Abstract
Objective
The purpose of this study was to investigate the clinical efficacy of dorsal root ganglion (DRG) pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b in the treatment of patients with acute herpes zoster neuralgia and its preventive effectiveness on postherpetic neuralgia (PHN).
Methods
A retrospective analysis of 62 patients with acute herpes zoster neuralgia was implemented. All patients were divided into two groups: pulsed radiofrequency paraspinal injection of recombinant human interferon-α2b (group P); pulsed radiofrequency combined with paravertebral injection of saline (group C). The numerical rating scales (NRS) scores were used for pain assessment, and the dose of the analgesic drug was recorded. Gal-3 and IL-6 levels in blood were compared between the two groups before treatment and at 1 week, 2, and 4 weeks after treatment. The incidence of PHN was recorded in both groups.
Results
The pain intensity, the levels of Gal-3 and IL-6 in blood and the dose of oral administration of gabapentin capsules and morphine were reduced in all patients after treatment. Compared with group C, patients in group P had lower NRS scores, blood levels of Gal-3 and IL-6, and dosages of oral gabapentin capsules and morphine hydrochloride immediate-release tablets after treatment. The incidence of PHN was significantly lower at 8 and 12 weeks.
Conclusion
DRG pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b for acute stage herpes zoster neuralgia is a more effective treatment, and can effectively prevent the incidence rate of PHN.
Keywords: pulsed radiofrequency, dorsal root ganglion, recombinant human interferon-α2b, herpes zoster, postherpetic neuralgia
Introduction
Herpes zoster is caused by varicella-zoster virus infection of the dorsal root ganglia or the trigeminal ganglia. It is more common in middle-aged and elderly people with reduced immunity. Some patients may have post-herpetic neuralgia for several months or even years. It seriously affects the quality of life of patients and increases the medical burden.1–3 Although there are many methods for treating herpes zoster neuralgia, the incidence of PHN is still high. A large number of clinical studies have shown that early effective intervention can shorten the acute phase. The duration and severity of herpes zoster neuralgia reduce the risk of developing PHN.4,5
A nerve block or epidural block in the treatment of acute/subacute herpes zoster neuralgia can significantly improve local blood circulation, nutritional nerves, and analgesia, and can accelerate herpes healing. However, the effectiveness of epidural block or nerve block to prevent PHN is controversial. Studies have reported that dorsal root ganglion (DRG) pulsed radiofrequency treatment for herpes zoster neuralgia is superior to continuous epidural block.4,6
Pulsed radiofrequency uses pulse current to limit heat generation to below 42°C, causing almost no thermal damage or nerve damage. Pulsed radiofrequency does not cause complications such as skin sensation, skin numbness, motor nerve damage, etc., and it is minimally invasive and easy to repeat. It is widely used in clinical practice. Since the DRG is an important target for the treatment of herpes zoster neuralgia, there have been a large number of reports on the acute dorsal herpes zoster neuralgia in the DRG pulsed radiofrequency treatment. The study for PHN also confirmed the effectiveness and safety of pulsed radiofrequency in DRG, and achieved immediate effect, but it failed to significantly reduce the incidence of PHN.7,8 Although pulsed radiofrequency can cause analgesia, there is no treatment for antiviral and immune system regulation at the target, which may be the main reason for its unsustainable efficacy.
Interferon is a cytokine synthesized and secreted by immune cells, glial cells, and neuronal cells, and has antiviral, antitumor and immunomodulatory effects.9 At present, the clinical use of intramuscular or subcutaneous injection for herpes zoster has a certain therapeutic effect, but systemic medication often has adverse reactions such as fever, allergy, and cytopenia. Guo et al10 reported low-dose recombinant human interferon-α2b is used for the treatment of herpes zoster in patients with herpes zoster. The clinical efficacy of the patient is improved. The immune function of the patient can be improved after treatment. The systemic effect is small, almost no systemic adverse reactions and the prevention of PHN can be effectively prevented. The possible mechanism of antiviral and enhanced immunity of recombinant human interferon-α2b is to induce the cells to produce a variety of antiviral proteins and inhibit the virus from multiplying in cells by binding to cell surface receptors.11 Therefore, the DRG injection of recombinant human interferon-α2b is effective in treating acute herpes zoster by antiviral and enhanced immunity, and also can avoid adverse reactions caused by systemic administration. However, the single therapy of DRG injection of recombinant human interferon-α2b is not enough to treat herpes zoster neuralgia. Gao et al and others reported that the incidence of PHN was still not less than 10% after Herpes zoster patients treated with recombinant human interferon-α2b after paravertebral injection.7 Although this method can effectively have antiviral effect and improve the immunity of patients, it cannot significantly reduce the incidence of PHN.
