Abstract
Purpose of review:
While the gut microbiome plays a crucial role in the maintenance of health, it is hypothesized to drive morbidity and mortality in critically ill patients. This review describes the relationship between the gut microbiome and the immune system in critical illness.
Recent findings:
The gut microbiome is converted to a pathobiome in the intensive care unit, characterized by decreased microbial diversity and pathogen predominance. These changes are induced by a pathologic microenvironment and are further exacerbated by common medical treatments initiated in the intensive care unit. The conversion of the microbiome to a pathobiome has direct consequences on the regulation of inflammation and immunity by loss of beneficial host responses and initiation of maladaptive changes that can further propagate critical illness.
Summary:
The gut microbiome is dramatically altered in the intensive care unit. In light of constant crosstalk between the microbiome and the host immune system, the pathobiome may play a key mechanistic role in driving a maladaptive response in critically ill patients. The pathobiome represents a potential therapeutic target in the management of critical illness whereby restoration of a healthier microbiome may directly alter the host inflammatory response, which could lead to improved patient outcomes.
Keywords: gut, microbiome, pathobiome, immunity, critical illness
INTRODUCTION
The intestinal microenvironment is comprised of the microbiome, the mucosal immune system, and the intestinal epithelium (1). Each host has at least as many microbial cells residing within their gut lumen as they do cells of human origin in the entire body, and the gut microbiome is essential in maintaining host health and homeostasis via a variety of functions including fermentation of non-digestible substrates such as dietary fiber (2). Remarkably, this massive microbial population -- many of which can be pathogenic if they were to translocate outside of the gut lumen -- is separated from the host by a single cell layer epithelium. This close inter-species contact leads to constant crosstalk between the gut microbiome and the immune system. Critical illness disrupts the gut microbiome, and the resultant changes are associated with adverse clinical outcomes (3, 4, 5). While the relationship between the microbiome and outcomes in patients is correlative, pre-clinical studies suggest that the microbiome may play a causative role in morbidity and mortality in the intensive care unit via mechanisms that involve secondary changes to the host immune responses that are maladaptive in nature. This review discusses how changes in the gut microbiome influence the immune system during critical illness.
The gut microbiome
Next-generation sequencing and metabolomics have propelled the study of the gut microbiome, providing detailed identification of gut microbes and their metabolites (6, 7). There are approximately 40 trillion bacterial cells found within the gut comprised predominantly of the two phylogenic types, Bacteroidetes and Firmacutes (8–10).
Gut microbiota play a critical role in a multitude of functions. Gut bacteria are involved in nutrient absorption as well as maintenance and protection of the gut epithelium (8). Gut bacteria also degrade complex polysaccharides to crucial energy in the form of short-chain fatty acids (11). These short chain fatty acids along with the gut bacteria are vital to local immune system development and function. Further, gut bacteria supply the host with key amino acids and vitamins (6).
Owing to their wide-ranging functions, gut microbiota support human health and homeostasis. Consequently, disruption of the gut microbial environment is implicated in a wide range of diseases. Critical illness transforms the health-promoting gut microbiome into a disease-promoting pathobiome (12, 13).
The pathobiome
Critical illness disrupts the microbiota across multiple body sites in both pediatric and adult patient populations (14–17). This collapse of the healthy microbiome is multifactorial. There are certain factors that cannot be manipulated, including the impact of critical illness on the host microenvironment, genetics and age (18). Next, there are factors that are manipulatable long-term such as prior exposure to smoking and alcohol; however, once a patient becomes critically ill, these are also not changeable. Finally, multiple components of medical management in the intensive care unit directly impact the microbiome including antibiotics, other medications (opioids and stress ulcer prophylaxis), nutritional management (tube feedings, total parenteral nutrition), healthcare interventions (intubation and the placement of invasive devices), and emotional stressors (13, 19–21, 22).
