than that from spores of strain KGB114 (lane 2).
Fig. 2.
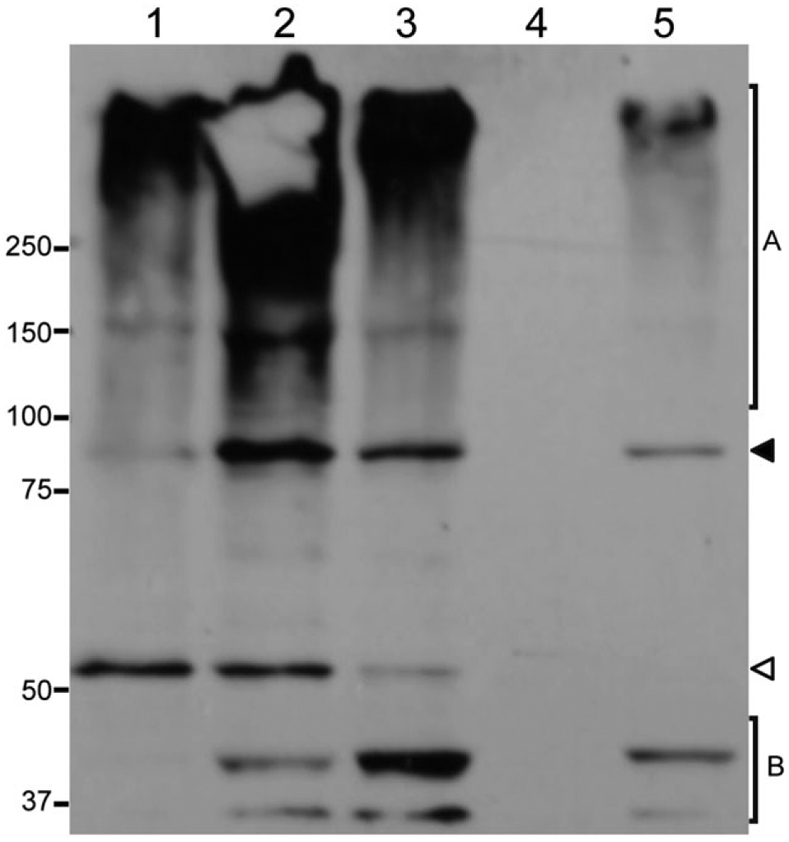
GerAA-mCherry is detected in the inner membrane of dormant spores. The inner membrane fraction from equal amounts (15 OD600 units) of spores of the following strains were isolated, run on an SDS-PAGE gel, transferred to a PVDF membrane and detected with antibodies to GerAA: control (PS4150; lane 1), PS4150 amyE::gerAA-mCherry (KGB114; lane 2), PS4150 ΔgerF amyE::gerAA-mCherry (KGB129; lane 3), PS4150 ΔgerA (KGB136; lane 4), and PS4150 ΔgerA amyE::gerAA-mCherry (KGB138; lane 5). PS4150 spores carried only the wild-type gerAA gene, KGB114 and KGB129 spore carried both wild-type and fusion copies of the gerAA gene, KGB138 spores carried only the gerAA-mCherry fusion gene, and KGB136 had neither wild-type nor fusion gene. The white and black arrowheads point to the location of the full-length GerAA and GerAA-mCherry bands respectively. The total amounts of protein present in the samples loaded in each lane were determined using the EZQ Protein Quantitation Kit, and their relative amounts were: 1.0 (lane 1), 1.0 (lane 2), 0.7 (lane 3), 0.5 (lane 4) and 0.3 (lane 5). The relatively low intensity of the GerAA-mCherry signal from KGB138 spores (lane 5) is most likely because approximately threefold less total protein was present in this lane compared with that in the PS4150 lane (lane 1). High molecular weight bands (region A) appeared to be derived from GerAA because they were not present in the inner membranes from ΔgerA spores, while low molecular weight bands (region B) were only present in spores expressing GerAA-mCherry and could represent degradation products.
GRs localize to a single focus
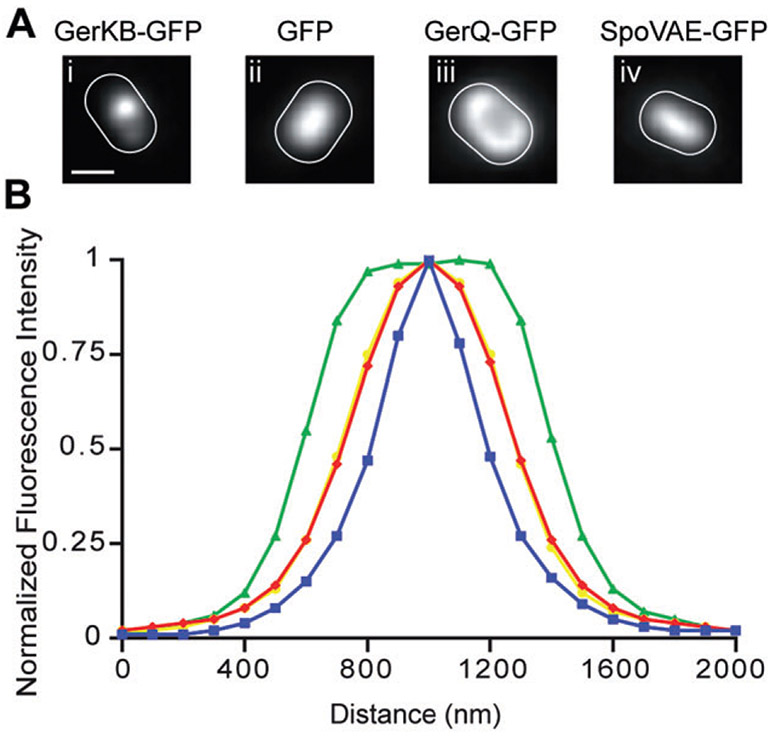
Germinant receptors in spores are likely present in very low numbers of molecules (Paidhungat and Setlow, 2001), so it was expected that the signal from fluorescent protein constructs of GRs would represent a small increase over the relatively high background observed in the spore during fluorescence imaging. To improve the signal-to-noise ratio in these images, we developed a protocol in which 150–200 images were obtained in succession and averaged, resulting in an overall integration time of up to 50 s. Remarkably, the resulting images of spores containing GerAA-mCherry, GerBA-GFP, GerKA-GFP or GerKB-GFP showed that the fluorescence signal was limited to discrete foci (Figs 3B and 4). This result was particularly striking given the relatively few molecules of GRs likely present in spores. In each case, foci were distinguishable from background autofluorescence in > 88% of the spores examined (Table 1), while discrete foci were never observed in PS4150 spores (Fig. 3A). Discrete foci were also not observed in spores that expressed: (i) soluble GFP in the spore core (Cowan et al., 2003), (ii) GerQ-GFP in the spore coat (Ragkousi et al., 2003) or (iii) a SpoVAE-GFP fusion in the spore inner membrane – instead the fluorescence signal was distributed over much larger regions of the spore (Fig. 4). The homogeneous distribution of the SpoVAE-GFP fusion protein in the central region of the spore is not inconsistent with a location for this protein throughout the spore inner membrane, given the fluorescence microscopy methodology used (see below), and such a distribution is consistent with the release of DPA during spore germination around the entire spore inner membrane, and not just from one region of this membrane, as observed recently by confocal Raman imaging (Kong et al., 2011).
Fig. 3. Nutrient germination proteins localize in foci in dormant spores.
