Abstract
BACKGROUND:
Hypertensive disorders of pregnancy (HDPs) are leading causes of maternal and perinatal morbidity and mortality. However, it is uncertain whether HDPs are associated with long-term risk of premature mortality (before age 70 years).
OBJECTIVES:
To evaluate whether HDPs are associated with premature mortality.
METHODS:
Between 1989 and 2017, we followed 88,395 parous female nurses participating in the Nurses’ Health Study II. We focused on gestational hypertension and preeclampsia within the term HDPs. Hazard ratios (HR) and 95% confidence intervals (CI) for the associations between HDPs and premature mortality were estimated using Cox proportional hazards models, adjusting for relevant confounders.
RESULTS:
We documented that 2,387 women died before age 70, including 1,141 cancer deaths and 212 CVD deaths. The occurrence of HDPs, either gestational hypertension or preeclampsia, was associated with an HR of 1.31 (95% CI:1.18, 1.46) for premature death during follow-up. When specific causes of death were examined, these relations were strongest for CVD-related mortality (HR=2.26, 95% CI:1.67 to 3.07). The association between HDPs and all-cause premature death persisted, regardless of the subsequent development of chronic hypertension (HR=1.20, 95% CI: 1.02 to 1.40 for HDPs only and 2.02, 95% CI: 1.75 to 2.33 for both HDPs and subsequent chronic hypertension).
CONCLUSIONS:
An occurrence of HDPs, either gestational hypertension or preeclampsia, was associated with an increased risk of premature mortality, particularly CVD mortality, even in the absence of chronic hypertension.
Keywords: Hypertensive disorders, pregnancy, mortality, women, cardiovascular diseases
Condensed Abstract:
Among 88,395 parous female nurses, we found that experiencing hypertensive disorders of pregnancy (HDPs), either gestational hypertension or preeclampsia, was associated with a greater risk of premature mortality, especially CVD-related deaths, even in the absence of chronic hypertension. Our results highlight the need for clinicians to screen for HDPs when evaluating CVD morbidity and mortality risk of their patients. Chronic hypertension may not be the main factor responsible for the long-term adverse impact of pregnancy-related hypertension, warranting exploration of other contributing factors.
INTRODUCTION
The dramatic worldwide increase in the standard of living, coupled with medical and public health advances during the last half of the 20th century, has resulted in a steady reduction in age-specific mortality at a global scale (1). Yet premature mortality (death before age 70) from non-communicable diseases (NCDs), also known as chronic diseases, remains a major public health challenge. According to the World Health Organization, while the age-standardized rates of NCD causes of death have decreased, the absolute number of deaths due to NCDs in people aged 30–69 years has increased from 12.5 million in 2000 to 15.2 million in 2016 (2,3).
Hypertensive disorders of pregnancy (HDPs), which occur in approximately 10% of all pregnancies worldwide, are the most common medical problem encountered in pregnancy (4). There are 4 types of HDPs: chronic hypertension (i.e., hypertension predating pregnancy or diagnosed before 20 weeks of pregnancy), gestational hypertension (GHTN), preeclampsia, and chronic hypertension with superimposed preeclampsia (5). GHTN and preeclampsia, which occur at or after 20 weeks’ gestation, are leading causes of maternal and perinatal morbidity and mortality (6). Several retrospective cohorts and disease registry studies have documented an association of GHTN and preeclampsia with the risk of all-cause and cardiovascular disease (CVD) mortality (7–14). Nevertheless, the relationship between HDPs with all-cause and cause-specific mortality has not been evaluated in a prospective cohort with careful control for common confounding factors and updated lifestyle characteristics. Moreover, while women with a history of HDPs had 3–5 times higher risk of developing chronic hypertension (15), it is unclear whether the association between HDPs and premature mortality is fully explained by the subsequent development of chronic hypertension and whether this association exists among women who do not develop chronic hypertension. Recurrent preeclampsia and complicated preeclampsia (e.g., with preterm birth and low birthweight) have been associated with greater CVD risk (16); some studies also suggest particularly increased CVD mortality among women whose last pregnancy was complicated by hypertension (7). However, it is unclear whether these associations pertain to premature mortality. To address these important questions, we focused on GHTN and preeclampsia within the term HDPs and examined the associations between HDPs and all-cause and cause-specific premature mortality among parous women participating in a large ongoing cohort study, the Nurses’ Health Study II (NHS-II), which has prospectively collected information on reproductive characteristics and repeatedly ascertained of numerous lifestyle and health-related characteristics over three decades.
METHODS
Study population
We included parous women from the ongoing Nurses’ Health Study II (NHS-II) cohort, which was established in 1989 by recruiting 116,429 U.S. female registered nurses aged 25–42 years. Biennial questionnaires are used to collect information on participants’ lifestyles and health. Participants were eligible for inclusion in this analysis if they reported a pregnancy (≥6 months) at or after age 18 years on the baseline questionnaire or became pregnant for the first time during follow-up through 2009 when most participants had completed their reproductive careers. We further excluded women who had missing data on date of birth (n=17) and chronic hypertension diagnosis (n=1,875), received a diagnosis of cancer, type 2 diabetes, or CVD before baseline (1989) or before their first pregnancy (n=1,633), or never returned follow-up questionnaires (n=1,324). After exclusions, 88,395 women were retained in our current analyses.
Ethics
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required (protocol number: 2009-P-002375).
