Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a common condition with comorbid insomnia reported in >70% of children and adults. These patients demonstrate delays in sleep-wake rhythms, nocturnal rise in melatonin, and early morning rise in cortisol. Given that standard psychopharmacologic treatments for ADHD often do not completely control symptoms in participants with circadian rhythm delay, we sought to test whether bright light therapy (BLT) advances circadian rhythms and further reduces ADHD symptoms over standard treatments. In addition to standard of care, participants with ADHD diagnosis underwent 1 week of baseline assessment followed by 2-weeks of 30-minute morning 10,000-lux BLT beginning 3h after mid-sleep time. Participants minimized overhead light after 4 pm, wore an actigraphy watch, and recorded BLT time on daily sleep logs. Dim Light Melatonin Onset (DLMO) was assessed at baseline and after 2-week treatment. ADHD symptoms were measured by the ADHD-Rating Scales (ADHD-RS). BLT significantly advanced the phase of DLMO by 31 min [mean time (SEM), 20:36 (0:21) advanced to 20:05 (0:20)] and mid-sleep time by 57 min [4:37 (0:22) advanced to 3:40 (0:16); paired t-tests, p = 0.002 and 0.004, respectively). Phase advances (in DLMO or mid-sleep time) were significantly correlated with decreased ADHD-RS total scores (p = 0.027 and 0.044) and Hyperactive-Impulsive sub-scores (p = 0.014 and 0.013, respectively). Actigraphy analysis for a subset of 8 participants with significant DLMO phase advance revealed no significant changes in total sleep time, sleep efficiency, wake after sleep onset, or percent wake during sleep interval. This is the first successful use of BLT for advancing melatonin phase and improving ADHD symptoms in adults. BLT may be a complementary treatment for both delayed sleep timing and ADHD symptoms in adults.
Keywords: ADHD, Bright Light Therapy, Delayed Circadian Phase, Dim Light Melatonin Onset
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by problems with attention, impulsivity and over-activity. The syndrome affects 4.5% of adults (Kessler et al., 2006), resulting in an estimated $3.6 billion loss in work costs (Birnbaum et al., 2005). More than 70% of children and adults with ADHD also report insomnia (Fargason et al., 2013; Fargason, 2011, Gruber et al., 2012; Kessler and Adler, 2006; Van der Heijden et al., 2005; Van Veen et al., 2010a). The sleep disturbance is often due to circadian rhythm disruption characterized by delayed sleep in both children and adults, and psychiatric disorders, including ADHD, have been found to be associated with phase delay (Gamble et al., 2013; Lewy et al., 2006). Patients with ADHD demonstrate delays in: sleep-wake rhythms, nocturnal rise in melatonin, and early morning rise in cortisol (Baird et al., 2012; Novakova et al., 2011; Van der Heijden, Smits, 2005; Van Veen and Kooij, 2010a). Despite the significant comorbidity of ADHD and sleep disturbances, common treatment regimens such as stimulants do not address these sleep problems, and hence fail to achieve full symptom control of core ADHD symptoms. In fact, treatment with long-acting stimulant medications can shorten sleep duration, delay sleep-onset, and reduce circadian amplitude, all of which may negatively impact executive function and attention (Morash-Conway, Gendron, Corkum, 2016; Ironside, Davidson, Corkum, 2010). Additionally, circadian dysfunction and resultant disturbed sleep are associated with learning problems (Kopasz et al., 2010; Van der Heijden, Smits, 2005), poor driving performance (Anderson and Horne, 2013; Ftouni et al., 2013), depression (Lall et al. , 2012; McCall et al., 2010; Taylor et al., 2005), and occupational dysfunction (Adan et al., 2012; Dagan, 2002), all of which are ADHD co-morbidities. As a result, there is an urgent need to develop non-pharmacological approaches to treat sleep disturbances, which often worsen attention deficits and impulsive behavior in adults with ADHD diagnosis.
When presented at the appropriate time-of-day, bright light therapy (BLT) has shown success for the treatment of a variety of sleep conditions (Crowley et al., 2004; Revell and Eastman, 2005; Smith et al., 2009; Wilhelmsen-Langeland et al., 2013; Wilhelmsen-Langeland et al., 2014; Wirz-Justice et al., 2013). A key characteristic of the endogenous circadian clock is the ability to synchronize 24-hour rhythms to the light-dark cycle, such that early evening light exposure shifts wake-time to a later time (phase delay) while early morning light exposure shifts wake-time to an earlier time (phase advance) (Klein and Weller, 1970; Tamarkin et al., 1979). Well-timed light exposure can be used to shift sleep-wake rhythms earlier or later before/after transmeridian travel or before/after a change in shift schedule (Honma et al., 1991; Revell and Eastman, 2005). Therapeutic use of BLT in the morning to extend the light exposure period during the winter (mimicking summer-like conditions) alleviates depression in seasonal affective disorder (Burgess et al., 2004; Lieverse et al., 2011; Terman, 2006). Insomnia associated with delayed sleep phase syndrome is improved after administration of morning BLT (30 min) in a randomized controlled trial (Wilhelmsen-Langeland and Saxvig, 2013). Finally, combining BLT with total sleep deprivation and sleep phase advance (‘Triple Chronotherapy’) significantly reduced depression and suicidal ideation in an open-label, adjunctive therapy trial (Sahlem et al., 2014).
