Social and emotional changes are key features of frontotemporal dementia but remain challenging to characterise and measure. Marshall et al. report the functional neuroanatomy of aberrant emotion processing across the spectrum of frontotemporal dementia, demonstrating abnormalities at multiple hierarchical levels including sensory processing, emotion categorisation and autonomic reactivity.
Keywords: frontotemporal dementia, functional MRI, emotion, autonomic, cardiac
Abstract
Impaired processing of emotional signals is a core feature of frontotemporal dementia syndromes, but the underlying neural mechanisms have proved challenging to characterize and measure. Progress in this field may depend on detecting functional changes in the working brain, and disentangling components of emotion processing that include sensory decoding, emotion categorization and emotional contagion. We addressed this using functional MRI of naturalistic, dynamic facial emotion processing with concurrent indices of autonomic arousal, in a cohort of patients representing all major frontotemporal dementia syndromes relative to healthy age-matched individuals. Seventeen patients with behavioural variant frontotemporal dementia [four female; mean (standard deviation) age 64.8 (6.8) years], 12 with semantic variant primary progressive aphasia [four female; 66.9 (7.0) years], nine with non-fluent variant primary progressive aphasia [five female; 67.4 (8.1) years] and 22 healthy controls [12 female; 68.6 (6.8) years] passively viewed videos of universal facial expressions during functional MRI acquisition, with simultaneous heart rate and pupillometric recordings; emotion identification accuracy was assessed in a post-scan behavioural task. Relative to healthy controls, patient groups showed significant impairments (analysis of variance models, all P < 0.05) of facial emotion identification (all syndromes) and cardiac (all syndromes) and pupillary (non-fluent variant only) reactivity. Group-level functional neuroanatomical changes were assessed using statistical parametric mapping, thresholded at P < 0.05 after correction for multiple comparisons over the whole brain or within pre-specified regions of interest. In response to viewing facial expressions, all participant groups showed comparable activation of primary visual cortex while patient groups showed differential hypo-activation of fusiform and posterior temporo-occipital junctional cortices. Bi-hemispheric, syndrome-specific activations predicting facial emotion identification performance were identified (behavioural variant, anterior insula and caudate; semantic variant, anterior temporal cortex; non-fluent variant, frontal operculum). The semantic and non-fluent variant groups additionally showed complex profiles of central parasympathetic and sympathetic autonomic involvement that overlapped signatures of emotional visual and categorization processing and extended (in the non-fluent group) to brainstem effector pathways. These findings open a window on the functional cerebral mechanisms underpinning complex socio-emotional phenotypes of frontotemporal dementia, with implications for novel physiological biomarker development.
Introduction
Impaired responses to emotional signals are a striking feature of the frontotemporal dementias (FTD) and profoundly disrupt social functioning in these diseases (Rohrer et al., 2012; Hsieh et al., 2013; Warren et al., 2013a; Marshall et al., 2018c). In the healthy brain, processing of socio-emotional signals such as facial expressions engages four principal, large-scale and hierarchically organized neural networks (Alcalá-López et al., 2017): a ‘visual-sensory’ network of face and biological motion-responsive areas, mediating analysis of stimulus features; a ‘limbic’ network of mesial temporal and ventromedial prefrontal structures, mediating affective valuation of stimuli; an ‘intermediate’ fronto-parietal and cingulo-insular network, integrating salient environmental and bodily states; and a ‘higher associative’ network of temporo-parietal junctional, temporal polar and dorsomedial prefrontal cortices, engaged in interpreting and responding to mental states. Classical models of face processing (Bruce and Young, 1986; Hutchings et al., 2017) map onto these networks, with fractionated systems subserving initial perceptual encoding of faces, subsequent identification of face identity and emotional expression and programming of an appropriate behavioural response. Autonomic reactivity to viewing facial emotions in health engages both visual association areas and the central autonomic control network, including anterior cingulate and insula (Critchley et al., 2005). Targeting of similar brain networks by the proteinopathies of FTD leads, predictably, to diverse socio-emotional symptoms: deficits of face recognition, emotional categorization, motoric and autonomic reactivity and emotional theory of mind have all been demonstrated in FTD and attributed to regional grey matter loss in distributed fronto-temporo-parietal circuitry (Rosen et al., 2002; Kipps and Hodges, 2006; Omar et al., 2011a, b; Rohrer et al., 2012; Couto et al., 2013; Downey et al., 2013; Oliver et al., 2015; Hazelton et al., 2016; Hutchings et al., 2017; Marshall et al., 2018a, b). However, the pathophysiological mechanisms that translate neural circuit disintegration to complex socio-emotional phenotypes in these diseases have not been examined directly.
The three major clinico-anatomical syndromes of FTD are each associated with characteristic (though overlapping) behavioural phenotypes and signature atrophy profiles. Considering these profiles in the light of emerging models of the healthy socio-emotional brain (Critchley et al., 2005; Alcalá-López et al., 2017), candidate neural mechanisms of socio-emotional dysfunction in particular FTD syndromes can be proposed. The behavioural variant of FTD (bvFTD) is a heterogeneous syndrome led by changes in social judgment and awareness, with variable profiles of fronto-insular and temporal lobe atrophy (Snowden et al., 2001; Warren et al., 2013a). Deficient processing of socio-emotional signals in bvFTD may arise from various levels of the processing hierarchy, encompassing sensory representation, autonomic responses, motor routines, emotional appraisal, and theory of mind (Kipps and Hodges, 2006; Fernandez-Duque et al., 2010; Couto et al., 2013; Baez et al., 2014; Joshi et al., 2014; Cohen et al., 2015; Oliver et al., 2015; Hutchings et al., 2017; Marshall et al., 2018a, b). Semantic variant primary progressive aphasia (svPPA) is led by multimodal disintegration of semantic knowledge associated with asymmetric, predominantly antero-medial temporal lobe atrophy (Marshall et al., 2018c). In svPPA, socio-emotional deficits have been related to erosion of social and emotional concepts and aberrant, overgeneralized or abnormally coupled autonomic and motoric responses to social signals despite retained capacity for emotional reactivity (Fletcher et al., 2015b; Marshall et al., 2017, 2018a). Non-fluent variant primary progressive aphasia (nfvPPA) is led by breakdown of motor speech output and programming, and is associated with atrophy predominantly affecting left inferior frontal cortex and insula (Marshall et al., 2018c). Though typically less prominent than language impairment, socio-emotional deficits are a feature of nfvPPA (Rohrer and Warren, 2010; Hazelton et al., 2016) and may reflect reduced sensorimotor processing of social signals and autonomic arousal (Couto et al., 2013; Cohen et al., 2015; Fletcher et al., 2015b; Marshall et al., 2018a, b).
