Abstract
Background:
Standard blood pressure control metrics may not account for changes in blood pressure over time.
Objective:
This study sought to estimate the independent association between time in systolic blood pressure target range and major adverse cardiovascular events among adults with hypertension.
Methods:
This study was a post-hoc analysis of SPRINT, a randomized clinical trial that compared intensive (<120 mm Hg) and standard (<140 mm Hg) systolic blood pressure treatment interventions in adults with hypertension and high cardiovascular risk. Target range was defined as 110–130 mm Hg and 120–140 mm Hg for the intensive and standard arms, respectively. Time in target range was estimated over the first 3 months of follow-up using linear interpolation. The association between time in target range with major adverse cardiovascular events was estimated using adjusted Cox proportional hazards regression models.
Results:
Participants with greater time in target range were younger, had lower 10-year cardiovascular risk and lower baseline systolic blood pressure and were more likely women and statin users. Each 1-standard deviation increase in time in target range associated significantly with a decreased risk of first major adverse cardiovascular event in fully adjusted models. Time in target range remained significantly associated with major adverse cardiovascular events despite adjustment for mean systolic blood pressure or systolic blood pressure variability. Among participants with mean systolic blood pressure at or below target, time in target range remained associated with major adverse cardiovascular events.
Conclusions:
Time in systolic blood pressure target range independently predicts major adverse cardiovascular event risk.
Keywords: hypertension, time in target range, quality of care
Condensed Abstract
Each 1-standard deviation increase in time in target range associated significantly with a decreased risk of first major adverse cardiovascular event in fully adjusted models, after further adjustment for mean systolic blood pressure and among participants with mean systolic blood pressure at or below target. Time in systolic blood pressure target range independently predicts major adverse cardiovascular event risk. Time in target range may be a useful metric of blood pressure control for population-based quality assessment and clinical trial interventions.
Introduction
Hypertension is the leading modifiable risk factor for cardiovascular disease and premature death in the world(1). Despite decades of public awareness campaigns, guideline statements, and widely available medications, blood pressure control rates remain suboptimal and have actually worsened in recent years(2). Blood pressure control is often defined by blood pressure(s) taken during a single clinical encounter. From a performance measure perspective, blood pressure control is often determined using only the last recorded blood pressure of the calendar year(3). This is problematic as blood pressure is a continuous measure that fluctuates over time and the last recorded measure may not adequately reflect control, or lack thereof, over time. Furthermore, several studies have demonstrated that greater variability in systolic blood pressure is associated with coronary atheroma progression, diastolic and systolic dysfunction, coronary heart disease, stroke, cardiovascular mortality, and all-cause mortality(4–6). This reinforces the need for additional measures of blood pressure control that may serve as a more appropriate performance measure.
The optimal measure of systolic blood pressure control would account for variation within- and out-of-target range. The mean of multiple systolic blood pressure measurements over a specified period may be within target range even if none of the individual systolic blood pressure measurements fall within that range. Similarly, systolic blood pressure variability over the course of days, weeks or months may be narrow even if all systolic blood pressure measures exceed the target range. This study sought to estimate the association between time in systolic blood pressure target range and major adverse cardiovascular events among adults with hypertension and to determine whether systolic blood pressure time in target range predicts major adverse cardiovascular events independent of mean systolic blood pressure and systolic blood pressure variability.
Methods
This study was a retrospective cohort study of participants of the Systolic Blood Pressure Intervention Trial (SPRINT).(7) We utilized deidentified, limited access datasets for each study available through the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Coordinating Center. The SPRINT study protocol was approved by Institutional Review Boards at each site and all participants provided informed consent.
Briefly, SPRINT was an open-label, randomized, controlled trial that compared an intensive systolic blood pressure treatment targeting a goal of less than 120 mm Hg to a standard systolic blood pressure treatment targeting a goal of less than 140 mm Hg. SPRINT included 9361 adults with hypertension and increased cardiovascular risk, excluding those with diabetes or prior stroke.
Population
The cohort for this analysis included all adults in SPRINT with at least two systolic blood pressure measurements during the exposure period (months 0 to 3) and excluding those with systolic blood pressure within target range at baseline before randomization (110–130 mm Hg and 120–140 mm Hg for the intensive and standard arms, respectively). Additionally, participants were excluded from certain time-to-event analyses if they experienced that event during the exposure period or were lost-to-follow-up. For example, participants who experienced a non-fatal myocardial infarction during the exposure period were excluded from time to first major adverse cardiovascular event analyses and included in time to first heart failure hospitalization.
Systolic Blood Pressure Control Measures
We defined three longitudinal measures of systolic blood pressure control during months 0–3 and, for the sensitivity analysis, months 0–6. Systolic blood pressure time in target range was estimated using linear interpolation.(8) The target range was defined as 110 to 130 mm Hg for the intensive treatment arm and 120 to 140 mm Hg for the standard treatment arm. The upper limit of 130 mm Hg in the intensive treatment arm was selected to match current guideline-recommended systolic blood pressure target.(9) Mean systolic blood pressure and systolic blood pressure variability were calculated as the weighted mean and the standard deviation of all systolic blood pressure measurements during months 0–3 (or 0–6), respectively.