Ascertainment of HDPs
Self-reports of physician diagnosis of preeclampsia [“pregnancy-related high blood pressure” (i.e., gestational hypertension) or “preeclampsia/ toxemia”] were ascertained on the baseline questionnaire in 1989 and updated every 2 years through 2001. GHTN was ascertained on the 1993 questionnaire and then updated every 2 years through 2001. Reports of GHTN and preeclampsia from 2002 to 2009 were ascertained in the 2009 questionnaire, where participants retrospectively reported information on all previous pregnancies, including the sequence and year of birth, as well as pregnancy complications such as HDPs, preterm birth, and low birthweight. Self-reports of preeclampsia have been validated against medical records in a subgroup of 598 NHS-II participants who received a diagnosis of preeclampsia or had evidence of GHTN and proteinuria (protein excretion ≥300 mg/24 hr urine, protein-creatinine ratio ≥0.3, or dipstick reading of ≥1+) (17). The positive predictive value of self-reported preeclampsia was 89% when compared against medical records with sufficient information to establish a diagnosis (18).
Ascertainment of chronic hypertension
The 1989 questionnaire retrospectively captured information of any physician-diagnosed chronic hypertension (i.e., high blood pressure outside of pregnancy) and the year of diagnosis, which was then updated every 2 years. Women were not considered exposed to GHTN or preeclampsia if chronic hypertension was reported before pregnancy. Participants were considered exposed from the age reporting a diagnosis of chronic hypertension. Self-reports of chronic hypertension have been validated against medical records in a subgroup of NHS-II participants, with a sensitivity and specificity of 94% and 85%, respectively (19). Anti-hypertensive medication use (e.g., thiazide diuretics, beta-blockers, and angiotensin-converting enzyme inhibitors) was ascertained at baseline and updated every 2 years.
Outcome ascertainment
Deaths were identified through the National Death Index, state vital statistics records, or by reports from the postal authorities or close relatives. The validity of this method was evaluated in a subgroup of participants from the Nurses’ Health Study (NHS). Among 179 participants known to be dead and 1997 known to be alive, 98.0% and 100% of the “true” dead and alive participants were correctly identified, respectively (20). The outcome of interest was premature mortality, defined as death before age 70 years (21). The cause of death was ascertained by physician review of medical records, autopsy reports, or death certificates. The International Classification of Diseases, Eighth Revision (ICD-8) was applied to distinguish deaths from CVD (ICD codes: 390–458), cancers (ICD codes: 140–207), and any other reasons (Supplemental Table 1).
Assessment of covariates
Participants reported their race/ethnicity, height, current weight, and weight at age 18 years in the 1989 questionnaire. Body weight was self-reported every 2 years. We calculated body mass index (BMI) at age 18 years and during each follow-up cycle. Lifestyle characteristics and health-related conditions such as smoking status, medication use, menopausal status, and parental histories of diabetes, myocardial infarction (MI) or stroke were updated every 2 years. Physical activity and alcohol consumption were ascertained at baseline and updated every 4–6 years. Dietary intake was assessed every 4 years using a validated semiquantitative food frequency questionnaire (22). We calculated the Alternate Healthy Eating Index (AHEI) 2010 to evaluate the overall dietary quality of each participant (23).
Data analysis
Participants’ follow-up time was calculated from the return date of baseline or follow-up questionnaires when the woman reported a pregnancy lasting at least 6 months until the date of death or the end of follow-up (June 30, 2017), whichever occurred first. Five women who died at or after age 70 years were treated as censored observations. Exposure status (the occurrence of HDPs) was updated every 2 years through 2009; participants were considered exposed from the age at first report of a pregnancy complicated by HDPs, regardless of occurrence in subsequent pregnancies. Thus, a woman with normotensive pregnancies who subsequently developed HDPs would contribute to both unexposed (initially, following the unaffected pregnancies) and exposed (after her first report of an affected pregnancy) person-time over follow-up.
We fitted Cox proportional hazards models to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between the occurrence of GHTN and/or preeclampsia with all-cause and cause-specific premature mortality, while simultaneously adjusting for confounders and risk factors (see Supplemental Appendix). To control as finely as possible for confounding by age, calendar time, and any possible interactions between these two timescales, we stratified the analysis jointly by age in months at the start of follow-up and calendar year for the current questionnaire cycle. The timescale for the analysis was months since the start of the current questionnaire cycle, which is equivalent to age in months. Multivariable models were adjusted for race/ethnicity and pre-pregnancy BMI, as well as time-varying menopausal status, current hormone therapy use, daily aspirin use, and parental history of MI or stroke. In the second set of multivariable models, we further adjusted for time-varying breastfeeding duration, parity, alcohol consumption, smoking status, total physical activity, BMI, and AHEI-2010 diet score. The Anderson-Gill data structure was used to efficiently handle time-varying covariates (24), when a new data record is created for every questionnaire cycle at which a participant is at risk, with covariates set to the values at the time the questionnaire is returned. For the covariates with missing values at a given time point (<5% for any covariates), data from the prior questionnaire was carried forward; otherwise, missing indicator variables were used in the analysis. We also assessed the risk of premature mortality by jointly classifying GHTN and preeclampsia.