To establish the pathophysiology of socio-emotional deficits in FTD requires functional neuroanatomical studies that dissect the multiple dimensions of emotion processing. From a clinical perspective, because socio-emotional alterations occur early in the evolution of FTD, improved understanding of the brain dysfunction that underpins these alterations could potentially drive development of new diagnostic and therapeutic biomarkers for disease detection and tracking, prior to the onset of irrecoverable brain damage. Two previous functional MRI studies of facial emotion processing in bvFTD have revealed reduced activity in face-responsive visual cortices, proposed to reflect disrupted top-down influences (Virani et al., 2013; De Winter et al., 2016). However, patterns of neural network dysfunction responsible for socio-emotional symptoms across the FTD spectrum have not yet been elucidated. Moreover, despite mounting evidence that autonomic regulation plays a key role in emotional reactivity in health and in FTD (Marshall et al., 2017, 2018a, b), the functional neuroanatomy of altered autonomic responses to affective stimuli has not been characterized in these diseases. Relatedly, capturing the pathophysiology of facial emotion processing in FTD is likely to require dynamic stimuli that more closely simulate the naturalistic socio-emotional signals of daily life, rather than conventional static images.
Here we addressed these issues using functional MRI of dynamic facial expressions with simultaneous recording of autonomic (cardiac and pupillary) responses in a cohort of patients representing all major FTD syndromes, and healthy age-matched individuals. Facial expressions were referenced to a comparably complex dynamic, affectively neutral visual baseline and to a simple fixation condition, allowing us to dissect visual sensory from emotion decoding responses. We used a passive viewing functional MRI design with no in-scanner output task to avoid potentially confounding task difficulty or performance monitoring effects; post-scanner behavioural data were collected to assess the accuracy of participants’ emotion identification. Based on available data in the healthy brain and in FTD (Virani et al., 2013; De Winter et al., 2016; Alcalá-López et al., 2017; Hutchings et al., 2017), we hypothesized that visual processing of dynamic facial expressions would be associated with activation of face and biological motion-responsive cortices and that FTD syndromes would be associated with attenuated activation of cortical mechanisms encoding emotions despite normal early visual processing. We further hypothesized that all FTD syndromes would be associated with impaired emotion identification but that syndromes would be differentiated based on their relative involvement of emotion evaluation and categorization mechanisms. Finally, we hypothesized that syndromic profiles of altered cardiac and pupillary reactivity would predict differential engagement of visual association and central autonomic control and effector pathways (Critchley et al., 2005; Marshall et al., 2018a, b).
Materials and methods
Participants
Sixty participants were included, comprising 38 patients fulfilling consensus criteria for a syndrome of FTD (Gorno-Tempini et al., 2011; Rascovsky et al., 2011) (17 with bvFTD, 12 with svPPA, nine with nfvPPA) of mild to moderate severity and 22 healthy older individuals with no history of neurological or psychiatric illness. No participant had a significant burden of cerebrovascular disease or visual loss. In all patients the syndromic diagnosis was endorsed by clinical and neuropsychological assessment and volumetric T1 brain MRI (see Supplementary Fig. 1 for patient group atrophy profiles). Five bvFTD patients had an identified disease-causing mutation (three MAPT, one C9orf72, one GRN). Demographic, clinical and neuropsychological characteristics of the participant groups are summarized in Table 1.
Table 1.
Clinical and neuropsychological characteristics of participant groups
| Characteristic | Controls | bvFTD | svPPA | nfvPPA |
|---|---|---|---|---|
| Demographic and clinical | ||||
| n, male:female | 10:12 | 13:4 | 8:4 | 4:5 |
| Age, years | 68.6 (6.8) | 64.8 (6.8) | 66.9 (7.0) | 67.4 (8.1) |
| Handedness, right:left:ambidextrous | 22:0 | 15:1 | 12:0 | 8:0 |
| Years of education | 16.1 (2.5) | 13.9 (5.0) | 15.6 (2.7) | 13.0 (3.4) |
| MMSE (/30) | 29.8 (0.4) | 23.7 (4.8)a | 23.8 (7.4)a | 16.9 (10.9)a,b,c |
| Duration, years | N/A | 7.2 (6.3) | 6.0 (2.6) | 3.8 (1.7) |
| General neuropsychological | ||||
| General intellect | ||||
| WASI verbal IQ | 122 (8.6) | 92 (31.5)a | 74 (20.1)a | 69 (17.7)a |
| WASI performance IQ | 124 (12.9) | 96 (18.3)a,c | 119 (15.4) | 94 (20.8)a,c |
| Episodic memory | ||||
| RMT words (/50) | 48.9 (1.4) | 37.6 (10.2)a | 33.8 (7.3)a | 39.2 (10.8)a |
| RMT faces (/50) | 44.8 (4.7) | 37.3 (7.0)a | 32.1 (5.0)a | 39.0 (7.9) |
| Camden PAL (/24) | 20.6 (2.8) | 13.7 (6.1)a | 6.5 (8.0)a,b,d | 16.5 (2.1) |
| Executive skills | ||||
| WASI Block Design (/71) | 46.8 (11.0) | 26.9 (15.1)a | 38.5 (15.6) | 20.5 (20.5)a |