The above-mentioned DRG pulsed radiofrequency technique and paravertebral injection of recombinant human interferon-α2b can effectively control the condition of acute herpes zoster neuralgia through different mechanisms of action and can reduce the incidence of PHN. Whether the application can effectively reduce the incidence of PHN has not been reported. This study was to investigate the clinical efficacy of DRG pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b in the treatment of patients with acute herpes zoster and the preventive effect on PHN.
Method
Participant
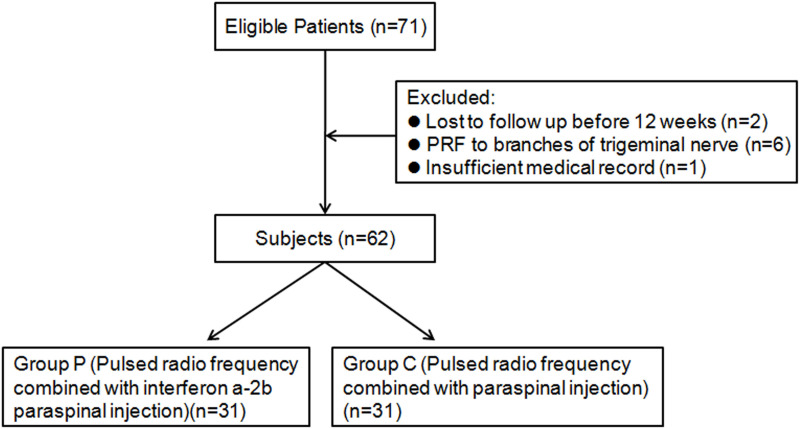
In this retrospective analysis, the inclusion criteria were: patients with acute herpes zoster neuropathy with neck, thoracic or lumbar ganglion involvement, duration ≤1 month, numerical rating scales (NRS) score >3 points; the exclusion criteria were as follows: patients with infection or tumor at the puncture site, those who are allergic to recombinant human interferon-α2b, compound methicone, severe cardiovascular and cerebrovascular diseases, liver and kidney dysfunction, patients with abnormal coagulation function, and poor glycemic control in patients with diabetes those who have long-term use of immunosuppressants or systemic failure, have psychiatric diseases, and cannot cooperate with the surgeon. Seventy-one patients with acute herpes zoster neuropathic pain who were treated in our hospital from January 2019 to August 2019 were enrolled. A random number table method was used to classify two groups of patients with different treatment methods for research, and the baseline data (gender, age, disease history) of the two groups were not statistically different. Six patients underwent pulsed radiofrequency to the peripheral branches of the trigeminal nerve. Two patients failed to follow up at 12 weeks after treatment. One patient had insufficient medical records. After excluding these patients, the medical records of 62 patients were analyzed. Thirty-one patients underwent pulsed radiofrequency combined with paravertebral injection of recombinant interferon-α2b (group P), and another 31 patients underwent pulsed radiofrequency combined with paravertebral injection (group C) (Figure 1). All patients had no history of treatment with epidural or nerve block before this treatment. This study was approved by the Ethics Committee of the Affiliated Hospital of Jiaxing University (LS2018-240), and conducted by the Declaration of Helsinki. All patient informed consents were obtained.
Figure 1.
Flow diagram of the study participants.
Procedure
All patients were treated with oral analgesics before treatment, and the pain level was still moderate to severe. In the case of conventional drug therapy, which can only temporarily relieve pain, pulsed radiofrequency combined with paravertebral injection was performed on the relevant level of DRG. All pulsed radiofrequency combined with paravertebral injection procedures were performed by a physician with experience in pain management.