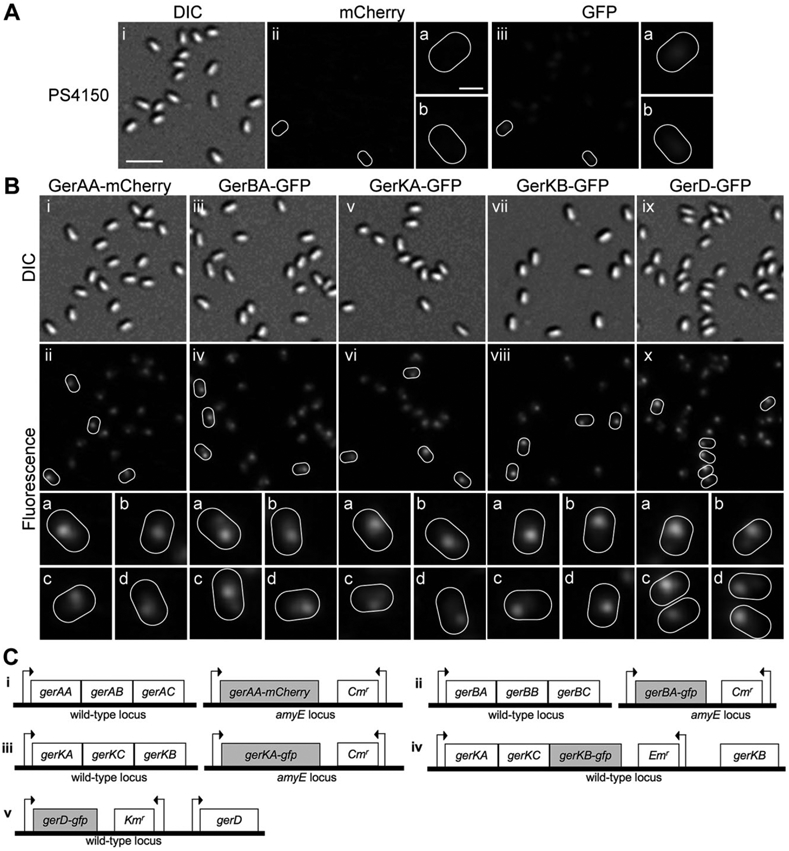
A. Background autofluorescence in PS4150 spores in the (ii) mCherry and (iii) GFP channels, respectively, with some spores outlined in white and magnified images of these spores shown in the corresponding panels (a) and (b); differential interference contrast (DIC) images of the same spores are shown in (i).
B. Localization of germination proteins in dormant spores. Panels show fluorescence images of spores expressing (ii) GerAA-mCherry (KGB114 – Ci) (iv) GerBA-GFP (KGB162 –Cii) (vi) GerKA-GFP (KGB98 – Ciii) (viii) GerKB-GFP (KGB04 – Civ) and (x) GerD-GFP (KGB73 – Cv); some spores are outlined in white and magnified images of these spores are shown in smaller panels (a–d) in the corresponding column. Panels (i, iii, v, vii and ix) are DIC images of the same spores in each column. All fluorescence images in this figure are shown with the same intensity scale. The scale bar in panel Ai is 2 μm, and panels (i–x) are at the same magnification; the scale bar in Aiia is 0.5 μm and all panels (a–d) are at the same magnification.
C. Genotypes of strains used in localization experiments are shown, arrows indicate the relative position of promoters as well as the direction of transcription. Note that in spores carrying the GerKB-GFP fusion the wild-type gerKB gene is present; however, it lacks a promoter and thus is unlikely to be expressed.
Fig. 4. Germination protein foci are diffraction-limited spots.
A. Fluorescence images of a single spore outlined in white based on the corresponding DIC image from strains (strains carried both wild-type and fusion copies of the genes of interest): (i) GerKB-GFP (KGB04) (ii) GFP (PS3518) (iii) GerQ-GFP (KB48), and (iv) SpoVAE-GFP (KGB165) (note that this latter strain almost certainly lacks any wild-type SpoVAE protein). Scale bar = 0.5 μm in panels (i–iv). These images are representative of those analysed in (B).
B. Plots show the normalized average distribution of the fluorescence signal along the long axis of spores of the following strains: GerKB-GFP (KGB04; 23 spores) is shown in blue, soluble GFP (PS3518; 19 spores) is shown in red, GerQ-GFP (KB48; 32 spores) is shown in green and SpoVAE-GFP (KGB165; 25 spores) is shown in yellow. Note that the yellow line representing data from SpoVAE-GFP spores overlaps the red line representing data from GerQ-GFP spores. The full width at half maximal intensity for GeKB-GFP signal was ~300 nm, which is considerably smaller than that for the soluble GFP, GerQ-GFP and SpoVAE-GFP signals, which were not present in discrete foci.
Table 1.
Presence of fluorescent foci in spores of various B. subtilis strains.
| Straina | Spores counted |
Foci per spore (%)b |
|||
|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥ 3 | ||
| GerAA-mCherry (KGB114) | 233 | 0 | 96 | 4 | 0 |
| GerBA-GFP (KGB162) | 302 | 12 | 84 | 4 | 0 |
| GerKA-GFP (KGB98) | 162 | 11 | 88 | 1 | 0 |
| GerKB-GFP (KGB04) | 182 | 5 | 93 | 2 | 0 |
| GerD-GFP (KGB73) | 290 | 6 | 85 | 9 | 0 |
The strains examined are listed according to the fusion protein phenotype, with strain numbers in parentheses. Strains carried both wild-type and fusion copies of the genes of interest.
The number of foci present in a population of spores of each strain was determined as described in Experimental procedures. Spores in which the fluorescence signal obtained could not be distinguished from the autofluorescence signal typically obtained from spores of the PS4150 background strain were scored as having no foci.
The results noted above indicate that the discrete foci of germination proteins in spores are not an artefact of fluorescent protein expression in dormant spores. One focus per spore was observed in 84–96% of spores with fusions to GRs, with 1–4% exhibiting two foci per spore and no spores exhibiting more than two foci (Table 1). In the remaining spores, the fluorescence intensity was below the threshold applied in our experiments, and these spores were classified as exhibiting no foci (Table 1). We observed no gross movement of GR foci in the 50 s time scale of time-lapse fluorescence microscopy experiments, consistent with the relative immobility of lipid molecules in the inner membrane of spores (Cowan et al., 2004). In addition, the GR foci did not appear to localize exclusively to spores’ polar or non-polar regions, although they may have exhibited a slight preference for non-polar regions (Table 2).
Table 2.
Germination protein clusters in dormant spores do not localize preferentially to the spore poles.
| Straina | Spores counted |
Positionb (%) |
|
|---|---|---|---|
| Non-Polar | Polar | ||
| GerAA-mCherry (KGB114) | 57 | 68 | 32 |
| GerBA-GFP (KGB162) | 82 | 80 | 20 |
| GerKA-GFP (KGB98) | 64 | 70 | 30 |
| GerKB-GFP (KGB04) | 52 | 70 | 30 |
| GerD-GFP (KGB73) | 52 | 75 | 25 |
The strains investigated are listed according to the fusion protein phenotype, with strain numbers in parentheses. Strains carried both wild-type and fusion copies of the genes of interest.
Foci were scored as polar if the maximum intensity pixel was within two pixel of either pole of the dormant spore and were scored as non-polar if the maximum intensity pixel was greater that two pixel from the pole of the spore. Based on these criteria ~60% of the spore area is polar, while the remaining ~40% is non-polar. Spores counted had one focus per spore and were oriented such that their long-axes were parallel to the microscope slide.
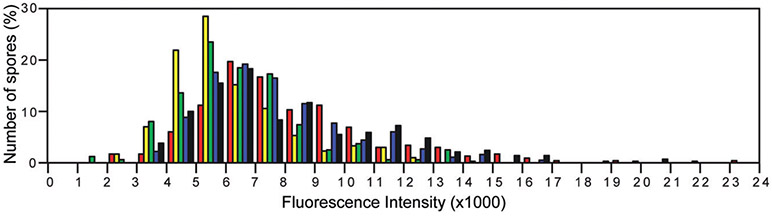
The fluorescence intensities of the GR foci were significantly higher than the background autofluorescence in PS4150 spores, and after correcting for this autofluorescence the majority of spores had little to no fluorescence outside the foci. These results are consistent with most if not all GR proteins residing in the foci, especially because there are so few GRs per spore. We also observed significant variation in the intensity of the fluorescent GR foci in spore populations (Table 3, Fig. 5), suggesting there likely is similar variation in the numbers of GR molecules per spore.
Table 3.
Variation in maximum fluorescence intensities of foci in spores of various B. subtilis strains.
| Fluorescence intensityb |
||||
|---|---|---|---|---|
| Straina | Spores counted | Mean | Maximum | Minimum |
| mCherry fluorescence (532 nm) | ||||
| (PS4150) | 203 | 585 | 3026 | 191 |
| GerAA-mCherry (KGB114) | 233 | 7227 | 22161 | 1538 |
| GFP fluorescence (488 nm) | ||||
| (PS4150) | 203 | 1886 | 5390 | 776 |
| GerBA-GFP (KGB162) | 302 | 5112 | 12411 | 645 |
| GerKA-GFP (KGB98) | 162 | 5479 | 12637 | 412 |
| GerKB-GFP (KGB04) | 182 | 6576 | 15187 | 2156 |
| GerD-GFP (KGB73) | 290 | 7228 | 20305 | 2048 |
The strains investigated are listed according to the fusion protein phenotype, with strain numbers in parentheses. Strains carried both wild-type and fusion copies of the genes of interest.