Intervention
Participants in SPRINT were randomized to either an intensive systolic blood pressure target of less than 120 mm Hg or a standard target of less than 140 mm Hg. Based upon a previous intensive blood pressure lowering trial, the SPRINT investigators anticipated that participants in the intensive and standard treatment arms would achieve the target systolic blood pressure within 8–12 and 3–6 months, respectively. Investigators were encouraged to use chlorthalidone or other thiazide-type diuretics (or loop diuretics for those with chronic kidney disease), angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, amlodipine or other dihydropyridine calcium channel blockers, and a beta-blocker for patients with compelling indications. Medication non-adherence was identified using the Adherence Scale. Study personnel were trained and re-trained periodically to identify and address barriers to medication non-adherence. All participants were advised to implement standard-of-care lifestyle modifications, such as weight loss, the DASH diet, decreased alcohol consumption, increased exercise and smoking cessation.
Blood pressure was measured in the clinic by trained staff according to standardized procedures.(10,11) The mean of three measurements was taken using an appropriately sized automated blood pressure cuff (Omron-HEM-707 XL, Omron Healthcare, Inc. Bannockburn, IL) while the participant was seated with back support. Blood pressure measurements were to occur early in the study visit before stressful procedures and after 5 minutes of rest. Study staff were absent from the participant’s during the rest period and the measurement period.
Blood pressure was measured at monthly intervals for the first three months and every three months thereafter in all participants irrespective of treatment allocation. For participants in the intensive blood pressure lowering arm, blood pressure was measured monthly until the target was reached or medication titration ceased while additional “milepost” visits mandated the initiation of a medication from a new class if systolic blood pressure exceeded 120 mm Hg at 6-month intervals. For the standard arm, blood pressure was measured monthly after each systolic blood pressure greater than or equal to 160 mm Hg. Medication intensification occurred in the standard treatment arm if systolic blood pressure was 160 mm Hg or greater at a single visit or at least 140 mm Hg at two consecutive visits.
Site investigators reviewed all seated systolic blood pressure measurements below 90 mm Hg and those below 100 mm Hg if decreased by at least 10 mm Hg from the last visit. Management of low seated systolic blood pressure was left to the discretion of the site investigator and medication down-titration was not mandated. Orthostatic hypotension was assessed at baseline, 1-month, 6-months, 12-months and yearly thereafter. Symptomatic orthostatic hypotension, defined as a decrease of systolic blood pressure of at least 20 mm Hg or systolic blood pressure of at least 10 mm Hg within 3 minutes of standing. Alternative causes of symptomatic orthostatic hypotension (e.g., dehydration, medications, etc.) were addressed and patients were provided strategies to minimize symptomatic orthostasis. In the standard treatment arm, medication down-titration occurred if systolic blood pressure was less than 130 mm Hg at a single visit or less than 135 mm Hg at two consecutive visits in the absence of symptomatic orthostatic hypotension or other adverse effects.
Cardiovascular Outcomes
Outcomes included major adverse cardiovascular events (cardiovascular death, myocardial infarction, non-myocardial infarction acute coronary syndrome, stroke or acute decompensated heart failure), individual major adverse cardiovascular events components, and treatment-related serious adverse events. The safety outcomes were hypotension, injurious falls, bradycardia, syncope, electrolyte imbalances, acute kidney injury and the composite of these outcomes (serious adverse events). Structured interviews were performed every 3 months to identify potential events. Relevant data were gathered according to a standard protocol and events were adjudicated by an independent committee blinded to treatment allocation.
Statistical Analysis
Categorical data were summarized as counts and percentages and continuous data were expressed as either mean and standard deviation or median and interquartile range. Continuous baseline data were compared across the four time in target range groups (0 to <25%, 25 to <50%, 50 to <75%, 75 to 100%) using the analysis of variance test and categorical baseline data were compared using chi-squared test. The relationships between systolic blood pressure time in target range, mean systolic blood pressure and systolic blood pressure variability were assessed. Scatter plots and correlations were stratified according to baseline systolic blood pressure above (>130 mm Hg for intensive and >140 mm Hg for standard) or below (<110 mm Hg for intensive and <130 mm Hg for standard) target range to account for differences in systolic blood pressure trajectory during the randomization period.
The associations of systolic blood pressure time in target range, mean systolic blood pressure and systolic blood pressure variability to first occurrence of an efficacy or safety outcome were estimated using hazard ratios (HR) and 95% confidence intervals (CI) derived from Cox proportional regression models. All models were stratified by intensive or standard SBP target and sequentially adjusted for predetermined sets of demographic variables (age, sex, race) and then medical history (10-year cardiovascular risk score, body mass index, estimated glomerular filtration rate and baseline systolic blood pressure). Hazard ratios for each systolic blood pressure exposure were estimated per 1-standard deviation increase (systolic blood pressure time in target range) or per 1-standard deviation decrease (mean systolic blood pressure and systolic blood pressure variability). Visual inspection and testing of the Schoenfeld residuals as a function of time did not suggest deviation from proportionality for any model.