| WASI Matrices (/32) | 25.5 (4.4) | 16.7 (8.7)a,c | 26.6 (3.5) | 15.4 (10.2)a,c |
| WMS-R digit span forward (max) | 7.1 (1.1) | 5.7 (1.1)a | 6.6 (0.9) | 4.3 (1.4)a,c |
| WMS-R digit span reverse (max) | 5.4 (1.3) | 4.6 (1.4) | 5.3 (1.3) | 3.2 (0.8)a,c |
| D-KEFS Stroop colour naming (s) | 29.6 (4.8) | 45.3 (19.5)a | 37.8 (8.9) | 70.0 (18.7)a,b,c |
| D-KEFS Stroop word reading (s) | 22.3 (3.4) | 28.2 (7.5) | 25.6 (10.7) | 61.4 (16.2)a,b,c |
| D-KEFS Stroop interference (s) | 55.9 (16.7) | 101.1 (52.6)a | 67.3 (19.0) | 123.3 (44.3)a,c |
| Letter fluency (F: total) | 17.4 (5.0) | 9.0 (5.6)a | 9.6 (3.8)a | 5.8 (3.3)a |
| Category fluency (animals: total) | 23.7 (4.2) | 13.0 (8.0)a | 6.5 (4.5)a,b | 12.6 (4.7)a |
| Trails A (s) | 31.9 (9.3) | 58.1 (36.3)a | 46.7 (16.1) | 65.3 (45.4)a |
| Trails B (s) | 66.3 (28.6) | 143.7 (81.6)a | 130.5 (18.8)a | 160.1 (89.7)a |
| Language skills | ||||
| WASI vocabulary | 70.3 (3.4) | 40.9 (24.8)a | 30.6 (18.9)a | 21.8 (21.3)a |
| BPVS | 148.0 (1.4) | 126.2 (30.6)a | 74.8 (37.1)a,b | 106.4 (52.8)a |
| GNT | 26.9 (2.3) | 16.7 (10.2)a | 2.0 (5.6)a,b | 9.0 (7.3)a |
| Other skills | ||||
| GDA (/24) | 14.1 (5.4) | 9.3 (6.1) | 12.8 (5.0) | 4.8 (5.1)a |
| VOSP Object Decision (/20) | 18.9 (1.1) | 15.7 (3.4)a | 15.9 (2.0)a | 15.5 (3.9)a |
| Emotion identificatione | ||||
| Anger | 4.43 (1.9) | 3.31 (2.0) | 2.50 (2.0) | 3.88 (1.5) |
| Disgust | 8.81 (1.1) | 6.13 (2.9)a | 5.33 (2.0)a | 5.00 (3.9)a |
| Fear | 5.48 (2.4) | 3.69 (2.9) | 3.42 (2.2) | 4.88 (2.9) |
| Happiness | 9.43 (0.8) | 8.44 (2.3) | 9.08 (0.9) | 7.13 (3.1) |
| Surprise | 7.76 (1.4) | 5.00 (3.2)a | 3.75 (2.5)a | 4.00 (3.3)a |
| Total (/50) | 35.9 (4.1) | 26.6 (9.5)a | 24.1 (5.7)a | 24.9 (12.6)a |
Mean (standard deviation) scores are shown unless otherwise indicated; maximum scores are shown after tests (in parentheses).
aSignificantly less than controls; bsignificantly less than bvFTD; csignificantly less than svPPA; dsignificantly less than nfvPPA, (all P < 0.05).
ePost-scanner experimental test of facial expression identification (see main text and Fig. 3).
BPVS = British Picture Vocabulary Scale (Dunn and Whetton, 1982); Category fluency totals for animal category and letter fluency for the letter F in 1 min (Gladsjo et al., 1999); D-KEFS = Delis Kaplan Executive System (Delis et al., 2001); GDA = Graded Difficulty Arithmetic (Jackson and Warrington, 1986); GNT = Graded Naming Test (McKenna and Warrington, 1980); MMSE = Mini-Mental State Examination score (Folstein et al., 1975); N/A = not assessed; PAL = Paired Associate Learning test (Warrington, 1996); RMT = Recognition Memory Test (Warrington, 1984); Trails-making task based on maximum time achievable 2.5 min on task A, 5 min on task B (Lezak, 2004); VOSP = Visual Object and Spatial Perception Battery – Object Decision test (Warrington and James, 1991); WASI = Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997); WMS = Wechsler Memory Scale (Wechsler, 1987).
This study was approved by the University College London institutional ethics committee and all participants gave informed consent in accordance with the Declaration of Helsinki.
Experimental stimuli
Videos of dynamic emotional facial expressions were obtained from the Face and Gesture Recognition Research Network (FG-NET) database (Wallhoff, 2006–2015). This database comprises silent recordings of young adults viewing emotional scenarios, designed to elicit spontaneous, naturalistic facial expressions but presented without any instruction to pose particular expressions. For each of the canonical emotions of anger, disgust, fear, happiness and surprise (Ekman et al., 1969) we selected 10 videos (50 stimuli in total) that clearly conveyed the relevant expression (sadness was omitted because its more diffuse time course sets it apart from other emotional expressions). Each video stimulus lasted between 4 and 8 s (mean 4.9 s), commencing as a neutral facial expression and evolving into an emotional expression. We did not include a neutral face condition because so-called ‘neutral’ faces are often interpreted as displaying negative affect (Rich et al., 2006; Suess et al., 2014). Using dynamic stimuli would tend to exaggerate this effect: in that context, an immobile face would appear hostile, while facial muscle movements not included in canonical emotional expressions nevertheless frequently transmit emotional content (Wallbott and Ricci-Bitti, 1993). For this reason, other studies of dynamic facial emotions have often used an abstract visual baseline (Grosbras and Paus, 2005; Sato et al., 2015). To provide a complex visual baseline without facial emotion features, we created 20 dynamic mosaics from the videos by dividing each video frame into 400 equal rectangles (20 × 20), and then randomizing the position of the rectangles within each video (the positions then remained consistent across all frames for a given stimulus). These dynamic mosaics were thus matched with the original videos for luminance, colour, contrast, motion, and duration, but without discernible face or emotional content, i.e. the same physical information was present, but the global configuration was radically altered.