The maximum fluorescence intensities in arbitrary units of individual spores in populations of 162–302 spores were determined for each strain. All intensity values are expressed with the same scale.
Fig. 5.
Variation of maximum fluorescence intensities of spores of various strains. The maximum fluorescence intensities in arbitrary units of individual spores in populations of 162–302 spores were determined for each strain. GerAA-mCherry (KGB114) is shown in red, GerBA-GFP (KGB162) is shown in yellow, GerKA-GFP (KGB98) is shown in green, GerKB-GFP (KGB04) is shown in blue and GerD-GFP (KGB73) is shown in black (strains carried both wild-type and fusion copies of the genes of interest).
GerD localizes to a single focus
GerD is also located in the spore’s inner membrane, although at ~50-fold higher levels than the GRs (Pelczar and Setlow, 2008). We expressed GerD-GFP from the normal chromosomal locus and under the native promoter in PS4150 spores, and GerD-GFP was at least partially functional (Fig. 1). We again observed discrete GerD-GFP fluorescent foci (Fig. 3) in 94% of spores, with one focus per spore being most common (Table 1). The GerD-GFP foci were on average slightly more intense than those of the GR foci (Table 3), consistent with more GerD than GR molecules in spores. However, the increase in fluorescence intensity was not consistent with a 50-fold increase in protein numbers. This may indicate that a significant population of the GerD-GFP fusion protein is not properly folded, thus making quantitative comparisons of gene expression impossible. Poor folding of at least some GerD-GFP molecules may be partly responsible for the inability of GerD-GFP to facilitate wild-type levels of nutrient germination, although the GFP moiety might also interfere with GerD’s interactions with either other GerD molecules or other proteins (Fig. 1). Like the GRs, GerD foci exhibited no gross movement in the 50 s time scale of time-lapse fluorescence microscopy experiments and GerD foci also did not preferentially localize to spores’ polar or non-polar regions (Table 2). As with the GRs, the majority of GerD-GFP spores had little fluorescence outside the foci suggesting that most GerD-GFP protein is clustered. There was also heterogeneity in the intensities of the GerD-GFP foci across the spore population (Table 3, Fig. 5).
Given the small physical dimension of B. subtilis spores (~1000 nm in length and ~500 nm in width), the GR and GerD foci appear to cover a significant portion of the spore. However, the foci observed are diffraction-limited spots given that on average their fluorescence signal fit a Gaussian function with full width at half maximal intensity of ~300 nm (Fig. 4B), which corresponds to the optical resolution of the microscope used. These data indicate that the germination proteins are likely present in subresolution structures in the spore inner membrane. In addition, because the thickness of the spore (~500 nm) is less than the depth-of-the-field (~700 nm) of the microscopy system, the images obtained reflect the fluorescence signal from the whole spore, including the top and bottom surfaces. Thus, even though the GRs, GerD and most likely SpoVAE are present in the spore inner membrane, they are not always peripherally located in the resulting images.
GRs and GerD foci colocalize
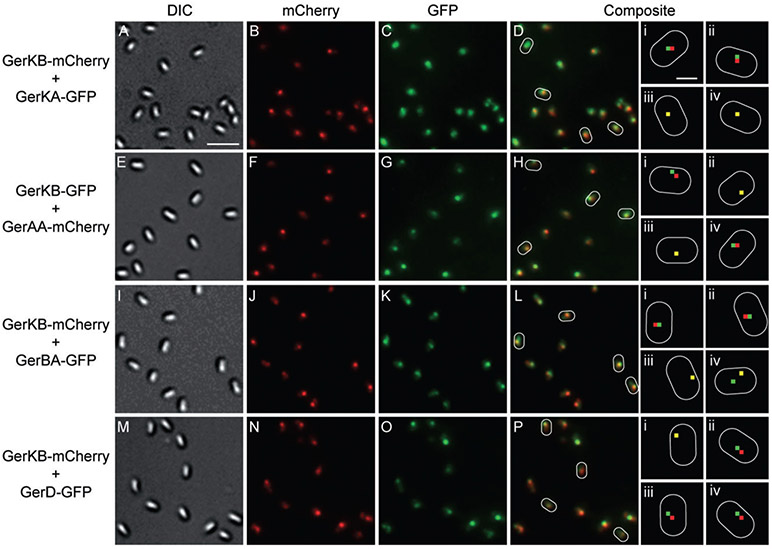
Because GRs and GerD may well interact and all GRs and GerD form foci, we examined whether these proteins are colocalized in dormant spores by coexpressing each protein of interest with either GerKB-GFP or GerKB-mCherry (strains carried both wild-type and fusion copies of the genes of interest). In colocalization experiments only those spores in which both proteins exhibited clear foci were used to determine the extent of colocalization. Foci were scored as colocalized if the pixels of maximum intensity from the GFP and mCherry signals were ≤ 1 pixel (100 nm) apart. This limit was chosen because the measured error in alignment of the dichroic mirrors used to see the GFP and mCherry signals was ± 100 nm. When GerKB-mCherry was expressed from the normal chromosomal locus and GerKA-GFP was coexpressed from the amyE locus, the fluorescent foci for each protein overlapped in 89% of the spores counted (Table 4, Fig. 6). This finding strongly supports previous evidence that individual proteins of the same GR interact in the dormant spore (Igarashi and Setlow, 2005; Vepachedu and Setlow, 2007). The intensities of the GerKB and GerKA foci in a given spore were not always similar, suggesting that there is variation in the numbers of GerKA and GerKB molecules in any given spore, perhaps because the fusion genes are expressed from different chromosomal locations or because of stochastic variations in translation efficiency.
Table 4.
Colocalization of fluorescent foci in spores of various B. subtilis strains.
| Pixels between fluorescence intensity maxima (%)b |
|||||||
|---|---|---|---|---|---|---|---|
| Straina | Spores counted | 0 | 1 | 2 | 3 | 4 | 5 |
| GerKB-mCherry + GerKA-GFP (KGB108) | 310 | 24 | 65 | 10 | 1 | 0 | 0 |
| GerKB-GFP + GerAA-mCherry (KGB115) | 246 | 31 | 67 | 2 | 0 | 0 | 0 |
| GerKB-mCherry + GerBA-GFP (KGB216) | 167 | 36 | 55 | 8 | 1 | 0 | 0 |
| GerKB-mCherry + GerD-GFP (KGB80) | 242 | 24 | 66 | 8 | 2 | 0 | 0 |
| GerKB-mCherry + YuaG-GFP (KGB156) | 142 | 11 | 34 | 35 | 15 | 4 | 1 |
The strains examined are listed according to the fusion protein phenotype, with strain numbers in parentheses. Strains carried both wild-type and fusion copies of the genes of interest.
Fluorescent foci were defined as colocalized if pixels of maximum intensity from the two signals were ≤ 1 pixel apart, and were not colocalized if this distance was ≥ 2 pixels apart.
Fig. 6.
Nutrient germination proteins colocalize in dormant spores. (A–D, E–H, I–L and M–P) are, respectively, images of spores expressing (strains carried both wild-type and fusion copies of the genes of interest): GerKB-mCherry + GerKA-GFP (KGB108); GerKB-GFP + GerAA-mCherry (KGB115); GerKB-mCherry + GerBA-GFP (KGB216); and GerKB-mCherry + GerD-GFP (KGB80). (A, E, I and M) are DIC images; (B, F, J and N) are mCherry fluorescence images in red; (C, G, K and O) are GFP fluorescence images in green and (D, H, L and P) are composite images of the corresponding mCherry and GFP fluorescence images. All fluorescence images were scaled such that the maximum fluorescence intensity of the 8-bit image was 254. Some spores are outlined in white and smaller panels (i–iv) are magnified from the corresponding composite fluorescence images of these spores in which the red and green dots denote the locations of the maximum intensity pixels in each spore. Where the maximum intensity red and green pixels overlap, the pixel appears yellow. The scale bar in panel A is 2 μm and panels (A–P) are at the same magnification; the scale bar in the top panel Di is 0.5 μm and all panels (i–iv) are at the same magnification.
When genes encoding GerAA-mCherry or GerBA-GFP at the amyE locus were coexpressed with gerKB-gfp at the normal locus, the foci colocalized in 98% and 91% of the spores counted respectively (Table 4, Fig. 6). These data indicate that all GRs are almost always in the same location in the dormant spore and thus have the potential for direct interactions. We also observed that in spores expressing two fluorescent fusion proteins the intensities of the foci for each protein were variable suggesting again that there are differences in the numbers of molecules of each GR in individual spores. When we coexpressed genes encoding GerD-GFP and GerKB-mCherry from their normal chromosomal loci, there again was colocalization in 90% of the spores (Table 4, Fig. 6), indicating that these two germination proteins are also in the same location in dormant spores.