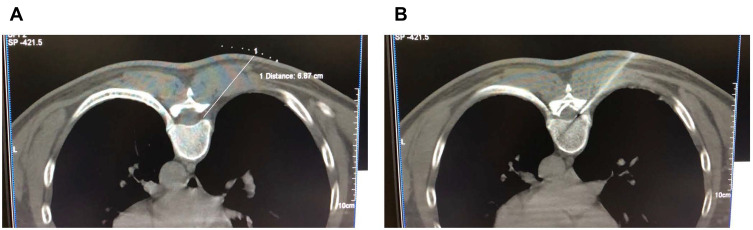
The patient was lying prone on the CT treatment bed, centered on the most severe segment of the pain, and expanded one segment at the top and bottom, and performed pulsed radiofrequency of the DRG with three segments each time. CT positioning selects the upper edge of the ventral intervertebral foramen as the puncture needlepoint, and designs the puncture path (Figure 2A), and disinfects the drape. Lidocaine hydrochloride (1.0%) was infiltrated with local anesthesia, and the RF trocar (20 G, length 150 mm, active end length 10 mm) was slowly advanced under CT. Finally, the needle tip is located in the upper quadrant of the vertebral foramen, and root pain can occur before the puncture site to the chest wall. The radiofrequency test (Baylis Medical Inc., Montreal, Canada) is sensory test, setting parameters, voltage: 0.1–0.3 V, frequency: 50 Hz, can induce discomfort such as acid, swelling, numbness or tingling in the original pain area. The exercise test uses low-frequency current, setting parameters, voltage: 0.4–1.0 V, frequency: 2 Hz, no corresponding trunk muscle fiber fibrillation and pulsation in the corresponding segment. We set the temperature, time, pulse width and frequency to 42°C, 360 s, 20 ms, 2 Hz. After the end of the pulsed radiofrequency, the electrode core is unplugged, and the puncture needle is free of blood, gas or liquid. Each section is injected with 5 mL of therapeutic solution, usually 3 segments (Figure 2B). The formulation of the treatment solution is: 2% lidocaine hydrochloride 100 mg, mecobalamin injection 1 mg, compound dexamethasone 1 mg and recombinant human interferon-α2b injection 1 million U and 30% iohexol 3 mL diluted to 15 mL by adding 0.9% physiological saline. The puncture point is pressed after the needle is removed. After 15 minutes of observation, patients with normal vital signs returned to the ward. This method is treated once a week for 3 times (the second and third times do not do pulsed radiofrequency treatment, only paravertebral nerve block, the treatment solution is the same as before). Patients in group C only underwent pulsed radiofrequency and paravertebral nerve block without recombinant human interferon-α2b injection. The course of treatment was the same as group P. Both groups of patients were given morphine hydrochloride immediate-release tablets 5 mg orally during the onset of pain during treatment.
Figure 2.
(A) CT locates the puncture path and the puncture target. CT positioning selects the upper edge of the ventral intervertebral foramen as the puncture needlepoint and designs the puncture path. (B) Distribution of the liquid in the paravertebral injection after pulsed radiofrequency. CT guided puncture needle to the ventral upper quadrant of the intervertebral foramen. After a successful test, the pulsed radiofrequency was applied. After the end of the pulsed radiofrequency, the paravertebral injection was distributed to the epidural space.
Data Collection
The following data were collected from the medical records for analysis: age, gender, potential disease, targeted DRG levels, days from herpes zoster to pulsed radiofrequency therapy, NRS before treatment and 1 week, 2 weeks, 4 weeks, 8 weeks and 12 weeks after treatment, recording the incidence of PHN in both groups and dosage of anticonvulsants and analgesics before and 1 week, 2 weeks, 4 weeks, 8 weeks and 12 weeks after treatment. Gal-3 and IL-6 levels in blood were measured before treatment and at 1 week, 2 weeks and 4 weeks after treatment.
Result Measurement
The analgesic effect of pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b on patients with acute herpes zoster neuralgia was evaluated by NRS score and the amount of anticonvulsant and analgesic. For ease of analysis, participants converted the doses of anticonvulsants and analgesics to equivalent doses of galanthin capsules12,13 and oral morphine equivalent doses.14
In various studies, clinically significant PHN was defined as persistent pain on NRS with an intensity of 3 or more.15–17 The proportion of clinically significant PHN at 4 weeks, 8 weeks, and 12 weeks after surgery was compared by the same criteria in this study.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) of continuous variables. The data normality was tested using the Kolmogorov–Smirnov test. The continuous variable group was compared using the Mann–Whitney U-test or the independent t-test, and the categorical variable group was analyzed by the chi-square test or Fisher’s exact test. Repeated measures analysis of variance was used to assess Gal-3 and IL-6 levels in the blood as well as pain intensity and drug dose over time. All data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered statistically significant.
