Abstract
FTY720 is an immunosuppressive multiple sclerosis (MS) drug that stimulates the expression of neuroprotective brain-derived-neurotrophic-factor (BDNF). In vivo preclinical data suggest that FTY720 could be beneficial for treating Parkinson’s patients, though its immunosuppressive effects might limit its efficacy. Two novel FTY720-derivatives, FTY720-C2 and FTY720-Mitoxy, also stimulate BDNF expression and enter brain like FTY720 but are not phosphorylated, suggesting they will not produce FTY720-like immunosuppression. Using FTY720 as a positive control, we measured low and high dose FTY720-derivatives, which did not stimulate FTY720-like lymphopenia or immunosuppressive signaling. These findings support the further preclinical assessment of the derivatives as potential novel Parkinson’s therapies.
Keywords: Anti-inflammatory, Fingolimod, Neuroprotection
Parkinson’s disease (PD) is a neurodegenerative disorder in which motor symptoms develop after the loss of substantia nigra dopaminergic neurons (1). The substantia nigra in PD brain also exhibits loss of brain-derived neurotrophic factors (BDNF); while increasing BDNF in PD models protects nigral neurons (2). Furthermore, PD duration correlates with low serum BDNF levels, and exercise can stimulate BDNF expression which may help slow PD progression (3). New data reveal that treating A53T parkinsonian mice with FTY720 (fingolimod) increases gut BDNF protein and mRNA levels while reducing constipation (4), a common premotor PD symptom. In that same report, blocking BDNF signaling in mice accelerates constipation and α-synuclein aggregation that is counteracted by FTY720 (4). Moreover, FTY720 significantly improves motor behavior and reduces dopaminergic neurotoxicity in cell and mouse PD models (5,6). Thus, therapies that increase endogenous BDNF levels hold promise for treating both non-motor and motor symptoms of PD. Such therapies may also slow disease progression; though there are currently no BDNF-stimulatory drugs in the clinic. Many laboratories have shown that FTY720 improves endogenous BDNF expression, promotes neuroprotection, and enhances neurogenesis in animal models (4,5,7–10). Furthermore, FTY720 increases BDNF in dopaminergic cells and protects them from neurotoxicity (5,11). Additionally, upregulating BDNF with FTY720 in a Rett syndrome mouse model (10), encouraged fast-tracking of FTY720 to treat Rett syndrome children (ClinicalTrials.gov identifier NCT02061137), raising the possibility that the neurotrophin stimulating activity of FTY720 could benefit patients with PD.
While much recent work has focused on BDNF modulation by FTY720, the drug was originally approved as an immunomodulator that reduces ~80% of circulating blood lymphocytes (12,13), producing a condition called lymphopenia. FTY720-induced lymphopenia results after the prodrug is phosphorylated by ubiquitous sphingosine kinases to form FTY720-P, a sphingosine-1 phosphate analog (14). FTY720-P can bind sphingosine-1 phosphate receptor 1 (S1PR1) on T cells to prevent their egress from lymphoid tissues into circulating blood (13). This has long-been considered the main therapeutic effect of FTY720 for treating autoimmune-related neurological disorders like MS (12) or chronic-inflammatory-demyelinating-polyneuropathy (ClinicalTrials.gov identifier NCT01625182). And though more than 130,000 MS patients worldwide have safely used FTY720; the immunosuppressive effects of the drug can lead to reactivation of dormant infections or a more serious condition, progressive multifocal leukoencephalopathy. This activity contraindicates FTY720 use in certain individuals (12). It also raises concern about using FTY720 to stimulate BDNF expression in patients with non-autoimmune conditions, like PD. This makes it important to identify new compounds with FTY720-like neuroprotective benefits that will not induce lymphopenia.