Under basal conditions, there are approximately 1000 different microbial species in the gut microbiome. However, the pathobiome is characterized by decreased microbial diversity (as few as 4 species identified in some critically ill patients) and pathogen domination (23, 24). For example, a study using shotgun metagenomics in 24 critically ill adult patients demonstrated that two thirds of patients experienced a marked drop in gut microbial diversity at some point during their stay in the intensive care unit, and that the predominant pathogens were typically resistant to antibiotics or had genes associated with antimicrobial resistance (3).
Critical illness influences the gut microbial landscape beyond microbial diversity and antibiotic resistance, as the composition of gut metabolites is also affected. A study of 60 critically ill pediatric patients and 55 age-matched healthy children was conducted using 1H-nuclear magnetic resonance (1H-NMR) spectroscopy and mass spectrometry to assess the functional capacity of the intestinal microbiome. Results demonstrated strong differences in the 1H-NMR metabolic profiles of fecal (and urine) samples of critically ill children compared to healthy children with reduced fecal excretion of short-chain fatty acids including butyrate, propionate and acetate (25). The study further demonstrated that fecal butyrate was associated with days free of intensive care. These findings are consistent with studies in critically ill adults. An analysis 140 adults in the intensive care unit not only demonstrated decreased levels of short chain fatty acids in critically ill patients but also showed that gut complications including enteritis and dysmotility occur more frequently in patients with low metabolite levels (26).
Changes in the gut microbiome have also been demonstrated to be associated with morbidity and mortality in critical illness. In a study of 301 adult intensive care unit patients, pathogen predominance based on a rectal swab at ICU admission predicted later infection with that organism for Escherichia coli, Pseudomonas species., Klebsiella species., and Clostridium difficile,. Further, having Enterococcus as the dominant pathogen on rectal swab testing was associated with increased risk for death or all-cause infection (4). In a different study of 98 neurocritically ill patients and 84 age and sex-matched healthy subjects, neurocritically ill patients had a significantly different composition of intestinal microbiota (5). Notably, increases in intestinal Enterobacteriales and Enterobacteriaceae during the first week in the intensive care unit were associated with a 92% increased risk of 180-day adjusted mortality. These studies suggest that certain pathogens may predict infection and mortality in critically ill patients, although it is unclear how generalizable these studies are.
Due to the association between the pathobiome and negative outcomes, the gut microbiome has been proposed as a potential target for therapeutic interventions in critical illness. Interventions under investigation include prebiotics, probiotics, synbiotics, short chain fatty acids, vitamin supplementation, dietary fiber, fecal microbial transplantation, and selective decontamination of the digestive tract (27, 28–34). There is insufficient evidence thus far to support the standard use of any of these therapies in the intensive care unit although many show promise in select patient populations. Further research is required to more definitively study the impact of manipulating the microbiome for therapeutic benefit in critically ill patients, and which patient populations would be most likely to benefit from microbiome manipulation.
What is the relationship between the gut microbiome and the immune system?
Under basal conditions, the gut microbiome maintains a mutualistic relationship with the host and is essential to the development and maintenance of the immune system. This is best demonstrated in germ-free mice, which lack commensal microbes. These mice never develop a mature immune system and therefore have increased susceptibility to many pathogens (35, 36). Specifically, germ-free neonatal mice have decreased development of bone marrow myeloid precursors and subsequent decreased myeloid lineage cells in the spleen rendering them susceptible to E. coli and Staphylococcus aureus, but intestinal re-colonization reduces immunologic dysfunction that predisposes to sepsis susceptibility (37).
Innate Immune System:
Mechanical barriers and innate immunological responses are critical in protecting the host against the trillions of bacteria inhabiting the gastrointestinal tract as well as from infection by pathogens. The intestinal epithelial cells, tight junctions, and the intestinal epithelium’s associated mucus layer, serve to physically separate the host from commensal microbes and pathogens (1). Numerous changes take place in the setting of critical illness that seriously compromise the intestinal epithelium. These changes include upregulation of intestinal epithelial cell apoptosis, decreased crypt cell proliferation, slowed cell migration from the crypt (resulting in shortened villus length), increased intestinal permeability, and disruption of the mucus layer (1, 38, 39).
