Abstract
Introduction
EGFR exon 20 insertion mutations account for 10% of all EGFR mutations and are mostly insensitive to approved EGFR tyrosine kinase inhibitors (EGFR TKIs). Novel EGFR TKIs have been developed or repurposed for these mutants. A limited number of preclinical studies have detailed these EGFR TKIs. We sought to use commercially available mobocertinib (TAK-788) to characterize the preclinical therapeutic window of this EGFR TKI against EGFR mutations and to probe possible on-target mechanisms of resistance (EGFR-C797S).
Methods
We used models of EGFR mutations to probe representative first, second, third generation, and in-development EGFR exon 20-active (poziotinib, mobocertinib) TKIs. We also introduced EGFR-C797S to these models to identify mechanisms of resistance.
Results
Cells driven by the most common EGFR exon 20 insertion mutations (A767_V769dupASV, D770_N771insSVD, H773_V774insH, and others) were inhibited by in-development EGFR TKIs at doses below those affecting EGFR-wildtype; albeit more common EGFR mutations (exon 19 deletions and L858R) were inhibited more readily by mobocertinib and poziotinib. Mobocertinib was able to inhibit the phosphorylation of EGFR in multiple preclinical models. The presence of EGFR-C797S led to greater than 200-fold resistance in proliferation assays probing mobocertinib and osimertinib. A review of clinical studies of mobocertinib disclosed responses that could be lasting.
Conclusions
This is one of the initial reports to characterize the novel EGFR TKI mobocertinib and highlights its broad activity against EGFR mutants plus the therapeutic window to EGFR exon 20 insertion mutations; and EGFR-C797S as a possible mechanism of resistance. Further clinical development of mobocertinib merits continuation.
Keywords: Lung cancer, EGFR exon 20 insertion, Mobocertinib, C797S, ERBB2
Introduction
The most common EGFR exon 20 and the structurally similar ERBB2 exon 20 insertion mutations—representing 10% of all EGFR mutations—are unique among ErbB family members in that they activate the adenosine triphosphate binding pocket of these kinases without the typical conformational changes that enhance sensitivity to the most EGFR and/or ERBB2 tyrosine kinase inhibitors (TKIs) approved for clinical use in mid-2020.1,2 Over the past half a decade, novel EGFR and ERBB2 TKIs or repurposed TKIs have been reported to have a slightly favorable therapeutic window for EGFR exon 20 insertion mutations in comparison with EGFR-wildtype (WT).3, 4, 5, 6 The ones that have reached clinical trial development for EGFR exon 20 insertion–mutated NSCLC include poziotinib, CLN-081, high dose osimertinib, and mobocertinib; with differing levels of activity reported to date.
Mobocertinib, previously named AP32788 (ARIAD Pharmaceuticals) and subsequently TAK-788 (Takeda/Millennium Pharmaceuticals), was developed to have a broad level of inhibition against EGFR kinase domain mutations on the basis of the crystal structure of EGFR mutants and its covalent binding to EGFR.6,7 The initial report of the preclinical activity of the drug was presented in abstract form in 2016 with basic assays disclosing lower inhibitory concentrations to a limited panel of EGFR and ERBB2 mutants, including exon 20 insertions, tested in comparison with EGFR-WT and ERBB2-WT proteins.6 The initial clinical study of mobocertinib (ClinicalTrials.gov Identifier: NCT02716116) was initiated March 2016 and evolved to an extension cohort (named EXCLAIM) at the maximum tolerated dose of 160 mg a day8—the basis of the April 2020 Food and Drug Administration (FDA)’s breakthrough therapy designation of mobocertinib for the treatment of EGFR exon 20 insertion–mutated NSCLC.
Our report sought to use commercially available mobocertinib to characterize the preclinical therapeutic window of this TKI against representative EGFR mutations and to probe possible on-target mechanisms of resistance (including EGFR-C797S), placing these results into the context of the reported clinical activity of this drug and other in-development EGFR TKIs.
Materials and Methods
Drugs
Erlotinib, afatinib, osimertinib (LC Laboratories), poziotinib (Adooq BioScience), and mobocertinib (MedChemExpress) were dissolved in DMSO and stored at −80°C.