To explore whether the association between HDPs and premature mortality was fully explained by the subsequent development of chronic hypertension and whether this association exists among women who didn’t develop chronic hypertension, we categorized exposure by taking into account the subsequent development of chronic hypertension and classified women as no HDPs, HDPs only without subsequent chronic hypertension, HDPs only, or both HDPs and subsequent chronic hypertension. We updated the exposure status prospectively during follow-up; participants without an occurrence of either HDPs or chronic hypertension served as the reference group. We also tested for effect modification by performing analyses stratified by age at first birth (≤25 vs. >25 years), breastfeeding duration (≤3 vs. >3 months), parity (1 vs. ≥2), parental history of CVD (yes vs. no), prepregnancy BMI (≥25 vs. <25 kg/m2), current BMI (≥30, 25–29.9, or <25 kg/m2), subsequent development of type 2 diabetes (yes vs. no), anti-hypertensive medicine use (yes vs. no), AHEI-2010 dietary score (in the up two-fifths vs. bottom three-fifths), smoking status (never/past vs. current), and physical activity in moderate-to-vigorous intensity (<150 vs. ≥150 min/week). Interaction on the multiplicative scale was assessed using likelihood ratio tests comparing models with and without a multiplicative interaction term between HDPs and the potential effect modifier. Finally, we explored the influence of changes in HDP status in the first and subsequent pregnancies, the number of pregnancies affected by HDPs, and co-exposure to preterm birth and low birth weight. We restricted the analysis to participants who responded to the 2009 questionnaire, because that questionnaire captured total reproductive history without risk of double-counting any pregnancies reported on biennial questionnaires. Participants’ follow-up time was calculated from the return date of the 2009 questionnaire.
Three sensitivity analyses were conducted. First, we re-analyzed the association between HDPs and premature mortality by excluding deaths due to complications of pregnancy, childbirth, and the puerperium. Second, women were considered exposed to HDPs even if previous chronic hypertension was reported. Third, we excluded multiple pregnancies (e.g., twins, triplets) from the analysis. All data were analyzed using SAS 9.3 for UNIX (SAS Institute Inc).
RESULTS
Participants’ age-standardized characteristics
We followed 88,395 parous women during 28 years of follow-up. They had a mean (SD) age at first birth of 26.7 (±4.7) years and a mean (SD) pre-pregnancy BMI of 21.0 (±3.0) kg/m2. In total, 12,405 women (14.0%) experienced HDPs in at least one of their pregnancies. Participants’ age-standardized characteristics at the analytic baseline according to HDPs either reported at baseline or during follow-up are shown in Table 1. Compared with women without HDPs, women who experienced GHTN and/or preeclampsia had a greater baseline BMI and higher baseline prevalence of gestational diabetes, and parental history of diabetes and MI/stroke. Women who reported GHTN and/or preeclampsia at baseline also had a higher baseline prevalence of chronic hypertension than women without HDPs (10% vs. 3%; Supplemental Table 2).
Table 1.
Age-standardized baseline (1989) characteristics according to the occurrence of HDPs either at baseline or during follow-up among 88,395 parous women (NHS-II, 1989-2017).*
| With an occurrence of HDPs | ||||
|---|---|---|---|---|
| Characteristic | No HDPs | HDPs (either GHTN or preeclampsia)§ | GHTN | Preeclampsia |
| No. | 75,990 | 12,405 | 7,253 | 10,742 |
| Age at first birth, mean (SD), y† | 26.7 (4.6) | 26.2 (4.4) | 26.5 (4.3) | 26.1 (4.4) |
| Parity (pregnancies ≥6 mo), mean (SD) | 1.8 (1.1) | 1.9 (1.0) | 1.9 (1.0) | 1.9 (1.0) |
| Parous, % | 87 | 92 | 93 | 92 |
| History of gestational diabetes, % | 3 | 7 | 8 | 7 |
| Breastfeeding duration, months | ||||
| <3.0 | 27 | 30 | 28 | 30 |
| 3.0–12.0 | 31 | 32 | 33 | 32 |
| >12.0 | 42 | 38 | 39 | 38 |
| Prepregnancy BMI, kg/m2 | 20.9 (2.9) | 21.8 (3.4) | 21.9 (3.5) | 21.7 (3.4) |
| Baseline age, y† | 34.9 (4.7) | 34.6 (4.6) | 34.4 (4.5) | 34.6 (4.6) |
| Baseline BMI, mean (SD) kg/m2 | 23.7 (4.4) | 26.0 (5.6) | 26.4 (5.8) | 26.0 (5.6) |
| White, % | 92 | 92 | 93 | 92 |
| Total physical activity, mean (SD), h/wk | 3.3 (4.9) | 3.1 (4.8) | 3.0 (4.5) | 3.1 (4.8) |
| AHEI-2010 dietary score, mean (SD) | 47.4 (10.6) | 47.0 (10.5) | 46.9 (10.5) | 47.0 (10.4) |
| Alcohol intake, mean (SD), g/d | 2.9 (5.6) | 2.5 (5.4) | 2.5 (5.4) | 2.5 (5.4) |
| Current regular aspirin use, %‡ | 10 | 12 | 11 | 12 |
| Parental history of diabetes, % | 16 | 20 | 20 | 20 |
| Parental history of MI or stroke, % | 14 | 18 | 18 | 18 |
| Smoking status, % | ||||
| Never | 66 | 65 | 68 | 64 |
| Past | 21 | 22 | 21 | 22 |
| Current | 13 | 13 | 11 | 13 |
Values of means (SDs) and percentages were standardized to the age distribution of the study population in 1989.
Value is not age-adjusted.
Aspirin or aspirin-containing products used regularly at least once per week in the past 2 years.
Participants could experience both GHTN and preeclampsia across their reproductive lifespan. HDPs= Hypertensive disorders of pregnancy; GHTN =gestational hypertension; MI= myocardial infarction.