The main analysis of the association of systolic blood pressure time in target range with cardiovascular outcomes was performed in the complete study cohort and then separately in the intensive and standard treatment arms. Multiplicative interaction terms were used to assess an interaction between randomization arm and association of systolic blood pressure time in target range with cardiovascular outcomes. Since the main analysis did not find evidence of an interaction, all subsequent analyses were performed in the complete study cohort to maximize statistical power.
We performed sensitivity analyses to test the value of adding systolic blood pressure time in target range to mean systolic blood pressure and systolic blood pressure variability and to test assumptions of the overall analyses. First, mean systolic blood pressure and systolic blood pressure variability were added to fully adjusted Cox proportional hazards regression models. Second, the association of time in target range with cardiovascular outcomes was assessed only in participants who achieved a mean systolic blood pressure within the target range. Third, the association of mean systolic blood pressure and cardiovascular outcomes was estimated across quartiles of time in target range. Last, we extended the exposure period from month 3 to month 6, which added an additional systolic blood pressure measurement at the expense of shorter follow-up and fewer events, to assess whether the association of time in target range with cardiovascular outcomes was modified by the choice of exposure period.
A p-value < 0.05 was considered statistically significant. All analyses were performed using Stata 15.1 (StataCorp, College Station, Texas).
Results
Overall Study Population Characteristics
After excluding 18 participants with only one systolic blood pressure measurement, 3,120 participants with systolic blood pressure within target range at baseline, and 61 participants with missing covariate data, the final study cohort included 6,162 participants (out of a total population of 9,361 SPRINT participants, Supplemental Figure 1). The mean age of the study population was 68±10 years, 2,319 (38%) were female and 1,027 (17%) had a history of clinical cardiovascular disease.
Time in Target Range, Mean Systolic Blood Pressure and Systolic Blood Pressure Standard Deviation
The overall median (IQR) systolic blood pressure time in target range was 47 (18–72)% for the intensive target arm and 51 (25–75)% for the standard target arm. The mean systolic blood pressure achieved during months 0–3 for the entire study population was 127±12 mm Hg for the intensive target arm and 135±11 mm Hg in the standard target arm. The mean (SD) systolic blood pressure variability was 11 (6) mm Hg.
Participants with time in target range of 75 to <100% were younger, had lower 10-year cardiovascular risk and lower baseline systolic blood pressure than participants with time in target range of 0 to <25% (Table 1). Females and statin users comprised a larger proportion of the group with time in target range of 75 to 100% than of the group with time in target range of 0 to <25% (Table 1). The proportions of participants with clinical cardiovascular disease and usage of blood pressure lowering agents did not differ across time in target range groups (Table 1).
Table 1.
Characteristics of Participants According to Systolic Blood Pressure Time in Target Range
| TTR Group | |||||
|---|---|---|---|---|---|
| 0 to <25% (n=1742) | 25 to <50% (n=1392) | 50 to <75% (n=1585) | 75 to <100% (n=1443) | P-Value | |
| Age | 68 (10) | 69 (10) | 68 (9) | 67 (9) | <.001 |
| Female | 701 (40%) | 558 (40%) | 591 (37%) | 469 (33%) | <.001 |
| White | 968 (56%) | 829 (60%) | 915 (58%) | 825 (57%) | .028 |
| Black | 559 (32%) | 413 (30%) | 446 (28%) | 419 (29%) | |
| Clinical Cardiovascular Disease | 287 (17%) | 262 (19%) | 257 (16%) | 221 (15%) | .08 |
| 10-Year ASCVD Risk | 23 (12) | 22 (12) | 22 (11) | 20 (11) | <.001 |
| Baseline SBP | 149 (17) | 147 (16) | 145 (15) | 139 (12) | <.001 |
| Number of BP Lowering Agents | .15 | ||||
| 0 | 193 (11%) | 133 (10%) | 177 (11%) | 155 (11%) | |
| 1 | 458 (26%) | 425 (31%) | 482 (30%) | 438 (30%) | |
| 2 | 598 (34%) | 460 (33%) | 526 (33%) | 502 (35%) | |
| 3 or more | 493 (28%) | 374 (27%) | 400 (25%) | 348 (24%) | |
| Statin | 660 (38%) | 589 (43%) | 679 (43%) | 634 (44%) | <.002 |
| Time in Target Range | 8 (9) | 38 (7) | 62 (8) | 86 (8) | <.001 |
| Mean SBP | 140 (15) | 130 (10) | 126 (8) | 124 (7) | <.001 |
| SBP Standard Deviation | 10 (7) | 14 (6) | 12 (5) | 9 (4) | <.001 |
In both the intensive and standard arms, participants with greater time in target range tended to have lower mean systolic blood pressure. Time in target range correlated significantly with mean systolic blood pressure among participants in the intensive treatment arm whether baseline systolic blood pressure exceeded 130 mm Hg (R=−0.70;P<.001) or was less than 110 mm Hg (R=0.29; P<0.01). Similarly, time in target range correlated significantly with mean systolic blood pressure among participants in the standard treatment arm with baseline systolic blood pressure greater than 140 mm Hg (R=−0.60; P<.001) or less than 120 mm Hg (R=0.46; P<.001). Importantly, mean systolic blood pressure varied above, below and within the target systolic blood pressure range at any given time in target range. In the intensive treatment arm, mean systolic blood pressure varied within, above and below target range of 110 to 130 mm Hg once time in target range values decreased below 40%. In the standard treatment arm, mean systolic blood pressure began to deviate from the target range of 120 to 140 mm Hg once time in target range decreased below 60%. The associations between systolic blood pressure time in target range and variability were weak or non-significant within the intensive arm for baseline systolic blood pressure above (R=0.04; P=.02) and below (R=−0.10; P=.38) target range and weak within the standard arm for baseline systolic blood pressure above (R=−0.05; P=0.02) and below (−0.10; P=0.05) the target range (Figure 1). Below a systolic blood pressure time in target range of 80%, systolic blood pressure variability ranged from less than 5 mm Hg to greater than 30 mm Hg. The relationship between mean systolic blood pressure and systolic blood pressure variability is shown in Supplemental Figure 2.
