Abstract
Simple Summary
Raman Spectroscopy (RS) is an optical technique able to determine biochemical differences within a biological tissue, yet there are few reports of its application in fresh tissue. This potential could be relevant to detect glioma infiltration and to guide surgery to achieve a total resection. We deployed RS with machine learning algorithms in a surgical scenario to probe the diagnostic accuracy ex-vivo in discriminating between normal-appearing and fresh neoplastic tissue in patients undergoing glioma resection. Analyzing 3450 spectra from 63 samples, we identified 19 novel RS shifts. These 19 novel shifts of known biological relevance were included in the analytical workflow, leading to 83% and 82% accuracy and precision, respectively, in discriminating between normal-appearing and neoplastic tissue. This study further supported the translational development of real-time tissue analysis with RS in oncological brain surgery and yielded novel findings in the biochemical features of the brain tissue affected by glioma.
Abstract
Identifying tumor cells infiltrating normal-appearing brain tissue is critical to achieve a total glioma resection. Raman spectroscopy (RS) is an optical technique with potential for real-time glioma detection. Most RS reports are based on formalin-fixed or frozen samples, with only a few studies deployed on fresh untreated tissue. We aimed to probe RS on untreated brain biopsies exploring novel Raman bands useful in distinguishing glioma and normal brain tissue. Sixty-three fresh tissue biopsies were analyzed within few minutes after resection. A total of 3450 spectra were collected, with 1377 labelled as Healthy and 2073 as Tumor. Machine learning methods were used to classify spectra compared to the histo-pathological standard. The algorithms extracted information from 60 different Raman peaks identified as the most representative among 135 peaks screened. We were able to distinguish between tumor and healthy brain tissue with accuracy and precision of 83% and 82%, respectively. We identified 19 new Raman shifts with known biological significance. Raman spectroscopy was effective and accurate in discriminating glioma tissue from healthy brain ex-vivo in fresh samples. This study added new spectroscopic data that can contribute to further develop Raman Spectroscopy as an intraoperative tool for in-vivo glioma detection.
Keywords: Raman spectroscopy, neuro-oncology, classification, glioma, machine learning
1. Introduction
Gliomas have an infiltrative growth pattern. A key challenge is to discriminate tumor and healthy tissue during surgery, and to identify neoplastic cells infiltrating beyond evident pathological borders [1]. Identifying the residual tumor burden is crucial as the total resection represents a favorable prognostic factor [2,3]. Unlike extracranial oncological surgery procedures, the intra-axial masses cannot be removed by applying large safety margins based only on visible landmarks, due to the proximity to eloquent structures. Damaging functional tissue could cause neurological deficits with a negative impact on the subject [4]. The definition itself of tumor border is also demanding as glioma cells are known to infiltrate normal-appearing white matter and be undetectable with current diagnostic methods in-vivo [5]. Imaging and fluorescence-guidance are currently used to delineate the boundaries of the resection. However, these methods have limited sensitivity, specificity and spatial resolution in vivo [6].
Raman spectroscopy (RS), and other optical techniques such as Fourier Transform Infrared Spectroscopy (FTIR) [7,8], emerged as a tool to study the biochemical differences between tumor and healthy tissue. The rapid and non-destructive features of RS made it an engaging method to detect tumor cells intraoperatively. A quick, unbiased brain tumor detection technique during glioma resection is of paramount relevance to achieve additional quantitative diagnostic intra-operative information.
Previous studies investigated the use of the RS to discriminate between human and animal brain tissue [8,9,10,11,12,13] in vitro and in-vivo [8] and tumor and healthy tissue on sections obtained from preprocessed tissue blocks [8]. However, tissue processing (de-waxing, washing, and clearing with solvents) can affect tumors’ biochemical phenotype by altering different cellular lipid constituents [14]. Changes following a chemical treatment can alter the spectral signatures and reduce the spectral differences between normal and malignant tissue [15,16]. Only a few studies are available with data from fresh tissue [5,17,18] or in-vivo [11]. Most studies are performed on frozen or Formalin-Fixed Paraffin Preserved (FFPP) samples with known artefacts [19]. The use of fresh tissue obtained during surgery and analyzed right after excision with engineering tools [18] for spectra analysis was thus advocated for improving Raman measurements and ease clinical translation.
We aimed to evaluate RS’s ability to distinguish unaffected tissue from glioma ex-vivo on fresh tissue samples from biopsies of supratentorial glioma and to explore whether additional Raman peaks could be detected onto untreated brain tissue.
2. Materials and Methods
2.1. Experimental Design
This study deployed single-point Raman spectroscopy on 63 biopsies from adult subjects undergoing surgery for WHO grade II-IV gliomas. The number of spectra and histological groups are reported in (Table 1). The ethics committee approved the study (Study n. 595) performed according to the 1964 Declaration of Helsinki and later amendments. Safe supra-marginal resection was pursued as previously described [20] with imaging and neurophysiological guidance [6,21].
Table 1.
Numbers of specimens and histological diagnosis. n°: numbers.
| n° of Samples | n° of Spectra | |
| Glioma tissue | 38 | 2073 |
| Grade II | ||
| Astrocytoma, WHO grade II (IDH-mutant) | 0 (0) | - |
| Oligodendroglioma, WHO grade II (IDH-mutant) | 4 (10.5) | 220 |
| Grade III | ||
| Astrocytoma, WHO grade III (IDH-mutant) | 5 (13.2) | 259 |
| Oligodendroglioma, WHO grade III (IDH-mutant) | 12 (31.6) | 642 |
| Grade IV | ||
| Glioblastoma, WHO grade IV (IDH wild-type) | 14 (36.8) | 773 |
| Glioblastoma, WHO grade IV (IDH mutant) | 3 (7.9) | 179 |
| Normal Tissue | 25 | 1377 |
| Total | 63 | 3450 |
2.2. Sample Preparation and Analysis
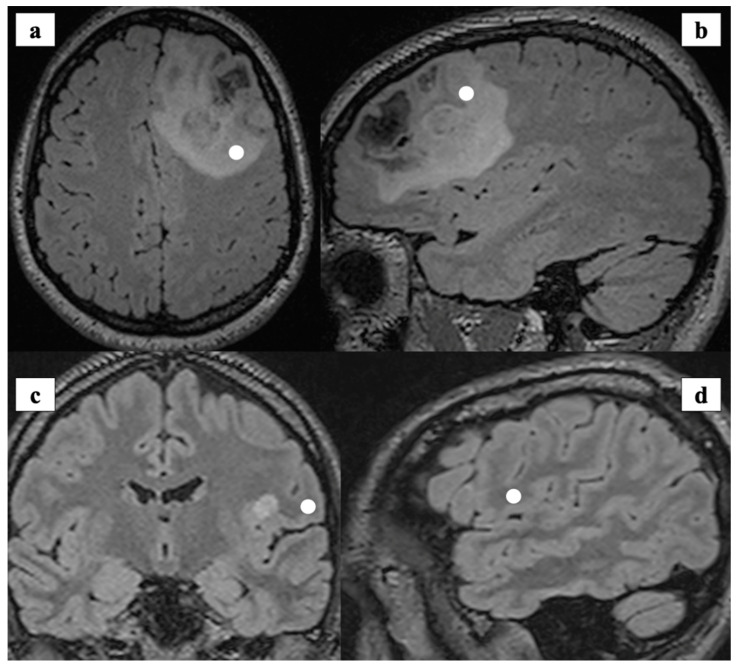
The bulk of the tumor tissue was sent for pathological and molecular analysis, according to the WHO classification [22]. The neurosurgeon collected different samples (tumor or healthy tissue) with image-guidance (Figure 1).