HPDs and mortality
During 2,355,049 person-years of follow-up, we documented 2,387 premature deaths during follow-up, including 1,141 cancer deaths and 212 CVD deaths (Table S1). Age-adjusted Cox proportional models showed that the occurrence of GHTN or preeclampsia was associated with an HR of 1.42 (95% CI 1.28 to 1.58) for premature death during follow-up (Central Illustration). These associations were slightly attenuated but remained statistically significant after additional adjustment for potential confounding factors (HR=1.32, 95% CI: 1.19 to 1.47), as well as time-varying (post-pregnancy) dietary, lifestyle, and reproductive characteristics (HR=1.31, 95% CI: 1.18 to 1.46) (Central Illustration). There was no evidence of effect modification by age at first birth, breastfeeding duration, parity, parental history of CVD, anti-hypertension treatment, subsequent development of type 2 diabetes, prepregnancy/curent BMI, physical activity, diet quality, and smoking status (Supplemental Table 3).
Central Illustration. Hazard ratios (HRs) for the risk of all-cause premature mortality according to the occurrence of HDPs.
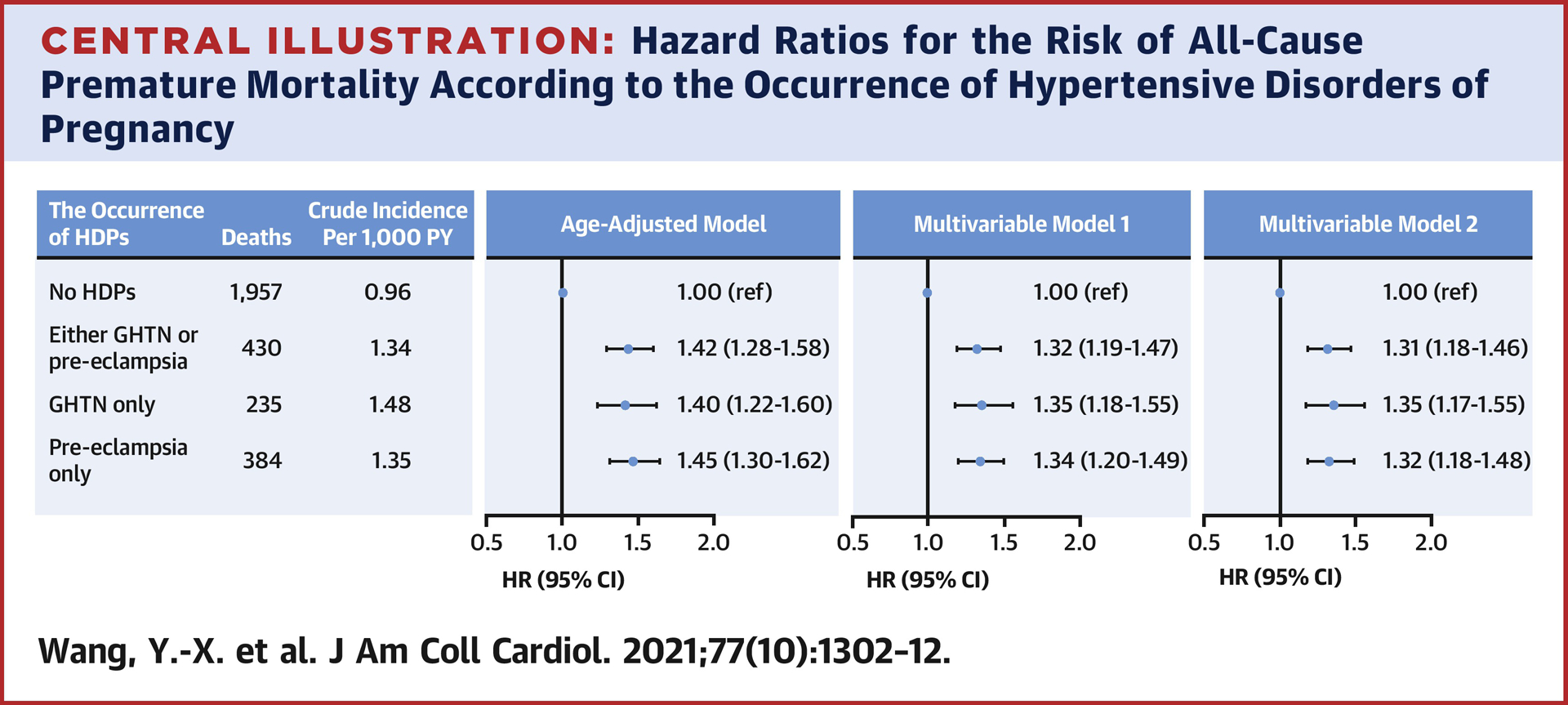
In the age-adjusted model, age in months at the start of follow-up and calendar year of the current questionnaire cycle was included as a stratified variable. Multivariable model 1 was further adjusted for White race/ethnicity, pre-pregnancy BMI, and time-varying menopausal status, current hormone therapy use, daily aspirin use, and parental history of MI or stroke. Multivariable model 2 was further adjusted for time-varying breastfeeding duration, parity, alcohol intake, smoking status, physical activity, AHEI-2010 dietary score, and current BMI. HDPs= Hypertensive disorders of pregnancy; GHTN=gestational hypertension; PY= person year; CI = Confidence intervals. The associations of GHTN and pre-eclampsia with premature mortality were assessed separately and always against normotensive pregnancies.