Stimulus presentation
During functional MRI scanning, stimuli were presented in a pseudorandomized block design (five stimuli per block) via a notebook computer using Eyelink Experiment Builder software (SR-Research, Ottawa, Canada). Each stimulus trial was triggered by the magnetic resonance scanner at the onset of a gradient echo-echo planar imaging (GE-EPI) volume acquisition. Visual stimuli were presented on a screen placed outside the bore of the MRI scanner, visible to participants in a periscopic mirror affixed to the radiofrequency (RF) head coil. A total of 90 trials were delivered, comprising 50 dynamic facial stimuli, 20 dynamic scrambled visual mosaics and 20 fixation cross trials (to allow estimation of primary visual sensory processing). Interstimulus interval was 11.72 s for video trials and 8.79 s for fixation cross trials. Following the end of each stimulus, a grey screen was presented until the onset of the next trial. To avoid potentially confounding effects from task preparation, difficulty and performance monitoring, participants were simply instructed to lie still and concentrate on the stimuli with their eyes open; no responses from the participants were obtained during scanning. All participants were remotely monitored via an MRI-compatible Eyelink 1000Plus eyetracker (SR-Research) to ensure they had their eyes open and were fixating on the stimuli.
Brain MRI acquisition
Functional MRI data were acquired using a 3 T Siemens Prisma scanner with a 12-channel RF head coil. A continuous acquisition GE-EPI sequence was used comprising 48 oblique axial slices covering the whole brain. The angle of acquisition was set at −30° from the intercomissural plane to minimize susceptibility-induced signal dropout in orbitofrontal cortex and anterior temporal lobes because of the proximity of these regions to the skull base. Interleaved slices of 2-mm thickness were obtained in descending order with voxel size 2 × 2 × 2 mm, field of view 192 mm, repetition time 2930 ms and echo time 30 ms. For each participant, 340 EPI volumes covering all 90 stimulus presentation trials were obtained for analysis (four volumes for each video trial, and three for each fixation cross trial), with a total scanning time of 16 min 40 s. Following acquisition of the functional MRI scan, a B0 field map was acquired to allow geometric correction of EPI data for field inhomogeneity distortions (field of view 192 mm, slice thickness 3 mm interleaved, voxel size 2.4 × 2.4 × 3 mm, repetition time 688 ms, echo time 1 4.92 ms, echo time 2 7.38 ms).
To enable structural co-registration of functional MRI data, volumetric brain MRIs were acquired for all patients in the same 3 T Siemens Prisma MRI scanner, using a 64-channel head-and-neck RF coil with a T1-weighted sagittal 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (echo time = 2.93 ms, inversion time = 850 ms, repetition time = 2000 ms), with matrix size 256 × 256 × 208 and voxel dimensions 1.1 × 1.1 × 1.1 mm. Parallel imaging (GRAPPA) was used with acceleration factor 2, resulting in an overall scan time of 5 min 6 s.
Autonomic recordings
Simultaneously with functional MRI data acquisition, heart rate was recorded continuously from the left index finger during scanning using an MRI-compatible pulse oximeter (Siemens, Erlangen, Germany). Pulse oximetry is typically the modality of choice for in-scanner heart rate measurement due to problems with scanner artefact in ECG recordings (Critchley et al., 2005; Guo et al., 2016). In addition, a long-range mount positioned within the bore of the scanner captured the participant’s right eye in the periscopic mirror; pupil size was recorded throughout scanning using the eyetracker.
Post-scan behavioural testing
Following the scanning session, each participant was shown the 50 facial emotion stimuli presented during scanning, using the Eyelink Experiment Builder software package on a notebook computer. After each video, they were asked to identify the emotion from a list of the five emotions used in the experiment. Responses were recorded for offline analysis. No time limits were imposed on responses, and no feedback was given during the task.
Analysis of autonomic and behavioural data
Raw heart rate data were analysed offline in MATLAB using a custom script to identify local maxima corresponding to pulse peaks in the waveform. All data were manually inspected to ensure consistency and accuracy of pulse detection. Data from participants with cardiac arrhythmias (e.g. atrial fibrillation) or of insufficient quality were excluded from subsequent heart rate analyses (three healthy controls, four patients with bvFTD, two with svPPA and one with nfvPPA). For each participant, a continuous smoothed heart rate trace was generated by converting each data point to the heart rate corresponding to the inter-beat interval in which it occurred, and then smoothing with a 1-s sliding filter. A heart rate reactivity trace was then generated for each trial by normalizing to the baseline heart rate for that trial, so that all values represented percentage heart rate change from trial baseline. Heart rate change was analysed across eight time-bins at 500-ms intervals from 0.5 s to 4 s from stimulus onset. This heart rate reactivity measure was analysed as the dependent variable in an ANOVA model, incorporating stimulus type and diagnostic group as fixed factors. Post hoc tests with Bonferroni correction were performed when main effects were found. Visualization of the mean trial heart rate trace for healthy controls showed that there was a consistent cardiac deceleration, with a nadir between 3 and 4 s from stimulus onset (Supplementary Fig. 2). A mean heart rate reactivity measure for each participant was therefore defined as the mean change in heart rate from baseline at 3 s from stimulus onset, and this value was entered into the second-level functional MRI analysis to establish the neural basis for between-participant variance in heart rate reactivity.
Pupillometry data were analysed offline using the SR Research Data Viewer software. Pupil reactivity was calculated for each trial as follows:
100 × max pupil size during 5 s post stimulus onset / mean pupil size during 1 s prior to stimulus onset (1)
Trials with pupil reactivity values over two standard deviations above the experimental mean (and therefore potentially contaminated by large artefacts, e.g. blinks) and trials with insufficient pupil capture were removed; overall, 17% of trials were excluded from subsequent analysis. Pupil reactivity was analysed for facial emotion and scrambled videos, but not for fixation cross trials, as the large difference in luminance between the video conditions and fixation cross conditions made them unsuitable for direct comparison. An ANOVA model was used to assess main effects on pupil size change of participant group, stimulus condition type and the interaction between the two. Post hoc tests with Bonferroni correction were performed when significant main effects were found. Mean pupil reactivity for each participant was entered into the second-level functional MRI analysis to establish the neural basis for between-participant variance in pupil reactivity.
Emotion identification scores were compared among groups using an ANOVA model, with Bonferroni-corrected post hoc t-tests when main effects were found. To explore the effect of deficits in other cognitive domains on emotion identification ability, cardiac reactivity and pupil reactivity, correlations were tested between these parameters and performance on tests of working memory (forward digit span), non-verbal intelligence (WASI Matrices), general executive function (Trail-making B test) and semantic knowledge (British Picture Vocabulary Scale).
A statistical threshold P < 0.05 (Bonferroni-corrected where appropriate for post hoc multiple comparisons) was accepted for all tests.