Figure 1.
(a,b): Preoperative MRI (axial and sagittal view) showing tumor consistent with Anaplastic Oligodendroglioma IDH-1 mutant, 1p/19q co-deleted grade III (WHO 2016). The white spots show the intraoperative site of tissue biopsy registered with Neuronavigation system and labelled as tumor. (c,d): Preoperative MRI (axial and sagittal view) showing the intraoperative location of tissue collection that was labelled as healthy. The specimen was collected (white spot) and analyzed during the surgical approach to a peri-insular ganglioglioma (Grade I, WHO 2016).
Tissue was defined as healthy if outside the pathological margins, as depicted by preoperative MR available during surgery on a neuronavigation platform (Curve, Brainlab, Munich, Germany). In particular, to collect healthy tissue, the surgeon targeted subcortical sites along the approach for deep-seated lesions, before encountering pathological areas, or at the boundaries of supratotal resection, as depicted by the neuronavigation. Conversely, tumor tissue was collected inside the lesion’s pathological margins, avoiding, when present, necrotic areas, as defined by the neuronavigation.
A sample section deemed surplus to diagnostic requirements was provided immediately after surgical resection to Raman analyst, washed with isotonic NaCl solution and placed under a CaF2 window for the analysis. At the end of Raman spectral extraction, samples were sent to a board-certified pathologist for histological and molecular evaluation. The board-certified pathologist was blinded to the RS findings and assessed the nature of the provided sample as either healthy or neoplastic. Each sample was marked as ‘‘Tumor’’ if diagnostic for the presence of glioma cells or ‘‘Healthy” if containing no tumor cells.
2.3. Raman Spectroscopic Acquisition
A benchtop RA800 Renishaw spectrometer system (Wotton-under-edge, Gloucestershire, UK) collected the Raman spectra. The system runs a Renishaw’s WiRE 4.0 software and is equipped with a near-infrared (NIR) laser (785 nm) with a maximum power of 500 mW. The initial spectral region of interest was 90–1.800 cm−1. We used an exposure time ranging from 0.5 to 2.5 s per spectrum. A range from 1 to 4 spectral accumulations for each acquisition was obtained. The power of the laser was kept at 100% for all the measurements. Spectra were obtained from randomly located points across the sample. The line-focus laser spreads the laser intensity across a line to minimize potential photodamage or fluorescence induction. An automatic performance quality check was performed on silicon and polystyrene internal standards for each measurement to reduce sample-to-sample variation. During the spectral acquisitions, we used the most optimal temperature and humidity conditions and the experimental parameters, such as exposure time, laser power and numbers of accumulations were iteratively modified for each point or area of interest, to optimize the signal and the spectra quality.
For each sample, we sought the maximum number of spectra with a higher signal-to-noise ratio with a punctual acquisition mode. We accomplished the analysis of each sample within 60 min from resection to minimize biological and chemical changing and to best simulate an in-vivo analysis [23].
2.4. Data Processing
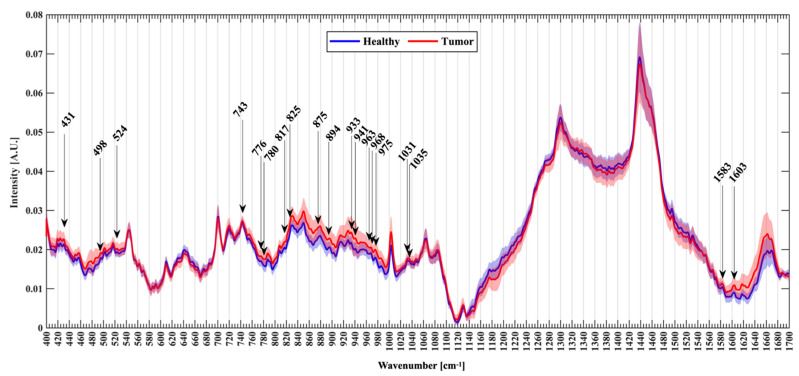
Pre-preprocessing to remove signal’s baseline drifts [24], cosmic rays and background artefacts [25] was performed before model classification. The data preprocessing (Matlab 2019b Mathworks, USA) followed these steps: (1) truncation of the spectral range to 400–1700 cm−1; (2) removal of saturated signal from the dataset using median filtering; (3) selection of spectra with Signal-to-Noise (S2N) ratio ≥3.5 around the phenylalanine 1004 cm−1 peak (4) background signal subtraction via Vancouver Raman Algorithm (VRA) method [26]; (5) outlier removal using interquartile range method and (6) signals normalization via root-mean-square (RMS). The resulting number of spectra for the classification were respectively 752 for healthy samples and 665 for tumors samples. (Figure 2) reported the normalized mean spectra with standard deviation for each group.
Figure 2.
Normalized mean spectra with standard deviation for healthy (blu) and tumor patients (red). Arrows mark the new Raman peaks identified.