Gut microbiota interact with components of the innate immune system in an antigen nonspecific way via activation of pathogen recognition receptors. This elicits release of cytokines that then trigger the production of antimicrobial peptides (40). Gut microbiota also interact with the recently defined innate lymphoid cells (ILCs) in an antigen nonspecific way. ILCs are found at mucosal sites and are involved in the maintenance of the intestinal epithelium, regulation of the gut microbial composition, and protection against infection (41). ILCs are classified into five subsets: type 1 ILCs, type 2 ILCs, type 3 ILCs, lymphoid tissue inducer cells (sometimes classified as a subset of ILC3s), and natural killer cells. These cells differ based on their phenotypes and cytokine signatures. There also exist a subpopulation of ILCs called regulatory ILCs, which are responsible for controlling intestinal inflammation (42).
Recent studies have shed light on how the gut microbiota may influence the ILCs, though there remains a general paucity of research in this area and a need for close attention to confounding effects on gut microbial composition. A mouse model demonstrated that Id2, a transcriptional regulator expressed by type 3 ILCs, is important in pathogen colonization resistance and defense against the mouse pathogen Citrobacter rodentium. Further, Id2 dependent microbiota control pathogen colonization resistance via interleukin (IL)-22 dependent regulation (43). This study supports that the gut microbiota, ILCs, and their crosstalk are essential to host defense in critical illness. Although outside the scope of this review, it is also important to note that the airway and lung microbiomes also impact the immune response in critical illness (44).
Adaptive Immune System:
The adaptive immune system is comprised largely of lymphocytes and antigen-presenting cells, with the gut containing 80% of the body’s total lymphocyte population (45). Commensal microbiota activate antigen presenting cells and stimulate the differentiation of CD4+ T cells into T helper (Th) and T regulatory (Treg) cells (46). A subset of Th cells, called Th17 cells, and Treg cells are of particular importance in the gut. Th17 cells produce the inflammatory cytokine IL-17. In mice, segmented filamentous bacteria (SFB) stimulate differentiation of Th17 cells (47). These cells subsequently stimulate intestinal epithelial cells to produce antimicrobial peptides, thereby acting as a bridge between the innate and adaptive immune systems (48). Treg cells are involved in suppression of inflammation, and Treg cells that express transcription factor forkhead box P3 are vital in immune tolerance of commensal microbes (49, 50).
Th17 and Treg cells have been demonstrated to play a role in the pathogenesis of multiple diseases (51). As an example, germ-free mice were colonized with the microbiota from healthy hosts or hosts with inflammatory bowel disease. T cell responses to each microbiota were then assessed. Results demonstrated that mice with the microbiota from the inflammatory bowel disease hosts had higher levels of Th17 and T helper 2 (Th2) cells and lower levels of a subset of Treg cells with retinoic acid-related orphan receptor γt, compared to healthy hosts. Additionally, inflammatory bowel disease hosts developed worse disease severity in a model of colitis. Though critical illness is pathologically distinct from inflammatory bowel disease, both are characterized by dysregulated inflammation and immune function. As such, research on the relationship between the gut microbiota and immune system in inflammatory bowel disease may provide insights into gut pathophysiology in critical illness.
