Abstract
Human genetic association and brain expression studies, and mouse behavioral and molecular studies implicate a role for the histidine triad nucleotide-binding protein 1 (HINT1) in schizophrenia, bipolar disorder, depression and anxiety. The high comorbidity between smoking and psychiatric disorders, schizophrenia in particular, is well established. Associations with schizophrenia and HINT1 are also sex specific, with effects more predominant in males; however, it is unknown if sex differences associated with the gene extend to other phenotypes. Thus, in this study, using a battery of behavioral tests, we elucidated the role of HINT1 in acute nicotine-mediated behaviors using male and female HINT1 wild-type (+/+) and knockout (−/−) mice. The results show that male HINT1 −/− mice were less sensitive to acute nicotine-induced antinociception in the tail-flick, but not hot-plate test. At low nicotine doses, male and female HINT1 −/− mice were less sensitive to nicotine-induced hypomotility, although the effect was more pronounced in females. Baseline differences in locomotor activity observed in male HINT1 +/+ and −/− mice were absent in females. Nicotine did not produce an anxiolytic effect in male HINT1 −/− mice, but rather an anxiogenic response. Diazepam also failed to induce an anxiolytic response in these mice, suggesting a general anxiety phenotype not specific to nicotine. Differences in anxiety-like behavior were not observed in female mice. These results further support a role for HINT1 in nicotine-mediated behaviors and suggest that alterations in the gene may have differential effects on phenotype in males and females.
Keywords: Acute nicotine responses, anxiety, HINT1, nicotine, protein kinase C interacting protein (PKCI), sex differences
The histidine triad nucleotide-binding protein 1 (HINT1), previously known as the protein kinase C interacting protein, is widely expressed in liver, kidney and brain, including mesocortical and mesostriatal regions (Liu et al. 2008), yet little is known about its physiological function. Human genetic association and brain expression studies implicate involvement of the HINT1 gene in risk for schizophrenia (Chen et al. 2008; Kurotaki et al. 2011; Varadarajulu et al. 2012; Vawter et al. 2001, 2002, 2004) and bipolar disorder (Elashoff et al. 2007; Konradi 2005). Behavioral studies using HINT1 knockout (−/−) mice also suggest a role for HINT1 in antidepressant and anxiety-like behaviors (Barbier & Wang 2009; Varadarajulu et al. 2011). Taken together, the aforementioned studies support a possible role for the HINT1 gene in mood regulation, anxiety-like behavior and stress-coping mechanisms.
Recently, we found that genetic variation in the HINT1 gene is protective against nicotine dependence, and HINT1 protein levels are altered in the nucleus accumbens after chronic nicotine exposure, suggesting a role for HINT1 in nicotine-mediated responses (Jackson et al. 2011). Indeed, the high comorbidity between smoking and psychiatric disorders, schizophrenia in particular, is well known, leading us to propose that HINT1, in addition to mood regulation, may also in part regulate nicotine responses. Additionally, associations with schizophrenia and HINT1 are sex specific, where effects are more prevalent in males than females (Chen et al. 2008; Vawter et al. 2004). It is unclear if the sex-specific effects associated with this gene are specific to schizophrenia, or if they are also prevalent in nicotine-mediated behaviors. Thus, we provide the first known study to elucidate the role of HINT1 in acute nicotine-mediated behaviors in male and female mice. Antinociception, hypothermia, locomotor activity and anxiety-like behavior were measured in different groups of male and female HINT1 wild-type (+/+) and −/− mice after treatment with acute injections of various nicotine doses. In this study, we focus on behaviors after a single injection of nicotine, as acute nicotine responses are important measures of initial nicotinic acetylcholine receptor (nAChR) responses, in particular β2*-containing nAChRs (where * denotes the possible inclusion of additional nAChR subunits), which play an important role in nicotine dependence (Jackson et al. 2008, 2009; Picciotto et al. 1998; Walters et al. 2006). Thus, acute responses may be related to vulnerability for future repeated drug use, abuse or dependence (Collins et al. 1988).
Materials and methods
Animals
Male and female HINT1 −/− and +/+ littermates were generated as previously described (Su et al. 2003). In brief, embryonic stem cells heterozygous for the targeted mutation were microinjected into C57BL/6 blastocysts and implanted into pseudopregnant females. The resulting male chimeras were mated with female C57BL/6 mice. The F1 generation mice resulting from this pairing were backcrossed to the 129X1/Sv mouse strain for over six generations, so that over 96% of their background is from the 129X1/Sv strain. Breeding pairs were supplied by Dr Jia Bei Wang from the University of Maryland (Baltimore, MD, USA). Mutant and +/+ controls were obtained from breeding heterozygote mice. Mice were bred for one to two additional generations before use in our studies. Animals were 10–14 weeks of age at the start of the experiments and were group-housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with ad libitum access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Drugs
(−)-Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO, USA). Diazepam was obtained from the National Institute on Drug Abuse (Bethesda, MD, USA). Nicotine was dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously (s.c.) at a volume of 10 ml/kg body weight unless noted otherwise. Diazepam was dissolved in 1:1:18 vehicle [5% ethanol, 5% emulphor-620 (Sanofi-Aventis, Bridgewater, NJ, USA) and 90% distilled water] and was injected via the intraperitoneal (i.p.) route of administration. All doses are expressed as the free base of the drug.
Acute nicotine studies
Independent groups of naïve male and female HINT1 +/+ and −/− mice (n= 6–12 per group) were injected with various doses of nicotine and tested at different time points after injection. Various responses to nicotine were measured: Antinociception using the tail-flick and hot-plate tests, changes in body temperature, changes in locomotor activity and plus maze performance. Independent groups of mice were used for each test and for each nicotine dose.
Antinociception
Antinociception was assessed by the tail-flick method of D’Amour and Smith (1941) and the hot-plate test. For the tail-flick test, mice were lightly restrained while a radiant heat source was directed onto the upper portion of the tail. A control response (2–4 seconds) was determined for each mouse before treatment, and test latency was determined 5 min after nicotine administration. The apparatus has an automatic cutoff of 10 seconds to minimize tissue damage. In the hot-plate test, mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH, USA). The hot plate is a rectangular heated surface surrounded by Plexiglass and maintained at 55°C. The device is connected to a manually operated timer that records the amount of time the mouse spends on the heated surface before showing signs of nociception (e.g. jumping and paw licking). A control response (8–12 seconds) was determined for each mouse before treatment, and test latency was determined 5 min after nicotine administration. The timer has an automatic cutoff of 40 seconds to avoid tissue damage. Antinociceptive response for both the tests was calculated as percentage of maximum possible effect (%MPE), where %MPE = [(test − control)/(10 (40 for the hot-plate) − control)] × 100. Increased latency in either test is indicative of antinociception.