The wavenumbers of all visible Raman peaks and slopes were identified on the mean Raman plots and compared to those previously reported in the literature, if present, to enhance the reliability of the current findings. We also recorded new prominent peaks at wavenumbers that were not present in the previous study on healthy vs. tumor tissue. We identified 135 Raman frequencies, and we extracted the intensity for each group as input features for the classification algorithms. The algorithms further refined the selection by identifying the minimum number of RS wavenumbers allowing to discriminate between the two groups with satisfying performance thresholds.
Two algorithms developed using Scikit-learn (http://scikit-learn.org, accessed on 26 October 2020) were used to assess classification performance with Leave-one-patient-out cross-validation: Random Forest (RF) and Gradient boosting trees (GB). A threshold between classification performance and robustness was achieved via hyperparameter tuning for each algorithm, along with a statistical feature selection (SciKitLearn FClassif—ANOVA test—top 60 features). An initial hyper-parameters optimization was performed for each classifier in a LOPO cross-validation. Each classifier was validated using a grid-search and 5-fold cross-validation. Parameters for the RF were set: (6,8,10,12) for the maximum tree depth, (50,100,150,200,250,300,350,400,450) for the maximum number of trees. Grid-search parameters for GB were set: estimators (100,150,200), max depth (5,8,10). As results of the optimization, optimal global parameters for classifiers were: RF numbers of trees: 150, max depth 5; while for GB were: numbers of estimators: 200 and max depth 5, learning rate at 0.1.
2.5. Statistical Analysis
Statistical analysis was performed among a subset of the top 60 Raman shifts provided by the classification algorithm to define potential new Raman shift useful to evaluate glioma tumoral tissue vs. healthy tissue. A Mann-Whitney test (two-tailed, α = 0.05) was performed, after checking normality using the Shapiro–Wilk test. (software SPSS statistics 24.0; IBM SPSS Inc., Chicago, IL, USA).
3. Results
We collected 3450 spectra from 63 different specimens: 1377 labelled as healthy and 2073 labelled as tumor (Table 1).
The results are reported in three sections. The mean spectra of the two groups were initially screened (i) to detect significant Raman peaks with possible biological relevance. The two classification algorithms (GB and RF) employed the intensity of the peaks identified at each shift as discrimination features. These algorithms were then (ii) tested for their discriminative power between neoplastic and normal tissue according to these features. Diagnostic accuracy, sensitivity, and specificity were computed. Finally, the algorithms identified 60 Raman peaks with the best discrimination power between the two groups. These 60 spectra were screened (iii) to analyze potential new peaks as compared to the published literature [5,19,27,28,29,30,31,32,33,34,35].
3.1. Classification Features and Algorithms
The algorithm analyzed the 135 Raman Peaks identified at the step (i), and the cross-validation loop identified 60 Peaks with the highest statistical significance and able to provide the highest performance in the two groups distinction. (Table 2) reported Raman Peaks with their biological assignments.
Table 2.
Tentative assignment of the main Raman shifts according to previous studies [5,18,26,27,28,29,30,31,32,33,34,35,36,37].
| Raman Shift (s−1) | Proposed Assignments |
|---|---|
| 431 * | Cholesterol/cholesterol ester |
| 450 | Ring torsion of phenyl |
| 457 | Proteins and cholesterol |
| 460 | Undefined |
| 474 | Glycogen and polysaccharides |
| 478 | Polysaccharides |
| 495 | Undefined |
| 498 * | Nucleic acids/nucleotides |
| 502 | Undefined |
| 517 | Undefined |
| 524 * | S-S disulfide stretching in proteins |
| 540 | n(S-S) trans-gauche-trans(amino acid cysteine) |
| 743 * | Heme (blood) |
| 754 | Symmetric breathing of tryptophan |
| 776 * | Phosphatidylinositol |
| 780 * | Uracil based ring breathing mode |
| 808 | Undefined |
| 817 * | C-C stretching/collagen assignment |
| 825 * | Phosphodiester |
| 826 | Tyr, proline |
| 837 | Undefined |
| 850 | Glycogen (high-grade tumors) |
| 853 | Glycogen (high-grade tumors) |
| 875 * | Choline and phospholipids |
| 880 | Tryptophan, d(ring) |
| 881 | Hydroxyproline and tryptophan (collagen); sterol ring stretch of cholesterol; asymmetric stretching of choline |
| 883 | ρ (CH2) (protein assignment) |
| 893/4 * | Phosphodiester (nucleic acids) |
| 903 | Undefined |
| 916 | Undefined |
| 927 | Undefined |
| 928 | Amino acids proline & valine (protein band) |
| 933 * | Proline, hydroxyproline |
| 934 | C-C backbone (collagen assignment) |
| 941 * | Glycogen |
| 950 | single bond stretching vibrations for the amino acids proline and valine and polysaccharides |
| 954 | Undefined |
| 958 | Stretching vibrations of PO4 in hydroxyapatite |
| 963 * | Protein assignments |
| 968 * | Lipids |
| 975 * and 977 | Tricalcium phosphate Ca3(PO4) calcification seen in schwannoma and necrosis |
| 1003 | Ring breathing mode of phenylalanine of protein |
| 1030 | Phenylalanine (protein assignment) and collagen |
| 1031 * | Phenylalanine (protein assignment) |
| 1035 * | Collagen |
| 1036 | Undefined |
| 1122 | Glycogen |
| 1337 | Aliphatic amino acids, including tryptophan, nucleic acids. Glycogen in necrosis |
| 1342 | Aliphatic amino acids, including tryptophan, nucleic acids. Glycogen |
| 1578 | C-C stretch of protein, nucleic acids |
| 1581 | C-C stretch of protein, nucleic acids |
| 1583 * | C-C stretch of protein, phenylalanine, nucleic acids |
| 1603 * | Phenylalanine & tyrosine/Oxygenated haemoglobin |
| 1603 | Cytosine, tyrosine, phenylalanine |
| 1614 | Aromatic amino acids (protein); Tyrosine e proline |
| 1616 | C-C stretching mode of tyrosine & tryptophan |
| 1657 | Amide I (a-helix) (protein) |
| 1659 | Amide I (a-helix) |
| 1660 | Amide I band |
Asterisk (*) marks new Raman peaks used for the first time for discrimination between glioma and healthy tissue in fresh samples.