Preprocessing and analysis of functional MRI data
Functional MRI data were processed using SPM12 software (www.fil.ion.ucl.ac.uk/spm) in MATLAB R2014b. The EPI series for each participant was realigned to the first image and unwarped with incorporation of B0 distortion information to correct for field inhomogeneities. The T1 volumetric image for each participant was registered to their EPI images before segmentation into grey matter, white matter and CSF using the New Segment toolbox of SPM. Forward deformations from the segmentation step were then used to normalize the EPI images into MNI space before smoothing the normalized unwarped EPI images with a 6 mm full-width at half-maximum Gaussian kernel. Each registration and normalization step was visually checked for quality control; in five participants, preprocessing was repeated with an additional skull-stripping step prior to registration.
Preprocessed GE-EPI images were entered into a first-level analysis for each participant incorporating the experimental conditions as separate regressors, modelled as a boxcar across the duration of each individual trial, and convolved with the canonical haemodynamic response function. Six head motion parameters were included as covariates of no interest. A liberal masking threshold of 0.1 was used at first level, to ensure that regions showing atrophy in some participants were not entirely excluded from the second-level analysis, where a majority threshold mask was applied (see Supplementary material for more detail on preprocessing performance in the presence of atrophy). T contrasts between conditions were generated from the first-level analysis: the contrast of facial emotion > fixation cross conditions was used to assess sensory processing of dynamic facial expressions, and the contrast of facial emotion > scrambled video conditions was used to assess decoding of facial emotions. The contrasts of positive facial emotion > negative facial emotion and negative facial emotion > positive facial emotion were used to assess valence-specific activation patterns (happiness and surprise were defined as positive emotions, anger, disgust and fear were defined as negative emotions.
In the second-level analysis, T contrasts from the first-level analysis were entered into a full factorial model incorporating all participants, with diagnostic group as a level variable. Masking was performed with a study-specific majority threshold mask (Ridgway et al., 2009). The effects of experimental conditions were modelled by assessing T contrasts for effect of condition across all participants, and F contrasts to detect group differences. Where main effects of participant group were found in the F contrast, group differences were assessed by generating beta plots incorporating all voxels in the relevant cluster. Beta plots for primary visual cortex were also generated to examine whether there were any between-group differences in primary afferent processing.
To establish the neural basis for between-participant differences in emotion identification ability and autonomic responses, total emotion identification score or mean physiological response parameter for each participant was incorporated as a second-level covariate, assessing T contrasts within each participant group separately to establish haemodynamic responses that explained variance in these parameters within each disease group (i.e. syndromic-specific predictors of response rather than activation differences between groups). For emotion identification ability, British Picture Vocabulary Scale scores for each participant were included as a covariate to remove variance attributable to semantic deficits. For cardiac responses, both negative (parasympathetic) and positive (sympathetic) correlations with heart rate change were assessed (Beissner et al., 2013). Although the precise neural inputs responsible for heart rate changes could not be measured (e.g. cardiac acceleration could be due to increased sympathetic input or decreased parasympathetic input), we used cardiac acceleration as a proxy for an overall shift in favour of sympathetic tone and vice versa (Paulus et al., 2016).
For all functional MRI analyses, we applied a cluster-defining uncorrected significance threshold P < 0.005; this cluster-defining threshold was selected according to evidence that it provides the optimal balance between the risks of type I and type II errors (Lieberman and Cunningham, 2009). The significance of blood oxygen level-dependent (BOLD) changes was assessed at two thresholds: at cluster level P < 0.05, after family-wise error (FWE) correction for multiple comparisons over the whole brain; and at peak voxel level PFWE < 0.05 within pre-specified anatomical regions of interest. These thresholds are complementary, allowing detection of robust, potentially novel associations (over the whole brain) while also incorporating prior hypotheses about likely regional associations, informed by previous work. Anatomical regions of interest were defined separately for each analysis based on previous evidence in the healthy brain and in FTD cohorts: for sensory processing of dynamic facial expressions, this region comprised fusiform face area, MT/V5, posterior superior temporal sulcus and middle temporal gyrus (Haxby and Gobbini, 2011; Alcalá-López et al., 2017); for identification of facial emotions, fusiform gyrus, anterior cingulate, insula, frontal operculum and anteromedial temporal lobe (Zahn et al., 2007; Jabbi and Keysers, 2008; Alcalá-López et al., 2017); and for autonomic reactivity, fusiform gyrus, anteromedial temporal lobe, anterior cingulate and insula (Critchley et al., 2005; Beissner et al., 2013; Cersosimo and Benarroch, 2013; Fletcher et al., 2016).
A study-specific mean brain image generated from all participants’ normalized T1 MRIs was used to display SPM results thresholded at uncorrected threshold P < 0.005 for display purposes.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available as they include information that could compromise the privacy of the research participants.
Results
General characteristics of participant groups
Participant groups did not differ significantly in age, gender or years of education (suggesting they were likely to be well matched for premorbid IQ), and patient groups had similar symptom durations.
Identification of facial emotions
Performance data for the post-scan emotion identification task are presented in Table 1 and Fig. 3. There were main effects of participant group [F(3) = 49.9, P < 0.001] and emotion type [F(4) = 26.0, P < 0.001], but no significant interaction between group and emotion [F(12) = 1.55, P = 0.10]. Post hoc tests demonstrated impaired emotion identification in all disease groups relative to healthy controls (all PBonf < 0.001) and in the svPPA group relative to the bvFTD group (PBonf = 0.038). Across the combined participant cohort, identification scores were higher for disgust and happiness than for other emotions (all pairwise comparisons PBonf < 0.001); while scores for anger identification were lower than those for fear (PBonf = 0.046) and surprise (PBonf = 0.012). Overall emotion identification ability correlated significantly with working memory (forward digit span; P = 0.002), general executive function (Trail-making B score; P < 0.001), non-verbal intelligence (WASI Matrices score; P = 0.001) and semantic competence (British Picture Vocabulary Scale score; P < 0.001).
Figure 3.