Our prior clinical trial demonstrated that delayed sleep timing (circadian phase delay) predicts ADHD symptom severity and that standard psychopharmacologic treatments for ADHD often do not completely control symptoms in participants with circadian rhythm delay (Gamble et al., 2013). These findings have not been translated into practical treatment applications such as BLT in patients with ADHD diagnosis. Thus, the aim of our study was to use BLT to manipulate the well-validated endogenous clock phase marker dim-light melatonin onset (DLMO) to explore the relationship between delayed circadian phase and ADHD symptoms. Understanding this relationship may highlight complementary or alternative mechanisms for clinical treatment.
2. Materials and Methods
2.1. Participants
In this study, all participants were between ages 19 and 64 and met the DSM-IV-TR criteria for ADHD (more restrictive than DSM-5 criteria) with a mean age of 36.1±13.2. Patients taking psychoactive medication for any condition other than ADHD or who were diagnosed with a co-morbid psychiatric disorder, sleep apnea, light-sensitive skin condition, ocular disorder or were shift-workers were excluded. Participants were recruited from the UAB Outpatient Psychiatry clinic during November 2013 - March 2014. Clinic patients who qualified for the study were given a research brochure with research coordinator contacts in a neutral manner. A total of 28 potential participants were screened, with four meeting exclusion criteria (Figure 1). One participant was lost to follow-up, and the remaining 23 participants gave written informed consent and were enrolled in the study, which was approved by the UAB institutional review board (IRB). Of the sample of 23, one participant withdrew due to an unrelated illness, three participants were not included due to protocol noncompliance with either the Actiwatch (N=2) or the light therapy (N=1). DLMO data from three participants were not analyzable due to low melatonin levels (N=2) and light exposure during collection (N=1), leaving a final sample of 16 participants who completed the protocol. The final sample (N=16) included 7 (44%) men and 9 (56%) women with 15 (94%) Caucasian and 1 (6%) Hispanic participant. This was an adjunctive procedure to standard of care, including current stimulant medication (stable for ≥ 3 months), which varied in dosage and type. Patients presenting on medications for ADHD were allowed to continue standard regimens within PDR-approved dosage ranges (including, mixed amphetamine salts (MAS) and extended released MAS, or bupropion) provided all dosing occurred prior to noon. Participants on stimulant medication were strongly encouraged to take their medication by 8:00 a.m. (Table 1).
Figure 1.
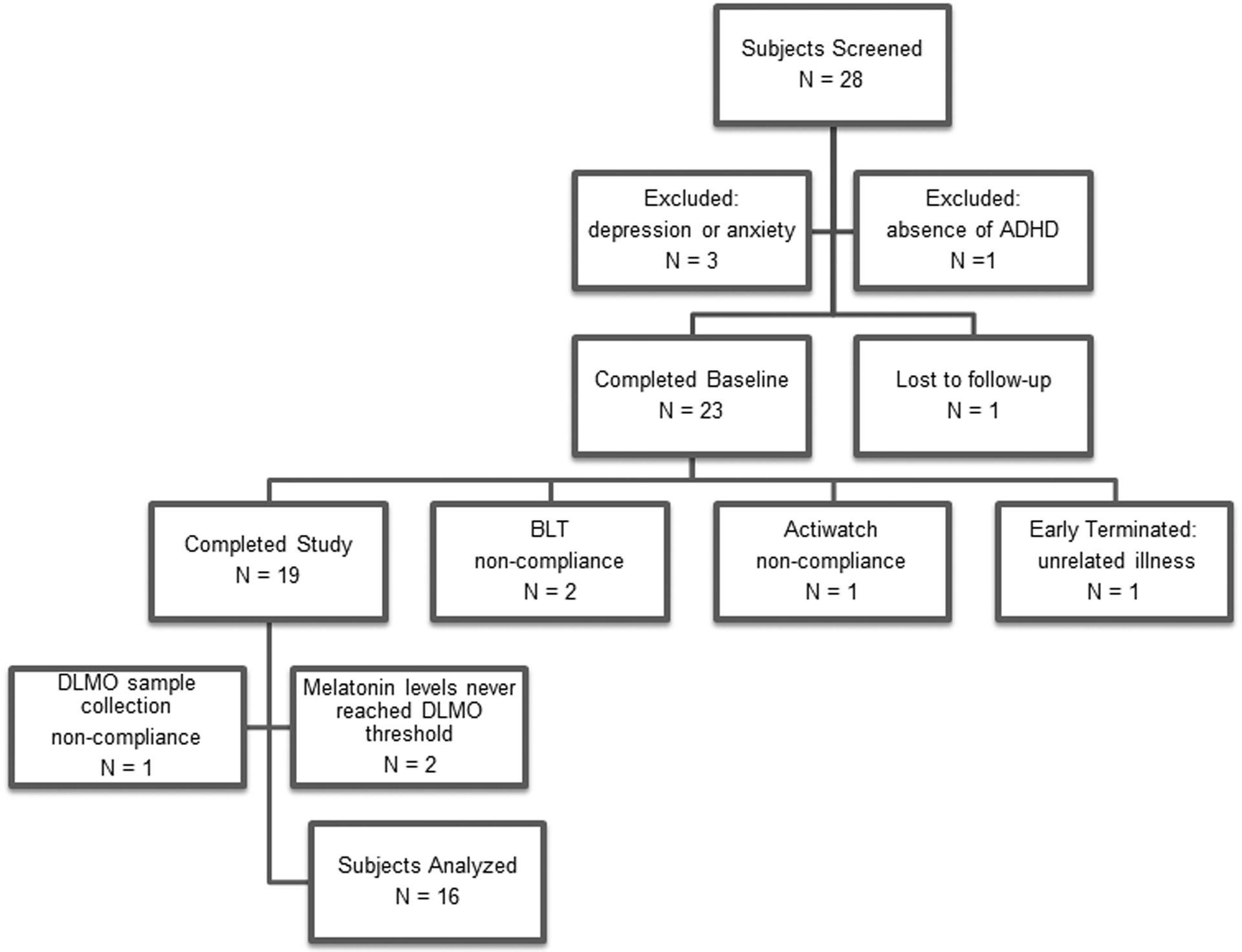
Twenty-eight potential participants were screened. Three were excluded for depression or anxierty diagnoses, and one was excluded for absence of ADHD diagnosis. One participant was lost to follow-up, and the remaining 23 participants were enrolled in the study. Of the sample of 23, one withdrew due to unrelated illness, and three participants were not included due to protocol noncompliance with either the Actiwatch (N=2) or the light therapy (N=1), leaving a final sample of 19 participants who completed the protocol.