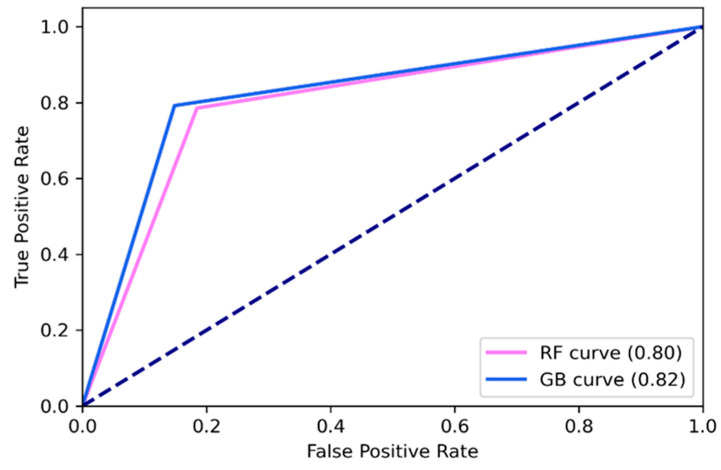
The GB method displayed better performance in distinguishing neoplastic from the normal tissue, with an accuracy of 83%, precision of 82%, recall 82% and F1_score 82%, computed based on tumor detection outcomes of True Positive (TP), True Negative (TN), False Positive (FP), and False Negative (FN). Conversely, The RF method displayed accuracy of 80%, precision of 79%, recall 80% and F1_score 80%.
Receiver Operating Characteristic (ROC) graphs were used to select models for their performance with respect to the False Positive Rate (FPR) and True positive Rate (TPR), and to compute the Area Under the Curve (AUC) to characterize the performance of the classification model (Figure 3). The ROC graphs showed that GB was the best to discriminate normal from tumor samples (all grades and types of glioma), with AUC of 0.82 respect to 0.80 of RF.
Figure 3.
Receiver operating characteristic curve for Random Forest (RF) and Gradient boosting trees (GB).
Table 3 showed a summary of the performance of the classification models.
Table 3.
Results of classification models for normal vs. glioma tissue biopsies using Raman Spectroscopy.
| Performance Metrics | Random Forest | Gradient Boosting |
|---|---|---|
| Accuracy | 0.80 | 0.83 |
| Precision | 0.79 | 0.82 |
| Recall | 0.80 | 0.82 |
| F1-score | 0.80 | 0.82 |
3.2. Spectral Analysis
The spectral analysis from the Raman active functional groups of nucleic acids, proteins and lipids allowed a thorough characterization of the neoplastic and normal brain biopsies. The average Raman spectra showed differences in the molecular signature between neoplastic and normal specimens (Figure 2). These differences are consistent with previous results from ex-vivo tissue samples [19,27,28,31,32,33,34,35,36].
The band 1003 cm−1 reflects the phenylalanine content of the sample as well as the band at 1030 and 1031 cm−1. In both groups, we easily recognize very intense bands around 1300, 1440 and a less intense peak around 1660 cm−1. These bands are related to protein and lipid. Other bands are visible between 1000 and 1100 cm−1, such as band at 1064 cm−1 (phospholipids and cholesterol stronger in normal than tumoral tissue), at 1080–1090 cm−1 (nucleic acids) and at 1126–1133 cm−1 (phospholipids (side-chains) and cholesterol). At lower wavenumbers the cluster of peaks between 419 cm−1 and 740 cm−1 showed contribution from cholesterol, cholesterol ester (427–430 cm−1, 457 cm−1, 545 cm−1) and choline (719 cm−1). Tryptophan contribution at 563 cm−1 is usually higher in glioblastoma and oligodendroglioma [37]. The 720:701 cm−1 intensity ratio of the mean spectra is increased in Tumor group (0.93 vs. 0.88) and probably reflecting the contribution of glioblastoma biopsies of the samples [32]. Raman bands related to heme are visible at 743 cm−1 while in 830, 850 cm−1 and 880 cm−1 are visible peaks respectively related to DNA, glycogen and tyrosine that are usually higher in high-grade tumors than in normal tissue.
The regions around 1300 cm−1 (p < 0.001), 1439–1441, and 1740 cm−1 (p < 0.001) are reduced in Tumor group and are related to fatty acids, CH2/CH3 deformation of lipids side chains, proteins, amino acids, cholesterol/cholesterol ester [30] usually present in normal with matter and ester group (1740 cm−1). The band at 1337 cm−1 is less intense in the Healthy specimens (p < 0.001) and is related to aliphatic amino acids, including tryptophan, nucleic acids and glycogen found in necrosis. The bands at 1003 cm−1 (p < 0.001) and 1031 cm−1 (p < 0.001) significantly different in phenylalanine in the two groups that are usually higher in glioma than normal tissue and astrocyte [30,38]. An increased DNA content related to band intensities at 498 cm−1 (p < 0.001), 830 cm−1 (p < 0.001) and 1087 cm−1 (p < 0.001) was also found in the Tumor group. A reduced lipid/cholesterol content on bands at 419, 427–430, 615, 968 cm−1 (all p < 0.001) is seen in the Healthy group. Intensities of bands related to proteins were significantly different (826, 880, 881, 883, 927, 934, 950, 963, 1035, 1583 1603–1616 cm−1 and 1660 cm−1, p < 0.001) between the two groups underling a changed protein profile related to tumoral proliferation.
3.3. Novel Raman Shifts
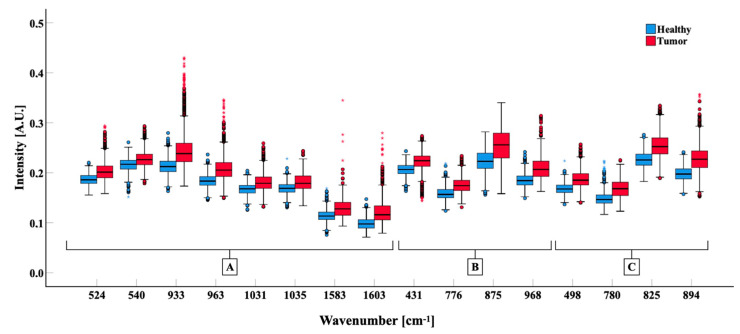
Among the top 60 Raman shifts features provided by the classification algorithm of the current study as highly significant in the distinction between normal and neoplastic tissues, 28 were previously described [5,8,11,17,18,19,33,39,40,41,42,43,44,45,46,47], corresponding at the following shifts: 457, 540, 754, 826, 850, 853, 880, 881, 883, 903, 927, 928, 934, 950, 958, 977, 1003, 1030, 1122, 1337, 1578, 1581, 1604, 1614, 1616, 1657, 1659, 1660 cm−1 (Table 2). We thus focused on the new 19 Raman Shifts with documented biological significance. These 19 bands were not used before in discriminating between glioma and healthy tissue. The intensity of Raman shift at each band resulted different between the two classes of samples, i.e., between tumor and normal tissues, underlining their biological difference [29,30]. The analysis confirmed statistically significant differences (p < 0.001) between normal and neoplastic tissue biopsies in the new 19 Raman shifts related to proteins (524, 933, 963, 1031, 1035, 1583, 1603 cm−1), nucleic acids (498, 780, 825, and 894 cm−1), lipids (431, 776, 875, 968 cm−1), collagen (817 cm−1), glycogen (941 cm−1), heme content (743 cm−1) and calcification (975 cm−1) (Figure 4).