Preclinical Models/Cell Lines
Ba/F3 (sequencing analysis was performed to confirm the presence of WT and mutant EGFR), BID007 (EGFR-A763_Y764insFQEA), BID019 (EGFR-N771_H772insH), PC-9 (EGFR-delE746_A750), and NCI-H1975 (H1975, EGFR-L858R+T790M) cell lines were maintained in RPMI 1640 medium (Mediatech) supplemented with 10% fetal bovine serum. In the case of EGFR-WT–driven Ba/F3 cells, EGF 10 ng/mL was added to support growth. All cells were grown at 37°C in a humidified atmosphere with 5% carbon dioxide and tested for mycoplasma contamination (MycoAlert Mycoplasma Detection Kit, Lonza) before the experiments (initiated within the initial 1–4 passages).
Generation of Compound EGFR-C797S Mutation
The EGFR-C797S mutation was introduced into the EGFR-L858R sequence construct in the context of the MigR1 retrovirus vector (Addgene) using the QuickChange XL site–directed mutagenesis kit (Stratagene), as described by our group for other mutations.2
Proliferation Assays
Cell viability was determined by CellTiter 96 AQueous One Solution proliferation kit (Promega) and/or Cell Counting Kit-8 (Dojindo Molecular Technologies) for Ba/F3 and other cells. Cells were plated in 96-well plates and then treated in the appropriate medium with or without EGFR TKIs for 3 days. Inhibitory proliferation curves and the 50% inhibitory concentration (IC50) were generated using GraphPad Prism (GraphPad software).
Protein-Level Analysis
Western blot lysates and preparation were performed as previously described.2,9 Total EGFR, β-actin antibodies (Santa Cruz Biotechnology), and phospho-EGFR (pT1068) antibody (ThermoFisher) were diluted to 1-to-1000, whereas secondary antibodies were diluted to 1-to-10,000.
Results
Preclinical Evaluation of EGFR Exon 20 Insertion Mutations Against Different Classes of EGFR TKIs in a Ba/F3 Isogenic System
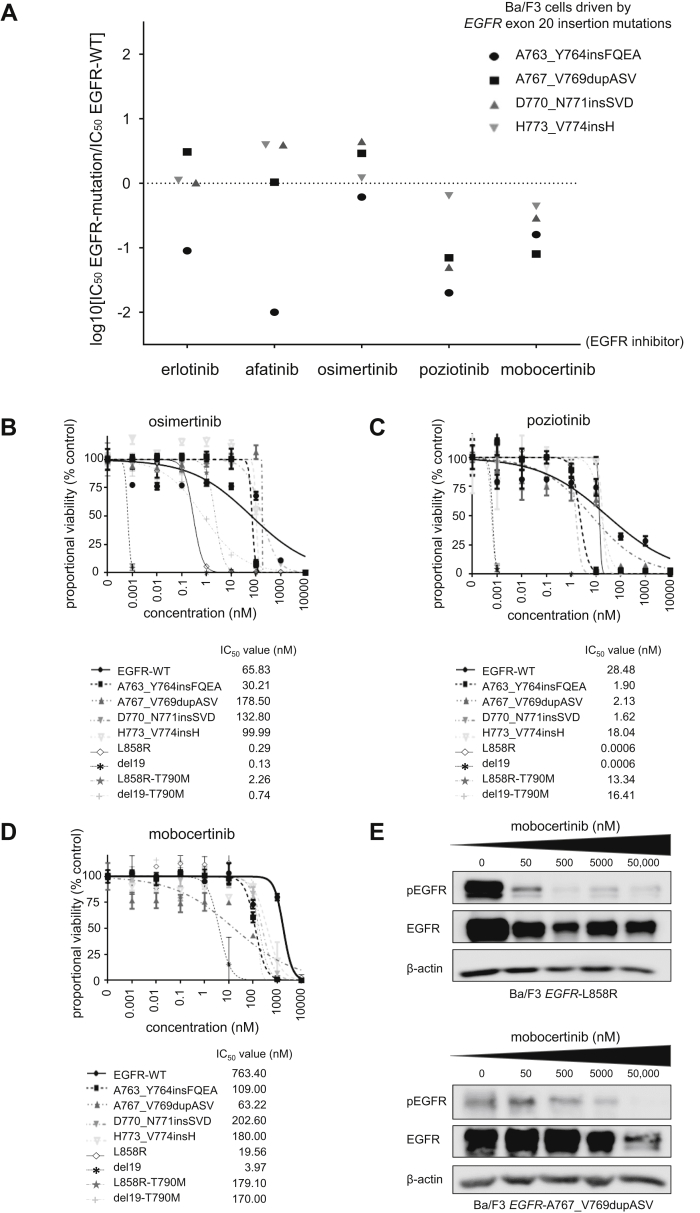
To highlight the potential therapeutic window of diverse classes of EGFR TKIs in different EGFR exon 20 insertion mutations, we selected four representative mutants (A763_Y764insFQEA, A767_V769dupASV, D770_N771insSVD, and H773_V774insH) and more typical EGFR TKI–sensitive or resistant EGFR mutants (exon 19 deletion delL747_P753insS [del19], exon 21 L858R, del19+T790M, and L858R+T790M) to contrast inhibitory concentrations with EGFR-WT (Fig. 1A–D). The EGFR exon 20 insertion mutations that match the most frequent aberrations (A767_V769dupASV, D770_N771insSVD, and H773_V774insH) in the Ba/F3 system displayed a favorable therapeutic window to poziotinib and mobocertinib (Fig. 1A), whereas the therapeutic windows (IC50 EGFR mutation divided by IC50 EGFR-WT) with erlotinib, afatinib, and osimertinib were unfavorable (Fig. 1A).