Table 1.
Demographics
| N = 16 | N (%) or Mean ± SEM |
|---|---|
| Age | 35.25 ± 3.39 |
| Gender | 9 (56%) Female 7 (44%) Male |
| Race/Ethnicity | 15 (94%) White, 1 (6%) Hispanic |
| Medication* | 4 (25%) no medication 1 (6.25%) Bupropion XL 5 (31.25%) short-acting mixed-amphetamine salt 4 (25%) long-acting mixed amphetamine salt 2 (12.5%) lisdexamfetamine |
Single morning dose only
2.2. BLT Procedure and Actigraphy
Participants were recruited by internal referral, fliers and local campus media. Trained research assistants performed a standard pre-screen interview when contacted by prospective participants. Qualifying individuals were invited for a screening visit. The initial screening visit included the consenting procedure; establishment of ADHD diagnosis by ADHD-RS and psychiatric clinical evaluation using DSM-IV-TR criteria; physical examination, and a Brief Sleep Disorder Screening Questionnaire. Research assistants conducted the Mini International Neuropsychiatric Interview for psychiatric conditions, and the HAM-A and HAM-D to exclude participants with depression or anxiety not in full remission (i.e. HAM-D score ≤ 7, HAM-A score ≤ 5 required). During this 3-week intervention study, consenting participants underwent one week of baseline assessment followed by 2-weeks of 30-minute morning BLT to advance sleep timing. A wrist accelerometer (Actiwatch Spectrum, Philips, Andover, MA) was worn continuously throughout the study for use in conjunction with a sleep-wake diary (Manber et al. , 1996) to monitor sleep-wake cycles for a baseline week and the two-week light treatment period. The intervention required home use of a standard UV-protected 10,000-lux Bright light therapy box (EnergyLight HF3318/60, Philips, Andover, MA) every morning. Data from the participant’s actigraphic baseline assessment week was used to determine the mid-sleep time as a basis for scheduling timing of morning light box use (3-hours after their calculated mid-sleep time). Participants were instructed to: (i) use the light box daily (for two weeks) for 30 minutes at the instructed time and record light box usage times on daily sleep logs (Manber et al., 1996), (ii) minimize overhead light and wear blue-wavelength blocking glasses outside after 4 pm to prevent Blue-wavelength light (460–480 nm range) from suppressing melatonin onset, and (iii) adhere to their usual schedule and record reasons for deviations in their sleep diary. For both baseline and post-light treatment DLMO assessment periods, participants stayed overnight in the UAB Hospital Research Unit to allow for strict control of light exposure, temperature and oral intake factors that could alter melatonin levels. Participants provided 2–3 ml of saliva on an hourly basis from 7 pm to 2 am, which were stored for DLMO assessment, and completed other outcome measures at both overnight visits. A daily sleep and light exposure diary was maintained for the entire study period. One subject did not complete sufficient sleep diary entries, resulting in N=15 for subsequent sleep analysis. Primary outcome measures included timing of the calculated Dim-light melatonin onset (DLMO) (see result section) obtained from salivary melatonin samples collected while in the temperature and light-controlled research unit and mid-sleep time by actigraphy (the mid-time point between sleep start and end time--an accurate measure of the circadian phase of sleep). Secondary validated outcome measures included: 1) The ADHD-Rating Scale (ADHD-RS); 2) PSQI-sleep quality scale (25); 3) sleep quality measures by actigraphy, and also; 4) “sleepiness” ratings on the daily sleep diary.