Figure 1. Relationships between Systolic Blood Pressure Time in Target Range, Mean Systolic Blood Pressure and Systolic Blood Pressure Standard Deviation.
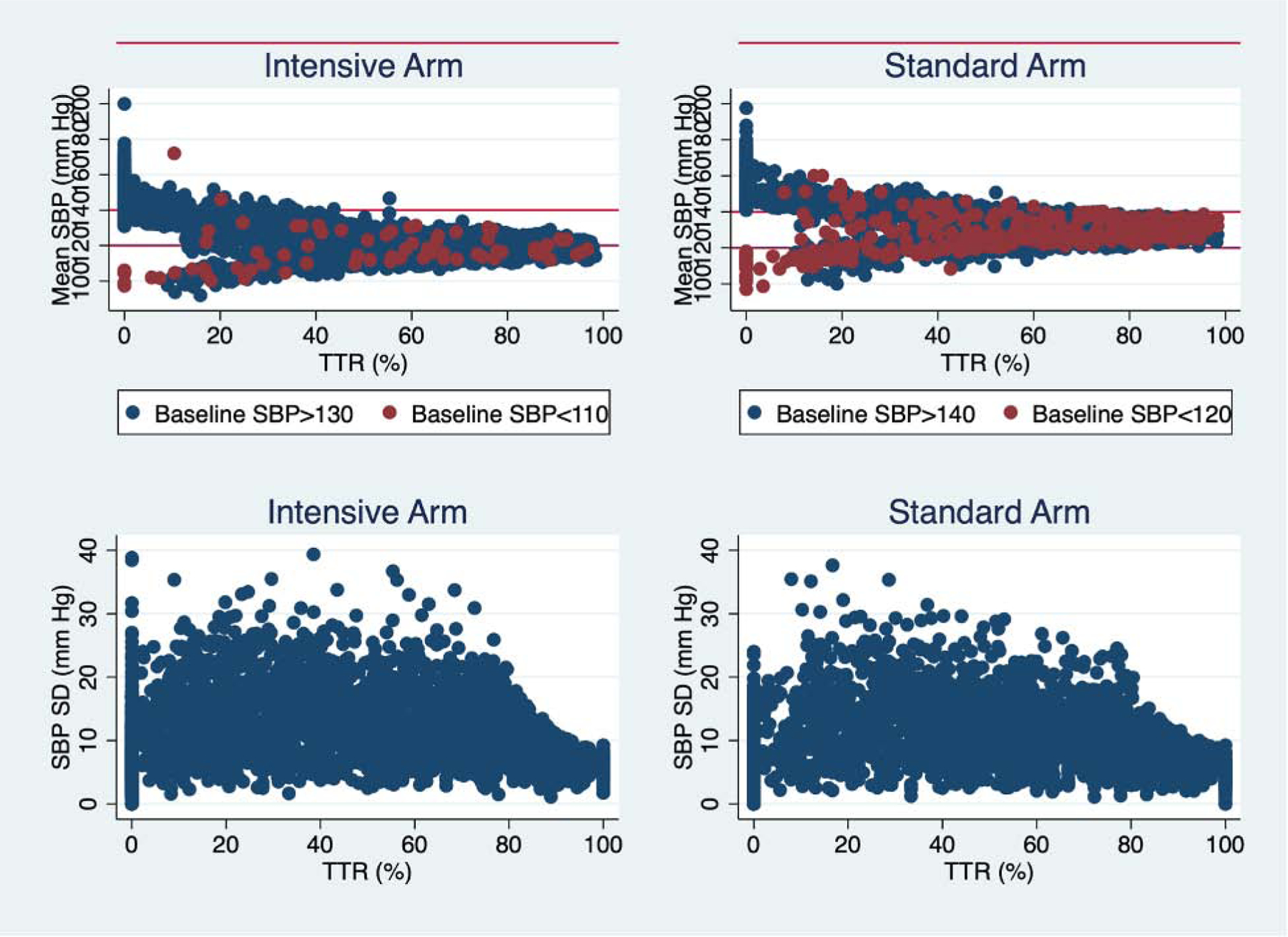
The relationships between systolic blood pressure control measures are depicted separately for intensive and standard blood pressure lowering arms.