We have created two FTY720-derivatives with modifications adjacent to the site that is phosphorylated on FTY720 (11), and both retain many positive characteristics of FTY720. For example, all three FTY720s stimulate BDNF expression in dopaminergic cells and protect against neurotoxicity (11). Like FTY720, both new derivatives also cross the brain blood barrier, yet unlike FTY720, neither is phosphorylated in human or rat liver hepatocytes (15). As mentioned above, phosphorylation of FTY720 leads to lymphopenia (13). Here we measured the effects of low dose and high dose FTY720-C2 and FTY720-Mitoxy on lymphopenia. Briefly, 6–12 week old C57BL/6 and Swiss Webster male and female mice were given single equivalent low molar doses by i.p. injection (1.25 mg/kg FTY720, 1.25 mg/kg FTY720-C2 or 2.5 mg/kg of FTY720-Mitoxy; 3 mice per drug, N = 9) modeling Morris et al. (13); or 10× subcutaneous high doses (12.5 mg/kg FTY720-C2 or 25 mg/kg of FTY720-Mitoxy; to 3 mice per drug, N = 6 total) to eliminate hepatic first pass effects. Blood was collected by tail-vein puncture into 4 heparinized micro-hematocrit tubes (VWR Micro Capillary Tubes, Radnor, PA, USA, 15401–560) per mouse per time point. Blood was collected just before drug (0 h) and at 24 h (Fig. 1A). Blood smears were prepared on glass microscope slides, air dried, and Wright-stained (Thermo Fisher, USA, Cat. 3121TS) (Fig. 1B). Lymphocytes and neutrophils were manually counted by bright field analysis of blood smear monolayers. For low dose experiments we counted 3 independent 2 mm2 fields on 3–5 slides per mouse per time point (0 h and 24 h). For high dose experiments we counted 100 total lymphocytes and neutrophils on 3–5 slides per mouse per time point (0 h and 24 h). Each set of data was evaluated by two different investigators, one of whom was blinded to treatment conditions. Data were analyzed using Student’s t-tests and normalized to % control ± SD for all experiments (GraphPad Prism 6, San Diego, CA, USA).
Fig. 1. FTY720-C2 or FTY720-Mitoxy treatment does not produce FTY720-like lymphopenia.
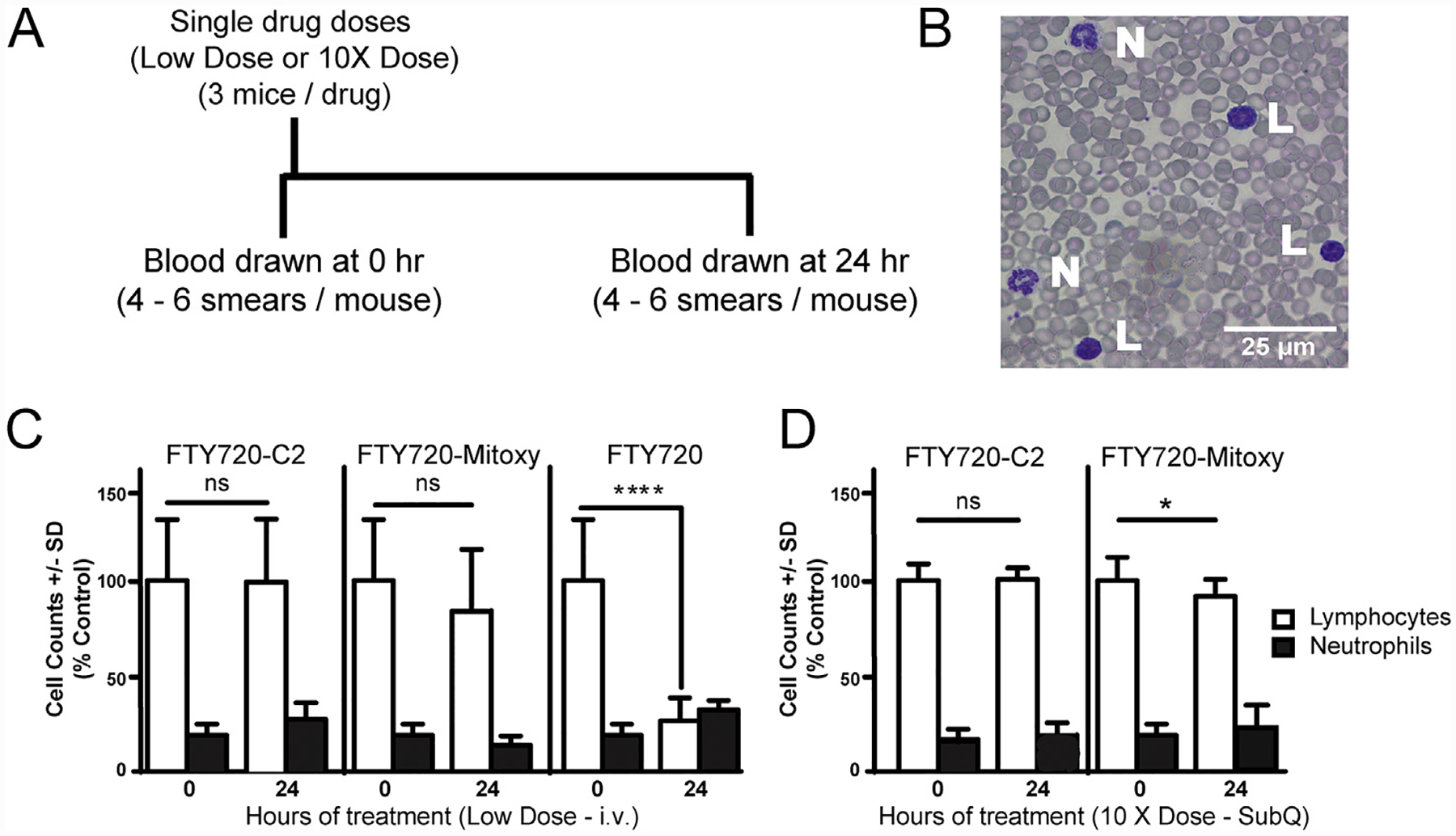