Body temperature
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI, Yellow Springs, OH, USA). Readings were taken just before and at 30 min after nicotine injection. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21 to 24°C from day to day.
Locomotor activity
Mice were placed into individual photocell activity cages (28 × 16.5 cm; Omnitech, Columbus, OH, USA) 5 min after saline or nicotine administration. Interruptions of the photocell beams (two banks of eight cells each) were then recorded for the next 30 min.
Data were measured as the average number of photocell interruptions during the 30-min test period.
Elevated plus maze
An elevated plus maze, prepared with gray Plexiglas, consisted of two open arms (23 × 6 cm) and two enclosed arms (23 × 6 × 15 cm in wall height) that extended from a central platform (5.5 × 5.5 cm). It was mounted on a base raised 60 cm above the floor. Fluorescent lights (350 lux intensity) located in the ceiling of the room provided the only source of light to the apparatus. The animals were placed in the center of the maze 5 min after saline or nicotine injection. For the diazepam study, baseline plus maze activity was measured, followed by a 30-min diazepam (0.25 mg/kg, i.p.) pretreatment. Time spent in the open arms was automatically recorded by a photocell beam system. The test lasted 5 min, and the apparatus was thoroughly cleaned with a 5% bleach cleaning solution after removal of each animal. The results were expressed as time spent in the open arms in seconds. Less time spent in the open arms of the plus maze was indicative of anxiety-like behavior. Stretched attend posture and the number of head dips were measured as risk assessment and exploratory behaviors. The number of arm crosses was also documented as a measure of locomotor activity.
Statistical analysis
Statistical analyses of acute nicotine behavioral studies were performed using three-way analysis of variance (ANOVA) with treatment, sex and genotype as the between-subject factors. ED50 and ED6° values with 95% confidence limits (CLs) were also calculated for the antinociception and body temperature studies, respectively, by unweighted least-squares linear regression as described by Tallarida and Murray (1987). If CL values did not overlap, the shift in the dose-response curve was considered significant. For the diazepam anxiety study, repeated-measures ANOVA with between-subjects factors was used to analyze within-subject differences (baseline vs. diazepam) and between-subject differences (genotype). P values <0.05 were considered to be statistically significant. Significant results were further analyzed using the Neuman-Keuls post hoc test.
Results
Nicotine-induced antinociception in acute thermal pain models
There was a significant main effect of dose for both the tail-flick (F(2,110) = 118.26, P < 0.001) and hot-plate (F(2,110) = 126.73, P < 0.001) tests, indicating that nicotine induced dose-dependent antinociception in both sexes and genotypes in both paradigms (Fig. 1). Significant genotype main effects showed that male HINT −/− mice were less sensitive to the acute antinociceptive effects of nicotine in the tail-flick test (F(1,110) = 27.05, P < 0.001) compared with +/+ littermates, as also indicated by a significant shift in the dose–response curve ([+/+] 0.95 (0.78–1.17) vs. [−/−] 1.8 (1.25–2.61); Table 1). Male HINT1 −/− mice exhibited a decreased latency in the tail-flick test after administration of all nicotine doses compared with HINT1 +/+ mice (Fig. 1a). There was no significant difference in nicotine-induced tail-flick antinociception between HINT1 +/+ and −/− females (Fig. 1b). Baseline antinociception did not differ between genotypes for any dose (average baseline: [+/+] 2.0 ± 0.1; [−/−] 2.2 ± 0.1). Overall, there were no significant main effects of sex. In contrast to the tail-flick results, in the hot-plate test, there were no significant main effects of genotype in either males (Fig. 1c) or females (Fig. 1d), indicating no significant difference between HINT1 +/+ and −/− mice in this paradigm. Nicotine potency ED50 values for both tests are presented in Table 1.
Figure 1: HINT1 mediates acute spinal antinociceptive effects of nicotine.
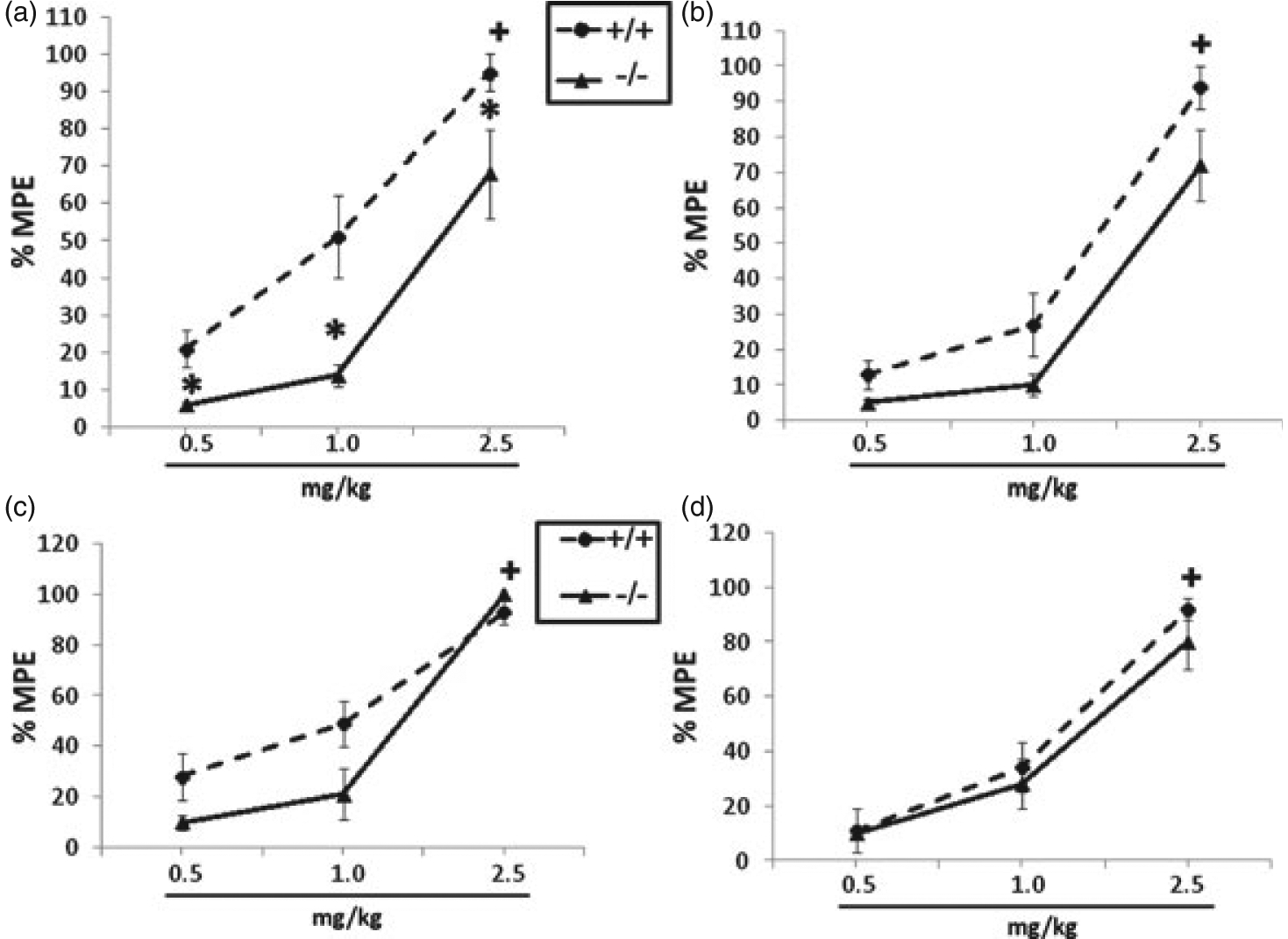
Nicotine-induced antinociception was observed in both sexes and genotypes in both tests. (a) Male HINT1−/− mice are less sensitive to nicotine-induced antinociception than their +/+ counterparts in the tail-flick test, but (c) not in the hot-plate test. (b) Female HINT1 +/+ and −/− mice do not significantly differ in their antinociceptive response to nicotine in the tail-flick or (d) hot-plate test. Each point represents the mean ± SEM for 10–12 mice per group. * denotes P < 0.05 vs. corresponding +/+ mouse. ‘+ ’ denotes P < 0.05 vs. lowest nicotine dose for corresponding genotype. The x-axis represents nicotine doses administered and the y-axis represents %MPE.
Table 1:
Summary of the potency of nicotine’s effects in the tail-flick, hot-plate and body temperature tests after acute nicotine administration (0.25, 0.5, 1 and 2.5 mg/kg, s.c.) in male and female HINT1 +/+ and −/− mice
| Test | Male HINT1 +/+ | Male HINT1 −/− | Female HINT1 +/+ | Female HINT1 −/− |
|---|---|---|---|---|
| Tail flick | 0.95 (0.78–1.17) | 1.8 (1.25–2.61)* | 1.2 (1.03–1.41) | 1.9 (1.32–2.66) |
| Hot plate (55°C) | 0.91 (0.71–1.18) | 1.2 (1.07–1.45) | 1.2 (1.03–1.36) | 1.5 (1.08–2.04) |
| Body temperature | 1.4 (1.11–1.72) | 1.6 (1.33–1.92) | 1.3 (1.08–1.29) | 1.5 (1.28–1.70) |
Potency is expressed as ED50 ± CLs (mg/kg) or ED6° ± CLs (mg/kg) for body temperature. Each group contained 10–12 mice.
Denotes significance vs. HINT1 +/+ and −/− mice of the same sex.
Nicotine-induced hypothermia
There was a main effect of dose in the body temperature assessment (F(2,110) = 162.88, P < 0.001). Acute nicotine dose dependently reduced body temperature in both male and female mice; however, there was no significant difference between HINT1 +/+ and −/− mice for either sex, suggesting that HINT1 is not involved in acute nicotine-induced hypothermia (Fig. 2). ED6° values are presented in Table 1.
Figure 2: HINT1 does not mediate acute nicotine-induced hypothermia.
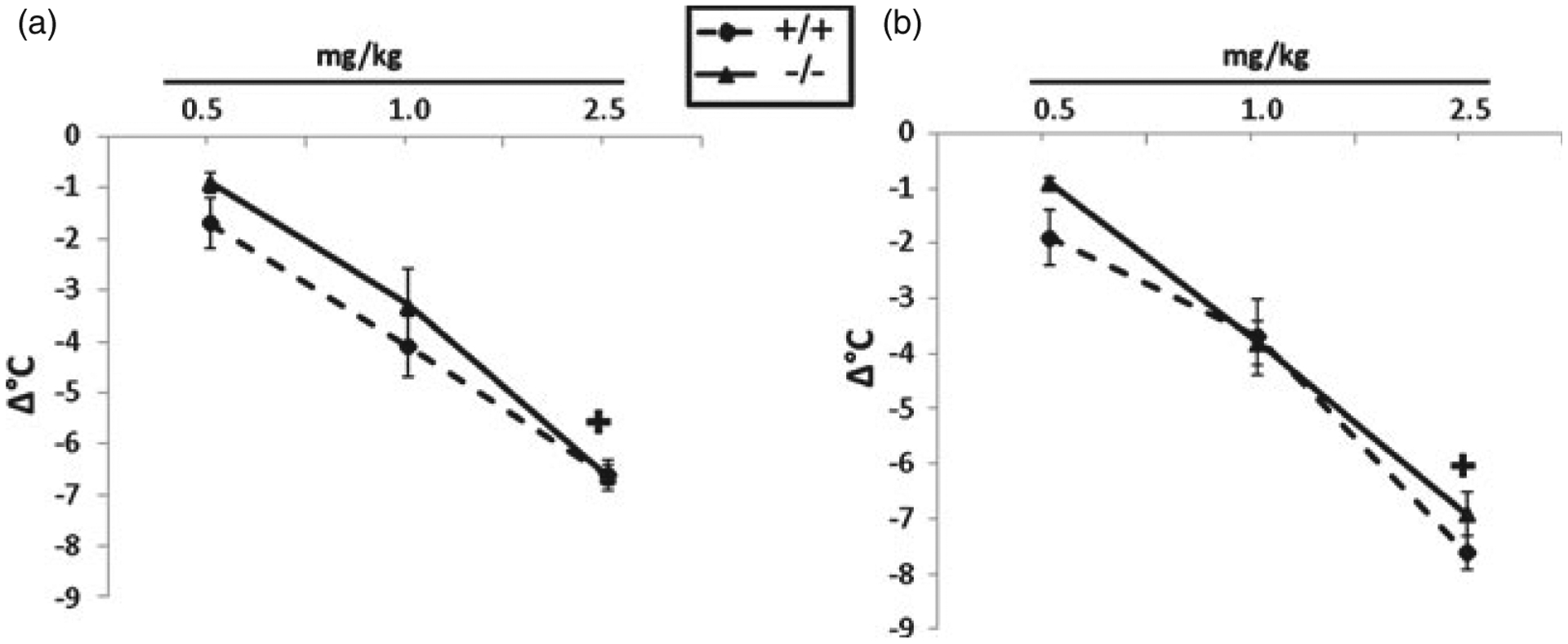
Nicotine induced a significant decrease in body temperature in both sexes and genotypes. (a) Male and (b) female HINT1 +/+ and −/− mice do not differ in their responses to nicotine-induced hypothermia. Each point represents the mean ± SEM for 10–12 mice per group. ‘+ ’ denotes P < 0.05 vs. lowest nicotine dose for corresponding genotype. The x-axis represents nicotine doses administered and the y-axis represents the change in body temperature (Δ°C).