2.3. Data collection
DLMO was assessed at baseline and after the 2-week treatment in the UAB Hospital Research Unit in order to control light exposure (< 10 lux) and oral intake factors. Hourly saliva samples (2–3 ml) from 7pm to 2am were assayed (Buhlmann Direct Saliva Melatonin ELISA 01-EK-DSM, ALPCO Diagnostics, Salem, NH) and DLMO defined as the time at which the melatonin exceeded a baseline threshold of 4 pg/ml and remained above that level. The precise clock time between sampling time-points was determined by linear interpolation (Voultsios et al., 1997; Crowley et al., 2016). Actigraphy data were determined after bed and wake times were entered into the Actiware analysis program, and used to determine sleep quality, efficiency, and fragmentation according to the software algorithms. Additional outcome measures were: mid-sleep time (the mid-time point between bed-time and wake-up time, according to the sleep diary); the ADHD-Rating Scale (ADHD-RS; DuPaul, 1998); actigraphy sleep measures (Gamble et al., 2013); sleep diary ‘sleepiness’ construct (Manber and Bootzin, 1996); and the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989).
2.4. Data analysis
Demographics and other data analysis were completed using SPSS 22. To compare any statistically significant changes between pre- and post-BLT outcomes, pairwise comparisons of the change from baseline were used. Following this initial analysis, correlations between the size of the phase shift (in either mid-sleep time or DLMO) and the reduction in ADHD symptoms were determined using a Pearson’s product moment correlation.
3. Results
A total of 19 participants completed the protocol. DLMO data from 3 participants were not analyzable due to low melatonin levels (N=2) and light exposure during collection (N=1). As predicted, BLT advanced the phase of DLMO by 31 min [mean time (SEM), 20:36 (0:21) advanced to 20:05 (0:20)] and mid-sleep time by 57 min [4:37 (0:22) advanced to 3:40 (0:16); paired t-tests, p = 0.002 and 0.004, respectively; Figure 2). The net effect of these two outcomes produced a significantly more narrow phase angle between DLMO and mid-sleep time [mean time (SEM), 8:11 (0:25) hours decreased to 7:44 (0:22) hours; paired t-tests, p = 0.001). Phase advances (in DLMO or mid-sleep time) were significantly correlated with decreased ADHD-RS total scores (r = −0.55 and r = −0.53, p = 0.027 and 0.044, respectively; Figure 3) and Hyperactive-Impulsive sub-scores (r = −0.60 and r = −0.62, p = 0.014 and 0.013, respectively). However, reductions in total and Hyperactive-Impulsive ADHD-RS scores were not correlated with decreased DLMO: mid-sleep phase angle (r = 0. 18 and r = 0.26, p = 0.515 and 0.356, respectively). Actigraphy analysis for a subset of 8 participants with significant DLMO phase advance (p = 0.01) revealed no significant changes in total sleep time, sleep efficiency, wake after sleep onset, or percent wake during sleep interval (Table 2). Although not statistically significant, there was a tendency for participants to have earlier sleep start times, wake-up times (sleep end), and increased sleep fragmentation (Table 2). Interestingly, ‘sleepiness’ ratings from the sleep dairy (Manber, Bootzin, 1996) were significantly reduced (mean ± SEM, 2.97 ± 0.13 to 2.63 ± 0.21; p = 0.033), and subjective sleep quality was improved as assessed by the PSQI (mean ± SEM, 10.1 ± 1.2 to 6.1 ± 1.3; p < 0.001).
Figure 2.
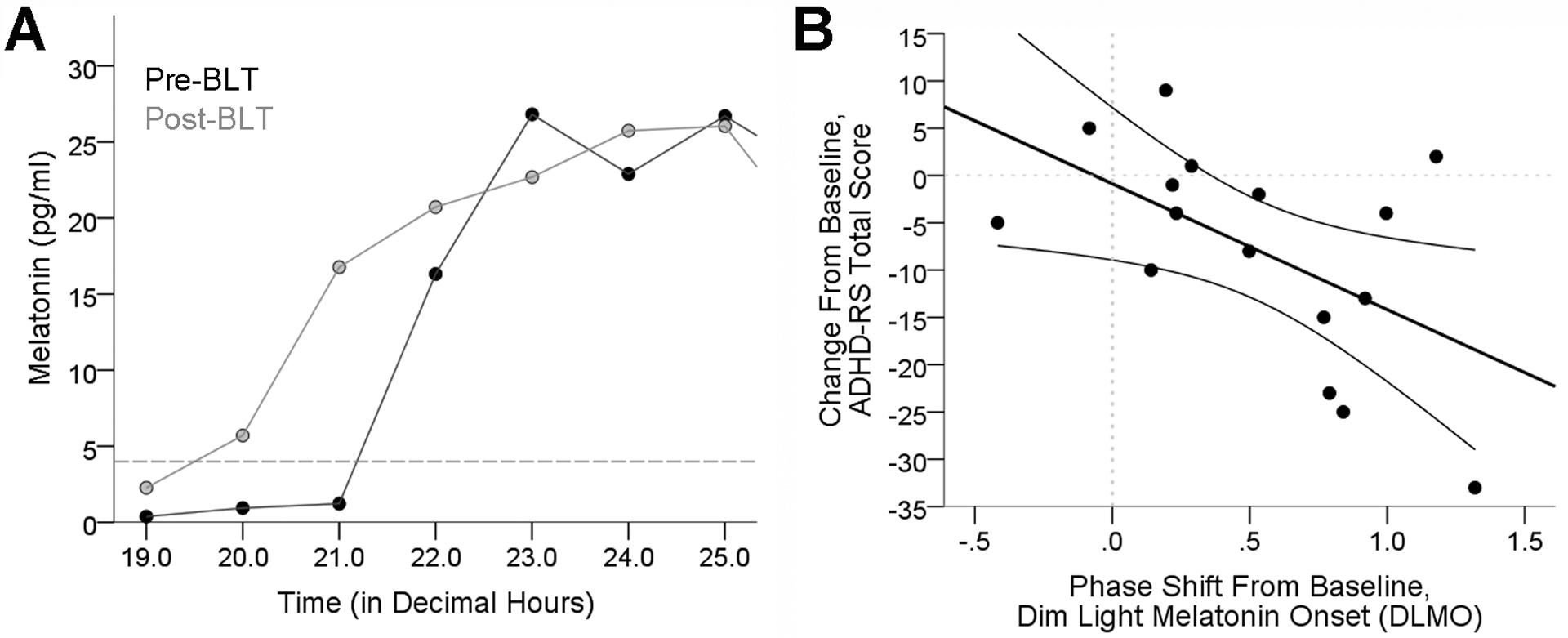
A) Chart showing time of DLMO Pre-BLT and Post-BLT for a representative participant. DLMO was determined for each participant according to the linear interpolation procedure described in the Methods. B) Chart demonstrating a significant association between the DLMO phase shift from baseline and corresponding change in ADHD-RS total score.