A preclinical study implicating the microbiome as causatively responsible for changes in mortality in sepsis demonstrated that altering the microbiome induced secondary changes in the adaptive immune system (52). Age and gender-matched, genetically identical mice from two different vendors were found to have markedly different gut microbiomes at baseline and following the onset of sepsis. After subjecting each of these to cecal ligation and puncture, mice with a more complex microbiome had a markedly improved survival, despite the fact that mice were genetically identical. This was associated with increased splenic IFNγ+CD4+ T cells, effector memory CD4+ T cells, and central memory CD4+ T cells and increased Peyer’s Patch effector memory CD4+ T cells in septic mice with the more complex microbiome. To determine whether differences in the microbiome were responsible for these differences, mice from different vendors were co-housed for three weeks, after which they assumed a similar microbiome. Co-housed mice had similar survival following sepsis regardless of vendor of origin, similar to mice that had a more complex microbiome at baseline. Notably, all differences in immunophenotype in the adaptive immune system that were dependent upon microbiome disappeared following co-housing. These results suggest that the gut microbiome directly affects immunophenotype and host survival from sepsis.
These results are complementary to a study comparing mice from different vendors, taking advantage of the fact that these mice differed in whether they had SFB (53). Mice containing SFB-specific CD4+ T cells underwent antigen-driven proliferation following polymicrobial sepsis whereas this did not occur in genetically identical mice that lacked this microbe. Co-housing mice prior to the onset of sepsis induced a similar phenotype in mice that initially lacked SFB but acquired it during co-housing. Additionally, mice containing SFB were resistant to a secondary lethal infection with an SFB antigen-expressing virulent Listeria, suggesting a role of the microbiome in mediating a protective role in sepsis via SFB-specific CD4+ T cells.
B lymphocytes also help modulate the host response to critical illness. Deep sequencing in germ-free mice demonstrates that microbial exposure at the intestinal mucosa generates oligoclonal responses that differ from either germ-free mice without microbial exposure or the diverse repertoire generated after systemic microbiota exposure (54). Notably, sequential systemic exposure to different microbial taxa diversified the IgG repertoire whereas sequential mucosal exposure produced limited overlapping repertoires and attrition of initial IgA binding specificities. These differences in systemic versus mucosal microbial exposure responses demonstrate a distinction between a flexible response to systemic exposure with the need to avoid sepsis, and a restricted response to mucosal exposure that reflects the mutualism present at the interface between the host and the gut microbiome.
B lymphocytes play a crucial role in the production of secretory IgA (sIgA) in the gut, which promote microbe colonization and neutralizes pathogens. Microbiota are important in stimulating both the amount and the diversity of sIgA produced (55, 56). IgA production, via T-dependent and T-independent pathways, is critical to maintaining gut microbial homeostasis. IgA binds to gut microbes and thereby prevents them from associating with the gut epithelium and from causing harm to host tissues (57). IgA coating of microbes also promotes commensal colonization, preventing invasion by competing bacterial species (58). The importance of this is highlighted in a murine model that demonstrated that gut microbial composition affects serum IgA concentration since exposing mice to a unique but natural microflora led to T-cell dependent increases in serum IgA levels and induction of large numbers of IgA-secreting plasma cells in the bone marrow (59). The resulting serum IgA bound to a restricted collection of bacterial taxa, and were induced after intestinal colonization with the commensal bacterium Helicobacter muridarum. Importantly, microbiota-induced serum IgA was protective against polymicrobial sepsis.
Although it is difficult to replicate the complex microbiome-immune interaction in vitro, cell culture experiments can give additional insights into how microbes impact adaptive immune responses. A study examining components of the gut microbiome found in probiotic therapies examined their impact on lymphocyte profiles (60). T-lymphocytes were cultured with Bacteroides fragilis, Clostridium perfringens, or Lactobacillus acidophilus. Lactobacillus induced IL-22 and IL-33 whereas Bacteroides induced IL-33 only under polymicrobial conditions. In contrast, PD-1+ expression was lowest with either monomicrobial Bacteroides or Bacteroides in a polymicrobial context whereas B- and T-lymphocyte attenuator-positive expression did not differ after individual microbes.