Analyses of cause-specific mortality showed that the occurrence of HDPs was related to a higher risk of premature CVD mortality (HR=2.26, 95% CI: 1.67 to 3.07) but was unrelated to premature cancer mortality (HR=0.97, 95% CI: 0.82 to 1.15; Figure 1). In analyses for less common causes of death (Supplemental Table 4), HDPs was associated with a greater risk of deaths due to infectious diseases (HR=2.77, 95% CI:1.38 to 5.54), respiratory disease (HR=2.26, 95% CI: 1.29 to 3.98), nervous system diseases (HR=2.51, 95% CI: 1.33 to 4.72), metabolic/immunity disorders (HR=4.85, 95% CI: 2.29 to 10.27), and symptoms, signs or ill-defined conditions (HR=1.72, 95% CI: 1.13 to 2.60). The risk of all-cause and cause-specific mortality was similar when GHTN and preeclampsia were examined separately (Central Illustration and Figure 1). Moreover, when GHTN and preeclampsia were jointly-classified, a similar risk of all-cause mortality was observed for GHTN only, preeclampsia only, or both (Supplemental Table 5).
Figure 1. Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for cause-specific premature mortality (before age 70 y) according to the occurrence of HDPs among 88,395 parous women (NHS-II, 1989–2017).

In the age-adjusted model, age in months at the start of follow-up and calendar year of the current questionnaire cycle was included as a stratified variable. Multivariable model 1 was further adjusted for White race/ethnicity, pre-pregnancy BMI, and time-varying menopausal status, current hormone therapy use, daily aspirin use, and parental history of MI or stroke. Multivariable model 2 was further adjusted for time-varying breastfeeding duration, parity, alcohol intake, smoking status, physical activity, AHEI-2010 dietary score, and current BMI. HDPs= Hypertensive disorders of pregnancy; GHTN=gestational hypertension; PY= person year; CI = Confidence intervals. The associations of GHTN and pre-eclampsia with premature mortality were assessed separately and always against normotensive pregnancies.
When we categorized exposure by taking into account the subsequent development of chronic hypertension, in the fully-adjusted models we found an elevated risk of all-cause premature mortality in relation to the occurrence of HDPs only (HR=1.20, 95% CI: 1.02 to 1.40), chronic hypertension only (HR=1.67, 95% CI:1.50 to 1.84), and both HDPs and subsequent chronic hypertension (HR=2.02, 95% CI: 1.75 to 2.33) (Table 2). A similar pattern of association was observed for mortality due to CVD (Table 2).
Table 2.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for the risk of all-cause and cause-specific premature mortality (before age 70 y) according to the joint-categories of HDPs and subsequent chronic hypertension among 88,395 parous women (NHS-II, 1989-2017).
| Cause of death | No HDPs and chronic hypertension | HDPs only | Chronic hypertension only | Both HDPs and chronic hypertension |
|---|---|---|---|---|
| All-cause | ||||
| Events, No. | 1,270 | 176 | 687 | 254 |
| PY, No. | 1,686,019 | 204,717 | 348,316 | 115,997 |
| Crude Incidence, per 1000 PY | 0.75 | 0.86 | 1.97 | 2.19 |
| HRs (95% CI) | ||||
| Age-adjusted model* | 1.00 (ref) | 1.27 (1.08–1.48) | 1.63 (1.47–1.79) | 2.05 (1.79–2.35) |
| Multivariable model 1† | 1.00 (ref) | 1.18 (1.01–1.38) | 1.64 (1.48–1.80) | 1.95 (1.70–2.24) |
| Multivariable model 2‡ | 1.00 (ref) | 1.20 (1.02–1.40) | 1.67 (1.50–1.84) | 2.02 (1.75–2.33) |
| CVD | ||||
| Events, No. | 78 | 17 | 71 | 46 |
| PY, No. | 1,687,129 | 204,858 | 348,873 | 116,178 |
| Crude Incidence, per 1000 PY | 0.05 | 0.08 | 0.20 | 0.40 |
| HRs (95% CI) | ||||
| Age-adjusted model* | 1.00 (ref) | 1.92 (1.14–3.26) | 3.36 (2.39–4.72) | 6.96 (4.78–10.13) |
| Multivariable model 1† | 1.00 (ref) | 1.70 (1.00–2.88) | 3.13 (2.21–4.42) | 6.04 (4.11–8.87) |
| Multivariable model 2‡ | 1.00 (ref) | 1.71 (1.01–2.92) | 3.23 (2.26–4.63) | 6.35 (4.22–9.54) |
| Cancer | ||||
| Events, No. | 722 | 75 | 266 | 78 |
| PY, No. | 1,686,525 | 204,810 | 348,681 | 116,148 |
| Crude Incidence, per 1000 PY | 0.43 | 0.37 | 0.76 | 0.67 |
| HRs (95% CI) | ||||
| Age-adjusted model* | 1.00 (ref) | 0.95 (0.75–1.20) | 1.19 (1.02–1.37) | 1.15 (0.91–1.46) |
| Multivariable model 1† | 1.00 (ref) | 0.90 (0.71–1.14) | 1.22 (1.05–1.42) | 1.14 (0.90–1.45) |
| Multivariable model 2‡ | 1.00 (ref) | 0.92 (0.73–1.17) | 1.27 (1.09–1.48) | 1.19 (0.93–1.52) |
| All other causes | ||||
| Events, No. | 340 | 61 | 211 | 98 |
| PY, No. | 1,686,866 | 204,816 | 348,743 | 116,134 |
| Crude Incidence, per 1000 PY | 0.20 | 0.30 | 0.61 | 0.84 |
| HRs (95% CI) | ||||
| Age-adjusted model* | 1.00 (ref) | 1.60 (1.22–2.11) | 2.13 (1.77–2.55) | 3.28 (2.61–4.13) |
| Multivariable model 1† | 1.00 (ref) | 1.46 (1.11–1.92) | 2.08 (1.73–2.51) | 2.99 (2.37–3.78) |
| Multivariable model 2‡ | 1.00 (ref) | 1.49 (1.13–1.96) | 2.08 (1.71–2.52) | 3.09 (2.42–3.94) |
In the age-adjusted model, age in months at the start of follow-up and calendar year of the current questionnaire cycle was included as a stratified variable.