Figure 3.
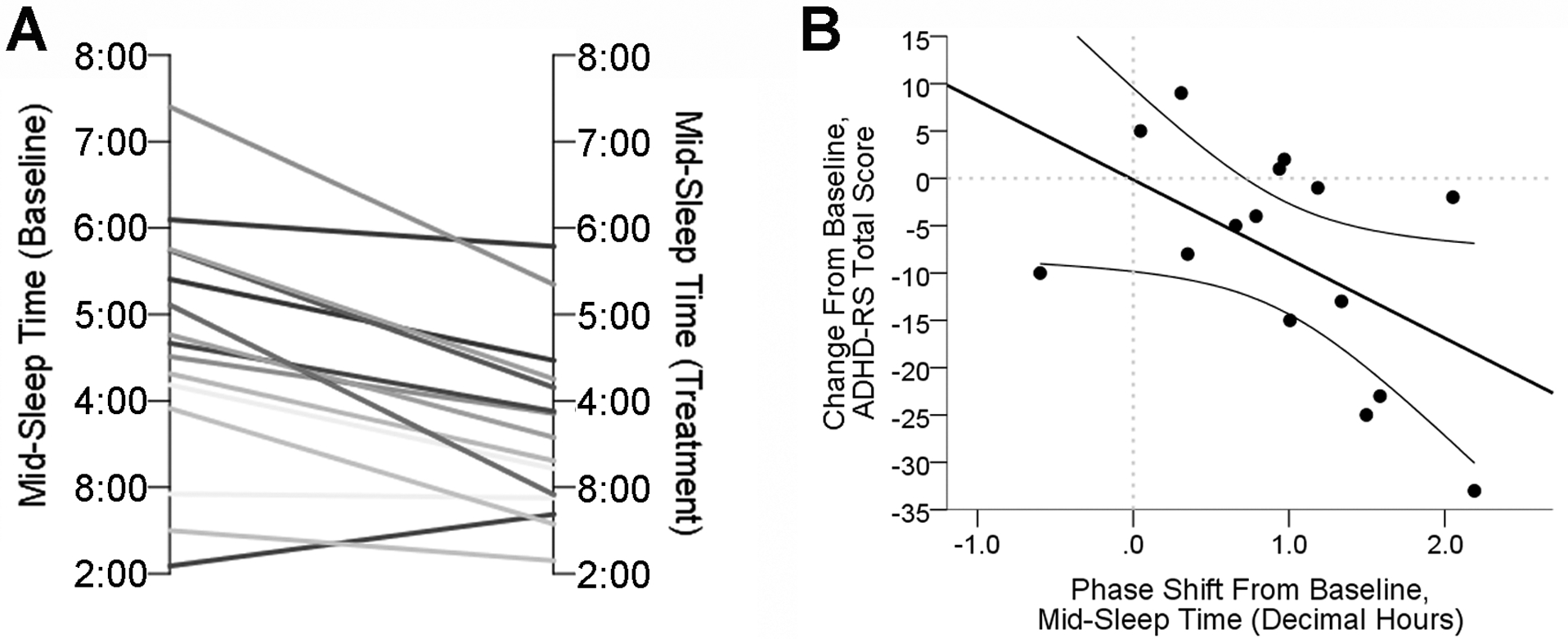
A) Chart comparing Mid-Sleep Time for each participant pre-BLT and post-BLT.
B) Chart demonstrating a significatn association between the shift in Mid-Sleep Time from baseline and corresponding change in ADHD-RS total score.
Table 2.