Nicotine-induced hypomotility
The results of the acute nicotine locomotor activity assessment are shown in Fig. 3. There were significant main effects of dose (F(4,144) = 22.61, P < 0.0001), genotype (F(1,144) = 6.97, P < 0.05) and sex (F(1,144) = 31.51, P < 0.0001), and significant dose × sex (F(4,144) = 14.68, P < 0.0001), dose × genotype (F(4,144) = 3.90, P < 0.05), sex × genotype (F(1,144) = 4.44, P < 0.05) and dose × sex × genotype interactions (F(4,144) = 4.08, P < 0.05). In the male locomotor assessment, saline activity scores were significantly higher in HINT1 −/− compared with +/+ mice ([+/+] 652.74 ± 40.1 vs. [−/−] 826.77 ± 25.4), suggesting baseline differences between genotypes in males; thus, data were expressed as a percentage of saline baseline to account for these differences. There was no significant difference in activity between female saline HINT1 +/+ and −/− mice ([+/+] 752.67 ± 43.7 vs. [−/−] 738.12 ± 38.5); though for consistency, these data were also expressed as a percentage of saline baseline. Nicotine significantly decreased locomotor activity in both male and female HINT1 +/+ and −/− mice (Fig. 3). HINT −/− mice were less sensitive to the locomotor depressant effects of nicotine, although this effect was only observed at the 0.1 mg/kg dose (Fig. 3a). Similarly, female HINT1 −/− mice were less sensitive to the locomotor depressive effects of nicotine at doses of 0.1 and 0.25 mg/kg (Fig. 3b). Male HINT1 +/+ and −/− mice exhibited significantly lower locomotor activity counts than their respective female counterparts, though only at the 0.1 mg/kg nicotine dose (Table 2).
Figure 3: HINT1 mediates nicotine-induced hypomotility at low nicotine doses.
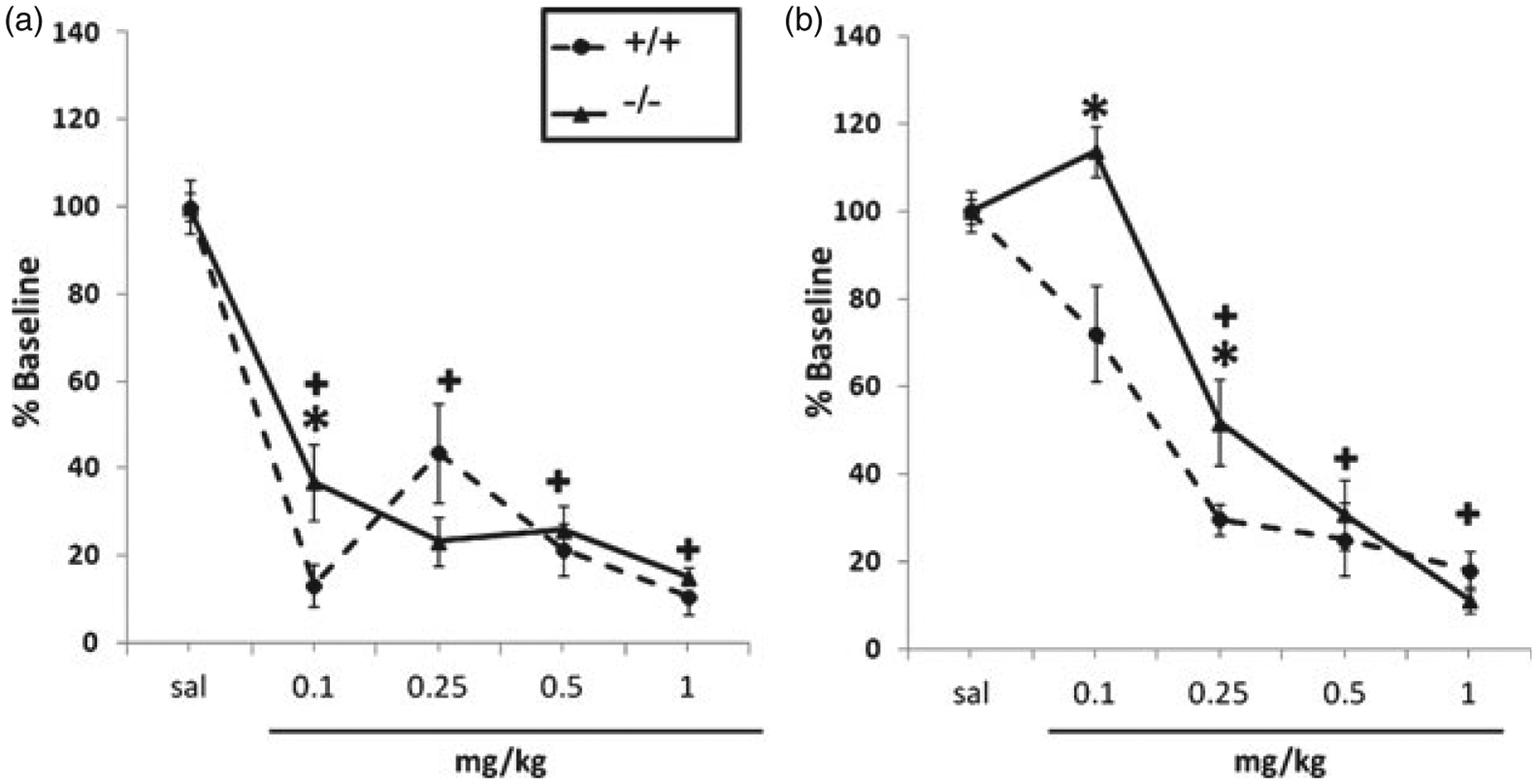
(a) Male HINT1 −/− mice are less sensitive to the locomotor depressant effects of nicotine compared with male HINT1 +/+ mice at the dose of 0.1 mg/kg. HINT1 −/− mice do not differ from +/+ counterparts at doses higher than 0.1 mg/kg. (b) Female HINT1 −/− mice are significantly less sensitive to the locomotor depressant effects of nicotine at 0.1 and 0.25 m/kg, but not at higher nicotine doses. Each point represents the mean ± SEM for 8–10 mice per group. * denotes P < 0.05 vs. corresponding +/+ group. ‘+ ’ denotes P < 0.05 vs. corresponding saline group for both genotypes. The x-axis represents nicotine doses administered and the y-axis represents the percentage of saline baseline.