Results
Comparison of General Clinical Data Between Group C and Group P
All patients were treated with conventional antiviral therapy (acyclovir cream, external application, 3–4 times a day combined with valacyclovir capsules, 0.3g po tid, 7–10 days of treatment) after the appearance of herpes zoster. All participants also underwent oral drug analgesia before pulsed radiofrequency combined with paravertebral injection. No significant differences were found in age, gender, treatment site, past medical history, and analgesic drug types between the two groups. NRS before treatment was 6.32 ± 0.945 and 5.81 ± 1.108, respectively. There was no significant difference between these two groups (P = 0.053) (Table 1).
Table 1.
Patient Demographic Data
| Group P | Group C | P-value | |
|---|---|---|---|
| Age,years,mean±SD | 64.71±10.374 | 66.74±11.699 | 0.472 |
| Gender, n, male/female | 12/19 | 16/15 | 0.307 |
| Days from zoster onset, mean±SD | 16.10±9.934 | 16.84±10.030 | 0.771 |
| Involved dermatome,n | |||
| Cervical, n | 4 | 6 | 0.710 |
| Thoracic, n | 19 | 19 | |
| Lumbosacral, n | 8 | 6 | |
| Underlying disease, n | |||
| Hypertension (HTN), n | 9 | 11 | 0.953 |
| Diabetes mellitus (DM), n | 7 | 6 | |
| HTN & DM, n | 6 | 6 | |
| None, n | 9 | 8 | |
| NRS before PRF, mean±SD | 6.32±0.945 | 5.81±1.108 | 0.053 |
| Analgesics at pre-PRF, n | |||
| Tramadol only, n | 2 | 2 | 0.957 |
| Tramadol with celecoxib | 3 | 4 | |
| Tramadol with opioid, n | 8 | 10 | |
| Opioid only, n | 1 | 1 | |
| Tramadol, celecoxib and opioids,n | 17 | 14 |
The NRS Scores and Incidence of PHN Were Lower in Group P
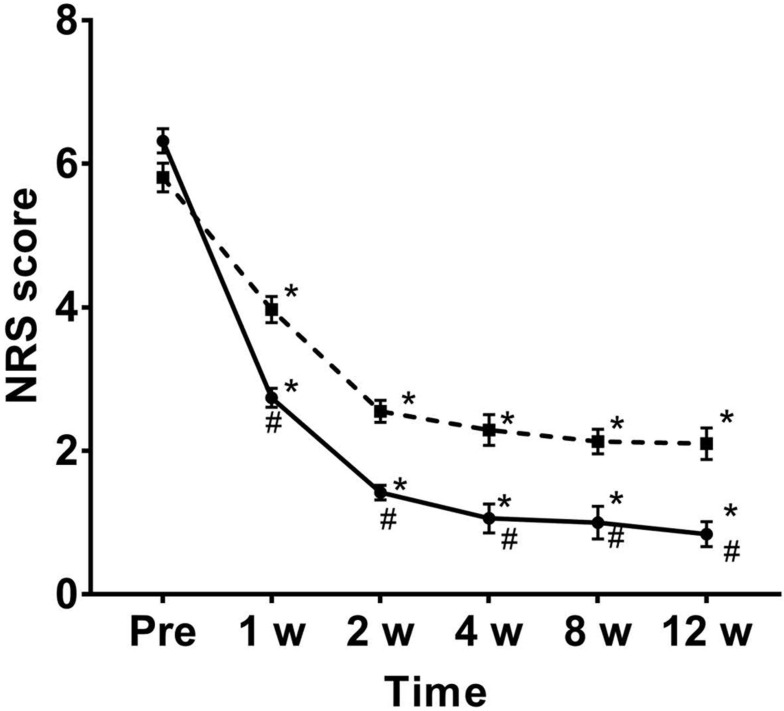
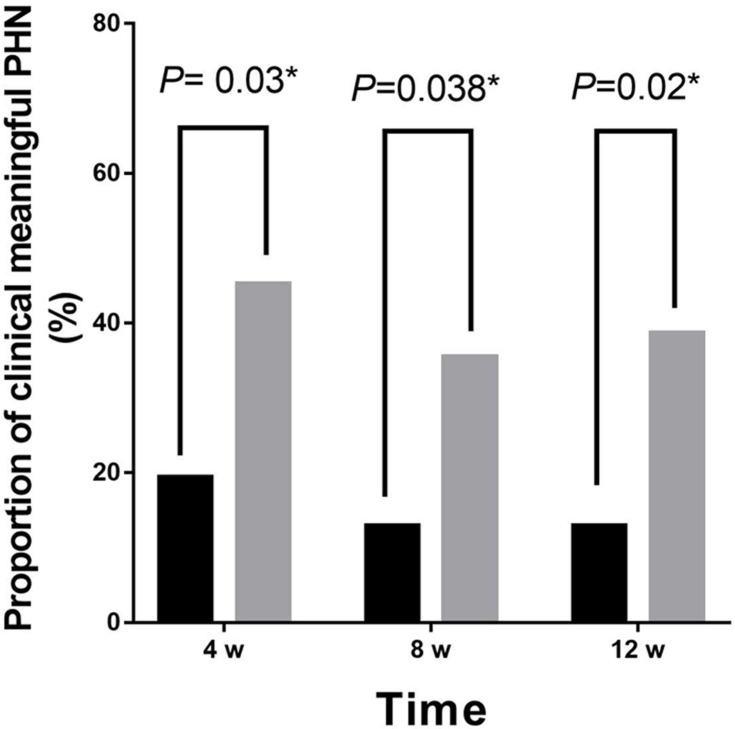
Over time, the NRS of both groups decreased significantly. Compared with group C, the NRS scores of patients were significantly lower at 1 week, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after treatment in group P (P = 0.04, 0.03,0.03, 0.03, 0.03) (Figure 3). The incidence of PHN at 4 weeks, 8 weeks, and 12 weeks after treatment was significantly lower in group P than in group C (P =0.03, 0.04, 0.02) (Figure 4).