Actigraphic sleep parameter estimates.
| Baseline | BLT | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | p | |||
| Sleep start (h) | 0:34 | 0:33 | 23:59 | 0:21 | 0.075 | ||
| Sleep end (h) | 7:47 | 0:34 | 7:04 | 0:20 | 0.064 | ||
| Mid-sleep time (h) | 4:11 | 0:32 | 3:31 | 0:18 | 0.048 | ||
| Total sleep duration (h) | 7.2 | 0.4 | 7.1 | 0.4 | 0.677 | ||
| Sleep efficiency (%) | 81.3 | 2.9 | 77.4 | 3.0 | 0.333 | ||
| % Wake | 7.4 | 0.9 | 8.0 | 0.7 | 0.243 | ||
| Wake After Sleep Onset (WASO) | 30.7 | 4.6 | 32.3 | 4.0 | 0.627 | ||
| Fragmentation Index (%) | 14.7 | 2.4 | 18.9 | 2.3 | 0.078 | ||
| DLMO (time; hours:min) | 19:49 | 0:14 | 19:21 | 0:16 | 0.028 | ||
Note: Sleep timing and duration are calculated based on the primary sleep session/day, excluding naps. All data were compared using paired samples t-tests (N=8), with significance indicated in bold (p < 0.05) or trends indicated in italics (p < 0.10).
4. Discussion
Despite the association of delayed sleep timing with ADHD symptom severity and the inadequacy of standard psychopharmacologic treatments for ADHD in participants with circadian rhythm delay (Gamble et al., 2013), the effectiveness of BLT as a treatment for patients with ADHD diagnosis has not previously been assessed. As hypothesized, BLT advanced DLMO and mid-sleep in adults with ADHD, and these advances were associated with decreased ADHD symptoms, including hyperactive-impulsive symptoms. This demonstrates the importance of addressing sleep-timing delays in preventing symptoms of impulsivity or hyperactivity (Gamble et al., 2013). This also supports BLT as a possible complementary treatment for both delayed sleep timing and ADHD symptoms in adults, especially given that the average reduction in ADHD-RS scores observed after BLT was ~8 points, which is comparable to other non-stimulant FDA approved agents determined from recent meta-analyses (Li et al., 2016).
One interesting outcome of this study is that the phase angle difference between evening DLMO and mid-sleep time significantly narrowed with BLT treatment. The “ideal” phase angle has been previously reported to be ~6 hours (Lewy et al., 2006). Before BLT, our delayed participants showed larger phase angles of ~8 hours, varying from 6 hours to over 12 hours. Participants with very large phase angles tended to have very late mid-sleep times (e.g., 5:30am). The change in mid-sleep time was greater than the change in DLMO, resulting in the timing of sleep occurring closer to DLMO than before BLT. Thus, we found that BLT was an effective treatment for restoring alignment between DLMO and mid-sleep phases even in extreme delayed types.
Consistent with these findings, symptoms of ADHD correlate with circadian disruption at multiple levels. Adults with ADHD diagnosis report excess daytime sleepiness, later bed/wake-up times, and have documented sleep-activity cycles that are delayed with reduced amplitude, suggesting that these physiological impairments may be due to deficits in the underlying circadian (24-h) clock (Bioulac et al., 2015; Van Veen et al., 2010b; Voinescu et al., 2012). Circadian rhythms in sleep-wake cycles, physiology, and hormones are intrinsically driven by a central clock in the hypothalamus (the suprachiasmatic nucleus). Cortisol and melatonin are the best markers of endogenous circadian phase in humans (Moore-Ede et al., 1982). Both the nocturnal rise in melatonin (measured as DLMO) and the early morning rise in cortisol appear later in children and adults diagnosed with ADHD, indicating a delayed circadian clock phase (Baird et al., 2011; Imeraj et al., 2012; Novakova et al., 2011; Van der Heijden K.B., 2005; Van Veen et al., 2010b). Additional evidence suggests that ADHD is associated with circadian impairment at the genetic/molecular level. For example, rhythmic expression of the circadian ‘clock genes’ (i.e., BMAL1 and PER2, the molecular ‘gears’ of the intracellular clock) is flattened in white blood cells from ADHD participants, and the amplitude of these oscillations is significantly correlated with increased nocturnal hyperactive behavior and delayed cortisol rhythms (Baird et al., 2011). Indeed, genetic polymorphisms of several circadian clock genes are associated with ADHD diagnosis (Brookes et al., 2006; Cao et al., 2012; Kissling et al., 2008; Xu et al., 2010). As result of a weakened and delayed intrinsic circadian clock, patients suffering from ADHD may experience difficulty waking up in the morning and going to bed at night, explaining why more than 70% of adults with ADHD have insomnia (Kessler et al., 2006) compared to estimates of around 30% in the adult, U.S. population (Roth et al., 2007). Unfortunately, insomnia associated with ADHD is largely unaddressed by current treatment regimens (Hamblin et al., 2007; Walsh et al., 2006), and is often exacerbated by administration of stimulants in the late day/evening (Fargason et al., 2012; Fargason et al., 2011). Taken together, this suggests that a delayed central circadian pacemaker underlies insomnia due to delayed sleep/activity rhythms in individuals with ADHD.
In the present study, BLT was found to be a feasible treatment for delayed sleep timing in adults with ADHD. ADHD symptoms sometimes make adherence to treatment difficult, and one subject was noncompliant with BLT and not included in the study. Participants used the light box ~10 out 14 days on average, and on these days, they were ~90% compliant with using the box within 9–12 hours of DLMO, which is necessary to produce a phase advance (Revell and Eastman, 2005). Participants also reported less subjective sleepiness and improved subjective sleep quality. This demonstrates the viability of using BLT with adults with ADHD.