Table 2:
HINT1 +/+ and −/− male and female locomotor activity counts at 0.1 mg/kg nicotine
| Groups | Locomotor activity counts ± SEM |
|---|---|
| HINT1 +/+ male | 86 ± 43 |
| HINT1 −/− male | 306 ± 28* |
| HINT1 +/+ female | 473 ± 43** |
| HINT1 −/− female | 849 ± 36** |
Denotes P < 0.05 vs. HINT1 +/+ male mice;
Denotes P < 0.05 vs. male counterparts.
Nicotine-induced anxiety-like behavior in the plus maze test
The results of the plus maze assessment are presented in Fig. 4 and Table 3. F and P values reported within the text correspond to the time spent on the open arms of the plus maze, which was our primary measure of anxiety-like behavior. There were significant main effects of treatment (F(3,79) = 6.29, P < 0.05) and genotype (F(3,79) = 7.89, P < 0.05); however, no significant main effects of sex or significant interactions were observed. At the doses tested in our model, nicotine induced a dose-dependent anxiolytic-like effect in male HINT1 +/+ mice, with significant effects at a dose of 0.05 mg/kg (Fig. 4a). Specifically, male HINT1 +/+ mice treated with nicotine (0.05 mg/kg, s.c.) spent significantly more time on the open arms of the plus maze and exhibited significantly more head dips at 0.05 and 0.1 mg/kg nicotine compared with saline-treated counterparts (Table 3). Alternatively, male HINT1 −/− mice did not exhibit an anxiolytic-like response at any nicotine dose tested and spent significantly less time on the open arms of the plus maze than male HINT1 +/+ mice at all nicotine doses tested (Fig. 4a). Head dips were also significantly less in male HINT1 −/− mice compared with +/+ counterparts at the 0.05 and 0.1 mg/kg nicotine doses (Table 3). Interestingly, ANOVA analysis on each sex separately showed that nicotine significantly impacted the anxiety-like response in HINT1 −/− male mice at the highest dose tested (0.1 mg/kg), where nicotine induced a significant anxiogenic-like response when compared with saline HINT1 −/− mice (F(3,40) = 9.68, P < 0.01), indicated by less time in the open arms and a decrease in the number of head dips (Fig. 4a; Table 3). In the female assessment, there were no main effects of genotype, but a significant main effect of treatment (F(3,40) = 6.01, P < 0.05). Nicotine did not produce anxiolytic effects at any dose tested in female HINT1 +/+ or −/− mice; however, a significant anxiogenic effect was observed in both genotypes at 0.1 mg/kg nicotine (Fig. 4b; Table 3). There was no significant difference between female HINT1 genotypes at any nicotine dose tested. There was no significant difference in baseline plus maze behavior or the number of arm crosses between genotypes or nicotine doses for either sex (male arm crosses: [+/+] 4.2 ± 0.7 vs. [−/−] 3.8 ± 0.7; female arm crosses: [+/+] 4.3 ± 0.3 vs. [−/−] 3.6 ± 0.4). Stretched attend posture counts did not significantly differ between either sex, dose or genotype in this assessment (Table 3).
Figure 4: HINT1 is involved in the nicotine-induced anxiety response in the plus maze test.
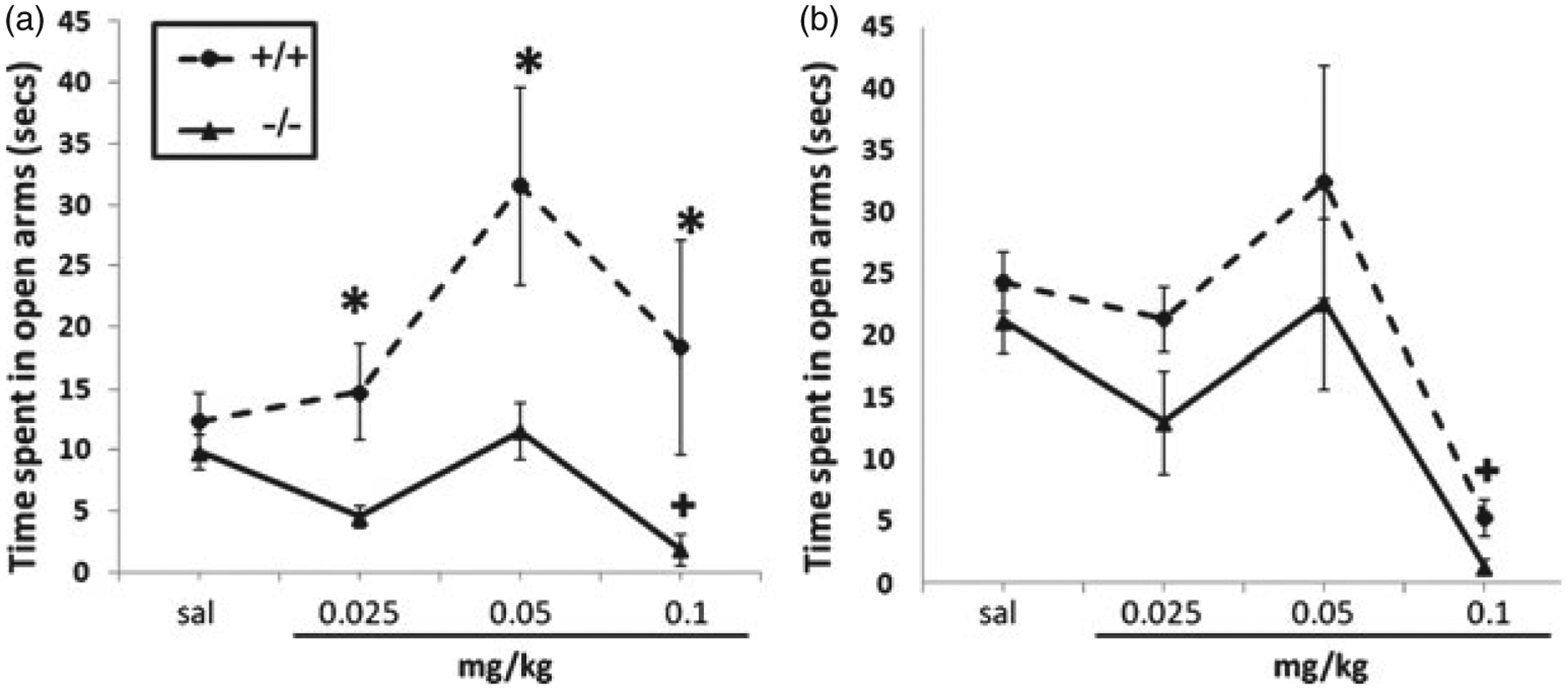
(a) Male HINT1 −/− mice did not exhibit a dose-dependent nicotine-induced anxiolytic response as observed in HINT1 +/+ mice; however, there was a significant anxiogenic response at 0.1 mg/kg compared with saline-treated HINT1 −/− mice (denoted by ‘+’ in a). (b) Female HINT1 +/+ and −/− mice do not differ in their response to nicotine in the plus maze. Each point represents the mean ± SEM for six mice per group. * denotes P < 0.05 compared with corresponding +/+ mice. In b, ‘+’ denotes P < 0.05 compared with saline, 0.025 and 0.05 mg/kg nicotine for both genotypes. The x-axis represents nicotine doses administered and the y-axis represents the average time spent in the open arms of the plus maze in seconds.