Figure 1.
Ba/F3 isogenic preclinical model of EGFR exon 20 insertions mutations to probe EGFR TKIs, including mobocertinib. (A) The therapeutic window of different EGFR TKIs to a set of EGFR exon 20 mutations. Cells were plated at a density of 5000 cells per well (96-well plates) and grown over 3 days after treatment. The logarithm of the IC50 of EGFR exon 20 mutants compared with EGFR-WT is plotted (three separate experiments are used to generate IC50). Values below zero indicate sensitivity, whereas values above zero indicate resistance to EGFR TKIs. (B) Dose-response proliferation assays (proportional percent viability) of osimertinib for all tested EGFR mutants. (C) Dose-response proliferation assays (proportional percent viability) of poziotinib for all tested EGFR mutants. (D) Dose-response proliferation assays (proportional percent viability) of mobocertinib for all tested EGFR mutants. (E) Western blotting of Ba/F3 cells driven by EGFR-L858R and EGFR-A767_V769dupASV. Cells were treated with the EGFR TKI mobocertinib for 8 hours at the indicated ascending concentrations. pEGFR at position 1068, total EGFR, and β-actin as a loading control are displayed. IC50, 50% inhibitory concentration; pEGFR, phosphorylated EGFR; TKI, tyrosine kinase inhibitor; WT, wildtype.
Erlotinib, afatinib (data not presented) were ineffective against compound EGFR-T790M–bearing cells. Poziotinib had values close to EGFR-WT (Fig. 1C), whereas osimertinib (Fig. 1B) and mobocertinib (Fig. 1D) had favorable therapeutic windows. Of note, all the tested EGFR TKIs had the lowest IC50 values for EGFR-del19 and -L858R–bearing cells (Fig. 1B–D). For mobocertinib, the values for IC50 were threefold to 51-fold higher for EGFR exon 20 insertions than del19 or L858R (Fig. 1D) but lower than the IC50 of EGFR-WT (Fig. 1A and D).
We also found that mobocertinib was able to induce, in a dose-dependent manner, inhibition of the phosphorylated state of EGFR in cells driven by EGFR-L858R and EGFR-A767_V769dupASV (Fig. 1E), corroborating on-target engagement of this EGFR TKI.