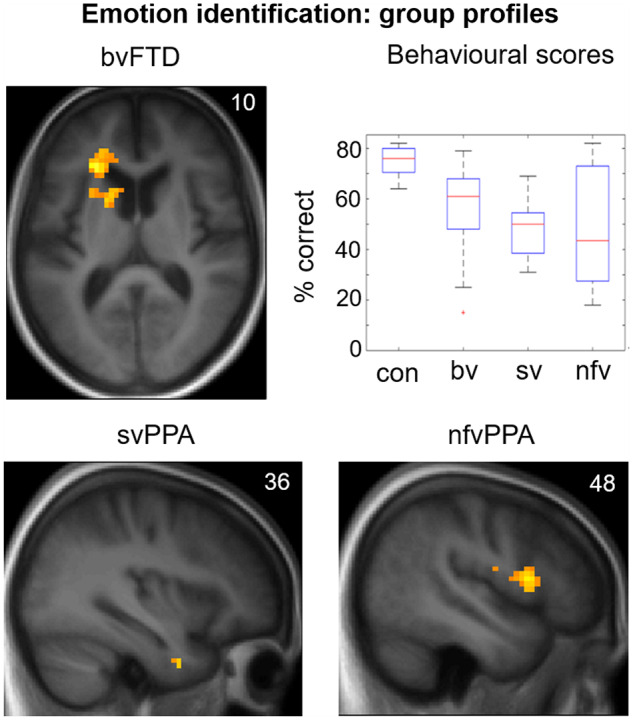
Emotion identification: behavioural results and functional neuroanatomy. The figure displays statistical parametric maps (SPMs) for the T-contrast (facial emotion > dynamic mosaic) in each patient group, with score on the post-scanner emotion identification task as predictor variable in order to show the key determinants of identification ability separately within each group (top left, bottom), together with a plot showing performance (per cent correct) on the emotion identification task by participant group (top right; box and whisker plots display median, interquartile range, minimum and maximum values, with outliers appearing as red crosses). SPMs are thresholded at the cluster-defining threshold of P < 0.005 uncorrected (all loci displayed on the sections shown were significant at PFWE < 0.05 at whole brain or in pre-specified regions of interest (see Table 3 for details) and displayed on sections of the structural group mean T1-weighted template brain image. The plane of each section (in mm in MNI space) is shown in the top right of each image; the axial section shows the left hemisphere on the left. bv = patient group with bvFTD; con = healthy control group; nfv = patient group with nfvPPA; sv = patient group with svPPA.
Cardiac reactivity
Participant groups did not differ in mean heart rate during the period of recording [F(3) = 1.23, P = 0.32], nor in overall heart rate variability [indexed as the variance of interbeat intervals; F(3) = 0.756, P = 0.525].
In the healthy control group, a consistent cardiac deceleration was shown for all stimulus conditions (one-sample t-test, P < 0.001). There was a main effect of stimulus condition on cardiac reactivity [F(2) = 6.3, P = 0.002], post hoc tests showing that greater cardiac deceleration occurred for emotional facial expressions than scrambled videos (PBonf = 0.033) and fixation crosses (PBonf = 0.009), with no significant difference between scrambled video and fixation cross conditions (PBonf = 1). Considering facial emotions separately, the healthy control group showed a main effect of emotion type on cardiac reactivity [F(6) = 11.35, P < 0.001]. Post hoc tests revealed that cardiac deceleration was greater for happiness than other emotions (all individual pairwise comparisons PBonf < 0.001). No other emotion-specific differences were identified in the healthy control group.
Across all participants, cardiac reactivity showed main effects of participant group [F(3) = 10.12 P < 0.001], stimulus type [F(6) = 12.89, P < 0.001] and a significant interaction of group and stimulus type [F(18) = 3.21, P < 0.001]. Relative to healthy controls, cardiac deceleration to visual stimuli was significantly attenuated in each patient group (all post hoc pairwise comparisons PBonf < 0.007). There were no significant differences between patient groups (all PBonf > 0.4). Mean cardiac responses to visual stimuli in each participant group are presented in Fig. 4; data for each stimulus type and participant group separately are presented in Supplementary Fig. 3.
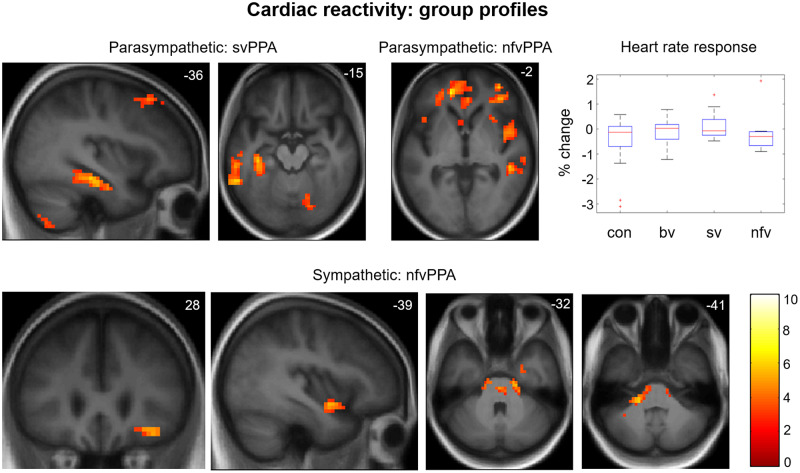
Figure 4.
Cardiac reactivity: heart rate modulation and functional neuroanatomy. Statistical parametric maps (SPMs) for the T-contrast (facial emotion > dynamic mosaic) in the svPPA and nfvPPA patient groups, with cardiac reactivity as predictor variable. Associations are shown separately for negative correlation with cardiac reactivity (i.e. BOLD signal predicting parasympathetic cardiac deceleration; top row) and positive correlation with cardiac reactivity (i.e. BOLD signal predicting sympathetic cardiac acceleration; bottom row). The plot (top right) shows mean cardiac reactivity (per cent change in heart rate from baseline) to facial expression stimuli by participant group (box and whisker plots display median, interquartile range, minimum and maximum values, with outliers appearing as red crosses). SPMs are thresholded at the cluster-defining threshold of P < 0.005 uncorrected and displayed on sections of the structural group mean T1-weighted template brain image. The plane of each section (in mm in MNI space) is shown in the top right of each image; axial sections show the right hemisphere on the right. The colour bar codes T-values. bv = patient group with bvFTD; con = healthy control group; nfv = patient group with nfvPPA; sv = patient group with svPPA.