Conclusions
Critical illness disrupts the gut microbiome with conversion to a pathobiome. This is associated with worsened mortality in critically ill patients, with pre-clinical studies suggesting a mechanistic link between alterations in the microbiome and survival. The microbiome plays a key role in both the development and maintenance of the host immune response. While data linking the pathobiome to an altered immune response are sparser, preliminary data suggests a direct link between the gut microbiome and a maladaptive immune response in critical illness. A schematic of the microbiome in health and disease is presented in figure 1. Based upon findings in critical illness, there is significant conceptual appeal to the concept of manipulating the microbiome for therapeutic gain – mediated in part by alterations in the host immune response. Future research directed at the relationship between the pathobiome and the host immune response may yield mechanistic insights that inform the development of novel therapeutics in critical illness.
Figure 1.
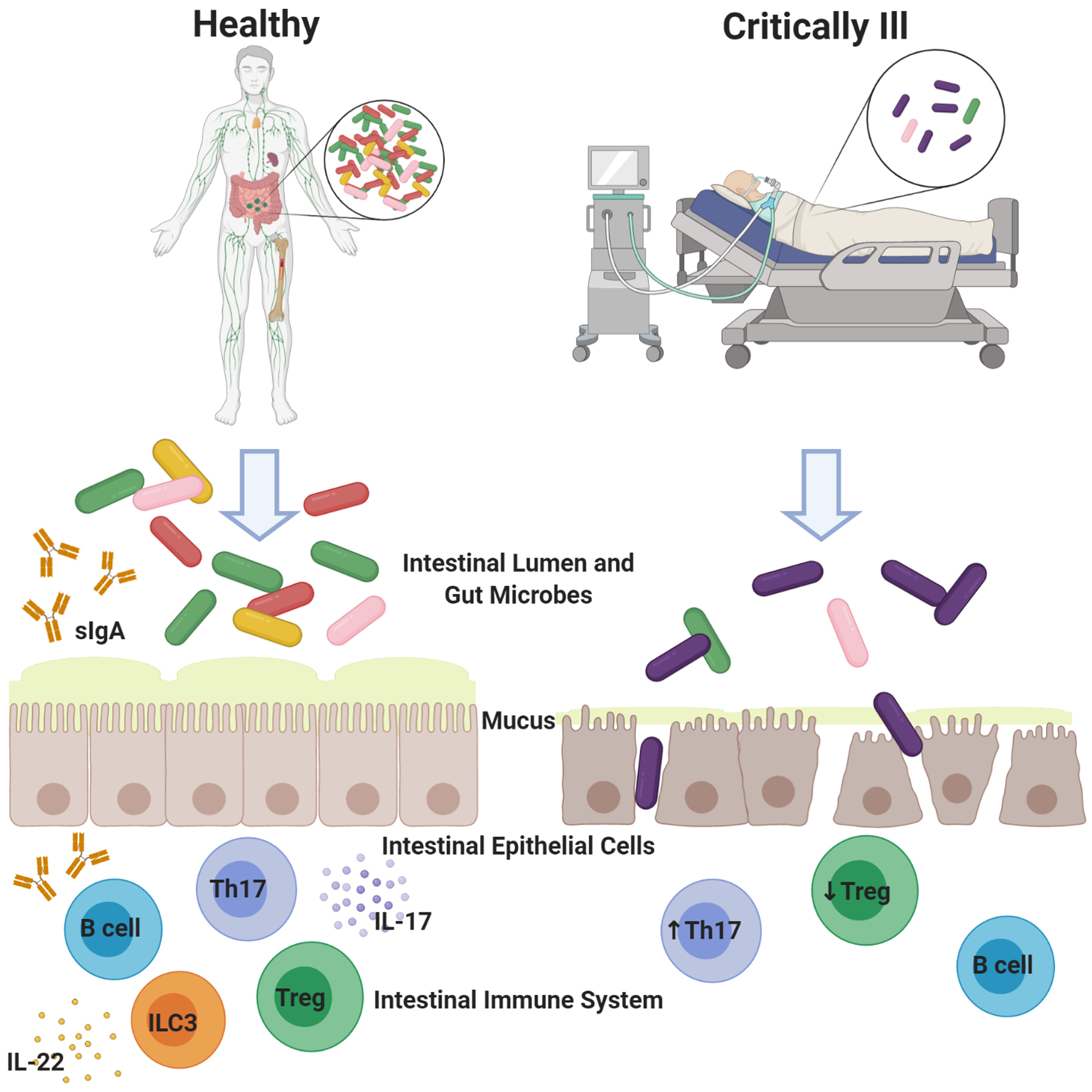
The healthy gut microbiome is characterized by microbial diversity and predominance of Bacteroidetes and Firmacutes. In the gut microbiome of the critically ill host, there is decreased microbial diversity and predominance of pathogenic bacteria. Bacteria in the healthy gut interact with pathogen recognition receptors and innate lymphoid cells to stimulate the innate immune response. Bacteria are also involved in stimulating differentiation of CD4+ T cells and antibody production. In contrast, conversion of the microbiome to a less diverse but more virulent pathobiome in the critically ill gut results in a dysregulated immune response. Figure created with BioRender.com
Key Points:
The healthy gut microbiome has a multitude of functions including immune homeostasis, nutrient absorption, maintenance of gut integrity, and host metabolism.