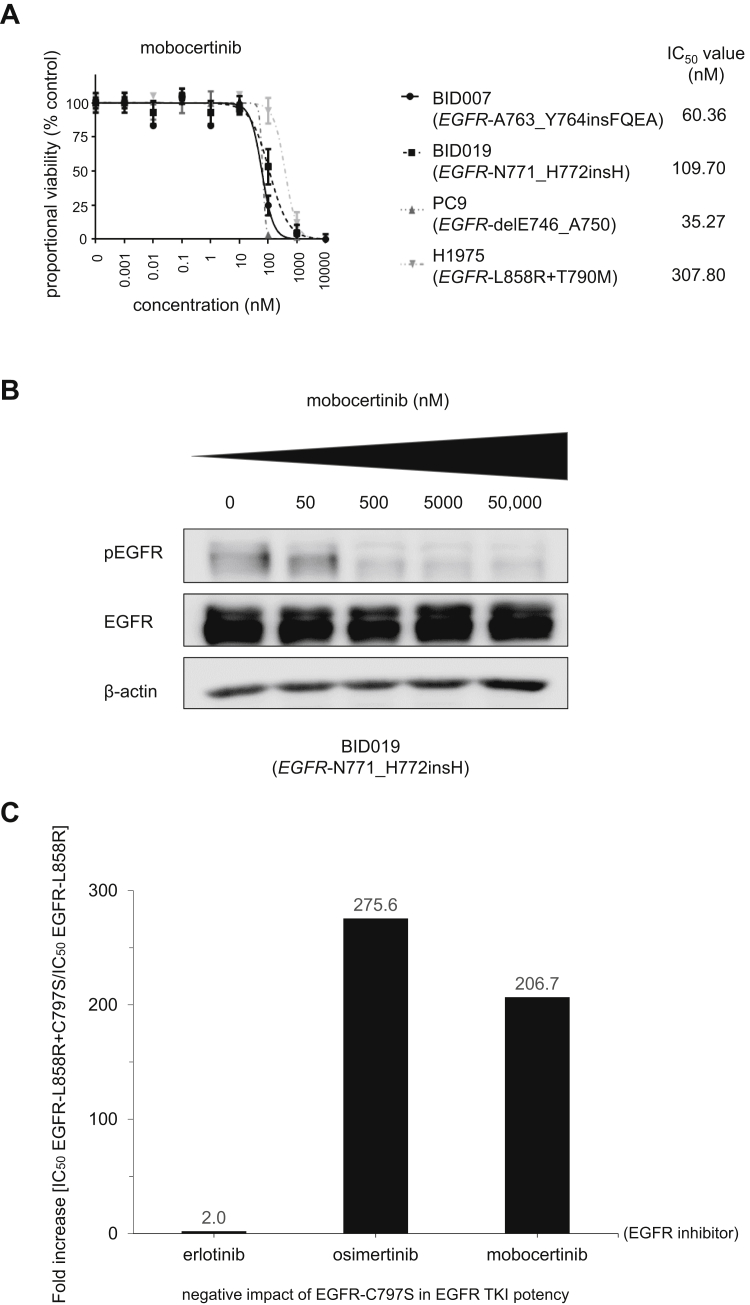
Preclinical Evaluation of Mobocertinib Against EGFR Exon 20 Insertion Mutations in Patient-Derived Lung Cancer Cell Lines
To confirm the Ba/F3 preclinical model findings, we used the second set of preclinical systems with patient-derived lung adenocarcinoma cell lines. The EGFR-A763_Y764insFQEA-bearing BID007 cells and the EGFR-N771_H772insH-bearing BID019 cells were inhibited by mobocertinib in a dose-dependent manner (Fig. 2A) similar to the Ba/F3 counterpart results (Fig. 1). Cell lines harboring the EGFR TKI sensitive EGFR-delE746_A750 mutation (PC-9) were the most sensitive, whereas lines harboring the erlotinib/afatinib/poziotinib-resistant EGFR-L858R+T790M mutation (H1975) were the least sensitive (Fig. 2A).
Figure 2.
Patient-derived cancer cell line preclinical models of EGFR exon 20 insertion mutations to probe mobocertinib and the role of EGFR-C797S as a mechanism of resistance. (A) Dose-response proliferation assays (proportional percent viability) of mobocertinib for all tested patient-derived EGFR-mutated lung adenocarcinoma cell lines. The main driver, EGFR mutation, is indicated for each cell. (B) Western blotting of cell line BID019 (EGFR- N771_H772insH). Cells were treated with the EGFR TKI mobocertinib for 8 hours at the indicated ascending concentrations. pEGFR at position 1068, total EGFR, and β-actin as a loading control are displayed. (C) Relative fold increase in the IC50 of EGFR-L858R+C797S compared with EGFR-L858R in the Ba/F3 system. Cells were plated at a density of 20,000 cells per well (96-well plates) and grown over 3 days after treatment. Three separate experiments were used to generate IC50s for each EGFR TKI tested, erlotinib, osimertinib, and mobocertinib. IC50, 50% inhibitory concentration; pEGFR, phosphorylated EGFR; TKI, tyrosine kinase inhibitor.
We also subjected BID019 to a dose-response exposure experiment to evaluate intracellular signaling and observed dose-dependent inhibition of phosphorylated EGFR (Fig. 2B) in this model.