Based on age-adjusted models, multivariable model 1 was further adjusted for White race/ethnicity (yes, no), pre-pregnancy BMI (<25, ≥25 kg/m2), and time-varying menopausal status (premenopausal, postmenopausal, unsure/biologically uncertain), current hormone therapy use (never, past, current), daily aspirin use (yes/no), and parental history of MI or stroke (yes, no).
Multivariable model 2 was further adjusted for time-varying breastfeeding duration (≤3.0, 3.1–12, >12 months), parity (≤1, 2, ≥3), alcohol intake (0, 1–14, ≥15 g/d), smoking status (never, former, current 1–34 cigarettes/day, current ≥35 cigarettes/day), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), AHEI-2010 dietary score (quintiles), and current BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2). HDPs= Hypertensive disorders of pregnancy; PY=person year.
When the influence of the sequence in which the affected pregnancies took place was tested among participants who responded to the 2009 questionnaire (Table 3), women who experienced HDPs after a normotensive pregnancy and those reporting HDPs both in the first and subsequent pregnancies were at the highest risk of dying prematurely than women with normotension in all pregnancies in the fully adjusted model (HR=1.82, 95% CI: 1.22 to 2.69; and 1.23, 95% CI: 0.74 to 2.04, respectively). Besides, the elevated risk of premature mortality also appeared to be driven by the small number of women who experienced HDPs in two or more pregnancies, and those who simultaneously reported HDPs and low birth weight (Table 3).
Table 3.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for the risk of all-cause premature mortality (before age 70 y) according to the occurrence of HDP status across multiple pregnancies among 61,806 parous women (NHS-II, 1989-2017).
| HDP status | Deaths/PY | Crude Incidence per 1000 PY | HRs (95% CI) | ||
|---|---|---|---|---|---|
| Age-adjusted model* | Multivariable model 1† | Multivariable model 2‡ | |||
| Change in HDP status | |||||
| Normotension in the first birth | |||||
| Normotensive subsequent births or no additional births | 530/415,338 | 1.28 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| HDPs in subsequent births | 27/11,767 | 2.29 | 1.86 (1.26–2.74) | 1.78 (1.21–2.63) | 1.82 (1.22–2.69) |
| HDP in the first birth | |||||
| Normotensive subsequent births or no additional births | 39/35,717 | 1.09 | 0.92 (0.66–1.27) | 0.88 (0.63–1.22) | 0.81 (0.58–1.13) |
| HDPs in subsequent births | 16/10,272 | 1.56 | 1.33 (0.81–2.19) | 1.27 (0.77–2.09) | 1.23 (0.74–2.04) |
| Total number of pregnancies with HDPs | |||||
| No HDPs | 530/415,338 | 1.28 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1 | 61/46,371 | 1.32 | 1.09 (0.84–1.43) | 1.05 (0.80–1.37) | 0.99 (0.76–1.30) |
| ≥2 | 21/11,384 | 1.84 | 1.59 (1.02–2.46) | 1.51 (0.97–2.35) | 1.47 (0.94–2.30) |
| Co-exposure to HDPs and preterm birth | |||||
| No HDPs | 530/415,338 | 1.28 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Only HDPs | 66/45,493 | 1.45 | 1.19 (0.92–1.54) | 1.14 (0.88–1.48) | 1.10 (0.84–1.42) |
| HDPs and preterm birth | 16/12,262 | 1.30 | 1.18 (0.72–1.94) | 1.13 (0.68–1.86) | 1.03 (0.62–1.69) |
| Co-exposure to HDPs and low birth weight | |||||
| No HDPs | 530/415,338 | 1.28 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Only HDPs | 67/48,197 | 1.39 | 1.16 (0.90–1.50) | 1.11 (0.86–1.43) | 1.06 (0.82–1.37) |
| HDPs and low birth weight | 15/9,558 | 1.57 | 1.32 (0.79–2.21) | 1.30 (0.78–2.18) | 1.20 (0.72–2.02) |
In the age-adjusted model, age in months at the start of follow-up and calendar year of the current questionnaire cycle was included as a stratified variable.
Based on age-adjusted models, multivariable model 1 was further adjusted for White race/ethnicity (yes, no), pre-pregnancy BMI (<25, ≥25 kg/m2), and time-varying menopausal status (premenopausal, postmenopausal, unsure/biologically uncertain), current hormone therapy use (never, past, current), daily aspirin use (yes/no), and parental history of MI or stroke (yes, no).
Multivariable model 2 was further adjusted for time-varying breastfeeding duration (≤3.0, 3.1–12, >12 months), parity (≤1, 2, ≥3), alcohol intake (0, 1–14, ≥15 g/d), smoking status (never, former, current 1–34 cigarettes/day, current ≥35 cigarettes/day), physical activity (0, 0.1–1.0, 1.1–2.4, 2.5–5.9, or ≥6 h/wk), AHEI-2010 dietary score (quintiles), and current BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2). HDPs= Hypertensive disorders of pregnancy; PY=person year.