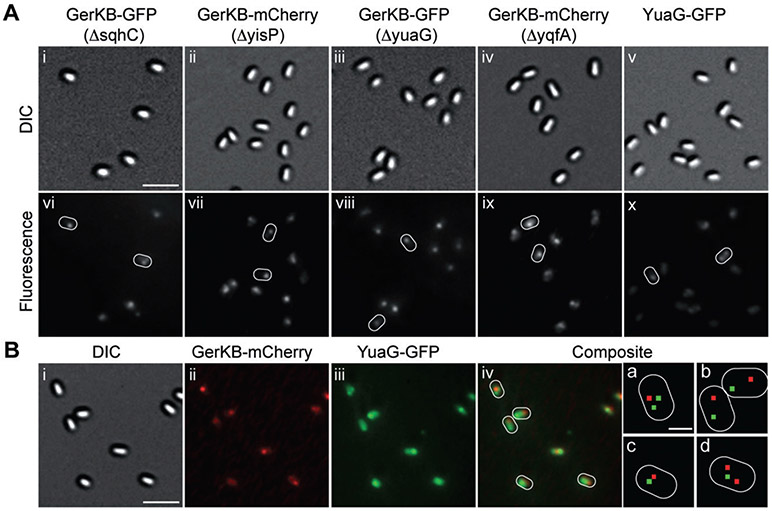
Recently, it was shown that specific polyisoprenoid lipids and flotillin homologues are localized in and essential for the formation of multiple discrete microdomains in B. subtilis cells’ plasma membrane (López and Kolter, 2010). We have determined that these proteins and lipids are unlikely to be involved in spore germination as deletion of either or both of the two B. subtilis genes encoding flotillin homologues (yuaG and yqfA) or enzymes involved in polyisoprenoid synthesis (sqhC or yisP) had no effect on spore germination or germination protein localization (Table 4, Fig. 7A) (strains carried both wild-type and fusion copies of the genes of interest). However, because YuaG-GFP seemed to form weak foci in dormant spores, we examined whether GerKB-mCherry colocalized with the unrelated YuaG-GFP protein. We observed that Yuag-GFP and GerKB-mCherry foci colocalized in only 44% of the spores examined, with many YuaG-GFP foci quite distant from those of GerKB-mCherry (Table 4, Fig. 7B). These data indicate that YuaG-GFP is randomly distributed with respect to the GRs. These results also strongly suggest that germination protein clustering in dormant spores is not dependent on formation of membrane microdomains enriched in polyisoprenoids and flotillin homologues.
Fig. 7. Polyisoprenoids and flotillin homologues are not involved in clustering of germination proteins.
A. Panels show DIC and corresponding fluorescence images of spores expressing (strains carried both wild-type and fusion copies of the genes of interest): (i, vi) GerKB-GFP in a ΔsqhC background (KGB150); (ii, vii) GerKB-mCherry in a ΔyisP background (KGB152); (iii, viii) GerKB-GFP in a ΔyuaG background (KGB147); (iv, ix) GerKB-mCherry in a ΔyqfA background (KGB154); and (v, x) YuaG-GFP (KGB155); some spores in the fluorescence images are outlined in white. All fluorescence images are shown with the same intensity scale. The scale bar in panel (Ai) is 2 μm, and all panels are at the same magnification.
B. Panels show the DIC image (i), mCherry fluorescence image in red (ii), GFP fluorescence image in green (iii), and a composite of mCherry and GFP fluorescence images (iv) of spores expressing GerKB-mCherry + YuaG-GFP (strain KGB156). All fluorescence images in this figure were scaled such that the maximum fluorescence intensity of the 8-bit image was 254. Some spores are outlined in white and smaller panels (a,b) are magnified from the corresponding composite fluorescence images of these spores in which the red and green dots denote the locations of the maximum intensity pixels in each spore. The scale bar in panel (i) is 2 μm and panels (i–iv) are at the same magnification; the scale bar in the panel a is 0.5 μm and all panels (a–d) are at the same magnification.
GerD is required for GR clustering
The GRs and GerD colocalize to a single focus in dormant spores and thus we were interested to see whether these proteins localized independently. To determine whether each GR would cluster in the absence of other GRs we examined spores expressing: (i) GerAA-mCherry in a ΔgerB ΔgerK strain, (ii) GerBA-mCherry in a ΔgerA ΔgerK strain and (iii) GerKB-GFP in a ΔgerA ΔgerB strain. To compare the degree of clustering in spores of the various strains the average Gaussian curvature at the peak of the fluorescence signal from spores of each strain was calculated. Spores with a fluorescent focus should have a higher curvature value than spores without a focus. In all cases single foci were observed in the majority of spores observed, indicating that the GRs did not depend on each other for recruitment to foci (Fig. 8) (GerAA and GerBA fusion strains carried both wild-type and fusion copies of the genes of interest, while the GerKB-GFP strain carried only the fusion gene). The calculated curvature of the fluorescence signal was either similar to that observed in the control strain or was slightly increased, also suggesting that the foci are maintained in these strains (Table 5). This result is not surprising especially in the case of GerA, as loss of GerB and GerK does not affect L-alanine germination. In addition, the requirement for both GerB and GerK in AGFK germination does not stem from interdependence for cluster formation. We also expressed GerAA-mCherry, GerBA-GFP, GerKA-GFP and GerKB-GFP in a ΔgerD background to determine whether GerD was necessary for GR clustering. In the absence of GerD, GRs no longer formed foci but were instead heterogeneously distributed throughout the spore, and consistent with this observation the calculated curvature of the fluorescence signal was significantly decreased (Table 5, Fig. 8) (GerAA, GerBA and GerKA fusion strains carried both wild-type and fusion copies of the genes of interest, while the GerKB-GFP strain carried only the fusion gene). These data indicate that GerD is required for the clustering of GRs and suggest that GerD’s role in nutrient germination may be to facilitate the organization of the GRs into clusters. To investigate whether GerD clustering was dependent on the GRs we examined the localization of GerD-GFP in the absence of all three GRs. In these spores GerD-GFP localized to single clusters in the majority of spores observed (Fig. 8). Thus GerD forms clusters independent of the GRs, which supports the role of GerD as a scaffold. Interestingly, the curvature of the fluorescence signal is almost doubled compared with the control strain. This could mean that GerD-GFP molecules cluster even more tightly in the absence of the GRs.
Fig. 8.
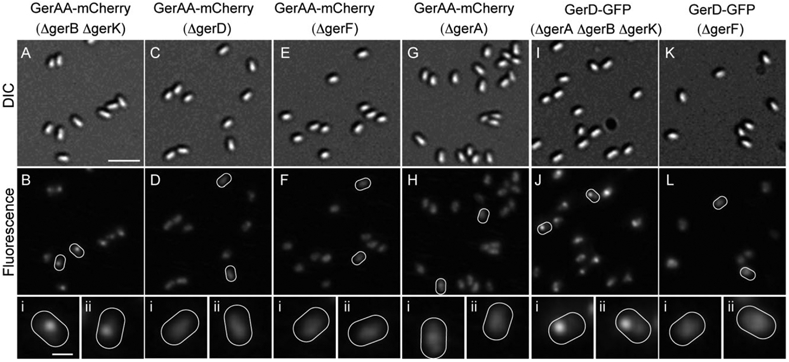
Effect of various mutations on the formation of germination protein foci. (A–B, C–D, E–F and G–H) are, respectively, images of spores expressing GerAA-mCherry (strains carried both wild-type and fusion copies of the genes of interest except in the case of KGB138 which only had the fusion gene) but with the following mutations: ΔgerB ΔgerK (KGB168); ΔgerD (KGB196); ΔgerF (KGB129); and ΔgerA (KGB138). (I–J and K–L) are, respectively, images of spores expressing GerD-GFP but with the following mutations: ΔgerA ΔgerB ΔgerK (KGB213); and ΔgerF (KGB125). Panels (A, C, E, G, I and K) are DIC images and panels (B, D, F, H, J and L) are the corresponding fluorescence images. Some spores in the fluorescence images are outlined in white and magnified images of these spores are shown in smaller panels (i–ii) in the corresponding column. The scale bar in panel A is 2 μm, and panels (A–L) are at the same magnification; the scale bar in (Ai) is 0.5 μm and all panels (i–ii) are at the same magnification. All fluorescence images in this figure are shown with the same intensity scale used for the fluorescence images in Fig. 1.
Table 5.