Associations of Systolic Blood Pressure Time in Target Range and Cardiovascular Outcomes
The primary outcome of first major adverse cardiovascular event occurred in 356 participants over a median follow-up of 3.3 years (incidence rate [95% confidence interval], 20.0 [18.0 to 22.2]). Each 1-standard deviation increase in time in target range associated significantly with a decreased risk of first major adverse cardiovascular event in the unadjusted model (HR, 95% [CI]: 0.78 [0.70 to 0.87]; P<.001) (Supplemental Figure 3) and after adjustment for age, sex and race (HR [95% CI], 0.78 [0.70 to 0.87]; P<.001). Further, greater time in target range significantly associated with decreased risk of first major adverse cardiovascular event after full adjustment for demographics, medical history and baseline systolic blood pressure (HR [95% CI], 0.78 [0.73 to 0.91]; P<.001).
In fully adjusted models, time in target range associated significantly with first non-fatal myocardial infarction (HR [95% CI], 0.75 [0.72 to 0.90]; P=.002) and first heart failure hospitalization (HR [95% CI], 0.79 [0.65 to 0.97]; P=.023). Time in target range associated with first non-fatal stroke in unadjusted and demographically adjusted models, but not in fully adjusted models (HR [95% CI], 0.80 [0.64 to 1.00]; P=.053) (Table 2). There were no associations between time in target range and non-myocardial infarction acute coronary syndrome, cardiovascular death or all-cause death (Table 2).
Table 2.
Associations of Systolic Blood Pressure Time in Target Range and Cardiovascular Outcomes
| Unadjusted | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N | IR (95% CI) | HR* (95% CI) | P | HR* (95% CI) | P | HR* (95% CI) | P |
| Major adverse cardiovascular outcomes | 356 | 20.0 (18.0 to 22.2) | 0.78 (0.70 to 0.87) | <.001 | 0.78 (0.70 to 0.87) | <.001 | 0.81 (0.73 to 0.91) | <.001 |
| Non-fatal myocardial infarction | 131 | 7.2 (6.1 to 8.6) | 0.70 (0.58 to 0.83) | <.001 | 0.69 (0.58 to 0.82) | <.001 | 0.75 (0.62 to 0.90) | .002 |
| Other acute coronary syndrome | 51 | 2.8 (2.1 to 3.7) | 0.83 (0.63 to 1.09) | .18 | 0.81 (0.61 to 1.07) | .13 | 0.81 (0.61 to 1.08) | .15 |
| Stroke | 91 | 5.0 (4.1 to 6.1) | 0.75 (0.60 to 0.92) | .006 | 0.75 (0.61 to 0.93) | .008 | 0.80 (0.64 to 1.00) | .053 |
| Heart failure hospitalization | 108 | 5.9 (4.9 to 7.2) | 0.75 (0.61 to 0.90) | .003 | 0.76 (0.63 to 0.93) | .007 | 0.79 (0.65 to 0.97) | .023 |
| Cardiovascular death | 68 | 3.7 (2.9 to 4.7) | 0.73 (0.57 to 0.93) | .010 | 0.73 (0.57 to 0.94) | .014 | 0.82 (0.63 to 1.06) | .12 |
| All-cause death | 236 | 12.8 (11.3 to 14.6) | 0.84 (0.74 to 0.96) | .009 | 0.86 (0.75 to 0.98) | .024 | 0.93 (0.81 to 1.06) | .27 |
| Major adverse cardiovascular event or death | 477 | 26.8 (24.5 to 29.3) | 0.81 (0.74 to 0.89) | <.001 | 0.82 (0.75 to 0.90) | <.001 | 0.85 (0.78 to 0.94) | .001 |
hazard ratio per 1-standard deviation increase in time in target range
The relationships between time in target range with major adverse cardiovascular events, the individual components of major adverse cardiovascular events and all-cause death did not significantly differ across the intensive and standard treatment arms (P-interaction>.16 for all). In fully adjusted models, systolic blood pressure time in target range associated with first major adverse cardiovascular event in the intensive (HR [95% CI], 0.85 [0.73 to 1.00]; P=.050) and standard (HR [95% CI], 0.78 [0.67 to 0.91]; P=.002) treatment arms (Supplemental Tables 1 and 2).
Associations of Mean Systolic Blood Pressure and Systolic Blood Pressure Variability with Cardiovascular Outcomes
Each 1-standard deviation decrease in mean systolic blood pressure associated significantly with a lower risk of a first major adverse cardiovascular event (HR [95% CI], 0.84 [0.75 to 0.94]; P=.003) and first non-fatal myocardial infarction (HR [95% CI], 0.81 [0.67 to 0.97]; P=.024) after adjustment for demographics, medical history and baseline systolic blood pressure. There were trends towards an association of mean systolic blood pressure and stroke (P=.087) and cardiovascular death (P=.071) in fully adjusted models (Supplemental Table 3). Systolic blood pressure standard deviation did not associate with first major cardiovascular event (HR [95% CI], 1.04 [0.92 to 1.16]; P=.55) or individual components of that composite outcome (Supplemental Table 4).