Figure 4.
Comparison of the band intensities of Healthy (blue) and Tumor (red) biopsies grouped for biological significance. Box-plots show 25°, 50°, 75° percentiles (box) and 5°–95° (whiskers) percentile of data distribution; the median of groups is indicated with a black bar. Mann–Whitney test was calculated for all bands showed (p < 0.001). (A) bands related to protein. (B) bands related to lipids. (C) Bands related to nucleic acids.
4. Discussion
This study demonstrated that a benchtop Raman Spectroscopy machine could distinguish between normal and neoplastic tissue in fresh tissue samples ex-vivo with good accuracy, sensitivity, and specificity when paired with supervised machine learning techniques. These findings added new evidence to preliminary results provided by the few available studies [5,18,42]. The study also identified 19 bands that had not been previously described that were different between pathological and normal tissue. About the two machine learning classification models, the GB had a better performance in classifying the tissue samples.
The balance between the patient’s functional integrity and the extent of tumor resection is fundamental: non-invasive tools providing rapid tissue assessment are thus advocated. Surgery is one of the most important prognostic factors in treatment as evidences that a more significant surgical safe resection leads to improved outcome are continuously emerging [20]. These goals cannot always be achieved due to the infiltrating behavior of gliomas. The challenge of contemporary neuro-oncological surgery is also to find the proper balance between tumor resection and the patient’s functional integrity [20] and to detect infiltrating neoplastic cells beyond pathological margins; an intra-operative tumor detecting technology can thus be strategic to enhance surgery, especially at the limit of resection where tumor infiltration can still be present but a level lower than the tumor core and the detection threshold of available methods. The decision regarding a further resection is to be critically balanced with an increased risk of new-onset neurological morbidity, that is more likely just at this limit, where surgery is at closer proximity to eloquent structures.
4.1. Raman Spectroscopy with Fresh Tissue Samples in an Ex-Vivo Scenario
The Raman system was next to the operating room, and the spectral measurements were acquired within 60 min from multiple regions of the fresh tissue immediately after surgical resection. The acquisition time was typically on the order of seconds for a single spectrum. No samples processing or manipulation was performed before optical analysis. In-vivo tissue discrimination between healthy and tumoral tissue needs data from fresh samples that can make Raman analysis more precise and capable to characterize the tissue even in the presence of biological contaminants and with a low number of total spectra acquired, to keep analytical time at minimum and be compatible with a regular surgical workflow. During surgery time is limited for spectral acquisition: an analytical algorithm able to extract as much information as possible from Raman spectra with reliable accuracy can thus improve the detection process. This study is among the few [5,18,42] available where untreated and unfrozen tissue is analyzed to best approximate an intra-operative scenario. The data from cryosection and FFPP tissue blocks can contain different biological artefact [19,48]. The presence of freeze artefact in samples can low the discrimination power, hampering an accurate analysis and changing the molecular structure of the biopsies [19]. Yet, frozen sections are currently more available and relatively rapid, but they can also be hampered by the compulsory availability of neuropathologic expertise. If robust, the RS herein reported has the potential to be automated and deployed directly by brain surgeons.
This study, consistently with previous results [49], demonstrated that Raman spectroscopy could detect changes in the chemical composition and molecular structure between pathological and healthy fresh tissue. The spectral characteristics of tumoral and healthy tissue properly matched with those established on the topic [49]. Machine learning algorithms allowed to find 60 best representative Raman shifts among over 135 bands analyzed.
4.2. Characterization of Raman Shifts
Out of 60 bands, 28 had already been previously described as different between healthy and tumor samples [5,8,11,17,18,19,33,39,40,41,42,43,44,45,46,47]. In particular, distribution in relative intensity of these bands was related to well-known differences in the biology and structural composition of tumoral and brain tissue such as DNA Amide I, protein and calcification that can be seen in the necrotic tumor core. Among these 60 top characterizing Raman bands, new shifts related to proteins (524, 933, 963, 1031, 1035, 1583, 1603 cm−1), nucleic acids (498, 780, 825 cm−1, and 894 cm−1), lipids (431, 776, 875, 968 cm−1), collagen (817 cm−1) and calcification within the samples (975 cm−1) were identified and characterized as different between healthy and glioma biopsies. Only once peaks at 498, 875, 934 cm−1 and 1031 cm−1 were previously reported [31], yet from the analysis of 6 frozen glioma samples and with no comparison with healthy tissue. The biological importance of these bands in gliomas samples is therefore strengthened by the findings of the current study. In the future, authors could include these 19 Raman shifts that are all related to well-known biological compounds with coherent biological differences between healthy and neoplastic tissue to improve the accuracy of Raman classification methods in untreated tissue and in an in-vivo scenario.
4.3. Diagnostic Performance of Machine Learning Classification Models
The RF and GB displayed an overall good performance and allowed to distinguish tumor from the normal brain with an accuracy respectively of 80% and 83%. This performance matched that reported in previous works [13,41], ranging from 85% to about 99%. However, it resulted lower to studies done in a similar ex- and in-vivo setting with untreated tissues to discriminate between tumor and normal tissue [5,11,18]. The performance is also lower than that obtained from datasets on frozen and FFFP section, where, however, a longer preparation step can make a potential intra-operative application less likely and alter the biochemical properties of the sample. Analyzing the distinct pre-analytical and analytical phases of our workflow, we collected evidence leading to arguing that well-controlled acquisition setting ex- and in-vivo and the tissue conservative status are an essential preprocessing step to grant optimal signal acquisition and, thus, final analytical output. The differences identified by the employed algorithms between the normal and the neoplastic tissues resulted consistent with previous findings, as for what machine learning as a separate step is concerned. The latter argument contributes to increasing the likelihood that this technique and workflow can be effectively reproduced.