Disruption of fluorescent foci in spores of various B. subtilis strains.
| Genotype (Strain)a | Spores counted | Curvatureb | Curvature relative to control |
|---|---|---|---|
| (PS4150) 488 nmc | 340 | 145 | – |
| (PS4150) 532 nm | 82 | 178 | – |
| GerAA-mCherry | |||
| Control (KGB114) | 513 | 874 | 1.0 |
| ΔgerB ΔgerK (KGB168) | 241 | 1028 | 1.2* |
| ΔgerD (KGB196) | 264 | 411 | 0.5*** |
| ΔgerF (KGB129) | 143 | 439 | 0.5*** |
| ΔgerA (KGB138) | 526 | 373 | 0.4*** |
| gerACA18 (KGB221) | 279 | 347 | 0.4*** |
| GerAA-mCherry 2X | |||
| Control (KGB174) | 122 | 1160 | 1.0 |
| ΔgerAB (KGB181) | 124 | 389 | 0.3** |
| ΔgerAC (KGB175) | 84 | 267 | 0.2** |
| GerBA-GFP | |||
| Control (KGB162) | 325 | 593 | 1.0 |
| ΔgerB (KGB189) | 217 | 323 | 0.5** |
| ΔgerD (KGB198) | 288 | 360 | 0.6** |
| GerBA-mCherry | |||
| Control (KGB103) | 233 | 601 | 1.0 |
| ΔgerA ΔgerK (KGB173) | 158 | 565 | 0.9 |
| ΔgerF (KGB128) | 252 | 390 | 0.6* |
| GerKA-GFP | |||
| Control (KGB98) | 215 | 735 | 1.0 |
| ΔgerD (KGB193) | 317 | 327 | 0.4** |
| ΔgerF (KGB127) | 415 | 428 | 0.6** |
| ΔgerK (KGB102) | 245 | 476 | 0.6* |
| GerKB-GFP | |||
| Control (KGB04) | 203 | 1000 | 1.0 |
| ΔgerA ΔgerB (KGB27) | 177 | 941 | 0.9 |
| ΔgerD (KGB26) | 344 | 358 | 0.4*** |
| GerKB-mCherry | |||
| Control (KGB100) | 327 | 777 | 1.0 |
| ΔgerF (KGB126) | 259 | 365 | 0.5** |
| ΔgerKA (KGB199) | 284 | 340 | 0.4*** |
| GerD-GFP | |||
| Control (KGB73) | 725 | 880 | 1.0 |
| ΔgerA ΔgerB ΔgerK (KGB213) | 465 | 1558 | 1.8*** |
| ΔgerF (KGB125) | 178 | 636 | 0.7* |
Denotes that the calculated average curvature relative to the corresponding control strain has a 1 × 10−15 ≤ P-value ≤ 1 × 10−3 as determined by the student’s t-test
Denotes that the calculated average curvature relative to the corresponding control strain has a 1 × 10−35 < P-value ≤ 1 × 10−15 as determined by the student’s t-test
Denotes that the calculated average curvature relative to the corresponding control strain has a P-value ≤ 1 × 10−35 as determined by the student’s t-test.
The fluorescent fusion protein of interest is listed in bold and the strains examined for each fusion protein are listed below. The control strain is listed first and contains the fusion protein in a PS4150 background. The strains listed after each control are derived from the control strain and contain the fusion protein of interest plus additional mutations indicated, the strain numbers are in parentheses. GerAA-mcherry 2X strains contain two copies of the fusion gene; these latter strains also lack the wild-type gene. Strains KGB27 and KGB26 contain only the gerKB-gfp fusion gene and the remaining GerKB fusion strains contain both fusion and wild-type genes, although the wild-type gene lacks a promoter and is unlikely to be expressed. Strains KGB138, KGB189 and KGB102 only contain the fusion copy of the gene of interest because the wild-type operon is deleted. All other strains contain both fusion and wild-type genes.
The average Gaussian curvature at the peak of the fluorescence signal from spores of each strain was calculated using ImageJ. Spores with a fluorescent focus should have a higher curvature value than spores without a focus.
PS4150 contains no fusion protein but has background autofluorescence and was thus examined in both GFP (488 nm) and mCherry (532 nm) channels as a baseline.
Lipid addition by GerF is essential for GR and GerD clustering
The C subunit of all the GRs as well as GerD are almost certainly lipoproteins and previous work has demonstrated that loss of GerF, the only known prelipoprotein diacylglycerol transferase in B. subtilis, greatly reduces the ability of spores to germinate (Igarashi et al., 2004). Thus we asked whether GerF was necessary for cluster formation by expressing GerAA-mCherry, GerBA-mCherry, GerKA-GFP, GerKB-mCherry and GerD-GFP in a ΔgerF background. Previous work has suggested that the loss of GerF could result in the loss of the germination lipoproteins (GerD and the GR C subunits) from the inner spore membrane (Pelczar and Setlow, 2008; Mongkolthanaruk et al., 2009; Cooper and Moir, 2011). In the absence of GerF the GRs and GerD were no longer in foci but were instead randomly distributed throughout the spore, but at least in the case of GerAA-mCherry the fusion protein was retained in the spore’s inner membrane (Table 5, Figs 2 and 8) (strains carried both wild-type and fusion copies of the genes of interest). These data indicate that lipid addition via GerF is essential for clustering of the germination proteins. The effect on GerD suggests that lipid addition is required for its organization into a cluster. However, the effect on the GRs could be directly due to the loss of lipid addition to their C subunits, or indirectly due to the effect of loss of lipid addition on GerD. Thus, we examined whether the localization of GerAA-mCherry would be altered in spores that contain gerACAla18 in which the diacylglycerylated cysteine is replaced with alanine. In these spores the localization of GerAA-mCherry is also disrupted, indicating that lipid addition to the C subunit of GRs is also important for GR cluster formation (Table 5).
All three subunits are required for clustering of a GR
The GRs are each composed of three subunits all of which are known to be required for spore germination; however, the specific role of each subunit is unknown (Errington, 1993). We thus investigated which of these subunits were essential for cluster formation. We first determined whether the A subunits could form clusters independently by deleting the wild-type operon in strains encoding A subunit fusions at the amyE locus. When we examined spores expressing GerAA-mCherry with ΔgerA, GerBA-GFP with ΔgerB and GerKA-GFP with ΔgerK we observed a heterogenous distribution of fluorescence instead of discrete foci, although at least in the case of GerAA-mCherry the fusion protein was retained in the spore’s inner membrane (Table 5, Figs 2 and 8). These data show that the A subunits of the GRs are not capable of forming clusters on their own and require either one or both of the B and C subunits, although insertion of GerAA-mCherry into the inner membrane of dormant spores occurs independently of GerAB and GerAC.
We then examined spores expressing GerAA-mCherry but with ΔgerAB or ΔgerAC to determine which of the B or C subunits were necessary for GerA clustering (spores lacked wild-type gerAA gene). In both cases the GerAA-mCherry fluorescence signal was again randomly distributed throughout the spore indicating that both GerAB and GerAC are required for GerAA clustering (Table 5). To determine whether the A subunit of a GR was required for clustering of the B subunit, we expressed GerKB-mCherry at the wild-type chromosomal locus in a ΔgerKA strain. We observed that GerKB no longer localized to foci suggesting that the B subunit of the GRs requires the A subunit for cluster formation (Table 5) (spores contained wild-type gerKB gene that lacks a promoter and is thus unlikely to be expressed). Although we were unsuccessful in making fusions to the C subunits of the GRs, our data suggest that all the subunits of the GRs are required for cluster formation.
Discussion
We have shown that fluorescent protein fusions to GerD and each of the GRs that are at the least partially functional typically localize to a single focus in the inner membrane of dormant B. subtilis spores. The germination protein foci are unlikely to be artefacts and unrelated to spore germination, because foci were not found in PS4150 spores with: (i) no germination protein-GFP fusions, (ii) fluorescent proteins in the core or coat and (iii) a functional SpoVAE-GFP protein fusion (Fig. 4). The germination protein foci are also unlikely to be inclusion bodies given that: (i) the GFP or mCherry components are fluorescent and thus properly folded, (ii) all of the germination protein fusions are at least partially functional and (iii) the fusion proteins are most likely in the spore’s inner membrane, which is unlikely to house inclusion bodies.