Independent Association of Systolic Blood Pressure Time in Target Range, Mean Systolic Blood Pressure and Systolic Blood Pressure Variability with Cardiovascular Outcomes
The associations of greater time in target range with decreased risk of cardiovascular outcomes remained significant across sensitivity analyses. After adjustment for mean systolic blood pressure, systolic blood pressure time in target range significantly associated with major adverse cardiovascular events (HR [95% CI], 0.85 [0.74 to 0.96]; P=.011) as well as with non-fatal myocardial infarction and heart failure hospitalization (Table 3). Adjustment for systolic blood pressure standard deviation had no effect on the associations of time in target range with cardiovascular outcomes (Table 3). In contrast, mean systolic blood pressure did not associate significantly with first major adverse cardiovascular event (HR [95% CI], 0.92 [0.81 to 1.05]; P=.22) or any other cardiovascular event (P>.28 for each) after adjustment for time in target range (Supplemental Table 3).
Table 3.
Association of Systolic Blood Pressure Time in Target Range and Cardiovascular Outcomes With and Without Adjustment for Mean Systolic Blood Pressure and Systolic Blood Pressure Standard Deviation
| Fully Adjusted | Fully Adjusted Plus Mean SBP | Fully Adjusted Plus SBP Standard Deviation | ||||
|---|---|---|---|---|---|---|
| Outcome | HR* (95% CI) | P | HR* (95% CI) | P | HR* (95% CI) | P |
| Major adverse cardiovascular outcomes | 0.81 (0.73 to 0.91) | <.001 | 0.85 (0.74 to 0.96) | .011 | 0.81 (0.73 to 0.91) | <.001 |
| Non-fatal myocardial infarction | 0.75 (0.62 to 0.90) | .002 | 0.78 (0.63 to 0.97) | .029 | 0.78 (0.65 to 0.94) | .010 |
| Other acute coronary syndrome | 0.81 (0.61 to 1.08) | .15 | 0.88 (0.63 to 1.24) | .47 | 0.80 (0.60 to 1.08) | .14 |
| Stroke | 0.80 (0.64 to 1.00) | .053 | 0.85 (0.65 to 1.11) | .23 | 0.80 (0.64 to 1.00) | .055 |
| Heart failure hospitalization | 0.79 (0.65 to 0.97) | .023 | 0.79 (0.62 to 0.99) | .041 | 0.78 (0.64 to 0.96) | .020 |
| Cardiovascular death | 0.82 (0.63 to 1.06) | .12 | 0.90 (0.65 to 1.24) | .52 | 0.80 (0.62 to 1.05) | .10 |
| All-cause death | 0.93 (0.81 to 1.06) | .27 | 0.94 (0.80 to 1.11) | .47 | 0.92 (0.80 to 1.06) | .23 |
| Major adverse cardiovascular event or death | 0.85 (0.78 to 0.94) | .001 | 0.87 (0.78 to 0.97) | .014 | 0.85 (0.77 to 0.94) | .001 |
hazard ratio per 1-standard deviation increase in time in target range, per 1-standard deviation decrease in mean systolic blood pressure or per 1-standard deviation decrease in systolic blood pressure standard deviation
Among those participants who achieved a mean systolic blood pressure at or below target (<130 mm Hg for the intensive arm or <140 for the standard arm), time in target range associated with first major adverse cardiovascular event (HR [95% CI], 0.81 [0.68 to 0.97]; P=.024) and heart failure hospitalization (HR [95% CI], 0.63 [0.46 to 0.86]; P=.004) (Table 4). In contrast, there was no association between mean systolic blood pressure and major adverse cardiovascular events in participants with the least time in target range (0–25%) or with the most time in target range (75–100%) (Table 5).
Table 4.
Associations of Systolic Blood Pressure Time in Target Range and Cardiovascular Outcomes With Mean Systolic Blood Pressure At or Below Target
| Unadjusted | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N | IR (95% CI) | HR* (95% CI) | P | HR* (95% CI) | P | HR* (95% CI) | P |
| Major adverse cardiovascular outcomes | 205 | 16.9 (14.8 to 19.4) | 0.76 (0.64 to 0.91) | .003 | 0.76 (0.63 to 0.90) | .002 | 0.81 (0.68 to 0.97) | .024 |
| Non-fatal myocardial infarction | 69 | 5.6 (4.4 to 7.1) | 0.79 (0.58 to 1.07) | .13 | 0.76 (0.56 to 1.04) | .084 | 0.82 (0.60 to 1.14) | .24 |
| Other acute coronary syndrome | 27 | 2.2 (1.5 to 3.2) | 0.79 (0.49 to 1.29) | .34 | 0.76 (0.46 to 1.23) | .26 | 0.90 (0.55 to 1.47) | .67 |
| Stroke | 49 | 4.0 (3.0 to 5.3) | 0.79 (0.55 to 1.13) | .20 | 0.79 (0.55 to 1.15) | .22 | 0.83 (0.57 to 1.21) | .34 |
| Heart failure hospitalization | 66 | 5.4 (4.2 to 6.8) | 0.60 (0.44 to 0.82) | .001 | 0.61 (0.44 to 0.83) | .002 | 0.63 (0.46 to 0.86) | .004 |
| Cardiovascular death | 36 | 2.9 (2.1 to 4.0) | 0.94 (0.61 to 1.45) | .79 | 0.94 (0.61 to 1.47) | .79 | 0.99 (0.64 to 1.54) | .98 |
| All-cause death | 139 | 11.2 (9.5 to 13.2) | 1.01 (0.81 to 1.26) | .92 | 1.02 (0.81 to 1.28) | .86 | 1.08 (0.85 to 1.36) | .54 |
| Major adverse cardiovascular event or death | 280 | 23.1 (20.5 to 25.9) | 0.84 (0.72 to 0.98) | .027 | 0.84 (0.71 to 0.98) | .023 | 0.89 (0.76 to 1.05) | .16 |
hazard ratio per 1-standard deviation increase in time in target range
Table 5.