4.4. Study Limitations
The time requested for analysis is higher than that previously described [5]. However, a learning effect was observed during the study, with lower analytical time with later samples. Data regarding diagnostic power in discriminating relevant molecular features are lacking [50] and should also be addressed to probe the diagnostic accuracy in this relevant issue. The limited number of cases also hamper a broader analysis of the data. However, given the few clinical series currently reported, even such a small clinical cohort can still represent a useful contribution to the field. It should also be remarked that no specific marker exists in IDH-wild type gliomas that absolutely rule out a single-cell infiltration; the definition of normal tissue should be deployed accordingly. Although developed in a real surgical scenario, this study was not powered and designed to assess how RS can impact the surgical practice itself and whether the RS can actually modify the surgical behavior, such as yielding to broader resection, a better outcome with lower morbidity and improved detection of neoplastic infiltration, for instance during recurrent disease after previous multimodal treatment or with non-enhancing gliomas, that show no intra-operative fluorescence [51].
We aimed at providing preliminary technical and clinical advances on dealing with untreated tissue, to explore whether this translational study can lead to further development of an intra-operative technology that could be considered safe, accurate and reliable to be ultimately deployed in-vivo. Future in-vivo studies should address these issues, evaluating the impact on surgical resection and outcome, upon findings provided by this and similar studies [5].
5. Conclusions
RS reliably discriminated glioma tumor and normal tissue on fresh tissue. Nineteen new Raman peaks useful to discriminate between glioma and healthy tissue were identified. The analysis of these new bands can support further development of real-time tissue analysis, increasing the accuracy and efficiency in a neurosurgical scenario. This study added a robust contribution to the application of this technology to oncological brain surgery. A refined investigation of the biochemical correspondences between the Raman results and the molecular biology of the lesion could provide insights into gliomagenesis and infiltrative behavior.
Acknowledgments
Martin Isabelle, David Reece and Paul Felder (Renishaw plc, Wotton-under-edge, Gloucestershire, UK) are kindly acknowledged for their contribution to the study. Spectroscopic equipment was loaned by Renishaw plc.
Abbreviations
1p19q codeletion: codeletion of chromosome arms 1p and 19q. AUC: Area under the curve. FFPP: Formalin-Fixed Paraffin Preserved. GB: Gradient Boosting Machine. IDH: isocitrate dehydrogenase. ROC: Receiver Operating Characteristic. RF: Random Forest Trees. WHO: World Health Organization. RS: Raman Spectroscopy
Author Contributions
Study Conception and Design: M.R. (Marco Riva), T.S., R.S., E.D.A., E.D.M., L.B. Acquisition of Data: E.D.A., T.S., M.R. (Marco Riva), M.C.N., L.G., L.B., B.F., M.R. (Marco Rossi). Analysis and Interpretation of Data: M.R. (Marco Riva), T.S., R.S., E.D.A., S.M. Drafting of Manuscript: M.R. (Marco Riva), T.S., R.S. Revision and approval of the manuscript: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s EU Research and Innovation program Horizon 2020 under grant agreement no 688279.
Institutional Review Board Statement
The institutional ethics committee approved the study (authorization no. 595) that was performed in accordance with the 1964 Declaration of Helsinki and later amendments.
Informed Consent Statement
All subjects signed a written informed consent before the surgical procedure to adhere to the study
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riva M., Lopci E., Gay L.G., Nibali M.C., Rossi M., Sciortino T., Castellano A., Bello L. Advancing Imaging to Enhance Surgery: From Image to Information Guidance. Neurosurg. Clin. North Am. 2020;32:31–46. doi: 10.1016/j.nec.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N., Polley M.-Y., McDermott M.W., Parsa A.T., Berger M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011:3–8. doi: 10.3171/2011.2.JNS10998. [DOI] [PubMed] [Google Scholar]
- 3.Smith J.S., Chang E.F., Lamborn K.R., Chang S.M., Prados M.D., Cha S., Tihan T., Vandenberg S., McDermott M.W., Berger M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 4.Talos I.-F., Zou K.H., Ohno-Machado L., Bhagwat J.G., Kikinis R., Black P.M., Jolesz F.A. Supratentorial low-grade glioma resectability: Statistical predictive analysis based on anatomic MR features and tumor characteristics. Radiology. 2006;239:506–513. doi: 10.1148/radiol.2392050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore L.J., Isabelle M., Bell I.M., Edgar O., Voets N.L., Stacey R., Ansorge O., Vallance C., Plaha P. Raman spectroscopy to differentiate between fresh tissue samples of glioma and normal brain: A comparison with 5-ALA–induced fluorescence-guided surgery. J. Neurosurg. 2020:1–11. doi: 10.3171/2020.5.JNS20376. [DOI] [PubMed] [Google Scholar]
- 6.Riva M., Hennersperger C., Milletari F., Katouzian A., Pessina F., Gutierrez-Becker B., Castellano A., Navab N., Bello L. 3D intra-operative ultrasound and MR image guidance: Pursuing an ultrasound-based management of brainshift to enhance neuronavigation. Int. J. Comput. Assist. Radiol. Surg. 2017 doi: 10.1007/s11548-017-1578-5. [DOI] [PubMed] [Google Scholar]