Table 3:
Head dip (HD) and stretched attend posture (SAP) counts in the elevated plus maze
| Group | Male HINT1 +/+ | Male HINT1 −/− | Female HINT +/+ | Female HINT1 −/− |
|---|---|---|---|---|
| Saline | HD: 2.8 ± 0.7 | HD: 4.0 ± 1.2 | HD: 4.1 ± 1.4 | HD: 2.4 ± 0.6 |
| SAP: 0.3 ± 0.2 | SAP: 0.5 ± 0.3 | SAP: 0.7 ± 0.3 | SAP: 0.5 ± 0.3 | |
| 0.025 mg/kg, nic | HD: 3.8 ± 0.8 | HD: 3.2 ± 1.4 | HD: 4.0 ± 0.7 | HD: 4.3 ± 1.3 |
| SAP: 0.3 ± 0.2 | SAP: 0.5 ± 0.2 | SAP: 0.3 ± 0.2 | SAP: 0.5 ± 0.3 | |
| 0.05 mg/kg, nic | HD: 8.3 ± 1.4*,** | HD: 1.8 ± 0.7 | HD: 5.3 ±2.0 | HD: 3.8 ± 1.2 |
| SAP: 0.2 ± 0.2 | SAP: 0.2 ± 0.2 | SAP: 0.3 ± 0.2 | SAP: 0.2 ± 0.2 | |
| 0.1 mg/kg, nic | HD: 7.1 ± 1.9*,** | HD: 0.4 ± 0.2 | HD: 0.8 ± 0.4*** | HD: 0.5 ± 0.3*** |
| SAP: 0.3 ± 0.2 | SAP: 0.2 ± 0.2 | SAP: 0.5 ± 0.2 | SAP: 0.3 ± 0.2 |
Each point represents the mean ± SEM of six mice per group. nic, nicotine.
Denotes P < 0.05 vs. corresponding saline group;
Denotes P < 0.05 vs. corresponding HINT1 −/− group;
Denotes P < 0.05 vs. all other groups.
Specificity of anxiety-related effect using diazepam
Male HINT1 +/+ and −/− mice, but not females, exhibited differential responses to nicotine in the plus maze assessment. Thus, to determine if the absence of an anxiolytic response in male HINT1 −/− mice was specific to nicotine, male HINT1 +/+ and −/− mice were treated with diazepam (0.25 mg/kg, i.p.) and tested in the plus maze 30 min after injection. The results are presented in Table 4. Statistical analysis showed significant main between-subject effects of genotype (F(1,14) = 5.85, P < 0.05), indicating significant differences between HINT1 +/+ and −/− mice in this assessment. While the number of head dips was significantly higher in HINT1 +/+ after diazepam treatment, main effects of treatment for time spent on the open arms, though marginal, were not significant (F(1,14) = 3.38, P = 0.08), suggesting no difference between baseline and diazepam treatment; however, a significant genotype × treatment interaction (F(1,14) = 4.67, P < 0.05) showed the true effects in this test. HINT1 +/+ and −/− mice did not differ in baseline responses in the plus maze; however, significant genotype differences were observed after diazepam treatment, as HINT1 +/+ mice spent significantly more time in the open arms than HINT1 −/− mice, indicative of an anxiolytic-like response in HINT1 +/+ mice that was not observed in −/− counterparts. Stretched attend posture did not significantly differ between groups.
Table 4:
The nicotine-induced anxiolytic effect in male HINT1 −/− mice is not specific to nicotine
| Groups | Time in open arms ± SEM (seconds) |
|---|---|
| Baseline- HINT1 +/+ | PM: 13.7 ± 2.6 |
| HD: 4.5 ± 0.9 | |
| SAP: 3.8 ± 1.0 | |
| Baseline- HINT1 −/− | PM: 10.8 ± 3.5 |
| HD: 4.2 ± 0.9 | |
| SAP: 2.5 ± 0.4 | |
| Diazepam-HINT1 +/+ | PM: 32.0 ± 9.0* |
| HD: 7.3 ± 0.8* | |
| SAP: 2.3 ± 0.3 | |
| Diazepam-HINT1 −/− | PM: 7.6 ± 3.6 |
| HD: 3.4 ± 0.5 | |
| SAP: 1.3 ± 0.3 |
Diazepam (0.25 mg/kg, i.p.) induced a significant anxiolytic response in HINT1 +/+ mice in the plus maze test, but failed to induce the response in HINT1 −/− mice. These results suggest a general anxiety-like phenotype in HINT1 −/− mice. Each point represents the mean ± SEM for seven to nine mice per group. HD, head dip; PM, plus maze time spent in open arms; SAP, stretched attend posture.
Denotes P < 0.05 vs. all other groups.
Discussion
In this study, using genetically modified HINT1 male and female mice, we found that HINT1 −/− mice exhibit differential responses compared with their +/+ counterparts after acute nicotine treatment. These differences appear to be sex specific and dose dependent. With the exception of the locomotor activity assessment, altered nicotine responses were more predominant in males than females. To our knowledge, this is the first study to assess nicotine responses in HINT1 mice and the first to explore sex differences in these mice.