Fully Adjusted Associations of Mean Systolic Blood Pressure and Cardiovascular Outcomes Stratified by Time in Target Range Groups
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | HR* (95% CI) | P | HR* (95% CI) | P | HR* (95% CI) | P | HR* (95% CI) | P |
| Major adverse cardiovascular outcomes | 0.99 (0.83 to 1.19) | .96 | 0.83 (0.64 to 1.08) | .16 | 0.87 (0.58 to 1.32) | .52 | 0.84 (0.38 to 1.88) | .68 |
| Non-fatal myocardial infarction | 0.98 (0.75 to 1.29) | .89 | 0.87 (0.54 to 1.38) | .54 | 0.99 (0.49 to 2.03) | .98 | 0.63 (0.15 to 2.58) | .52 |
| Other acute coronary syndrome | 1.08 (0.64 to 1.83) | .76 | 0.55 (0.26 to 1.16) | .12 | 1.79 (0.63 to 5.10) | .28 | 0.16 (0.02 to 1.36) | .093 |
| Stroke | 0.87 (0.60 to 1.26) | .46 | 0.91 (0.56 to 1.46) | .69 | 1.38 (0.56 to 3.39) | .48 | 0.58 (0.11 to 3.02) | .51 |
| Heart failure hospitalization | 1.17 (0.85 to 1.62) | .34 | 1.16 (0.74 to 1.81) | .52 | 0.57 (0.30 to 1.11) | .097 | 0.62 (0.58 to 3.49) | .58 |
| Cardiovascular death | 0.99 (0.65 to 1.51) | .96 | 0.49 (0.22 to 1.11) | .087 | 0.93 (0.33 to 2.61) | .90 | 4.25 (0.83 to 21.83) | .083 |
| All-cause death | 0.98 (0.78 to 1.24) | .86 | 1.04 (0.74 to 1.47) | .82 | 1.14 (0.69 to 1.90) | .61 | 1.33 (0.56 to 3.16) | .53 |
| Major adverse cardiovascular event or death | 1.02 (0.87 to 1.19) | .85 | 0.91 (0.72 to 1.14) | .39 | 0.99 (0.69 to 1.40) | .93 | 0.85 (0.44 to 1.64) | .62 |
hazard ratio per 1-standard deviation decrease in mean systolic blood pressure
In a sensitivity analysis that used time in target range, mean systolic blood pressure and systolic blood pressure standard deviation for months 0–6 instead of months 0–3, time in target range over months 0–6 significantly associated with a reduced risk of major adverse cardiovascular events, non-fatal myocardial infarction, heart failure hospitalization, cardiovascular death and all-cause death (Supplemental Table 5). After adjustment for mean systolic blood pressure over months 0–6, time in target range over months 0–6 did not associate with major adverse cardiovascular events but did associate with heart failure hospitalization, cardiovascular death and all-cause death (Supplemental Table 5). Systolic blood pressure standard deviation over months 0–6 had no effect on the relationships between time in target range over months 0–6 and cardiovascular outcomes (Supplemental Table 5). Neither mean systolic blood pressure over months 0–6 nor systolic blood pressure variability over months 0–6 associated with any cardiovascular outcome except for mean systolic blood pressure over months 0–6 with cardiovascular death (HR [95% CI], 0.75 [0.57 to 0.99]; P=.042) and all-cause death (HR [95% CI], 0.84 [0.72 to 0.98]; P=.028) in the sensitivity analyses with an extended exposure period. After adjustment for time in target range over months 0–6, there were no significant associations between mean systolic blood pressure over months 0–6 or systolic blood pressure variability over months 0–6 and any cardiovascular outcome (P>.11 for all).
Time in target range did not associate with the risk of any serious adverse event or any treatment-related serious adverse event (Supplemental Table 6). The risk of acute kidney injury significantly decreased with greater time in target range (Supplemental Table 6).
Discussion
In this analysis of adults with hypertension and high cardiovascular risk, greater time with a systolic blood pressure within target range predicted a decreased risk of major adverse cardiovascular events independent of traditional cardiovascular risk factors (Central Illustration). Systolic blood pressure time in target range remained a significant predictor of cardiovascular outcomes even after adjusting for mean systolic blood pressure and systolic blood pressure variability. Most important, systolic blood pressure time in target range predicted cardiovascular outcomes even among participants with a mean systolic blood pressure within target range. These results suggest that time in target range provides incremental value beyond mean systolic blood pressure to population-based hypertension quality monitoring and clinical trial-based blood pressure interventions.
Central Illustration. Systolic Blood Pressure Time in Target Range as an Independent Predictor of Cardiovascular Outcomes.