Figure 3.
Changes in NRS after treatment. Over time, NRS in both groups showed significant changes. However, compared with C group, the NRS of group P was significantly reduced. The dotted line of the box and the solid line of the round frame indicate the NRS changes of C group and P group, respectively. *P < 0.05 compared to pretreatment; #P < 0.05 compared to the group C.
Abbreviation: NRS, numerical rating scale.
Figure 4.
The proportion of clinically significant PHN at 4, 8 and 12 weeks after treatment in both groups. Gray and black indicate the proportion of clinically significant PHN in group C and group P, respectively. *P < 0.05 compared to the pretreatment.
Blood Gal-3 and IL-6 Levels, The Doses of Oral Gabapentin Capsule and Morphine Were All Lower in Group P
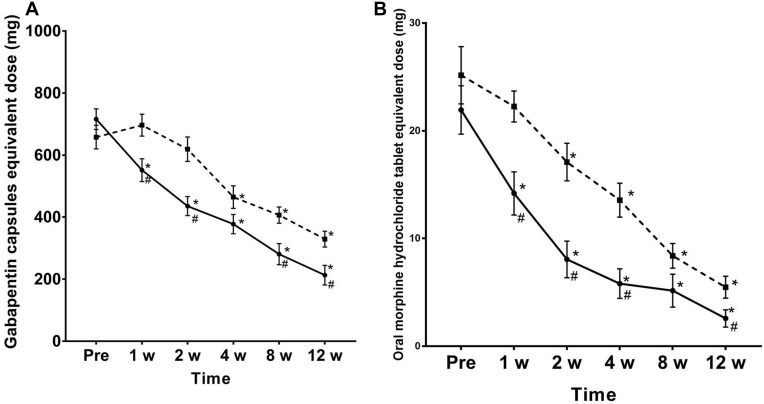
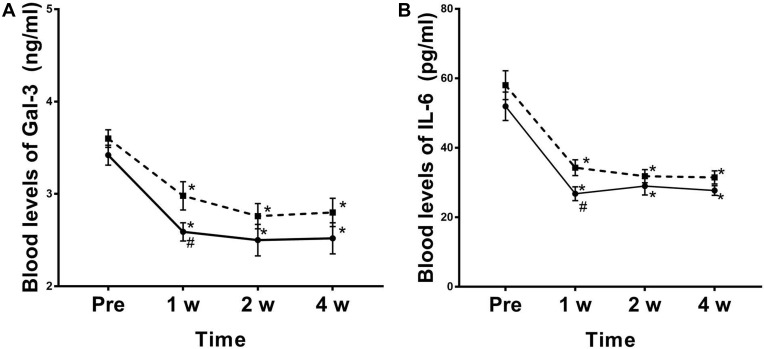
Compared with before treatment, the doses of oral gabapentin capsules and morphine hydrochloride were decreased in group P at 1 week, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after treatment, and the difference was statistically significant (P < 0.05). Compared with group C, the doses of oral administration of gabapentin capsules decreased in group P at 1 week, 2 weeks, 8 weeks, and 12 weeks after treatment, the difference was statistically significant (P <0.05), the doses of oral morphine decreased in group P at 1 week, 2 weeks, 4 weeks, and 12 weeks after treatment, and the difference was statistically significant (P < 0.05) (Figure 5). Compared with before treatment, the levels of Gal-3 and IL-6 in the blood decreased at 1 week, 2 weeks and 4 weeks after treatment, and the difference was statistically significant (P <0.05). Compared with group C, the levels of blood Gal-3 and IL-6 in group P decreased at 1 week after treatment, and the difference was statistically significant (P <0.05) (Figure 6).