- 7.Cameron J.M., Conn J.J.A., Rinaldi C., Sala A., Brennan P.M., Jenkinson M.D., Caldwell H., Cinque G., Syed K., Butler H.J., et al. Interrogation of IDH1 Status in Gliomas by Fourier Transform Infrared Spectroscopy. Cancers. 2020;12:3682. doi: 10.3390/cancers12123682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gajjar K., Heppenstall L.D., Pang W., Ashton K.M., Trevisan J., Patel I.I., Llabjani V., Stringfellow H.F., Martin-Hirsch P.L., Dawson T., et al. Diagnostic segregation of human brain tumours using Fourier-transform infrared and/or Raman spectroscopy coupled with discriminant analysis. Anal. Methods. 2013;5:89–102. doi: 10.1039/C2AY25544H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer T., Bergner N., Bielecki C., Krafft C., Akimov D., Romeike B.F.M., Reichart R., Kalff R., Dietzek B., Popp J. Nonlinear microscopy, infrared, and Raman microspectroscopy for brain tumor analysis. J. Biomed. Opt. 2011;16:021113. doi: 10.1117/1.3533268. [DOI] [PubMed] [Google Scholar]
- 10.Ji M., Orringer D.A., Freudiger C.W., Ramkissoon S., Liu X., Lau D., Golby A.J., Norton I., Hayashi M., Agar N.Y.R., et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 2013;5:201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jermyn M., Mok K., Mercier J., Desroches J., Pichette J., Saint-Arnaud K., Bernstein L., Guiot M.-C.C., Petrecca K., Leblond F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015;7:274ra19. doi: 10.1126/scitranslmed.aaa2384. [DOI] [PubMed] [Google Scholar]
- 12.Kircher M.F., de la Zerda A., Jokerst J.V., Zavaleta C.L., Kempen P.J., Mittra E., Pitter K., Huang R., Campos C., Habte F., et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong K., Kendall C., Stone N., Notingher I. Raman spectroscopy for medical diagnostics—From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015;89:121–134. doi: 10.1016/j.addr.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Surmacki J., Brozek-Pluska B., Kordek R., Abramczyk H. The lipid-reactive oxygen species phenotype of breast cancer. Raman spectroscopy and mapping, PCA and PLSDA for invasive ductal carcinoma and invasive lobular carcinoma. Molecular tumorigenic mechanisms beyond Warburg effect. Analyst. 2015;140:2121–2133. doi: 10.1039/C4AN01876A. [DOI] [PubMed] [Google Scholar]
- 15.Crow P., Ritchie A., Wright M., Kendall C., Stone N. Evaluation of Raman spectroscopy to provide a real time, optical method for discrimination between normal and abnormal tissue in the prostate. Eur. Urol. Suppl. 2002;1:92. doi: 10.1016/S1569-9056(02)80356-6. [DOI] [Google Scholar]
- 16.Krishna C.M., Sockalingum G.D., Venteo L., Bhat R.A., Kushtagi P., Pluot M., Manfait M. Evaluation of the suitability of ex vivo handled ovarian tissues for optical diagnosis by Raman microspectroscopy. Biopolymers. 2005;79:269–276. doi: 10.1002/bip.20346. [DOI] [PubMed] [Google Scholar]
- 17.Jermyn M., Desroches J., Mercier J., Tremblay M.-A., St-Arnaud K., Guiot M.-C., Petrecca K., Leblond F. Neural networks improve brain cancer detection with Raman spectroscopy in the presence of operating room light artifacts. J. Biomed. Opt. 2016;21:094002. doi: 10.1117/1.JBO.21.9.094002. [DOI] [PubMed] [Google Scholar]
- 18.Lemoine É., Dallaire F., Yadav R., Agarwal R., Kadoury S., Trudel D., Guiot M.C., Petrecca K., Leblond F. Feature engineering applied to intraoperative: In vivo Raman spectroscopy sheds light on molecular processes in brain cancer: A retrospective study of 65 patients. Analyst. 2019;144:6517–6532. doi: 10.1039/C9AN01144G. [DOI] [PubMed] [Google Scholar]
- 19.Kalkanis S.N., Kast R.E., Rosenblum M.L., Mikkelsen T., Yurgelevic S.M., Nelson K.M., Raghunathan A., Poisson L.M., Auner G.W. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neurooncol. 2014;116:477–485. doi: 10.1007/s11060-013-1326-9. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M., Gay L., Ambrogi F., Nibali M.C., Sciortino T., Puglisi G., Leonetti A., Mocellini C., Caroli M., Cordera S., et al. Association of Supratotal Resection with Progression-Free Survival, Malignant Transformation, and Overall Survival in Lower-Grade Gliomas. Neuro. Oncol. 2020 doi: 10.1093/neuonc/noaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riva M., Hiepe P., Frommert M., Divenuto I., Gay L.G., Sciortino T., Nibali M.C., Rossi M., Pessina F., Bello L. Intraoperative computed tomography and finite element modelling for multimodal image fusion in brain surgery. Oper. Neurosurg. 2020;18:531–541. doi: 10.1093/ons/opz196. [DOI] [PubMed] [Google Scholar]
- 22.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 23.Eiseman E., Bloom G., Brower J., Clancy N., Olmsted S.S. Case Studies of Existing Human Tissue Repositories “Best Practices” for a Biospecimen Resource for the Genomic and Proteomic Era. Rand Corporation; Santa Monica, CA, USA: 2013. [Google Scholar]
- 24.Barman I., Kong C.-R., Singh G.P., Dasari R.R. Effect of photobleaching on calibration model development in biological Raman spectroscopy. J. Biomed. Opt. 2011;16:011004. doi: 10.1117/1.3520131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Henson M.J. A practical algorithm to remove cosmic spikes in Raman imaging data for pharmaceutical applications. Appl. Spectrosc. 2007;61:1015–1020. doi: 10.1366/000370207781745847. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J., Lui H., McLean D.I., Zeng H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl. Spectrosc. 2007;61:1225–1232. doi: 10.1366/000370207782597003. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee H.N., Zhang L. Deciphering the finger Prints of Brain Cancer Astrocytoma in comparison to Astrocytes by using near infrared Raman Spectroscopy. Mol. Cell. Biochem. 2007;295:237–240. doi: 10.1007/s11010-006-9278-4. [DOI] [PubMed] [Google Scholar]
- 28.Krafft C., Sobottka S.B., Schackert G., Salzer R. Raman and infrared spectroscopic mapping of human primary intracranial tumors: A comparative study. J. Raman Spectrosc. 2006;37:367–375. doi: 10.1002/jrs.1450. [DOI] [Google Scholar]
- 29.Movasaghi Z., Rehman S., Rehman I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007;42:493–541. doi: 10.1080/05704920701551530. [DOI] [Google Scholar]