A. Timeline of drug dosing and blood collection for mice treated with low dose or high dose FTY720 or FTY720-derivatives. B. Bright field microscopic image from a representative blood smear in our studies, showing Wright-stained lymphocytes (L) and neutrophils (N) among many red blood cells in a single microscopic field. C. Cell counts from mice (n = 3/condition) treated with i.p. delivery of various FTY720s. FTY720, as a positive control, confirms that it can significantly decrease lymphocyte numbers in the blood within 24 h. No significant change in lymphocytes or neutrophils is noted in mice given low equivalent molar doses of intravenously delivered FTY720-C2 or FTY720-Mitoxy. D. Blood cell counts from mice (n = 3/condition) given high dose FTY720-C2 or FTY720-Mitoxy by subcutaneous delivery to avoid first-pass effects. No significant change in lymphocytes or neutrophils is noted after high dose FTY720-C2, and though lymphocyte numbers were significantly lower after high dose FTY720-Mitoxy, the effect does not replicate FTY720-associated lymphopenia. C and D, white bars = lymphocytes; black bars = neutrophils. SubQ = subcutaneous dosing. Scale bar = 25 μm. Student’s t-tests. Data represent the mean ± SD. *p < 0.05, ****p < 0.0001; ns = not significant.
As a positive control we used a dose of FTY720 that causes ~80% reduction in circulating lymphocytes at 24 h but has little effect on neutrophils (Fig. 1C, right data set), closely replicating the work of Morris et al. (13). Equivalent low molar doses of FTY720-C2 (Fig. 1C, left data set) or FTY720-Mitoxy (Fig. 1C, middle data set) also had little effect on lymphocytes or neutrophils. To eliminate hepatic first-pass metabolic effects related to i.p.-delivered drugs, we used 10× higher doses of FTY720-C2 and FTY720-Mitoxy, delivered subcutaneously. Overall, the high dose data (Fig. 1D) were similar to low dose data for both novel FTY720-derivatives (Fig. 1C), except that high dose FTY720-Mitoxy (Fig. 1D, right data set) reduced lymphocyte numbers by ~20%, which though significant, is within the normal range and far below the 80% loss induced by FTY720. High doses of FTY720-C2 and FTY720-Mitoxy were also well tolerated in mice.