Sensitivity analyses
The association of HDPs and all-cause premature mortality was not substantially changed when deaths due to complications of pregnancy, childbirth, and the puerperium were excluded, and when women were considered exposed to HDPs even if previous chronic hypertension was reported (Supplemental Table 6). The results were also robust when we excluded multiple pregnancies (e.g., twins, triplets) (Supplemental Table 6).
DISCUSSION
Results from this large prospective cohort show that HDPs, either GHTN or preeclampsia, was associated with an increased risk of premature mortality, particularly mortality from CVD. These associations persisted, regardless of the subsequent development of chronic hypertension. It is also noteworthy that the elevated risk of premature mortality appeared to be driven by the small number of women who experienced HDPs in two or more pregnancies, and those who simultaneously reported HDPs and low birth weight.
Comparison with other studies
Long-term cohort studies have well-documented that HDPs are positively associated with CVD, chronic hypertension, and type 2 diabetes (25), which are increasingly known to be related to a higher risk of mortality. To date, however, few prospective cohort studies have assessed the association between HDPs and mortality (particularly premature mortality). In support of our findings, Theilen and colleagues reported a significantly higher risk of mortality due to all-cause, ischemic heart disease, and stroke among women with a history of hypertensive diseases of pregnancy in a large retrospective study based on the Utah Population Database (10). Similarly, several retrospective studies and registry databases also showed positive associations between preeclampsia and risk of all-cause mortality (9,11), and CVD-related mortality (7–9,12–14,26). In contrast, in a small retrospective cohort, Wilson and colleagues reported that HDPs were unrelated to all-cause mortality among 3,593 Aberdeen women (27). A lack of association between preeclampsia and risk of all-cause mortality was also reported in a large retrospective study based on 129,290 registered births (16).
Methodological weaknesses of these retrospective studies and registry databases included retrospective assessment of HDPs in the long-distant past and a lack of detailed data on underlying risk factors of premature mortality before (e.g., age at first birth, parental history of chronic diseases, prepregnancy BMI) and after pregnancy (e.g., parity), as well as time-varying information on important covariates such as chronic hypertension, BMI, dietary quality, alcohol use, smoking status, and physical activity. In the present study, though there was no effect modification by reproductive characteristics, anti-hypertension treatment, dietary or lifestyle factors, we noted that the elevated risk of all-cause premature mortality was strongest for the occurrence of both HDPs and subsequent chronic hypertension. This is not surprising given that HDPs are strong risk factors for chronic hypertension (15), which, in turn, are associated with premature mortality, particularly CVD mortality (28). However, the association between HDPs and premature mortality also persisted, even in the absence of chronic hypertension. Together, these findings suggest that while the previously described progression of HDPs to chronic hypertension to increased cardiovascular morbidity and mortality is undoubtedly important (29), it may not be the primary pathway through which HDPs impact health and ultimately mortality. We found that HDPs was associated with a greater risk of deaths due to infectious diseases, respiratory disease, nervous system diseases, metabolic/immunity disorders. In support of our findings, previous studies showed that HDPs were associated with a higher risk of respiratory, central nervous system, and endocrine or metabolic morbidity (30,31). However, further studies are needed to verify these novel findings.
Our study is the first to explore the effect of change in HDPs status throughout a woman’s reproductive lifespan on mortality. We found that the highest risk of premature mortality was observed among women who experienced HDPs after a normotensive pregnancy and those reporting HDPs both in the first and subsequent pregnancies. This finding is in contrast to the results by Skjaerven and colleagues (7), which suggested that preeclampsia is a strong predictor of CVD mortality primarily among one-child mothers. However, the latter study followed women with a first singleton birth between 1967 and 2002 through linkage to the national Cause of Death Registry by 2009, which could result in exposure misclassification since not all subsequent births across the reproductive lifespan were included. Besides, we found that the elevated risk of premature mortality appeared to be driven by the small number of women who experienced HDPs in two or more pregnancies and those who simultaneously reported HDPs and low birth weight, which was in line with the growing evidence showing a particularly high risk for cardiovascular morbidity and mortality among women who had preeclampsia in two or more pregnancies (14,29), and those who reported preeclampsia complicated with low birth weight (16).
Underlying mechanisms of the observed relations
When specific causes of death were examined, we did not find any evidence of an association between HDPs and cancer mortality, supporting the existing evidence of no association between HDPs and cancer risk (32,33). Instead, we observed a strong association between HDPs and CVD mortality before age 70. The relation of HDPs and CVD mortality could be partly explained by some shared risk factors of HDPs and CVD, such as insulin resistance and systemic inflammation (34). Besides, the pathological processes implicated in HDPs, including angiogenic imbalance, complement activation, inflammation, and hemodynamic changes, may also contribute directly to cardiac stress that exceeds normal pregnancy, leading to overt cardiac damage (35,36). Finally, the association between HDPs and CVD may also be mediated by epigenomic changes. For instance, Julian and colleagues identified six differentially methylated regions predisposing offspring of HDPs to later-life vascular diseases (37). There is also evidence showing that these above-mentioned pathways are involved in the associations between HDPs and other chronic diseases (38).