The observation that GRs and GerD typically colocalize to the same focus suggests that these foci play an important role during spore germination. In addition, the germination protein foci described are likely specialized structures specific to germination as a GFP-fusion to YuaG, a membrane-associated protein not involved in spore germination but that also forms foci, did not associate with the germination protein foci. In our experiments, a loss of germination protein clustering was consistently correlated with a loss or significant reduction in the ability of spores to germinate in response to nutrients. This latter result strongly suggests, although does not prove that the germination protein foci are functional aggregates that are necessary for optimal germination and are almost certain to represent a dedicated germination centre or ‘germinosome’ in the spore inner membrane.
Our data show that the GRs depend on GerD for clustering, suggesting that the role for GerD in nutrient germination is at least in part to organize the germinosome. The previously noted ability of GerD to multimerize could be integral to its role as a scaffold (Pelczar and Setlow, 2008). GerD may also require the help of other unidentified protein or non-protein factors for forming the clusters. Linkage to diacylglycerol moieties in the inner membrane via GerF appears to be essential for GerD function. Previous analysis of the effect of loss of specific phospholipids on nutrient germination has shown only a rather weak dependence of spore germination on the presence of cardiolipin in the spore’s inner membrane (Griffiths and Setlow, 2009), suggesting that specific phospholipids are unlikely to be essential for germinosome formation. Because no GerD homologue has been found in sporeforming Clostridium species it would be interesting to investigate whether GRs in these species also form a germinosome; if so it is likely that other proteins are employed to organize these structures.
As mentioned above, all subunits of the GRs are required for responding to nutrients, although the function of each subunit remains unknown. We have found that all subunits are also required for GR clustering, indicating that regions of each subunit contribute to incorporation into the germinosome. Recent work has identified various mutations in GerAA and GerAB that alter GerA receptor function and possibly assembly (Cooper and Moir, 2011; Mongkolthanaruk et al., 2011). It will be important to examine which of the mutations identified affect cluster formation and thus are likely to affect global receptor assembly and which do not and thus are more likely to be more directly involved in ligand binding or modulating signal transduction. In addition, the structure of the GerBC subunit of the GerB receptor was recently solved by X-ray crystallography (Li et al., 2010); thus further analysis of the GerBC subunit by site-directed mutagenesis guided by the protein structure could provide information about what parts of this protein may be involved in protein clustering.
The numbers of GRs in spores is estimated to be very low, < 40 molecules per spore (Paidhungat and Setlow, 2001). However organization into clusters greatly increases the local concentration of the GRs and is likely to contribute to increased sensitivity and a more efficient response to nutrients. We have shown that loss of GerD disrupts GR clusters, and previous work indicates that the rate of nutrient germination in gerD spores is decreased greater than 12-fold compared with wild-type spores, although it is not abolished (Pelczar et al., 2007). The observation that simply overexpressing the GRs does not rescue the gerD phenotype (Pelczar et al., 2007), suggests that the germinosome serves to do more than simply increase the local GR concentration. It thus is possible that GerD also helps to recruit other proteins to the germinosome that play a role in the germination response. In addition, although our data show that GRs require all their subunits for clustering, it is not clear how GRs become associated with the clusters. We can conclude from our data that the GRs and GerD are at least in close proximity and there may well be a network of direct and indirect interactions among the various proteins, although to date there is no evidence for direct interaction between GerD and the GR subunits. Thus, more work will be needed to define what parts of GerD and the GR subunits are necessary for clustering and colocalization. Further experiments using protein cross-linking and fluorescence resonance energy transfer could determine whether there are indeed direct interactions between proteins in the foci and aid in identifying unknown components of the germinosome.
The clustering of GRs and GerD in dormant spores appears to be integral to spore germination. Thus, it will be interesting to determine when and how the proteins are recruited to the germinosome during sporulation. GRs and GerD are synthesized during sporulation well before DPA accumulation (Kemp et al., 1991; Igarashi and Setlow, 2006). Do the germination protein foci appear in parallel with these proteins’ synthesis or are they formed later? It is plausible that forming foci later in sporulation may preclude premature activation of the GRs in the developing spore. The unusual characteristics of the spore’s inner membrane (Cowan et al., 2004) may also be essential for association of GRs and GerD in foci.
In addition to GRs and GerD, it is possible that other inner membrane proteins that function in spore germination, especially at early stages, may be in the same foci. Proteins in this category could include some of the SpoVA proteins as well as: (i) receptors for as yet unidentified ligands, (ii) agents that may facilitate signal transduction from the GRs to GerD and (iii) agents that transduce information from GerD to SpoVA proteins. In addition, there may be specific proteins and lipids that are important only for cluster formation and stability. It is thus possible that we have uncovered only a few of the elements of a network of proteins that form the germinosome.
The fusion proteins used in this study can facilitate spore germination, and it would be interesting to examine the fate of the foci formed by these proteins during spore germination. Time-lapse fluorescence microscopy during germination would reveal whether fluorescent foci are retained during and after germination, and preliminary data suggest that at least some of the foci are no longer visible after germination (K.K. Griffiths et al., unpublished). Because GR and GerD functions are limited to early stages of spore germination, the foci of these proteins might only be necessary for early germination events. In cases where foci do disappear it may be interesting to examine how and at what stage of germination the changes in localization occur. Previous work has shown that GerBA remains in the inner membrane after spore germination while GerD does not (Paidhungat and Setlow, 2001; Pelczar and Setlow, 2008).
Assuming that the intensities of the fluorescent foci are directly proportional to levels of germination proteins in spores, we consistently observed that levels of all the proteins investigated varied widely from spore to spore, and that within a single spore the levels of different GRs also varied. Previous studies have shown that GR overexpression leads to increased rates of spore nutrient germination and that loss of GerD drastically reduces spore germination rates (Atluri et al., 2006; Pelczar et al., 2007). It is thus expected that spores with the highest levels of GRs and GerD will germinate fastest while those with the lowest levels will germinate slowest. However, there are endless permutations of the levels of each GR and GerD, which could explain the heterogeneity of germination rates that is typically observed across spore populations (Zhang et al., 2010). Recently, spores that are particularly resistant to germination, termed superdormant spores, have been isolated, and some data indicate that these spores may have low GR levels (Ghosh and Setlow, 2009). Certainly, there are likely to be multiple combinations of levels of GRs, GerD and possibly other unidentified proteins that could lead to superdormancy.
While the germination proteins investigated in this study are located in the spore’s inner membrane, little is known about their structure or their topology. The A and B components of the GRs are most likely polytopic integral transmembrane proteins, but the number of transmembrane domains and the orientation of their N- and C-termini with respect to the membrane have not been determined. Knowing which parts of the GRs face the extracytoplasmic space could provides clues to which parts of these various proteins bind nutrient ligands and are capable of interacting on either side of the spore inner membrane.
The germinosome described in this report in which GRs and GerD coexist is a novel structural component in spores. However, the clustering of proteins needed for recognition and transmittal of signals by small molecules is by no means unique to spores, because clusters of chemotaxis proteins are present in many bacteria. In these organisms, various chemoreceptors that respond to specific stimuli, as well as downstream signalling molecules and effector proteins all cluster together (Alley et al., 1992; Maddock and Shapiro, 1993). Studies on these systems have shown that interactions among and between these proteins are particularly important for the exquisite sensitivity of the receptors and for rapid and integrated responses to chemotactic stimuli (Falke, 2002; Sourjik, 2004). Similarly, we propose that clustering of GRs and GerD in dormant spores is a major contributor to the cooperativity observed in the nutrient germination of bacterial spores.
Experimental procedures
Strains and plasmids used, spore preparation and germination
Bacillus subtilis strains used in this work are listed in Table 6 and are isogenic derivatives of strain PS832, a prototrophic derivative of strain 168. Details of plasmid and strain construction are described in Supporting information and Table S1. Spores were prepared at 37°C in liquid 2 × SG medium without antibiotics, and spores were cleaned and stored as described (Nicholson and Setlow, 1990). Spores used in this work were > 98% free of vegetative or sporulating cells, germinated spores or cell debris as determined by phase contrast microscopy. Spores at an optical density at 600 nm (OD600) of > 10 in water were heat-shocked at 75°C for 30 min, and cooled on ice for ~15 min. Spores were germinated at an OD600 of 1 at 37°C in 1 ml of: (i) 10 mM L-alanine in 10 mM Tris-HCl (pH 8.6) or (ii) AGFK [10 mML-asparagine, 28 mMd-glucose, 28 mM d-fructose, 50 mM KCl in 10 mM Tris-HCl buffer (pH 8.6)]. Spore germination was assessed by measuring the OD600 of germinating cultures that falls ~60% upon completion of germination. At the end of germination experiments (3–7 h), phase-contrast microscopy was used to assess the extent of spore germination.
Table 6.