Male HINT1 −/− mice were less sensitive to nicotine-induced antinociception in the tail-flick test, but not the hot-plate test. The hot-plate test predominantly reflects a supraspinal antinociceptive response (Dennis et al. 1980; Ramabadran & Bansinath 1986), whereas the tail-flick test predominantly measures a spinal antinociceptive response (Dennis et al. 1980; King et al. 1997). Thus, it is likely our results suggest a differential role for the gene in spinal vs. supraspinal nicotine-induced pain mechanisms. Although we observed no genotype differences in nicotine-induced hot-plate antinociception, male HINT1 −/− mice show enhanced antinociception in response to acute morphine in the hot-plate test (Guang et al. 2004). This effect is also the opposite of that observed for nicotine in the tail-flick test. HINT1 binds to the C-terminus of the mu-opioid receptor and negatively regulates phosphorylation and desensitization of the receptor, which may result in altered responses to morphine in HINT −/− mice (Guang et al. 2004). To date, it is unknown whether HINT1 interacts with nAChRs involved in acute nicotine antinociceptive effects, such as α4β2 and α5 (Jackson et al. 2010; Marubio et al. 1999). While these studies suggest that HINT1 is involved in pain mechanisms, the response may differ depending on the given drug. Although female HINT1 −/− and +/+ mice showed nicotine-mediated dose-dependent increases in hot-plate latency, indicative of antinociception, there were no significant differences between genotypes.
Male and female HINT1 −/− mice were less sensitive to the nicotine-induced locomotor hypomotility at low nicotine doses, although locomotor activity counts were significantly higher in HINT1 −/− females compared with male counterparts after nicotine treatment, indicating that female HINT1 −/− mice are even less sensitive to the locomotor hypomotility effects of nicotine than male counterparts. While there was no significant difference in activity between saline-treated HINT1 −/− and +/+ females, saline-treated male HINT1 −/− had significantly higher activity than male HINT1 +/+ mice, suggesting baseline activity differences. Interestingly, the opposite was found in a previous study, where decreased basal locomotor activity was observed in HINT1 −/− mice relative to HINT1 +/+ mice (Barbier et al. 2007). There are, however, several differences that may account for this discrepancy. Our animals were tested 5 min after a s.c. saline injection; thus, it is possible that differences related to the stress of the injection may have occurred in this paradigm. We also tested mice for 30 min total, whereas in the Barbier et al. (2007) study, mice were exposed to the chamber for 90–120 min. Hypolocomotor effects may be observed in these mice with longer locomotor activity sessions. Further, we did not acclimate the mice to a novel environment in our study, which may have differential effects on the outcome. Compared with +/+ counterparts, HINT1 −/− mice are more sensitive to hyperlocomotor effects of amphetamine and the dopamine receptor agonist apomorphine, suggesting that absence of HINT1 may be associated with dysregulation of postsynaptic dopamine transmission (Barbier et al. 2007). It is possible that such alterations in dopamine transmission play a role in various drug responses and are involved in basal locomotor activity differences in male mice, but not in female mice. Altered dopaminergic transmission has been implicated in the pathophysiology of schizophrenia (Lewis & Lieberman 2000; Seeman 1987). Sex-specific effects were observed with HINT1 in schizophrenia patients, where more prominent effects were observed in males compared with females (Chen et al. 2008; Vawter et al. 2004). Thus, it is of interest to determine if HINT1 dopamine alterations are more prevalent in males, and if these alterations contribute to specific behavioral phenotypes related to drug abuse or other psychiatric disorders.
In the plus maze assessment, male HINT1 −/− mice did not exhibit anxiolytic-like behavior after acute nicotine, as observed in their +/+ counterparts, suggesting that HINT1 −/− mice are less sensitive to the anxiolytic effects of nicotine. This lack of anxiolytic response was not specific to nicotine, however, as these mice also showed no anxiolytic-like response to diazepam. Alternatively, an anxiogenic-like response was observed in HINT1 −/− mice at the highest nicotine dose tested in this paradigm. Nicotine has been reported to produce both anxiolytic and anxiogenic effects in the plus maze, depending on the dose (Biala & Budzynska 2006; O’Neill & Brioni 1994; Varani & Balerio 2012); thus, our results may suggest that HINT1 −/− are more sensitive to the anxiogenic effects of nicotine. Previous studies report that male HINT1 −/− mice are more anxious under basal conditions than HINT1 +/+ mice (Varadarajulu et al. 2011). Because both nicotine and the known anxiolytic medication diazepam failed to reverse the anxiety-like behavior in male HINT1 −/− mice, it appears that altered HINT1 expression is associated with a general anxiety-like phenotype in the mouse. Results from this study are in contrast to results of the Barbier and Wang (2009) study, where HINT1 −/− mice were less anxious than +/+ counterparts. Mice in this study were 10–14 weeks of age, similar to the age range used in the Varadarajulu et al. (2011) study, whereas mice were significantly older (6–9 months) during testing in the Barbier and Wang (2009) study, suggesting the possibility of age-related effects associated with the gene. Indeed, as observed in older primates, HINT1 is downregulated in the temporal cortex during brain aging (Abdel Rassoul et al. 2010). Older (2–3 years of age) HINT1 −/− mice are also more likely to develop spontaneous tumors than +/+ counterparts (Li et al. 2006). For these studies, it is possible that downstream mechanisms, with age, are significantly altered in HINT1 −/− mice, resulting in significant differences in behavior. With the use of genetically modified animals, we also cannot rule out the possibility of effects related to compensatory mechanisms in subsequent generations of HINT1 −/− mice. Interestingly, the anxiogenic-like response observed in HINT1 −/− mice was also observed in female HINT1 −/− and +/+ mice. It would be of interest in future studies to assess the role of HINT1 in anxiety phenotypes through a genetic association study in humans to determine if such sex differences with HINT1 extend to anxiety disorders, and if altered HINT1 expression in males results in greater resistance to anxiolytic medications.
In this study, HINT1 −/− mice are, in general, less sensitive to acute nicotine-mediated behaviors, and these effects were predominantly observed in male mice, suggesting that alteration of the HINT1 gene has a greater effect on acute nicotine-mediated responses in males than females. We also propose that the gene may have a larger role in anxiety-like phenotypes in males compared with females. Because sex differences in HINT1 genetic variation and brain expression were also observed in schizophrenia, phenotypes associated with HINT1 polymorphisms may differ depending on the sex of the individual. Further clarification of the role of HINT1 in nicotine-mediated behaviors and psychiatric disorders will provide additional understanding of mechanisms contributing to smoking and comorbid disorders.