Additionally, we evaluated FTY720-C2 and FTY720-Mitoxy in short term cell culture experiments in which we measured activation of the S1PR1 downstream kinase, ERK1/2 (10). In dopaminergic MN9D cells we modeled the work of Hait et al., who teased apart the effects of FTY720-P from FTY720 with regard to ERK1/2 (16). Briefly, MN9D cells were seeded 6 × 105 onto 6 well plates then treated 16–18 h later with Vehicle, or 1 μM FTY720-P, FTY720, FTY720-C2 or FTY720-Mitoxy prepared in the same vehicle solution. At 30 min, cells were lysed and equivalent amounts of total protein were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked, then incubated overnight in anti-pERK1/2 (Tyr204) (Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat. sc-7383) followed by anti-total ERK1/2 (Santa Cruz Biotechnology, Cat. sc-93) to quantify pERK1/2 to total ERK1/2 levels. Blots were imaged using Odyssey (LiCor Biosciences, Lincoln, NE, USA) and quantified using Image-Quant (GE Healthcare, Little Chalfont, UK). ERK1/2 data from 4 independent experiments were analyzed by ANOVA, using Dunnett’s multiple comparisons test for post-hoc analysis (Prism 6, GraphPad Inc.). This allowed us to show that while the positive control, FTY720-P, significantly increased pERK1/2 levels; unphosphorylated FTY720 and FTY720-derivatives did not significantly increase pERK1/2 levels (Fig. 2). These results also suggest that FTY720-C2 and FTY720-Mitoxy do not stimulate S1PR1 signaling in our cells, similarly to what Hait and colleagues saw using this method (16).
Fig. 2. Only FTY720-P significantly activates the S1PR1-downstream-kinase, ERK1/2 in 30 min treatments.
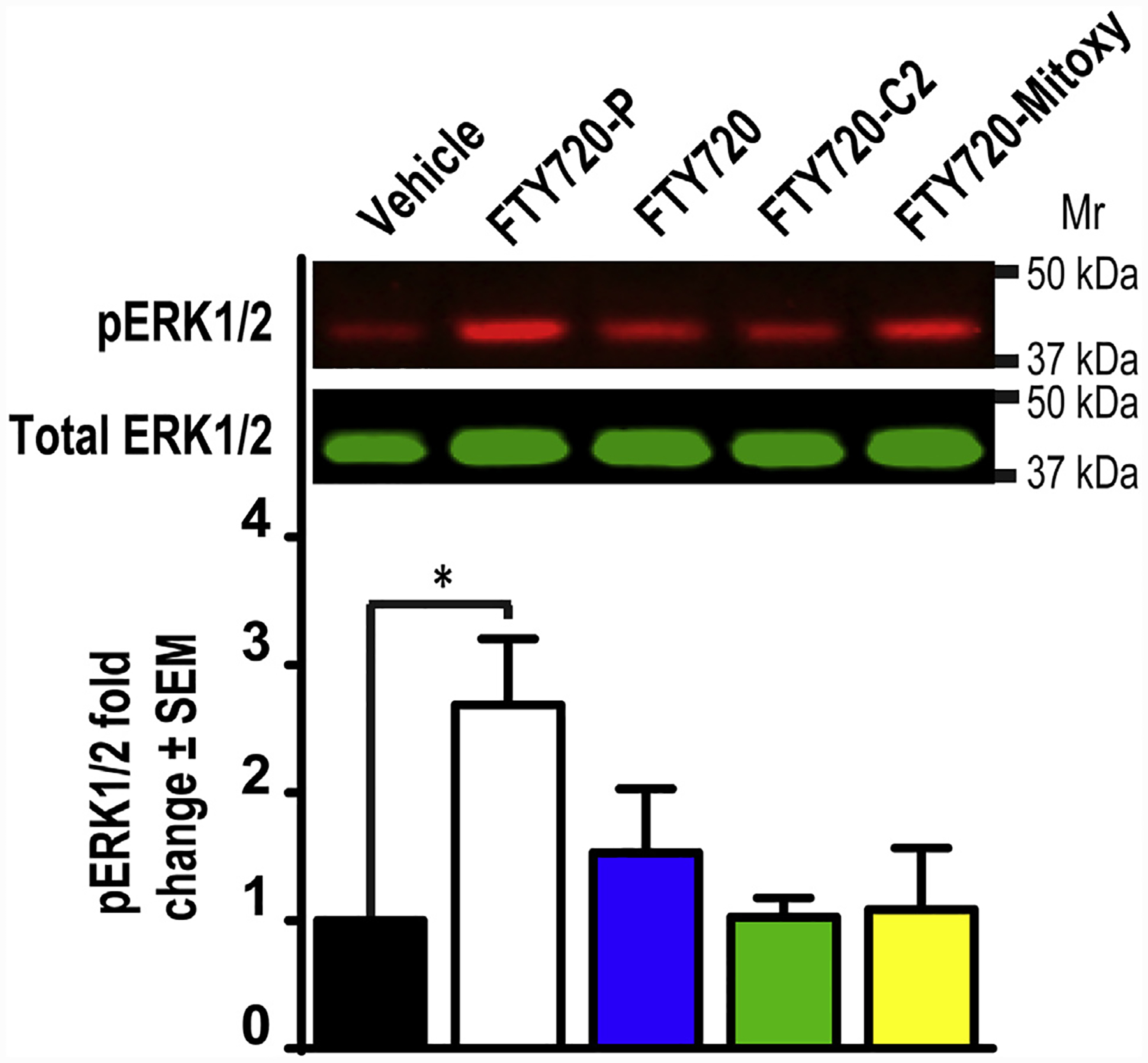
Representative immunoblots of MN9D lysates after 30 min treatment with Vehicle (control), 1 μM of FTY720-P, FTY720, FTY720-C2, or FTY720-Mitoxy prepared in the same control solution. ERK1/2 activation was assessed by probing immunoblots for pERK1/2 normalized to total ERK1/2. The histogram shows that only FTY720-P significantly increases pERK1/2 at the 30 min timepoint. Data were analyzed by ANOVA using Dunnett’s multiple comparisons test for post-hoc analysis. Data represent the mean ± SEM of 4 independent experiments. *p < 0.05.