In critical illness, the gut microbiome is converted to a pathobiome which is associated with increased host morbidity and mortality.
Crosstalk between the gut microbiome and the immune system occurs on a consistent basis
Alterations in the microbiome are associated with – and at times responsible for – changes to both the innate and adaptive immune system in critical illness.
Financial Support and Sponsorship:
This work was supported by funding from the National Institutes of Health (GM072808, GM095442, HL151897, GM104323, AA027396)
Footnotes
Conflicts of Interest:
None.
REFREENCES
- 1.Otani S, Coopersmith CM. Gut integrity in critical illness. Journal of intensive care. 2019;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. Bmj. 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi A, Halstead FD, Bamford A, et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb Genom. 2019;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]; **This is a prospective observational study of adult ICU patients in which the gut microbiome in critical illness was assessed in a novel way using shotgun metagenomic sequencing, phylogenetic profiling, and microbial genome analyses. Results demonstrated dramatic changes to the gut microbiome in critical illness and highlighted shotgun metagenomics as a potential tool for microbial surveillance.
- 4.Freedberg DE, Zhou MJ, Cohen ME, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44(8):1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu R, Tan C, Zhu J, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care. 2019;23(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is a prospective observational cohort study in a neurocritically ill patient population, in which characteristics of the gut microbiome and risk of death were evaluated. Results demonstrated significant differences between neurocritically ill and healthy groups, with regard to the gut microbiome and mortality risk.