Strengths and limitations
The strengths of this study include the large study population, extensive prospective follow-up, and rigorous ascertainment of key lifestyle and health outcomes. Our analysis was carefully adjusted for various confounding factors, including reproductive characteristics and dietary and lifestyle factors that were collected using validated questionnaires. Besides, information on all pregnancies from each woman enabled us to estimate the effect of change in HDP status during the reproductive lifespan on mortality. However, our study also has some limitations. First, the diagnosis of HDPs and chronic hypertension was self-reported which, despite validation against medical records in subgroup participants from this cohort, can result in misclassification of disease status and biased risk estimates. Second, although we have controlled for various confounding factors and predictors of premature mortality, residual confounding from unadjusted covariates cannot be completely ruled out. However, strong residual confounding is unlikely given that adjustment for covariates didn’t change the estimates substantially. Third, because our study population mainly consisted of professional non-Hispanic white women, our findings may not be generalizable to ethnic/racial minority groups. Fourth, the cause of death was uncertain in 15% of women, which may have resulted in imprecise estimates for disease-specific mortality.
CONCLUSIONS
In conclusion, our results suggest that HDPs, either GHTN or preeclampsia, was associated with a greater risk of premature mortality, especially CVD-related deaths, even in the absence of chronic hypertension. Our results highlight the need for clinicians to screen for the history of HDPs when evaluating CVD morbidity and mortality risk of their patients.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
Hypertension during pregnancy, even in women without chronic hypertension, is associated with accelerated long-term mortality, particularly cardiovascular death.
Translational Outlook:
Chronic hypertension may not be the main factor responsible for the long-term adverse impact of pregnancy-related hypertension, warranting exploration of other contributing factors.
Acknowledgment:
We thank the participants and staff of the Nurses’ Health Study II for their invaluable contributions as well as staff from the cancer registries in the following states for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding: This study was supported by grants U01-HL145386, U01-CA176726, R01-HL034594, and R01-HL088521 from the National Institutes of Health.
Disclosures: All authors declare: no support from any organization or industry for the submitted work; no financial relationships with any organizations or industries that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Abbreviations:
- AHEI
Alternative Healthy Eating Index
- BMI
Body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- GHTN
gestational hypertension
- HR
hazard ratio
- HDP
hypertensive disorders of pregnancy
- ICD
International Classification of Diseases
- MI
Myocardial infarction
- NCDs
non-communicable diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mortality GBD, Causes of Death C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). Global status report on noncommunicable diseases 2018. https://wwwwhoint/nmh/publications/ncd-profiles-2018/en/, 2018.
- 3.WHO (World Health Organization). Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva, WHO. 2018. [Google Scholar]
- 4.American College of Obstetricians and Gynecologists, Pregnancy TFoHi. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 5.Mammaro A, Carrara S, Cavaliere A et al. Hypertensive disorders of pregnancy. J Prenat Med 2009;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MA, Magee LA, Kenny LC et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018;13:291–310. [DOI] [PubMed] [Google Scholar]
- 7.Skjaerven R, Wilcox AJ, Klungsoyr K et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ 2012;345:e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol 2010;24:323–30. [DOI] [PubMed] [Google Scholar]
- 9.Funai EF, Friedlander Y, Paltiel O et al. Long-term mortality after preeclampsia. Epidemiology 2005;16:206–15. [DOI] [PubMed] [Google Scholar]
- 10.Theilen LH, Fraser A, Hollingshaus MS et al. All-Cause and Cause-Specific Mortality After Hypertensive Disease of Pregnancy. Obstet Gynecol 2016;128:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 2001;323:1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 2015;132:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010;56:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol 2018;219:107 e1–107 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–51. [DOI] [PubMed] [Google Scholar]
- 16.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 2001;357:2002–6. [DOI] [PubMed] [Google Scholar]
- 17.Stuart JJ, Tanz LJ, Missmer SA et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med 2018;169:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart JJ, Tanz LJ, Cook NR et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 21.Wang YX, Arvizu M, Rich-Edwards JW et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ 2020;371:m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C, Spiegelman D, Rimm EB et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol 2018;187:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YX, Shan ZL, Arvizu M et al. Associations of Menstrual Cycle Characteristics Across the Reproductive Life Span and Lifestyle Factors with Risk of Type 2 Diabetes. JAMA Netw Open 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin D, Fleming TR. Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis: Survival Analysis: Springer Science & Business Media, 2012. [Google Scholar]
- 25.Grandi SM, Filion KB, Yoon S et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]
- 26.Lin YS, Tang CH, Yang CY et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol 2011;107:325–30. [DOI] [PubMed] [Google Scholar]
- 27.Wilson BJ, Watson MS, Prescott GJ et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 2003;326:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forouzanfar MH, Liu P, Roth GA et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 29.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG 2018;125:1642–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol 2014;124:771–81. [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Ventura V, Li Y, Pasupuleti V, Roman YM, Hernandez AV, Perez-Lopez FR. Effects of preeclampsia and eclampsia on maternal metabolic and biochemical outcomes in later life: a systematic review and meta-analysis. Metabolism 2020;102:154012. [DOI] [PubMed] [Google Scholar]
- 32.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res 2017;40:213–220. [DOI] [PubMed] [Google Scholar]
- 34.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation 2010;122:579–84. [DOI] [PubMed] [Google Scholar]
- 35.Levine RJ, Maynard SE, Qian C et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83. [DOI] [PubMed] [Google Scholar]
- 36.Maynard SE, Moore Simas TA, Solitro MJ et al. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am J Obstet Gynecol 2008;198:200 e1–7. [DOI] [PubMed] [Google Scholar]
- 37.Julian CG, Pedersen BS, Salmon CS et al. Unique DNA Methylation Patterns in Offspring of Hypertensive Pregnancy. Clin Transl Sci 2015;8:740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mowry FE, Biancardi VC. Neuroinflammation in hypertension: the renin-angiotensin system versus pro-resolution pathways. Pharmacol Res 2019;144:279–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