We previously demonstrated that all three FTY720s increase BDNF mRNA (11) and our newly published data show that S1PR1-signaling through ERK1/2 activation is not necessary for FTY720-mediated increases in BDNF expression (17). This is also supported by our current findings in which only the positive control, FTY720, caused lymphopenia in vivo (Fig. 1C). As FTY720-mediated S1PR1 activation can also cause first-dose bradycardia in patients, our findings with FTY720-C2 and FTY720-Mitoxy further suggest that neither is likely to produce that effect. Finally, as both FTY720-C2 and FTY720-Mitoxy cross the blood brain barrier (15), they also have the potential to protect brain regions susceptible to neurodegeneration, such as substantia nigra in PD or frontal cortex in patients suffering from neuropathic pain. Taken together, the data encourage further preclinical assessment of FTY720-C2 and FTY720-Mitoxy for disorders where stimulating BDNF expression may counteract neuropathology and slow disease progression.
Acknowledgements
The authors thank Texas Tech University Health Sciences Center El Paso SABR Program (183119) (to TKB), and Hoy Family Research (243337) and Lizanell and Colbert Coldwell Foundation (243036) (RGP) for their generous support. We also thank Tania Fertl of the TTUHSC LARC for help with drug delivery and blood collection. This work is dedicated to D. Byer and M. J. Fox, and in loving memory of S. M. Hoy, J. Cordy, and L. “Rusty” Lanelli.
Footnotes
Conflict of interest statement
The corresponding author has filed a patent, “Compositions and Methods for the Treatment of Parkinson’s Disease”, US 20150290145, CA 2888634, which does not alter adherence to Journal of Pharmacological Sciences policies.