- 6.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff NS, Hugenholtz F, Wiersinga WJ. The emerging role of the microbiota in the ICU. Crit Care. 2018;22(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckburg PB, Bik EM, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164(3):337–40. [DOI] [PubMed] [Google Scholar]
- 10.Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochimica et biophysica acta Molecular basis of disease. 2017;1863(10 Pt B):2574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krezalek MA, DeFazio J, Zaborina O, et al. The Shift of an Intestinal “Microbiome” to a “Pathobiome” Governs the Course and Outcome of Sepsis Following Surgical Injury. Shock. 2016;45(5):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alverdy JC, Krezalek MA. Collapse of the Microbiome, Emergence of the Pathobiome, and the Immunopathology of Sepsis. Crit Care Med. 2017;45(2):337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers MB, Firek B, Shi M, et al. Disruption of the microbiota across multiple body sites in critically ill children. Microbiome. 2016;4(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh A, Rogers MB, Firek B, et al. Dysbiosis Across Multiple Body Sites in Critically Ill Adult Surgical Patients. Shock. 2016;46(6):649–54. [DOI] [PubMed] [Google Scholar]
- 16.Lankelma JM, van Vught LA, Belzer C, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarche D, Johnstone J, Zytaruk N, et al. Microbial dysbiosis and mortality during mechanical ventilation: a prospective observational study. Respir Res. 2018;19(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. [DOI] [PubMed] [Google Scholar]
- 19.Kitsios GD, Morowitz MJ, Dickson RP, et al. Dysbiosis in the intensive care unit: Microbiome science coming to the bedside. Journal of critical care. 2017;38:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23(2):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhalodi AA, van Engelen TSR, Virk HS, et al. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother. 2019;74(Suppl 1):i6–i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukovic E, Moitra VK, Freedberg DE. The microbiome: implications for perioperative and critical care. Curr Opin Anaesthesiol. 2019;32(3):412–20. [DOI] [PubMed] [Google Scholar]
- 23.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio. 2014;5(5):e01361–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald D, Ackermann G, Khailova L, et al. Extreme Dysbiosis of the Microbiome in Critical Illness. mSphere. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijeyesekera A, Wagner J, De Goffau M, et al. Multi-Compartment Profiling of Bacterial and Host Metabolites Identifies Intestinal Dysbiosis and Its Functional Consequences in the Critically Ill Child. Crit Care Med. 2019;47(9):e727–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This is a prospective multicenter cohort study that assessed the functional activity of the intestinal microbiome in critically ill and healthy pediatric patient populations using metabolic profiling. There were strong differences between the groups with regard to urinary and fecal metabolic profiles.
- 26.Yamada T, Shimizu K, Ogura H, et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J Parenter Enteral Nutr. 2015;39(5):569–77. [DOI] [PubMed] [Google Scholar]
- 27.Cantorna MT, Snyder L, Arora J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit Rev Biochem Mol Biol. 2019;54(2):184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng H, Li JG, Mao Z, et al. Probiotics for Preventing Ventilator-Associated Pneumonia in Mechanically Ventilated Patients: A Meta-Analysis with Trial Sequential Analysis. Front Pharmacol. 2017;8:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClave SA, Patel J, Bhutiani N. Should fecal microbial transplantation be used in the ICU? Current opinion in critical care. 2018;24(2):105–11. [DOI] [PubMed] [Google Scholar]
- 30.Price R, Maclennan G, Glen J. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedberg DE, Messina M, Lynch E, et al. Impact of Fiber-Based Enteral Nutrition on the Gut Microbiome of ICU Patients Receiving Broad-Spectrum Antibiotics: A Randomized Pilot Trial. Critical care explorations. 2020;2(6):e0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choy A, Freedberg DE. Impact of microbiome-based interventions on gastrointestinal pathogen colonization in the intensive care unit. Therapeutic advances in gastroenterology. 2020;13:1756284820939447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keskey R, Cone JT, DeFazio JR, et al. The use of fecal microbiota transplant in sepsis. Translational research : the journal of laboratory and clinical medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548(7668):407–12. [DOI] [PubMed] [Google Scholar]
- 35.Josefsdottir KS, Baldridge MT, Kadmon CS, et al. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129(6):729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi A, Yáñez A, Price JG, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adelman MW, Woodworth MH, Langelier C, et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit Care. 2020;24(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otani S, Oami T, Yoseph BP, et al. Overexpression of BCL-2 in the Intestinal Epithelium Prevents Sepsis-Induced Gut Barrier Dysfunction via Altering Tight Junction Protein Expression. Shock. 2020;54(3):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng M, Klingensmith NJ, Liang Z, et al. Regulators of Intestinal Epithelial Migration in Sepsis. Shock. 2019;51(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thaiss CA, Zmora N, Levy M, et al. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. [DOI] [PubMed] [Google Scholar]
- 41.Ganal-Vonarburg SC, Duerr CU. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology. 2020;159(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is a comprehensive review on the relationship between the microbiota and ILCs throughout life, with special focus on intestinal immunity.