Acknowledgments
The authors would like to thank Tie Shan-Han and Cindy Evans for their technical assistance in the studies. This work was supported by National Institute of Mental Health grant MH020030 to K.J.J., DA019498 to X.C. and DA018722 to J.B.W., and National Institute on Drug Abuse grant NIDA-05274 to M.I.D. There are no conflicts of interest to disclose for any author involved in this study.
References
- Abdel Rassoul R, Alves S, Pantesco V, De Vos J, Michel B, Perret M, Mestre-Francés N, Verdier JM & Devau G (2010) Distinct transcriptome expression of the temporal cortex of the primate microcebus murinus during brain aging versus Alzheimer’s disease-like pathology. PLoS One 5, pii: e12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E & Wang JB (2009) Anti-depressant and anxiolytic like behaviors in PKCI/HINT1 knockout mice associated with elevated plasma corticosterone level. BMC Neurosci 10, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Zapata A, Oh E, Liu Q, Zhu F, Undie A, Shippenberg T & Wang JB (2007) Supersensitivity to amphetamine in protein kinase-C interacting protein/HINT1 knockout mice. Neuropsychopharmacology 32, 1774–1782. [DOI] [PubMed] [Google Scholar]
- Biala G & Budzynska B (2006) Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels. Life Sci 79, 81–88. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang X, O’Neill FA, Walsh D, Kendler KS & Chen X (2008) Is the histidine triad nucleotide-binding protein (HINT1) gene a candidate for schizophrenia? Schizophr Res 106, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Miner LL & Marks MJ (1988) Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol Biochem Behav 30, 269–278. [DOI] [PubMed] [Google Scholar]
- D’Amour FE & Smith DL (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72, 74–79. [Google Scholar]
- Dennis SG, Melzack R, Gutman S & Boucher F (1980) Pain modulation by adrenergic agents and morphine as measured by three pain tests. Life Sci 26, 1247–1259. [DOI] [PubMed] [Google Scholar]
- Elashoff M, Higgs BW, Yolken RH, Knable MB, Weis S, Webster MJ, Barci BM & Torrey EF (2007) Meta-analysis of 12 genomic studies in bipolar disorder. J Mol Neurosci 31, 221–244. [DOI] [PubMed] [Google Scholar]
- Guang W, Wang H, Su T, Weinstein IB & Wang JB (2004) Role of mPKCI, a novel mu-opioid receptor interactive protein, in receptor desensitization, phosphorylation, and morphine-induced analgesia. Mol Pharmacol 66, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP & Damaj MI (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Kota DH, Martin BR & Damaj MI (2009) The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharmacology 56, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA & Damaj MI (2010) The role of α5 nicotinic acetylcholine receptors in the behavioral and pharmacological effects of nicotine in mice. J Pharmacol Exp Ther 334, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Chen Q, Chen J, Aggen SH, Kendler KS & Chen X (2011) Association of histidine-triad nucleotide binding protein 1 (HINT1) gene variants with nicotine dependence. Pharmacogenomics J 11, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE, Joynes RL & Grau JW (1997) Tail-flick test II. The role of supraspinal systems and avoidance learning. Behav Neurosci 111, 754–767. [DOI] [PubMed] [Google Scholar]
- Konradi C (2005) Gene expression microarray studies in polygenic disorders: applications and data analysis. Brain Res Brain Res Rev 50, 142–155. [DOI] [PubMed] [Google Scholar]
- Kurotaki N, Tasaki S, Mishima H, Ono S, Imamura A, Kikuchi T, Nishida N, Tokunaga K, Yoshiura K & Ozawa H (2011) Identification of novel schizophrenia loci by homozygosity mapping using DNA microarray analysis. PLoS One 6, e20589. DOI: 10.1371/journal.pone.0020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA & Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28, 325–334. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Y, Su T, Santella RM & Weinstein IB (2006) Hint1 is a haplo-insufficient tumor suppressor in mice. Oncogene 25, 713–721. [DOI] [PubMed] [Google Scholar]
- Liu Q, Puche AC & Wang JB (2008) Distribution and expression of protein kinase C interactive protein (PKCI/HINT1) in mouse central nervous system (CNS). Neurochem Res 33, 1263–1276. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novére N, de Kerchove d’Exaerde A, Huchet M, Damaj MI & Changeux JP (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398, 805–810. [DOI] [PubMed] [Google Scholar]
- O’Neill AB & Brioni JD (1994) Benzodiazepine receptor mediation of the anxiolytic-like effect of (−)-nicotine in mice. Pharmacol Biochem Behav 49, 755–757. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K & Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Ramabadran K & Bansinath M (1986) A critical analysis of the experimental evaluation of nociceptine reactions in animals. Pharm Res 3, 263–270. [DOI] [PubMed] [Google Scholar]
- Seeman P (1987) Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1, 133–152. [DOI] [PubMed] [Google Scholar]
- Su T, Suzui M, Wang L, Lin CS, Xing WQ & Weinstein IB (2003) Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci U S A 100, 7824–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ & Murray RB (1987). Manual of Pharmacological Calculations with Computer Programs. Springer, New York, pp. 29. [Google Scholar]
- Varadarajulu J, Lebar M, Krishnamoorthy G, Habelt S, Lu J, Bernard Weinstein I, Li H, Holsboer F, Turck CW & Touma C (2011) Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res 220, 305–311. [DOI] [PubMed] [Google Scholar]
- Varadarajulu J, Schmitt A, Falkai P, Alsaif M, Turck CW & Martins-de-Souza D (2012) Differential expression of HINT1 in schizophrenia brain tissue. Eur Arch Psychiatry Clin Neurosci 262, 167–172. [DOI] [PubMed] [Google Scholar]
- Varani AP & Balerio GN (2012) GABA(B) receptors involvement in the effects induced by nicotine on anxiety-related behaviour in mice. Pharmacol Res 65, 507–513. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WHIII, Donovan DM, Webster M, Freed WJ & Becker KG (2001) Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Res Bull 55, 641–650. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG & Freed WJ (2002) Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res 58, 11–20. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Shannon WC, Ferran E, Matsumoto M, Over-man K, Hyde TM, Weinberger DR, Bunney WE & Kleinman JE (2004) Gene expression of metabolic enzymes and a protease inhibitor in the prefrontal cortex are decreased in schizophrenia. Neurochem Res 29, 1245–1255. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B & Damaj MI (2006) The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 184, 339–344. [DOI] [PubMed] [Google Scholar]