B. subtilis strains used.
| Strain | Genotypea | Source |
|---|---|---|
| PS3518 | PS832 amyE::sspE-gfp | Cowan et al. (2003) |
| PS4150 | PS832 ΔgerE::spc, ΔcotE::tet | Ghosh et al. (2008) |
| KB48 | PS832 gerQ-gfp kan gerQ | Ragkousi et al. (2003) |
| KGB04 | PS4150 gerKA gerKC gerKB-gfp ermC gerKB | This study |
| KGB08 | PS4150 gerKA gerKC gerKB::gerKB-gfp ermC | This study |
| KGB26 | PS4150 gerKA gerKC gerKB::gerKB-gfp ermC, ΔgerD::cam | This study |
| KGB27 | PS4150 gerKA gerKC gerKB::gerKB-gfp ermC, ΔgerA::kan, ΔgerB::cat | This study |
| KGB34 | PS4150 gerKA gerKC gerKB::gerKB-mCherry ermC | This study |
| KGB52 | PS4150 gerD::gerD-gfp kan | This study |
| KGB73 | PS4150 gerD-gfp kan gerD | This study |
| KGB80 | PS4150 gerKA gerKC gerKB-mCherry cat gerKB, gerD-gfp kan gerD | This study |
| KGB98 | PS4150 amyE::gerKA-gfp | This study |
| KGB100 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB | This study |
| KGB102 | PS4150 amyE::gerKA-gfp, ΔgerK::ermC | This study |
| KGB103 | PS4150 amyE::gerBA-mCherry | This study |
| KGB108 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB, amyE::gerKA-gfp | This study |
| KGB114 | PS4150 amyE::gerAA-mCherry | This study |
| KGB115 | PS4150 gerKA gerKC gerKB-gfp ermC gerKB, amyE::gerAA-mCherry | This study |
| KGB125 | PS4150 gerD-gfp kan gerD, ΔgerF::ermC | This study |
| KGB126 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB, ΔgerF::ermC | This study |
| KGB127 | PS4150 amyE::gerKA-gfp, ΔgerF::ermC | This study |
| KGB128 | PS4150 amyE::gerBA-mCherry, ΔgerF::ermC | This study |
| KGB129 | PS4150 amyE::gerAA-mCherry, AgerF::ermC | This study |
| KGB136 | PS4150 ΔgerA::kan | This study |
| KGB138 | PS4150 ΔgerA::kan, amyE::gerAA-mCherry | This study |
| KGB147 | PS4150 gerKA gerKC gerKB-gfp ermC gerKB, ΔyuaG::kan | This study |
| KGB150 | PS4150 gerKA gerKC gerKB-gfp ermC gerKB, ΔsqhC::cat | This study |
| KGB152 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB, ΔyisP::cat | This study |
| KGB154 | PS4150 gerKA gerKC gerKB-mCherry cat gerKB, ΔyqfA::ermC | This study |
| KGB155 | PS4150 amyE::yuaG-gfp | This study |
| KGB156 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB, amyE::yuaG-gfp | This study |
| KGB157 | PS4150 gerKA gerKC gerKB-mCherry cat gerKB, ΔyqfA::ermC, ΔyuaG::kan | This study |
| KGB162 | PS4150 amyE::gerBA-gfp | This study |
| KGB165 | PS4150 spoVAA spoVAB spoVAC spoVAD spoVAE-gfp ermC spoVAE spoVAF | This study |
| KGB168 | PS4150 amyE::gerAA-mCherry, ΔgerB::kan, ΔgerK::ermC | This study |
| KGB173 | PS4150 amyE::gerBA-mCherry, ΔgerA::kan, ΔgerK::ermC | This study |
| KGB174 | KGB136 amyE::gerAA-mCherry gerAB gerAC ermC gerAA-mCherry cat | This study |
| KGB175 | KGB136 amyE::gerAA-mCherry gerAC ermC gerAA-mCherry cat | This study |
| KGB181 | KGB136 amyE::gerAA-mCherry gerAB ermC gerAA-mCherry cat | This study |
| KGB189 | PS832 ΔgerE::ermC, ΔcotE::tet, amyE::gerBA-gfp, ΔgerB::spc | This study |
| KGB193 | PS4150 amyE::gerKA-gfp, ΔgerD::kan | This study |
| KGB196 | PS4150 amyE::gerAA-mCherry, ΔgerD::kan | This study |
| KGB198 | PS4150 amyE::gerBA-gfp, ΔgerD::kan | This study |
| KGB199 | PS4150 gerKA::ermC gerKC gerKB-mCherry kan gerKB | This study |
| KGB202 | PS832 ΔgerE::ermC, ΔcotE::tet, ΔgerB::spc, amyE::gerBA-gfp gerBB gerBC kan gerBA-gfp cat | This study |
| KGB203 | PS4150 gerKA::ermC gerKC gerKB, amyE::gerKA-gfp | This study |
| KGB213 | PS832 gerD-gfp kan gerD, ΔgerE::mt spc, ΔcotE::mt tet, ΔgerA::kan, ΔgerB::spc, ΔgerK::tet | This study |
| KGB216 | PS4150 gerKA gerKC gerKB-mCherry kan gerKB, amyE::gerBA-gfp | This study |
| KGB221 | PS832 ΔgerE::mt spc, ΔcotE::tet, gerAA gerAB gerAC::gerACA18 spc, amyE::gerAA-mCherry | This study |
Emboldened genes do not have an associated promoter and thus are unlikely to be transcribed.
Spore fractionation and Western blot analysis
Spores of the various strains at an OD600 of 50 (2 ml) were decoated by incubating at 70°C for 45 min in 0.1 M NaOH, 0.1 M NaCl, 0.5% sodium dodecyl sulfate (SDS), 0.1 M dithiothreitol and washed at least 10 times with water (Griffiths and Setlow, 2009). To make spore lysates, spores were suspended at an OD600 of 75 in 1 ml TEP buffer [50 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride] containing 2 mg of lysozyme, 2 μg each of RNAse A and DNase I, and 40 μg of MgCl2, incubated at 37°C for 5 min, then kept on ice for 20 min. The spore suspension was sonicated with 100 mg of glass beads using 15 s bursts until > 80% lysis was observed by phase contrast microscopy (Paidhungat and Setlow, 2001). The sample was centrifuged at 4°C for 5 min in a microcentrifuge, the supernatant was saved and the pellet was washed with 0.5 ml of TEP buffer, centrifuged again and this second supernatant was pooled with the first. In this process the glass beads, unbroken spores and integument debris were removed. The pooled supernatant or lysate was then centrifuged at 100 000 g for 1 h, the resulting pellet (P100) was gently rinsed with 100 μl of TEP and resuspended in 75 μl TEP buffer containing 1% Triton X-100 (Paidhungat and Setlow, 2001). Samples were boiled in 1 × SDS containing sample buffer, volumes of each sample equivalent to an OD600 of decoated spores of 15 were separated using 7.5% SDS polyacrylamide gel electrophoresis, and proteins were transferred to an Immbilon-P membrane (Millipore, Billerica, MA, USA). These Western blots were probed with a 1:500 dilution of polyclonal antibodies to GerAA (made as described in Supporting information), followed by a 1:5000 dilution of bovine anti-rabbit immunoglobulin horse radish peroxidase conjugate (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and analysed for chemiluminescence using X-ray film. Although we attempted to load equivalent amounts of each protein sample based on the known starting quantity of decoated spores, it is likely that there was non-uniform loss of protein during the sample preparation process. We thus quantified the amount of total protein in our P100 samples from each strain and determined the relative amounts of total protein loaded during Western blot experiments using the EZQ Protein Quantitation Kit (Invitrogen, Carlsbad, CA, USA).
Microscopy
Spores were suspended in water at an OD600 of 50–70. For microscopy, 0.5 ml of spore suspension was spread between two glass coverslips that were cleaned by sonicating in 10% NaOH, 95% ethanol and distilled water. Images were collected using a modified epi-fluorescence microscope (IX81, Olympus, Center Valley, PA, USA) equipped with a 60× microscope objective (NA = 1.45, Olympus). Image acquisition software was built on top of the μManager platform (http://micro-manager.org). The fluorescence signals were captured with a TE-cooled EM-CCD camera (Princeton Instruments, Trenton, NJ, USA), and images were processed and analysed using ImageJ software. In each experiment 150–200 fluorescence images were taken consecutively with an acquisition time of 250 ms. For spores of strains PS3158 and KB48 the acquisition time was reduced to 15 and 50 ms, respectively, to prevent saturation of the fluorescence signal because the soluble GFP and GerQ-GFP in these strains are expressed at much higher levels than the GR fusions. Images used in subsequent analysis were generated by averaging 100 consecutive images in the acquisition sequence followed by subtraction of the background signal present in cell-free areas. Because PS4150 spores have some background autofluorescence, we determined the maximum fluorescence intensity of 203 PS4150 spores in both the mCherry and GFP channels (Table 3). Based on this data we set thresholds of 1400 and 3200 for the mCherry and GFP channels, respectively, for determining whether fluorescence in test strains was due to the expression of fusion proteins. The maximum autofluorescence of individual PS4150 spores was below these thresholds in 95% of the spores observed. Spores from test strains with fluorescence below these thresholds were scored as having no foci.