Role of EGFR-C797S As an On-Target Mechanism of Resistance to Mobocertinib
Mobocertinib is an irreversible EGFR inhibitor designed to covalently bind to amino acid cysteine at position 797 of the EGFR’s kinase domain.6,7 Therefore, we generated a system harboring a mobocertinib-sensitive EGFR mutation in cis to the EGFR-C797S mutation (Fig. 1C). The compound EGFR-C797S mutated protein product negated the inhibitory ability of mobocertinib and osimertinib, as indicated by the greater than 200-fold shift in measured IC50 values (Fig. 1C). Erlotinib, a reversible EGFR TKI that does not bind covalently to EGFR-C797, was minimally disturbed by the presence of the compound EGFR-C797S product (Fig. 1C).
Discussion
The clinical care of advanced NSCLC in 2020 is anchored on tumor-based genomic and immune biomarkers. EGFR was the first targetable driver oncogene, and perhaps one of the most clinically relevant; however, EGFR TKIs are only approved for tumors harboring EGFR-del19, -L858R, -L861Q, G719X, -S768I, and -T790M. Off label use of approved EGFR TKIs is further supported for EGFR exon 18 indels/E709X, exon 19 insertions, exon 20 A763_Y764insFQEA, exon 18 to 25 kinase domain duplications and rearrangements.1,2,5,9,10 The identification of EGFR TKIs or other therapies with a favorable therapeutic window for patients with EGFR exon 20 insertion–mutated tumors continues to be a significant unmet necessity.
We characterized the irreversible EGFR TKI mobocertinib in this study. Mobocertinib was generated using a structure-based design to increase the potency and selectivity to EGFR mutations in relation to EGFR-WT.6,7 It has a kinase selectivity profile for EGFR, ERBB2, and other ErbB family members with minimal selectivity against other common kinases.7 Initial studies disclosed a broad level of activity against most types of common EGFR mutations, including exon 20 insertion mutations, in isogeneic cell lines, and in vivo murine models.6 Our group was able to illustrate that, indeed, mobocertinib is a panactive EGFR TKI with a favorable therapeutic window (inhibitory profiles below those of EGFR-WT) for all EGFR mutations tested (Figs. 1 and 2). It is most potent against the canonical EGFR mutations, del19 and L858R (Figs. 1D and 2A), but still selective—albeit with a narrower therapeutic window—against the most common EGFR exon 20 insertion mutations, A767_V769dupASV, D770_N771insSVD, and H773_V774insH (Fig. 1A). We confirmed this selectivity potential using isogenic cell line models (Fig. 1), NSCLC cancer cell lines with EGFR exon 20 insertions (Fig. 2) and at the intracellular signaling level (Figs. 1E and 2B). Our data, to the best of our knowledge, revealed, for the first time, that mobocertinib is susceptible to on-target resistance mediated by the EGFR-C797S mutation that negates the drug’s covalent binding cysteine, in a pattern also seen with the third-generation first-in-class EGFR TKI osimertinib (Fig. 2C). These results form a sound preclinical complementary basis to support the clinical results of mobocertinib. The most updated results of the initial phase I/II and extension cohort (EXCLAIM) trial of mobocertinib (NCT02716116) were presented in September 2020 (Table 1). The recommended phase 2 dose was established at 160 mg/day, and 28 patients were treated in the expansion cohort at this initial dose.8 The overall response rate (ORR) was 43% (95% confidence interval [CI]:24%–63%), with a disease control rate of 86% (95% CI: 67%–96%) and median progression-free survival (PFS) of 7.3 months (95% CI: 4.4–15.6). Toxicities were noted in most cases and were mostly related to gastrointestinal events (i.e., diarrhea, nausea) and skin rash8 that are markers of EGFR-WT adverse events common to most EGFR TKIs. The narrow therapeutic window observed in our preclinical report (Fig. 1D) substantiates the pattern of adverse events seen in the clinical study. The drug’s observed activity led to an FDA breakthrough therapy designation of mobocertinib for the treatment of EGFR exon 20 insertion–mutated NSCLC in April 2020. These initial results also spawned a randomized phase III trial of mobocertinib versus the standard platinum-doublet chemotherapy in 318 treatment-naive advanced NSCLC with EGFR exon 20 insertion mutations (EXCLAIM-2, NCT04129502) that commenced in 2019. The results of the latter trial may help establish the evidence-based first-line treatment strategy for EGFR exon 20–mutated NSCLC in the decades to come.