Figure 5.
Dosage changes after adding saponin capsules and morphine hydrochloride after treatment. The dotted line of the box and the solid line of the round frame indicate the dose changes of the group C and D, respectively. The doses of group C plus gabapentin capsules (A) and morphine (B) were generally higher; *P < 0.05 compared to the pretreatment. #P < 0.05 compared to the C group.
Figure 6.
Changes in blood Gal-3 levels and IL-6 levels at 1 week, 2 and 4 weeks after treatment in both groups. The dotted line of the box and the solid line of the round frame indicate changes in blood Gal-3 levels (A) and IL-6 levels (B) in C and P groups, respectively. *P < 0.05 compared to the pretreatment. #P < 0.05 compared to the C group.
No Adverse Reactions Occurred in the Two Groups of Patients After Treatment
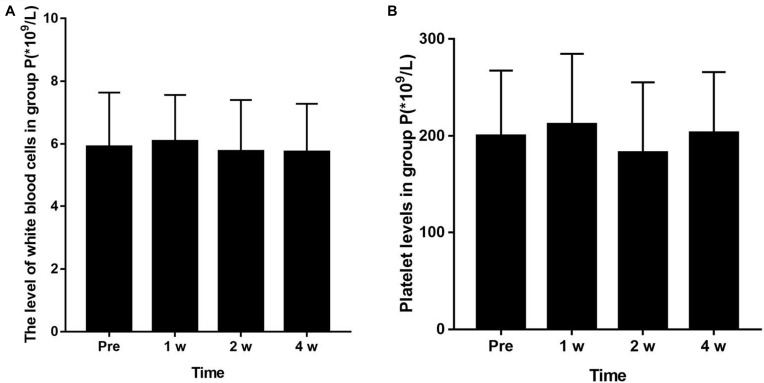
All patients had no adverse reactions such as fever, chills, fatigue, and myalgia after treatment. There was no significant change in the levels of leukocytes and platelets in group P before and 1 week, 2 weeks, and 4 weeks after treatment. The difference was not statistically significant (P > 0.05) (Figure 7).
Figure 7.
Changes of white cell and platelet levels in the blood before treatment and at 1 week, 2 and 4 weeks after treatment in group P (P < 0.05). There was no significant change in the levels of leukocytes (A) and platelets (B) in group P before and after 1 week, 2 and 4 weeks after treatment (P > 0.05).
Discussion
In this retrospective study, NRS scores were significantly reduced over time in both groups. The pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b showed a more significant reduction in pain scores at all time points after treatment. The doses of oral gabapentin capsules and morphine hydrochloride were significantly lower than that of the control group and after treatment. The incidence of PHN at 4 weeks, 8 weeks, and 12 weeks was also significantly reduced. In patients with herpes zoster, the application of pulsed radiofrequency to the dorsal root ganglia can reduce signal transduction of the central nervous system by regulating pain fibers. Therefore, further neuropathy processes can be blocked before severe neuropathy occurs. Pulsed radiofrequency causes tissue damage at the ultrastructural level of the dorsal root ganglia.18,19 Recent studies have shown that Na/K ATP enzyme and c-Fos expression are up-regulated in the spinal cord20 and increased synaptic transmission21 after pulsed radiofrequency therapy. These mechanisms may lead to changes in neural plasticity that may help to enhance the therapeutic effects of pulsed radiofrequency.