This figure depicts examples of high and low systolic blood pressure time in target range (left and right upper panels). Systolic blood pressure time in target range associates with a decreased risk of major cardiovascular outcomes after adjustment for cardiovascular risk factors and mean systolic blood pressure (lower panel).
Time in therapeutic range added incremental value to mean systolic blood pressure but adding mean systolic blood pressure to time in target range did not provide additional prognostic value. This result makes intuitive sense because mean systolic blood pressure cannot be within target range without time in target range also being high. While time in target range correlated significantly with mean systolic blood pressure, scatterplots indicate that mean systolic blood pressure varied widely above, below and within the target range, in particular at lower times in target ranges.
Time in target range may prove useful for population-level monitoring of blood pressure control and in clinical trials of blood pressure control interventions. At the population-level, these results indicate that mean systolic blood pressure fails to represent the full spectrum of hypertension-related cardiovascular risk. Time in target range may be a useful surrogate outcome to characterize the effect of blood pressure control interventions and new antihypertensive therapies.(12) This approach may also be useful for clinicians as it provides a more holistic, long view of an individual patient’s blood pressure control and inform treatment decisions. Further research is warranted to determine if treating to a specified time in target range would ensure maximal benefit while also reducing the risk of overtreatment with antihypertensives.
These results did not detect heterogeneity in effect between intensive and standard blood pressure control interventions. This lack of heterogeneity indicates that the decreased risk cannot be attributable to differences in blood pressure control or blood pressure management between the two treatment arms in SPRINT. Assessment of time in target range in routine practice with ambulatory or home blood pressure monitoring and without strict, protocol-directed blood pressure management is needed to determine the generalizability of these results.
Time in target range was estimated using linear interpolation rather than the proportion of measurements within range to account for differential time between measurements and because few blood pressure measurements were recorded during the exposure period. A previous analysis of Veterans with and without hypertension found that a higher proportion of systolic blood pressure measurements within 120 to 140 mm Hg associated with a decreased risk of all-cause mortality.(13) The present study builds upon these results with a more rigorous measure of time in target range, assessment of cardiovascular outcomes and adverse drug effects, definition of target range based upon current guideline recommendations and inclusion of a more diverse population.
This analysis has certain limitations. The study population included adults enrolled in a clinical trial that provided strict protocols for blood pressure management. This limitation would be expected to bias these results towards the null because strict blood pressure control would limit changes in blood pressure and force time in target range and mean blood pressure to converge. Despite a strong correlation with mean systolic blood pressure, time in target range remained independently prognostic of cardiovascular outcomes. The exposure period was limited to 3 months in the main analysis and 6 months in the sensitivity analysis to maximize statistical power. While this initial period captures most antihypertensive medication changes, assessment of time in therapeutic range over a longer period of time would be of interest. As previously mentioned, these results should be verified study cohorts derived from routinely collected clinical data.
Conclusions
Time in target range predicts major adverse cardiovascular events independent of mean achieved systolic blood pressure. Time in target range may be a useful metric of blood pressure control for population-based quality assessment and clinical trial interventions.
Supplementary Material
Clinical Perspectives.
Competency in Patient Care:
Time in target range represents an important measure of blood pressure control beyond the mean achieved level and correlates with clinical outcomes in patients with hypertension.
Translational Outlook:
Future studies should evaluate the impact of time in target range outside clinical trial settings and across defined patient subgroups.
Acknowledgements
The authors thank the SPRINT investigators, study teams and participants for making these data available for secondary analyses.
Abbreviations
- DASH
Dietary Approaches to Stop Hypertension
- SPRINT
Systolic Blood Pressure Intervention Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have nothing to disclose.
References
- 1.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Hardy ST, Fine LJ et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. Jama 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quality ID #236 (NQF 0018): Controlling High Blood Pressure. 2020.
- 4.Wang J, Shi X, Ma C et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hypertens 2017;35:10–17. [DOI] [PubMed] [Google Scholar]
- 5.Nwabuo CC, Yano Y, Moreira HT et al. Association Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life. JAMA Cardiology 2020;5:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark D 3rd, Nicholls SJ, St John J et al. Visit-to-Visit Blood Pressure Variability, Coronary Atheroma Progression, and Clinical Outcomes. JAMA Cardiol 2019;4:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The SRG, Wright JT, Williamson JD et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: Comparative evaluation of measures of time-in-therapeutic range. Journal of Thrombosis and Thrombolysis 2003;15:213–216. [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosius WT, Sink KM, Foy CG et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The systolic blood pressure intervention trial (SPRINT). Clinical Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman EZ, Ambrosius WT, Cushman WC et al. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Hypertension. Circulation 2017;136:440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon DL, Parod ED, Sisson EM, Van Tassell BW, Nadpara PA, Dow A. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. JACCP: JOURNAL OF THE AMERICAN COLLEGE OF CLINICAL PHARMACY 2020;3:404–409. [Google Scholar]
- 13.Doumas M, Tsioufis C, Fletcher R, Amdur R, Faselis C, Papademetriou V. Time in Therapeutic Range, as a Determinant of All-Cause Mortality in Patients With Hypertension. J Am Heart Assoc 2017;6:e007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