There were no significant correlations between cardiac reactivity and neuropsychological measures of working memory, general executive function, non-verbal intelligence or semantic competence (all P > 0.3).
Pupil reactivity
There were main effects on pupil responses to video stimuli from both participant group [F(3) = 8.714, P < 0.001] and stimulus condition [F(5) = 3.149, P = 0.008], but no significant interaction between group and condition [F(15) = 0.91, P = 0.55]. Post hoc tests revealed that pupil reactivity was significantly less for scrambled videos than for facial emotions (P < 0.001), but did not differ between facial emotions (all P > 0.08). Relative to healthy controls, pupil responses to visual stimuli were significantly reduced in the nfvPPA group (PBonf < 0.001) but not the svPPA group (PBonf = 0.078) or bvFTD group (PBonf = 1). Mean pupil responses to visual stimuli in each participant group are displayed in Fig. 5; pupillary responses in each stimulus condition are presented in Supplementary Fig. 4.
Figure 5.
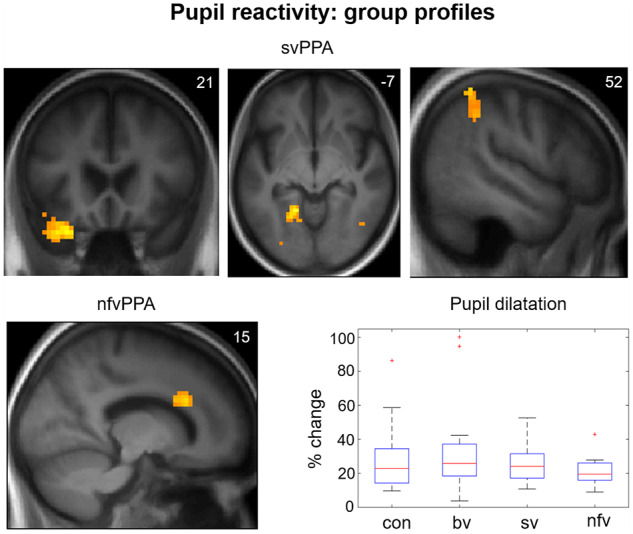
Pupil reactivity: pupil size change and functional neuroanatomy. The figure displays statistical parametric maps (SPMs) for the T-contrast (facial emotion > dynamic mosaic) in the svPPA and nfvPPA patient groups, with pupil reactivity as predictor variable. The plot (bottom right) shows mean pupil reactivity (per cent increase in pupil size from baseline) to facial expression stimuli by participant group (box and whisker plots display median, interquartile range, minimum and maximum values, with outliers appearing as red crosses). SPMs are thresholded at the cluster-defining threshold of P < 0.005 uncorrected and displayed on sections of the structural group mean T1-weighted template brain image. The plane of each section (in mm in MNI space) is shown in the top right of each image; axial and coronal sections show the left hemisphere on the left. bv = patient group with bvFTD; con = healthy control group; nfv = patient group with nfvPPA; sv = patient group with svPPA.
There were no significant correlations between pupil reactivity and neuropsychological measures of working memory, general executive function, non-verbal intelligence or semantic competence (all P > 0.12).
Functional neuroanatomy
Functional neuroanatomical correlates of viewing and identifying facial emotions are shown in Table 2 and Figs 1–3 and correlates of autonomic reactivity are shown in Table 3 and Figs 4 and 5.
Table 2.
Functional neuroanatomical associations of viewing dynamic facial emotions
| Group | Region | Side | Cluster (voxels) | Peak (mm) | PFWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Early visual processing: effect of conditiona | |||||||
| All | Primary visual cortex | Right | 279 | 15 | −94 | 14 | <0.001 |
| Left | – | −12 | 91 | 2 | – | ||
| Facial emotion processing: effect of conditionb | |||||||
| All | Area MT/V5 | Right | 345 | 51 | −70 | 2 | <0.001 |
| Superior temporal sulcus / middle temporal gyrus | Right | – | 57 | −34 | 2 | – | |
| Angular gyrus | Right | – | 63 | −58 | 14 | – | |
| Fusiform gyrus | Right | 71 | 42 | −46 | −16 | 0.001* | |
| Left | 62 | −42 | −52 | −19 | 0.021* | ||
| Area MT/V5 | Left | 87 | 45 | −58 | 11 | 0.010* | |
| Facial emotion processing: positive > negative emotionsc | |||||||
| All | Cuneus | Left | 254 | −3 | −88 | 23 | 0.001 |
| Cuneus | Right | – | 6 | −82 | 38 | – | |
| Facial emotion processing: negative > positive emotionsd | |||||||
| All | Inferior occipital gyrus | Right | 157 | 24 | −88 | 2 | 0.019 |
| Lingual gyrus | Right | – | 15 | −91 | −4 | – | |
| Fusiform gyrus | Right | – | 21 | −82 | −7 | – | |
| Area MT/V5 | Right | 25 | 48 | −64 | 2 | 0.045* | |
| Fusiform gyrus | Left | 32 | −27 | −88 | −10 | 0.031* | |
| Facial emotion processing: effect of groupe | |||||||
| All | Area MT/V5 | Right | 145 | 54 | −67 | −4 | 0.001 |
| Middle temporal gyrus | Right | – | 60 | −55 | 11 | – | |
| Fusiform gyrus | Right | 32 | 42 | −46 | −16 | 0.020* | |
The table presents functional MRI correlates for the individual specified contrasts across the combined participant cohort (all groups). Voxel coordinates of local maxima within significant clusters are in standard MNI stereotactic space. P-values represent cluster-level FWE-corrected values over the whole brain, except *peak level FWE-corrected within pre-specified regions of interest.
Key contrasts were formed as follows.
aT contrast facial emotion > fixation cross; bT contrast facial emotion > mosaic; cT contrast positive emotion > negative emotion; dT contrast negative emotion > positive emotion; eF contrast facial emotion > mosaic.