- 42.Wang S, Xia P, Chen Y, et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell. 2017;171(1):201–16.e18. [DOI] [PubMed] [Google Scholar]
- 43.Guo X, Liang Y, Zhang Y, et al. Innate Lymphoid Cells Control Early Colonization Resistance against Intestinal Pathogens through ID2-Dependent Regulation of the Microbiota. Immunity. 2015;42(4):731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Loeches I, Dickson R, Torres A, et al. The importance of airway and lung microbiome in the critically ill. Crit Care. 2020;24(1):537. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Although outside the scope of this review focusing on the gut microbiome, this is a nice overview of the lung and airway microbiome in critical illness
- 45.Thome JJ, Yudanin N, Ohmura Y, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atarashi K, Tanoue T, Ando M, et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163(2):367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver CT, Elson CO, Fouser LA, et al. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sujino T, London M, Hoytema van Konijnenburg DP, et al. Tissue adaptation of regulatory and intraepithelial CD4⁺ T cells controls gut inflammation. Science. 2016;352(6293):1581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraj P, Ignatowicz L. The mechanisms shaping the repertoire of CD4(+) Foxp3(+) regulatory T cells. Immunology. 2018;153(3):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Britton GJ, Contijoch EJ, Mogno I, et al. Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt(+) Regulatory T Cells and Exacerbate Colitis in Mice. Immunity. 2019;50(1):212–24.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is a study in which the microbiota from healthy and IBD hosts were transferred into germ-free mice, to assess T cell response to each microbiota. Results supported intestinal microbiota involvement in dysregulated immune response and IBD pathogenesis.
- 52.Fay KT, Klingensmith NJ, Chen CW, et al. The gut microbiome alters immunophenotype and survival from sepsis. Faseb j. 2019;33(10):11258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study, genetically identical mice from different vendors with different microbiomes were made septic. Results demonstrated a direct causal role for the microbiome in mediating mortality from sepsis, associated with changes in the immunophenotype of the adaptive immune system.
- 53.Cabrera-Perez J, Babcock JC, Dileepan T, et al. Gut Microbial Membership Modulates CD4 T Cell Reconstitution and Function after Sepsis. J Immunol. 2016;197(5):1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Limenitakis JP, Greiff V, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584(7820):274–8. [DOI] [PubMed] [Google Scholar]; **This study used deep sequencing in germ-free mice and determined that microbial exposure at the intestinal mucosa generates oligoclonal responses that differ from either germ-free mice witout microbial exposure or those given systemic microbiota. Systemic exposure led to a more flexible and robust response (likely to prevent sepsis) than mucosal expsoure, consistent with the constant crosstalk between the gut microbiome and host immune system
- 55.Moon C, Baldridge MT, Wallace MA, et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521(7550):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macpherson AJ, Yilmaz B, Limenitakis JP, et al. IgA Function in Relation to the Intestinal Microbiota. Annu Rev Immunol. 2018;36:359–81. [DOI] [PubMed] [Google Scholar]
- 57.Bunker JJ, Bendelac A. IgA Responses to Microbiota. Immunity. 2018;49(2):211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donaldson GP, Ladinsky MS, Yu KB, et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science. 2018;360(6390):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilmore JR, Gaudette BT, Gomez Atria D, et al. Commensal Microbes Induce Serum IgA Responses that Protect against Polymicrobial Sepsis. Cell host & microbe. 2018;23(3):302–11.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heffernan IM, McGeary JE, Chung CS, et al. Unmasking Unique Immune Altering Aspects of the Microbiome as a Tool to Correct Sepsis-Induced Immune Dysfunction. Surg Infect (Larchmt). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study examines the impact of different microbes commonly used in probiotics on the adaptive immune response in vivo and finds differential effects that a dependent on organism studied.