Limitations of this study include small sample size, some variability in treatment adherence and lower than desirable adherence. The lack of a placebo control is also a limitation; however, it is important to note that this was a pilot study. Additionally, the treatment response was significantly associated with the size of shift in DLMO, an intrinsic response, suggesting that the effect was not due to placebo. Finally, true blind placebo controls for light therapy are a major challenge for the chronobiology research with few alternatives that do not induce phase changes or introduce the possibility of incidental light exposure. Despite these limitations, this intervention still induced a modest (~ half hour) phase advance in DLMO for the majority of the participants primarily due to frequent usage of the light within 9–12 hours of DLMO.
This study demonstrates the utility of BLT when assessed with an objective circadian phase marker (DLMO). Future research should assess the effectiveness of using a morning BLT intervention to advance circadian phase of adults with ADHD and improve ADHD symptoms while reducing stimulant side effects.
4.1. Conclusion
This open-label pilot study suggests that BLT is a feasible treatment for adults with ADHD and may be a successful complementary treatment for delayed sleep timing and symptoms of ADHD in adults. A randomized, placebo-controlled trial is needed to assess futher treatment effects.
Acknowledgments
We would like to thank Dr. James Meador-Woodruff, M.D. (UAB Department of Psychiatry) for general support for this project, and Dr. Richard Shelton, M.D. (UAB Department of Psychiatry) for critical review of the study and for editing this letter. Drs. Meador-Woodruff and Shelton have no financial disclosures for this project.
Financial/Material support: UAB Health Services Foundation General Endowment Fund Actigraphy Program and UAB Psychiatry departmental funds (Birmingham, AL, USA)
Footnotes
Disclosures: All authors have no direct or indirect affiliations or financial interests in connection with the content of this paper to disclose.
References
- Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29:1153–75. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Driving drowsy also worsens driver distraction. Sleep Med. 2013;14:466–8. [DOI] [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Molecular psychiatry. 2011. [DOI] [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17:988–95. [DOI] [PubMed] [Google Scholar]
- Bioulac S, Chaufton C, Taillard J, Claret A, Sagaspe P, Fabrigoule C, et al. Excessive daytime sleepiness in adult patients with ADHD as measured by the Maintenance of Wakefulness Test, an electrophysiologic measure. J Clin Psychiatry. 2015. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Current medical research and opinion. 2005;21:195–206. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11:934–53. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF, Young MA, Eastman CI. Bright light therapy for winter depression--is phase advancing beneficial? Chronobiol Int. 2004;21:759–75. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- Cao YL, Cui QT, Tang CH, Chang X. [Association of CLOCK gene T3111C polymorphism with attention deficit hyperactivity disorder and related sleep disturbances in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:285–8. [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004;27:1077–87. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Suh C, Molina TA, Fogg LF, Sharkey KM, Carskadon MA. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Med. 2016; 20:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan Y Circadian Rhythm Sleep Disorders (CRSD) in psychiatry--a review. The Israel journal of psychiatry and related sciences. 2002;39:19–27. [PubMed] [Google Scholar]
- DuPaul GP T; Anastopoulos A; Reid R ADHD Rating Scale IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford Press; 1998. [Google Scholar]
- Fargason RE, Hollar AF, White S, Gamble K. Adults With ADHD-Without Insomnia History Have Subclinical Sleep Disturbance but Not Circadian Delay: An ADHD Phenotype? J Atten Disord. 2012. [DOI] [PubMed] [Google Scholar]
- Fargason RE, Hollar AF, White S, Gamble KL. Adults with ADHD-without insomnia history have subclinical sleep disturbance but not circadian delay: an ADHD phenotype? J Atten Disord. 2013;17:583–8. [DOI] [PubMed] [Google Scholar]
- Fargason REG KL; Avis KT; Besing RC; Jackson C; Cates M; May R Ramelteon for Insomnia Related to Attention-Deficit/Hyperactivity Disorder Psychopharmacol Bull. 2011;44:32–53. [PMC free article] [PubMed] [Google Scholar]
- Ftouni S, Sletten TL, Howard M, Anderson C, Lenne MG, Lockley SW, et al. Objective and subjective measures of sleepiness, and their associations with on-road driving events in shift workers. J Sleep Res. 2013;22:58–69. [DOI] [PubMed] [Google Scholar]
- Gamble KL, May RS, Besing RC, Tankersly AP, Fargason RE. Delayed sleep timing and symptoms in adults with attention-deficit/hyperactivity disorder: a controlled actigraphy study. Chronobiol Int. 2013;30:598–606. [DOI] [PubMed] [Google Scholar]
- Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep. 2004;27:261–6. [DOI] [PubMed] [Google Scholar]
- Gruber R, Fontil L, Bergmame L, Wiebe ST, Amsel R, Frenette S, et al. Contributions of circadian tendencies and behavioral problems to sleep onset problems of children with ADHD. BMC Psychiatry. 2012;12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin JE. Insomnia: an ignored health problem. Primary care. 2007;34:659–74, viii. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Sasaki M, Endo T. Bright lights accelerate the re-entrainment of circadian clock to 8-hour phase-advance shift of sleep-wake schedule: 1) Circadian rhythms in rectal temperature and plasma melatonin level. Jpn J Psychiatry Neurol. 1991;45:153–4. [PubMed] [Google Scholar]