Recombinant human interferon-α2b injection has a broad spectrum of antiviral, antitumor, inhibition of cell proliferation and immune enhancement. Recombinant human interferon-α2b binds to cell surface receptors, induces cells to produce a variety of antiviral proteins, inhibits virus propagation in cells, enhances immune function, enhances macrophage phagocytosis, and enhances lymphocyte cytotoxicity and natural killer cells. Although the most common adverse reactions in the application of recombinant human interferon include fever, headache, chills, fatigue, myalgia, nausea, vomiting or diarrhea, significant reduction of white blood cells and platelets, patients in group P did not show any adverse reactions during the follow-up period. There was no significant change in blood picture before and after treatment. For the safest and most minimal reduction of adverse drug reactions, reference. This study used low-dose recombinant human interferon-α2b injection for paravertebral injection. This topic will continue to study the optimal dose of recombinant human interferon-α2b injection for paresthetic neuralgia. DRG pulsed radiofrequency technique and paravertebral injection of recombinant human interferon-α2b combined with different mechanisms of action to control the condition of acute herpes zoster neuralgia, reduce the incidence of PHN, and obtain a better treatment with a single treatment.
The level of galectin-3 is a good biomarker for patients with inflammatory pain. Galectin-3 is a galactoside that binds to lectin and is also an important pro-inflammatory mediator, which is related to acute and chronic inflammation. Some scholars have studied that Gal-3 mRNA and protein are significantly increased in the spinal dorsal horn of mice after varicella-zoster virus infection. Gal-3 gene-deficient mice or intrathecal Gal-3 antibodies significantly reduce tactile pain, suggesting that Gal-3 is involved in the production of PHN.22 In addition, Gal-3 may mediate herpes zoster neuralgia via phagocytic cells and microglia, and the level of blood Gal-3 is positively correlated with the severity of neuropathic pain in patients.23 In the present study, the levels of Gal-3 in blood and were reduced in all patients after treatment. Compared with group C, blood levels of Gal-3 of patients in group P had lower. This is consistent with other study results.
The function of IL-6 is to regulate cell growth and differentiation, regulate immune response, acute-phase response, and play a very important role in the anti-infective immune response. Serum IL-6 levels are positively correlated with neurological damage, and high levels of IL-6 are key factors leading to the development of nerve damage and chronic pain and may play an important role in the formation of neuropathic pain.23 In this study, patients with pulsed radiofrequency combined with paravertebral injection of recombinant human interferon-α2b showed a significant decrease in blood Gal-3 and IL-6 levels 1 week after treatment, indicating that the patients’ pain was relieved and immunity increased; after treatment 8 weeks later, tend to be stable, indicating that after the condition improved, it tends to be stable, the nerve repair and the condition need to be improved for a long time, and it takes a long time to follow up.
This study is a retrospective analysis that relies on a chart review; therefore, the actual dose taken may differ from the dose recorded by the doctor’s advice, which is a limitation of our study. Other limitations of our study are the relatively small sample size and the short review time of the medical records. Further long-term observations are needed to determine the duration of the effect of DRG pulsed radiofrequency combined with injection of recombinant human interferon-α2b on paresthetic neuralgia.
Conclusion
In summary, the DRG pulsed radiofrequency combined with paravertebral injection of recombinant interferon-α2b is a more effective treatment for acute herpes zoster neuralgia, which can more effectively prevent the incidence of PHN.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (82001176), Natural Science Foundation of Zhejiang Province of China (LY20H090020, LGF20H090021, LQ19H090007), Medical and Health Science and Technology Research Program of Zhejiang Province (2021KY352, 2020RC124, 2020RC122, 2019KY687), Science and Technology Project of Jiaxing City (2020AY30009), Emergency Science and Technology Special Fund of Jiaxing City (2020GZ30001), Key Discipline Established by Zhejiang Province and Jiaxing City Jointly –Pain Medicine (2019-ss-ttyx), Key Discipline of Anesthesiology of Jiaxing City (2019-zc-06) and Jiaxing Key Laboratory of Neurology and Pain Medicine.