- 30.Brusatori M., Auner G., Noh T., Scarpace L., Broadbent B., Kalkanis S.N. Intraoperative Raman Spectroscopy. Neurosurg. Clin. N. Am. 2017;28:633–652. doi: 10.1016/j.nec.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Bergner N., Krafft C., Geiger K.D., Kirsch M., Schackert G., Popp J. Unsupervised unmixing of Raman microspectroscopic images for morphochemical analysis of non-dried brain tumor specimens. Proc. Anal. Bioanal. Chem. 2012;403:719–725. doi: 10.1007/s00216-012-5858-1. [DOI] [PubMed] [Google Scholar]
- 32.Köhler M., MacHill S., Salzer R., Krafft C. Characterization of lipid extracts from brain tissue and tumors using Raman spectroscopy and mass spectrometry. Anal. Bioanal. Chem. 2009;393 doi: 10.1007/s00216-008-2592-9. [DOI] [PubMed] [Google Scholar]
- 33.Krafft C., Shapoval L., Sobottka S.B., Geiger K.D., Schackert G., Salzer R. Identification of primary tumors of brain metastases by SIMCA classification of IR spectroscopic images. Biochim. Biophys. Acta Biomembr. 2006;1758:883–891. doi: 10.1016/j.bbamem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Koljenović S., Schut T.B., Vincent A., Kros J.M., Puppels G.J. Detection of meningioma in dura mater by Raman spectroscopy. Anal. Chem. 2005;77:7958–7965. doi: 10.1021/ac0512599. [DOI] [PubMed] [Google Scholar]
- 35.Kast R.E., Auner G.W., Rosenblum M.L., Mikkelsen T., Yurgelevic S.M., Raghunathan A., Poisson L.M., Kalkanis S.N. Raman molecular imaging of brain frozen tissue sections. J. Neurooncol. 2014;120:55–62. doi: 10.1007/s11060-014-1536-9. [DOI] [PubMed] [Google Scholar]
- 36.Beljebbar A., Dukic S., Amharref N., Manfait M. Ex vivo and in vivo diagnosis of C6 glioblastoma development by Raman spectroscopy coupled to a microprobe. Anal. Bioanal. Chem. 2010;398:477–487. doi: 10.1007/s00216-010-3910-6. [DOI] [PubMed] [Google Scholar]
- 37.Aydin O., Altaş M., Kahraman M., Bayrak O.F., Culha M. Differentiation of healthy brain tissue and tumors using surface-enhanced Raman scattering. Appl. Spectrosc. 2009;63:1095–1100. doi: 10.1366/000370209789553219. [DOI] [PubMed] [Google Scholar]
- 38.Tanahashi K., Natsume A., Ohka F., Momota H., Kato A., Motomura K., Watabe N., Muraishi S., Nakahara H., Saito Y., et al. Assessment of tumor cells in a mouse model of diffuse infiltrative glioma by Raman spectroscopy. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/860241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y., Liu C.-H., Sun Y., Pu Y., Boydston-White S., Liu Y., Alfano R.R. Human brain cancer studied by resonance Raman spectroscopy. J. Biomed. Opt. 2012;17:116021. doi: 10.1117/1.JBO.17.11.116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krafft C., Sobottka S.B., Schackert G., Salzer R. Near infrared Raman spectroscopic mapping of native brain tissue and intracranial tumors. Analyst. 2005;130:1070–1077. doi: 10.1039/b419232j. [DOI] [PubMed] [Google Scholar]
- 41.Bury D., Morais C.L.M., Martin F.L., Lima K.M.G., Ashton K.M., Baker M.J., Dawson T.P. Discrimination of fresh frozen non-tumour and tumour brain tissue using spectrochemical analyses and a classification model. Br. J. Neurosurg. 2020;34:40–45. doi: 10.1080/02688697.2019.1679352. [DOI] [PubMed] [Google Scholar]
- 42.Auner A.W., Kast R.E., Rabah R., Poulik J.M., Klein M.D. Conclusions and data analysis: A 6-year study of Raman spectroscopy of solid tumors at a major pediatric institute. Pediatr. Surg. Int. 2013;29:129–140. doi: 10.1007/s00383-012-3211-6. [DOI] [PubMed] [Google Scholar]
- 43.Beleites C., Geiger K., Kirsch M., Sobottka S.B., Schackert G., Salzer R. Raman spectroscopic grading of astrocytoma tissues: Using soft reference information. Anal. Bioanal. Chem. 2011;400:2801–2816. doi: 10.1007/s00216-011-4985-4. [DOI] [PubMed] [Google Scholar]
- 44.Aguiar R.P., Silveira L., Falcão E.T., Pacheco M.T.T., Zângaro R.A., Pasqualucci C.A. Discriminating neoplastic and normal brain tissues in vitro through raman spectroscopy: A principal components analysis classification model. Photomed. Laser Surg. 2013;31:595–604. doi: 10.1089/pho.2012.3460. [DOI] [PubMed] [Google Scholar]
- 45.Galli R., Meinhardt M., Koch E., Schackert G., Steiner G., Kirsch M., Uckermann O. Rapid Label-Free Analysis of Brain Tumor Biopsies by Near Infrared Raman and Fluorescence Spectroscopy—A Study of 209 Patients. Front. Oncol. 2019;9:1–13. doi: 10.3389/fonc.2019.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anna I., Bartosz P., Lech P., Halina A. Novel strategies of Raman imaging for brain tumor research. Oncotarget. 2017;8:85290–85310. doi: 10.18632/oncotarget.19668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kast R., Auner G., Yurgelevic S., Broadbent B., Raghunathan A., Poisson L.M., Mikkelsen T., Rosenblum M.L., Kalkanis S.N. Identification of regions of normal grey matter and white matter from pathologic glioblastoma and necrosis in frozen sections using Raman imaging. J. Neurooncol. 2015;125:287–295. doi: 10.1007/s11060-015-1929-4. [DOI] [PubMed] [Google Scholar]
- 48.Ali S.M., Bonnier F., Tfayli A., Lambkin H., Flynn K., McDonagh V., Healy C., Clive Lee T., Lyng F.M., Byrne H.J. Raman spectroscopic analysis of human skin tissue sections ex-vivo: Evaluation of the effects of tissue processing and dewaxing. Med. Opt. 2013;18:061202. doi: 10.1117/1.JBO.18.6.061202. [DOI] [PubMed] [Google Scholar]
- 49.Hollon T., Lewis S., Freudiger C.W., Sunney Xie X., Orringer D.A. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg. Focus. 2016 doi: 10.3171/2015.12.FOCUS15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livermore L.J., Isabelle M., Bell I.M., Scott C., Walsby-Tickle J., Gannon J., Plaha P., Vallance C., Ansorge O. Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neuro Oncol. Adv. 2019;1 doi: 10.1093/noajnl/vdz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendricks B.K., Sanai N., Stummer W. Fluorescence-guided surgery with aminolevulinic acid for low-grade gliomas. J. Neurooncol. 2019;141:13–18. doi: 10.1007/s11060-018-03026-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.