Table 1.
Summary of the Outcomes From Clinical Trials of Select EGFR TKIs for EGFR Exon 20 Insertion–Mutated Lung Cancer, Including Mobocertinib, Poziotinib, and High-Dose Osimertinib
| EGFR TKI/ Starting Dose/ No. of Cases | Trial Name/NCT No./Reference | ORR, % (95% CI) | DCR, % (95% CI) | PFS, mo, median (95% CI) |
|---|---|---|---|---|
| Osimertinib/160 mg/day/n = 21 | ECOG-ACRIN 5162/NCT03191149/ASCO 202012 | 24 | 82 | 9.6 (4.1–10.7) |
| Poziotinib/16 mg/day/n = 115 | ZENITH20/NCT03318939/ASCO 202011 | 14.8 (8.9–22.6) | 68.7 (59.4–77.0) | 4.2 (3.7–6.8) |
| Mobocertinib/160 mg/day/n = 28 | EXCLAIM/NCT02716116/ESMO 20208 | 43 (24–63) | 86 (67–96) | 7.3 (4.4–15.6) |
ASCO, American Society of Clinical Oncology; CI, confidence interval; DCR, disease control rate; ESMO, European Society for Medical Oncology; NCI, National Cancer Institute; NCT, ClinicalTrials.gov identifier; ORR, overall response rate; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
Other EGFR TKIs currently in clinical development—such as poziotinib and CLN-081—have also exhibited a reproducible therapeutic window in preclinical models.4,5,9 The clinical trial of poziotinib for EGFR exon 20 insertion–mutated NSCLC (ZENITH20, NCT03318939) has led to suboptimal initial efficacy results, with ORR below 15% and median PFS below 5 months (Table 1)—results that may be explained by dose-limiting skin and gastrointestinal adverse events at the 16 mg/day dosing.11 Further development of alternative dosing schemes of poziotinib is ongoing (NCT03318939). The initial dose escalation of CLN-0815 is ongoing, with some initial responses noted in the target cohort (NCT04036682). Another EGFR TKI strategy based on our group’s preclinical models3 is the use of a higher-than-the-standard dose of osimertinib. The ECOG-ACRIN 5162 trial (NCT03191149) reported that the dosing scheme of osimertinib 160 mg/day (double the FDA approved dose) was tolerable but only induced responses in the minority of cases,12 with an ORR below 25% (Table 1). Perhaps higher doses of osimertinib may be necessary for future studies. Of note, poziotinib and mobocertinib are also undergoing clinical trial evaluation for ERBB2 exon 20 insertion–mutated NSCLC in the aforementioned clinical studies. The ERBB2 TKI pyrotinib (NCT02535507) was recently reported to have a modest ORR of 30% (95% CI: 18.8%–43.2%) and median PFS of 6.9 months (95% CI: 5.5–8.3) in ERBB2-mutated NSCLC.13 It is likely other EGFR and/or ERBB2 TKIs will enter this burgeoning clinical trial space. Other strategies to target these tumors include antibody-drug conjugates (ADCs). The most robust results have been reported for ERBB2 ADCs, with ado-trastuzumab emtansine and trastuzumab deruxtecan exhibiting efficacy and safety in NSCLC.14 The dual EGFR-MET ADC amivantamab has been tested in EGFR-mutated NSCLCs (CHRYSALIS, NCT02609776), and initial results in 39 patients with EGFR exon 20 insertion–mutated NSCLC15 exhibited an ORR of 36% (95% CI: 21%–53%) and median PFS of 8.3 months (95% CI: 3.0–14.8)—the basis of an FDA breakthrough therapy designation in May 2020.
In summary, our preclinical results and the evolving clinical trial program of mobocertinib assert this panactive EGFR TKI as a promising treatment strategy for EGFR exon 20 insertion–mutated NSCLC. However, the same data highlight the potential for high rates of EGFR-WT–mediated adverse events and susceptibility to EGFR-C797S as a mechanism of resistance. The robust pipeline of EGFR TKIs and ADCs with preclinical and early clinical activity against EGFR and ERBB2 exon 20 insertion–mutated tumors hold the promise of a much-needed drug approval for these important cohorts of NSCLC.