Author Contributions
M. Yao contributed to design of research, edited and revised manuscript; L. Xu performed acquisition of data, or analysis and interpretation of data; Y Fei drafted manuscript and performed research; B Huang revising manuscript critically for important intellectual content; J Deng performed research and prepared figures; All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Meyers JL, Candrilli SD, Rausch DA, Yan S, Patterson BJ, Levin MJ. Costs of herpes zoster complications in older adults: a cohort study of US claims database. Vaccine. 2019;37(9):1235–1244. doi: 10.1016/j.vaccine.2018.11.079 [DOI] [PubMed] [Google Scholar]
- 2.Mareque M, Oyaguez I, Morano R, Casado MA. Systematic review of the evidence on the epidemiology of herpes zoster: incidence in the general population and specific subpopulations in Spain. Public Health. 2019;167:136–146. doi: 10.1016/j.puhe.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 3.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821. doi: 10.1016/j.mayocp.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31(4):235–243. doi: 10.3344/kjp.2018.31.4.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding Y, Li H, Hong T, Zhao R, Yao P, Zhao G. Efficacy and safety of computed tomography-guided pulsed radiofrequency modulation of thoracic dorsal root ganglion on herpes zoster neuralgia. Neuromodulation. 2019;22(1):108–114. doi: 10.1111/ner.12858 [DOI] [PubMed] [Google Scholar]
- 6.Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS One. 2017;12(8):e0183559. doi: 10.1371/journal.pone.0183559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo S, Shen M, Zhang L, et al. The effect of interventional pain management on treating postherpetic neuralgia. Indian J Dermatol. 2019;64(3):251. doi: 10.4103/ijd.IJD_130_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y, Yao P, Li H, et al. CT-guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi: 10.3389/fnins.2019.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye L, Xiang T, Zhu J, et al. Interferon consensus sequence-binding protein 8, a tumor suppressor, suppresses tumor growth and invasion of non-small cell lung cancer by interacting with the wnt/beta-catenin pathway. Cell Physiol Biochem. 2018;51(2):961–978. doi: 10.1159/000495399 [DOI] [PubMed] [Google Scholar]
- 10.Guo YY, Nan J, Guo SP, et al. Efficacy evaluation of interferon alpha-2b in prevention of postherpetic neuralgia by paravertebral nerve block. Chin J Pain Med. 2015;21:830–839. [Google Scholar]
- 11.Raza A, Ovais M, Aziz H, et al. Evaluation of temporal virological responses to interferon-alpha-2b plus ribavirin among genotype 3a hepatitis C virus-infected patients. Intervirology. 2017;60(3):75–81. doi: 10.1159/000481913 [DOI] [PubMed] [Google Scholar]
- 12.Toth C. Substitution of gabapentin therapy with pregabalin therapy in neuropathic pain due to peripheral neuropathy. Pain Med. 2010;11(3):456–465. doi: 10.1111/j.1526-4637.2009.00796.x [DOI] [PubMed] [Google Scholar]
- 13.Bockbrader HN, Budhwani MN, Wesche DL. Gabapentin to pregabalin therapy transition: a pharmacokinetic simulation. Am J Ther. 2013;20(1):32–36. doi: 10.1097/MJT.0b013e318250f80e [DOI] [PubMed] [Google Scholar]
- 14.Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers. 2019;11(4):510. doi: 10.3390/cancers11040510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–356. doi: 10.1016/j.jpain.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 16.Apalla Z, Sotiriou E, Lallas A, Lazaridou E, Ioannides D. Botulinum toxin A in postherpetic neuralgia: a parallel, randomized, double-blind, single-dose, placebo-controlled trial. Clin J Pain. 2013;29(10):857–864. doi: 10.1097/AJP.0b013e31827a72d2 [DOI] [PubMed] [Google Scholar]
- 17.Parsons B, Argoff CE, Clair A, Emir B. Improvement in pain severity category in clinical trials of pregabalin. J Pain Res. 2016;9:779–785. doi: 10.2147/JPR.S102696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20(3):E411–E418. doi: 10.36076/ppj.2017.E418 [DOI] [PubMed] [Google Scholar]
- 19.Erdine S, Bilir A, Cosman ER, Cosman ER Jr. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–417. doi: 10.1111/j.1533-2500.2009.00317.x [DOI] [PubMed] [Google Scholar]
- 20.Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–613. [PubMed] [Google Scholar]
- 21.Cahana A, Vutskits L, Muller D. Acute differential modulation of synaptic transmission and cell survival during exposure to pulsed and continuous radiofrequency energy. J Pain. 2003;4(4):197–202. doi: 10.1016/S1526-5900(03)00554-6 [DOI] [PubMed] [Google Scholar]
- 22.Takasaki I, Taniguchi K, Komatsu F, et al. Contribution of spinal galectin-3 to acute herpetic allodynia in mice. Pain. 2012;153(3):585–592. doi: 10.1016/j.pain.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 23.Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther. 2018;184:145–158. doi: 10.1016/j.pharmthera.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]