References
- (1).Kalia LV, Lang AE. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol. 2016;12(2):65–66. [DOI] [PubMed] [Google Scholar]
- (2).Rodrigues TM, Jeronimo-Santos A, Outeiro TF, Sebastiao AM, Diogenes MJ. Challenges and promises in the development of neurotrophic factor-based therapies for Parkinson’s disease. Drugs Aging. 2014;31(4):239–261. [DOI] [PubMed] [Google Scholar]
- (3).Hirsch MA, Iyer SS, Sanjak M. Exercise-induced neuroplasticity in human Parkinson’s disease: what is the evidence telling us? Parkinsonism Relat Disord. 2016;22 Suppl 1:S78–S81. [DOI] [PubMed] [Google Scholar]
- (4).Vidal-Martinez G, Vargas-Medrano J, Gil-Tommee C, Medina D, Garza NT, Yang B, et al. FTY720/fingolimod reduces synucleinopathy and improves gut motility in A53T mice: contributions of pro-brain-derived neurotrophic factor (Pro-BDNF) and mature BDNF. J Biol Chem. 2016;291(39):20811–20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ren M, Han M, Wei X, Guo Y, Shi H, Zhang X, et al. FTY720 attenuates 6-OHDA-associated dopaminergic degeneration in cellular and mouse Parkinsonian models. Neurochem Res 2016:1–11. [DOI] [PubMed] [Google Scholar]
- (6).Zhao P, Yang X, Yang L, Li M, Wood K, Liu Q, et al. Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB J. 2017;31(1): 172–179. [DOI] [PubMed] [Google Scholar]
- (7).Miguez A, Garcia-Diaz Barriga G, Brito V, Straccia M, Giralt A, Gines S, et al. Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75NTR up-regulation and astrocyte-mediated inflammation. Hum Mol Genet. 2015;24(17):4958–4970. [DOI] [PubMed] [Google Scholar]
- (8).Efstathopoulos P, Kourgiantaki A, Karali K, Sidiropoulou K, Margioris AN, Gravanis A, et al. Fingolimod induces neurogenesis in adult mouse hippocampus and improves contextual fear memory. Transl Psychiatry. 2015;5: e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Doi Y, Takeuchi H, Horiuchi H, Hanyu T, Kawanokuchi J, Jin S, et al. Fingolimod phosphate attenuates oligomeric amyloid beta-induced neurotoxicity via increased brain-derived neurotrophic factor expression in neurons. PLoS One. 2013;8(4):e61988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2012;109(35):14230–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Vargas-Medrano J, Krishnamachari S, Villanueva E, Godfrey WH, Lou H, Chinnasamy R, et al. Novel FTY720-based compounds stimulate neurotrophin expression and phosphatase activity in dopaminergic cells. ACS Med Chem Lett. 2014;5(7):782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ayzenberg I, Hoepner R, Kleiter I. Fingolimod for multiple sclerosis and emerging indications: appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag. 2016;12:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Morris MA, Gibb DR, Picard F, Brinkmann V, Straume M, Ley K. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur J Immunol. 2005;35(12):3570–3580. [DOI] [PubMed] [Google Scholar]
- (14).Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–897. [DOI] [PubMed] [Google Scholar]
- (15).Enoru JO, Yang B, Krishnamachari S, Villanueva E, DeMaio W, Watanyar A, et al. Preclinical metabolism, pharmacokinetics and in vivo analysis of new blood-brain-barrier penetrant fingolimod analogues: FTY720-C2 and FTY720-Mitoxy. PLoS One. 2016;11(9):e0162162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hait NC, Wise LE, Allegood JC, O’Brien M, Avni D, Reeves TM, et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci. 2014;17(7):971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Segura-Ulate I, Yang B, Vargas-Medrano J, Perez RG. FTY720 (Fingolimod) reverses alpha-synuclein-induced downregulation of brain-derived neurotrophic factor mRNA in OLN-93 oligodendroglial cells. Neuropharmacology 2017. 10.1016/j.neuropharm.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