- Imeraj L, Antrop I, Roeyers H, Swanson J, Swanson J, Deschepper E, Bal S, Deboutte D. Time-of-day effects in arousal: disrupted diurnal cortisol profiles in children with ADHD. J Child Psycholo Psychiatry. 2012; 53; 782–789. [DOI] [PubMed] [Google Scholar]
- Ironside S, Davidson F, Corkum P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J Sleep Res. 2010;19:546–51. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC AL, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. The American journal of psychiatry. 2006; April;163:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissling C, Retz W, Wiemann S, Coogan AN, Clement RM, Hunnerkopf R, et al. A polymorphism at the 3’-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147:333–8. [DOI] [PubMed] [Google Scholar]
- Klein DC, Weller J. Input and output signals in a model neural system: the regulation of melatonin production in the pineal gland. In Vitro. 1970;6:197–204. [DOI] [PubMed] [Google Scholar]
- Kopasz M, Loessl B, Hornyak M, Riemann D, Nissen C, Piosczyk H, et al. Sleep and memory in healthy children and adolescents - a critical review. Sleep Med Rev. 2010;14:167–77. [DOI] [PubMed] [Google Scholar]
- Lall GS, Atkinson LA, Corlett SA, Broadbridge PJ, Bonsall DR. Circadian entrainment and its role in depression: a mechanistic review. J Neural Transm. 2012;119:1085–96. [DOI] [PubMed] [Google Scholar]
- Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. Journal of child psychology and psychiatry, and allied disciplines. 2000;41:803–12. [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK The circadian basis of winter depression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao J, He S Wang Q. An evaluation on the efficacy and safety of treatments for ADHD in Children and adolescents: a Comparison of multiple treatments. Mol Neurolbiol. 2016; {Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. [DOI] [PubMed] [Google Scholar]
- Manber R, Bootzin RR, Acebo C, Carskadon MA. The effects of regularizing sleep-wake schedules on daytime sleepiness. Sleep. 1996;19:432–41. [DOI] [PubMed] [Google Scholar]
- McCall WV, Blocker JN, D’Agostino R Jr., Kimball J, Boggs N, Lasater B, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010;6:322–9. [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. The Clocks That Time Us: Physiology of the Circadian Timing System. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Morash-Conway J, Gendron M, Corkum P. The role of sleep quality in moderating the effectiveness of medication in the treatment of children with ADHD. Atten Defic Hyperact Disord. 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Novakova M, Paclt I, Ptacek R, Kuzelova H, Hajek I, Sumova A. Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiol Int. 2011;28:630–7. [DOI] [PubMed] [Google Scholar]
- Revell VL, Eastman CI. How to trick mother nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Franklin M, Bramley TJ. The state of insomnia and emerging trends. The American journal of managed care. 2007;13:S117–20. [PubMed] [Google Scholar]
- Sahlem GL, Kalivas B, Fox JB, Lamb K, Roper A, Williams EN, Williams NR, Korte JE, El Sabbagh S, Guille C, Barth KS, Uhde TW, George MS, Short EB. Adjunctive triple chronotherapy (combinted total sleep deprivation, sleep phase advance, and bright light therapy) rapidly improves mood and suicidality in suicidal depressed inpatients: an open label pilot study. J Psychiatr Res. 2014;58:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarkin L, Reppert SM, Klein DC. Regulation of pineal melatonin in the Syrian hamster. Endocrinology. 1979;104:385–9. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. [DOI] [PubMed] [Google Scholar]
- Terman M Review: light therapy is an effective treatment for seasonal affective disorder. Evidence-based mental health. 2006;9:21. [DOI] [PubMed] [Google Scholar]
- Van der Heijden KBSMG, Van Someren EJ,Gunning WB . Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22:559–70. [DOI] [PubMed] [Google Scholar]
- Van der Heijden KB, Smits MG, Van Someren EJ, Gunning WB. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int. 2005;22:559–70. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ. Delayed Circadian Rhythm in Adults with Attention-Deficit/Hyperactivity Disorder and Chronic Sleep-Onset Insomnia. Biol Psychiatry. 2010a. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biological psychiatry. 2010b;67:1091–6. [DOI] [PubMed] [Google Scholar]
- Voinescu BI, Szentagotai A, David D. Sleep disturbance, circadian preference and symptoms of adult attention deficit hyperactivity disorder (ADHD). J Neural Transm. 2012;119:1195–204. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. [DOI] [PubMed] [Google Scholar]
- Walsh JK. Insights into the public health burden of insomnia. Sleep. 2006;29:142–3. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, Nordhus IH, Vedaa O, Lundervold AJ, et al. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. J Biol Rhythms. 2013;28:306–21. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, Nordhus IH, Vedaa O, Sorensen E, et al. The personality profile of young adults with delayed sleep phase disorder. Behav Sleep Med. 2014;12:481–92. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders : a clinician’s manual for light and wake therapy. 2nd, rev. ed. Basel ; New York: Karger; 2013. [Google Scholar]
- Xu X, Breen G, Chen CK, Huang YS, Wu YY, Asherson P. Association study between a polymorphism at the 3’-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav Brain Funct. 2010;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]