Acknowledgments
This work was funded in part through the National Institutes of Health/National Cancer Institute with grants numbers R37 CA218707 (to Dr. Costa) and R01 CA240257 (to Dr. S.S. Kobayashi), and also the Department of Defense with grant number LC170223 (to Dr. S.S. Kobayashi).
Footnotes
Drs. Vasconcelos and Kobayashi contributed equally to this work.
Disclosure: Dr. Costa reports receiving personal fees (consulting fees and honoraria) and nonfinancial support (institutional research support) from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, and BluePrint Medicine; and nonfinancial support (institutional research support) from Merck Sharp and Dohme Corporation, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, and Tesaro, all outside of the submitted work. Dr. S.S. Kobayashi reports receiving research support from Boehringer Ingelheim, MiNA Therapeutics, and Taiho Therapeutics; and personal fees (honoraria) from Boehringer Ingelheim, Bristol-Myers Squibb, and Takeda Pharmaceuticals outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Yasuda H., Kobayashi S., Costa D.B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [published correction appears in Lancet Oncol. 2011;12:1182] [DOI] [PubMed] [Google Scholar]
- 2.Yasuda H., Park E., Yun C.H. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. doi: 10.1126/scitranslmed.3007205. [published correction appears in Sci Transl Med. 2014;6:225er1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano T., Yasuda H., Tani T. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015;6:38789–38803. doi: 10.18632/oncotarget.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robichaux J.P., Elamin Y.Y., Tan Z. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24:638–646. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udagawa H., Hasako S., Ohashi A. TAS6417/CLN-081 is a pan-mutation-selective EGFR tyrosine kinase inhibitor with a broad spectrum of preclinical activity against clinically relevant EGFR mutations. Mol Cancer Res. 2019;17:2233–2243. doi: 10.1158/1541-7786.MCR-19-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalvez F, Zhu X, Huang WS, et al. AP32788, a potent, selective inhibitor of EGFR and HER2 oncogenic mutants, including exon 20 insertions, in preclinical models. Presented at: 107th Annual Meeting of the American Association for Cancer Research. April 16–20, 2016; New Orleans, LA. Cancer Res76 (14 Suppl), 2016, abstract 2644.
- 7.Gonzalvez F. TAK 788: An EGFR inhibitor, currently in Phase II clinical trials, targeting lung cancers with Exon20 insertion mutations. Proceedings of the AACR Virtual Annual Meeting Vol. I; 2020; VSY. DDT02 - new drugs on the horizon: Part 2. https://www.abstractsonline.com/pp8/#!/9045/presentation/6845
- 8.Riely G., Neal J.W., Camidge D.R. 1261MO updated results from a phase I/II study of mobocertinib (TAK-788) in NSCLC with EGFR exon 20 insertions (exon20ins) Ann Oncol. 2020;31(suppl 4):S815–S816. [Google Scholar]
- 9.Vasconcelos P.E.N.S., Gergis C., Viray H. EGFR-A763_Y764insFQEA is a unique exon 20 insertion mutation that displays sensitivity to approved and in-development lung cancer EGFR tyrosine kinase inhibitors. JTO Clin Res Rep. 2020;1:100051. doi: 10.1016/j.jtocrr.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorge S.E., Lucena-Araujo A.R., Yasuda H. EGFR Exon 20 insertion mutations display sensitivity to Hsp90 inhibition in preclinical models and lung adenocarcinomas. Clin Cancer Res. 2018;24:6548–6555. doi: 10.1158/1078-0432.CCR-18-1541. [published correction appears in Clin Cancer Res. 2020;26:2277] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le X., Goldman J.W., Clarke J.M. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol. 2020;38(suppl 15) 9514–9514. [Google Scholar]
- 12.Piotrowska Z., Wang Y., Sequist L.V., Ramalingam S.S. ECOG-ACRIN 5162: a phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2020;38(suppl 15) 9513–9513. [Google Scholar]
- 13.Zhou C., Li X., Wang Q. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J Clin Oncol. 2020;38:2753–2761. doi: 10.1200/JCO.20.00297. [DOI] [PubMed] [Google Scholar]
- 14.Tsurutani J., Iwata H., Krop I. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10:688–701. doi: 10.1158/2159-8290.CD-19-1014. [published correction appears in Cancer Discov. 2020;10:1078] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K., John T., Kim S. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC) J Clin Oncol. 2020;38(suppl 15) 9512–9512. [Google Scholar]