In colocalization experiments images from mCherry and GFP channels processed as described above were merged and aligned using offset values determined using 500 nm fluorescent beads. To determine the extent of colocalization, the position of the pixel with maximum fluorescence intensity for each spore was determined in both mCherry and GFP channels. Foci were defined as colocalized if pixels of maximum intensity from the two signals were ≤ 1 pixel apart, and were not colocalized if this distance was ≥ 2 pixels apart. The positions of the mCherry foci and the GFP foci can be slightly shifted (one pixel is ~100 nm) because of the signal noise and because of the wavefront alteration caused by changing of dichroic mirrors. In cases where spores had two foci for one or both fusion proteins, the spore was counted as exhibiting colocalization if at least one focus from each fusion protein colocalized with the other according to the criteria described above.
To determine the distribution of the fluorescence signal along the length of spores expressing GerKB-GFP, soluble GFP, GerQ-GFP and SpoVAE-GFP, the average fluorescence signal as a function of the position along the long axis of the spore was determined for spores of each strain using ImageJ software. The spores analysed had one focus per spore and were oriented such that their long-axes were parallel to the microscope slide. The fluorescence distribution curves from spores of each strain were aligned and then averaged and normalized.
To quantify the tendency of the different strains to form foci, the fluorescence intensity of images processed as described above were normalized and the Gaussian curvatures were calculated for each spore at its peak fluorescence intensity using ImageJ and then averaged. Spores with a fluorescent focus should have a higher curvature value than spores without a focus.
Supplementary Material
Acknowledgements
This material is based upon work supported by a Department of Defense Multi-disciplinary University Research Initiative through the U. S. Army Research Laboratory and the U. S. Army Research Office under contract number W911NF-09–1-0286. We are grateful to J. Dworkin for plasmid pAF328 and to R. Kolter for sharing results prior to publication. We also thank G. Korza for preparing the GerAA antiserum and K. Stewart for determining the working dilutions of the GerAA antibody.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alley MR, Maddock JR, and Shapiro L (1992) Polar localization of a bacterial chemoreceptor. Genes Dev 6: 825–836. [DOI] [PubMed] [Google Scholar]
- Atluri S, Ragkousi K, Cortezzo DE, and Setlow P (2006) Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J Bacteriol 188: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GR, and Moir A (2011) Amino acid residues in the GerAB protein important in the function and assembly of the alanine spore germination receptor of Bacillus subtilis 168. J Bacteriol 193: 2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AE, Koppel DE, Setlow B, and Setlow P (2003) A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc Natl Acad Sci USA 100: 4209–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan AE, Olivastro EM, Koppel DE, Loshon CA, Setlow B, and Setlow P (2004) Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc Natl Acad Sci USA 101: 7733–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J (1993) Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev 57: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ (2002) Cooperativity between bacterial chemotaxis receptors. Proc Natl Acad Sci USA 99: 6530–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, and Errington J (1985) Nucleotide sequence and complementation analysis of a polycistronic sporulation operon, SpoVA in Bacillus subtilis. J Gen Microbiol 131: 1091–1105. [DOI] [PubMed] [Google Scholar]
- Ghosh S, and Setlow P (2009) Isolation and characterization of superdormant spores of Bacillus species. J Bacteriol 191: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Setlow B, Wahome PG, Cowan AE, Plomp M, Malkin AJ, and Setlow P (2008) Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190: 6741–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths KK, and Setlow P (2009) Effects of modification of membrane lipid composition on Bacilus subtilis sporulation and spore properties. J Appl Microbiol 106: 2064–2078. [DOI] [PubMed] [Google Scholar]
- Hudson KD, Corfe BM, Kemp EH, Feavers IM, Coote PJ, and Moir A (2001) Localization of GerAA and GerAC germination proteins in Bacillus subtilis spore. J Bacteriol 183: 4317–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, and Setlow P (2005) Interaction between individual protein components of the GerA and GerB nutrient receptors that trigger germination of Bacillus subtilis spores. J Bacteriol 187: 2513–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, and Setlow P (2006) Transcription of the Bacillus subtilis gerK operon, which encodes a spore germinant receptor, and comparison with that of operons encoding other germinant receptors. J Bacteriol 188: 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Setlow B, Paidhungat M, and Setlow P (2004) Effects of gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J Bacteriol 186: 2984–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp EH, Sammons RL, Moir A, Sun D, and Setlow P (1991) Analysis of transcriptional control of the gerD spore germination gene of Bacillus subtilis 168. J Bacteriol 173: 4646–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner D, and Sourjik V (2006) Spatial organization of the bacterial chemotaxis system. Curr Opin Microbiol 9: 619–624. [DOI] [PubMed] [Google Scholar]
- Kong L, Zhang P, Yu J, Setlow P, and Li Y (2011) Rapid confocal Raman imaging using a synchro multifoci-scan scheme for dynamic monitoring of single living cells. Appl Phys Lett 98: 213703–213705. [Google Scholar]
- Li Y, Setlow B, Setlow P, and Hao B (2010) Crystal structure of the GerBC component of a Bacillus subtilis spore germinant receptor. J Mol Biol 402: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López D, and Kolter R (2010) Functional microdomains in bacterial membranes. Genes Dev 24: 1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock JR, and Shapiro L (1993) Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259: 1717–1723. [DOI] [PubMed] [Google Scholar]
- Magge A, Setlow B, Cowan AE, and Setlow P (2009) Analysis of dye binding by and membrane potential in spores of Bacillus species. J Appl Microbiol 106: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolthanaruk W, Robinson C, and Moir A (2009) Localization of the GerD spore germination protein in the Bacillus subtilis spore. Microbiol 155: 1146–1151. [DOI] [PubMed] [Google Scholar]
- Mongkolthanaruk W, Cooper GR, Mawer JSP, Allan RN, and Moir A (2011) Effect of amino acid substitutions in the GerAA protein on the function of the alanine-responsive germinant receptor of Bacillus subtilis spores. J Bacteriol 193: 2268–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ, and Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol Rev 64: 548–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, and Setlow P (1990) Sporulation, germination and outgrowth. In Molecular Biological Methods for Bacillus. Harwood CR, and Cutting SM (eds). Chichester, United Kingdom: John Wiley and Sons, pp. 391–450. [Google Scholar]
- Paidhungat M, and Setlow P (2001) Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J Bacteriol 183: 3982–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidhungat M, Ragkousi K, and Setlow P (2001) Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol 183: 4886–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Sabja D, Setlow P, and Sarker MR (2010) Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19: 85–94. [DOI] [PubMed] [Google Scholar]
- Pelczar PL, and Setlow P (2008) Localization of the germination protein GerD to the inner membrane in Bacillus subtilis spores. J Bacteriol 190: 5635–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelczar PL, Igarashi T, Setlow B, and Setlow P (2007) Role of GerD in germination of Bacillus subtilis spores. J Bacteriol 189: 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragkousi K, Eichenberger P, van Ooij C, and Setlow P (2003) Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J Bacteriol 185: 2315–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P (2003) Spore germination. Curr Opin Microbiol 6: 550–556. [DOI] [PubMed] [Google Scholar]
- Setlow P (2006) Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101: 514–525. [DOI] [PubMed] [Google Scholar]
- Sourjik V (2004) Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol 12: 569–576. [DOI] [PubMed] [Google Scholar]
- Vepachedu VR, and Setlow P (2004) Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol Lett 239: 71–77. [DOI] [PubMed] [Google Scholar]
- Vepachedu VR, and Setlow P (2005) Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J Bacteriol 187: 5677–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vepachedu VR, and Setlow P (2007) Analysis of interactions between nutrient germinant receptors and SpoVA proteins of Bacillus subtilis spores. FEMS Microbiol Lett 274: 42–47. [DOI] [PubMed] [Google Scholar]
- Zhang P, Garner W, Yi X, Yu J, Li YQ, and Setlow P (2010) Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192: 3608–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.