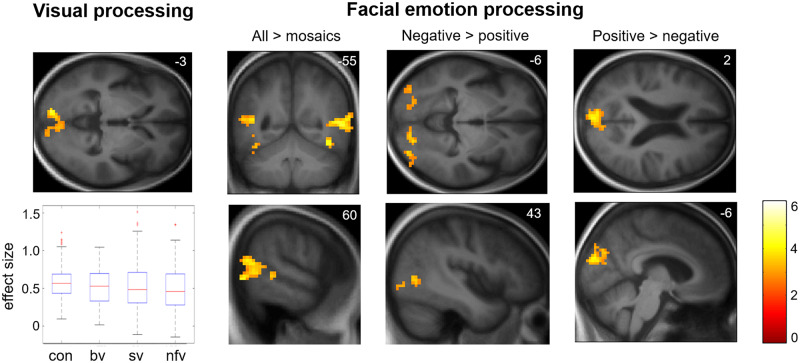
Figure 1.
Functional neuroanatomy of facial emotion viewing: effect of condition. Statistical parametric maps (SPMs) of T contrasts for effect of condition across all participants for early visual processing (visual stimulus > fixation cross contrast; left) and facial emotion processing (contrasts for all facial expressions > dynamic mosaic baseline, positive facial expressions > negative expressions, negative facial expressions > positive expressions) together with a plot (bottom left) of effect sizes (beta-values) demonstrating consistent activation of bilateral primary visual cortex across participant groups (box and whisker plots display median, interquartile range, minimum and maximum values, with outliers appearing as red crosses). SPMs are thresholded at the cluster-defining threshold of P < 0.005 uncorrected and displayed on sections of the structural group mean T1-weighted template brain image. The plane of each section (in mm in MNI space) is shown in the top right of each image; axial sections show the left hemisphere on the top and the coronal section shows the left hemisphere on the left. The colour bar codes T-values (the same range applies to SPMs in other figures, unless otherwise indicated). bv = patient group with bvFTD; con = healthy control group; nfv = patient group with nfvPPA; sv = patient group with svPPA.
Table 3.
Functional neuroanatomical associations of emotion identification and physiological responses
| Group | Region | Side | Cluster (voxels) | Peak (mm) | PFWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Emotion identification performance (after covarying for semantic ability) | |||||||
| bvFTD | Anterior insula | Left | 167 | −24 | 29 | 11 | 0.009 |
| Caudate | Left | – | −15 | 11 | 8 | – | |
| svPPA | Temporal pole | Right | 4 | 36 | 2 | −37 | 0.015* |
| nfvPPA | Frontal operculum | Right | 100 | 48 | 11 | 11 | 0.023* |
| Cardiac parasympathetic activitya | |||||||
| svPPA | Fusiform gyrus | Left | 166 | −36 | −28 | −16 | 0.008 |
| Middle temporal gyrus | Left | 142 | −57 | −49 | −16 | 0.019 | |
| Superior frontal gyrus | Left | 131 | −18 | −1 | 68 | 0.028 | |
| Fusiform gyrus | Right | 49 | 18 | −76 | −16 | 0.033* | |
| nfvPPA | Dorsolateral prefrontal | Right | 3023 | 36 | 38 | 17 | <0.001 |
| Medial prefrontal | Right | – | 18 | 22 | 49 | – | |
| Left | – | −6 | 42 | 33 | – | ||
| Anterior cingulate | Right | – | 10 | 45 | 11 | – | |
| Left | – | −10 | 48 | 16 | – | ||
| Caudate | Left | – | −9 | 2 | 14 | – | |
| Insula | Right | – | 43 | 2 | −2 | – | |
| Frontal operculum | Left | 343 | −42 | 20 | 8 | <0.001 | |
| Superior temporal sulcus | Right | 122 | 48 | −34 | 1 | 0.040 | |
| Cardiac sympathetic activityb | |||||||
| nfvPPA | Orbitofrontal cortex | Right | 346 | 15 | 5 | −19 | <0.001 |
| Temporoparietal junction | Right | 160 | 45 | −34 | 29 | 0.010 | |
| Pons | Right | 119 | 1 | −25 | −32 | 0.045 | |
| Lateral medulla | Right | – | 14 | −29 | −44 | – | |
| Left | – | −7 | −27 | −44 | – | ||
| Insula | Left | 76 | −36 | −1 | −13 | <0.001* | |
| Pupil activity | |||||||
| svPPA | Angular gyrus | Left | 186 | −42 | −64 | 59 | 0.004 |
| Right | 122 | 45 | −40 | 41 | 0.039 | ||
| Fusiform gyrus | Left | 129 | −18 | −52 | −7 | 0.030 | |
| Right | 37 | 42 | −67 | −16 | 0.017* | ||
| Temporal pole | Left | 68 | −27 | 14 | −31 | 0.001* | |
| nfvPPA | Anterior cingulate | Right | 62 | 12 | 17 | 23 | 0.045* |
The table presents functional MRI correlates for the specified response measures at second level within each syndromic group. Voxel coordinates of local maxima within significant clusters are in standard MNI stereotactic space. P-values represent cluster-level FWE-corrected values over the whole brain, except *peak-level FWE-corrected within pre-specified regions of interest.
aNegative association with heart rate change.
bPositive association with heart rate change.
Across the combined participant cohort, early visual processing (video>fixation cross condition) was associated with bi-hemispheric activation of primary visual cortex, while facial emotion-specific sensory processing (facial emotion > scrambled mosaic condition) was associated with bi-hemispheric activation of fusiform face area (Kanwisher et al., 1997) and a cluster of association cortices including MT/V5 (Dumoulin et al., 2000), angular gyrus, posterior superior temporal sulcus and middle temporal gyrus (Fig. 1). Valence-specific contrasts revealed greater activation of early visual processing areas by positive emotions (bilateral cuneus; positive emotion > negative emotion contrast), and greater activation of higher visual processing areas associated with face and biological motion detection by negative emotions (bilateral fusiform, right lingual gyrus and MT/V5; negative emotion>positive emotion contrast).
Activation of primary visual cortex did not differ between participant groups. However, activation of right fusiform and temporo-occipital junctional cortices showed a main effect of participant group: beta plots (Fig. 2) revealed reduced posterior middle temporal gyrus activation relative to healthy controls in the bvFTD and nfvPPA groups, and reduced fusiform activation relative to healthy controls in all syndromic groups.
Figure 2.
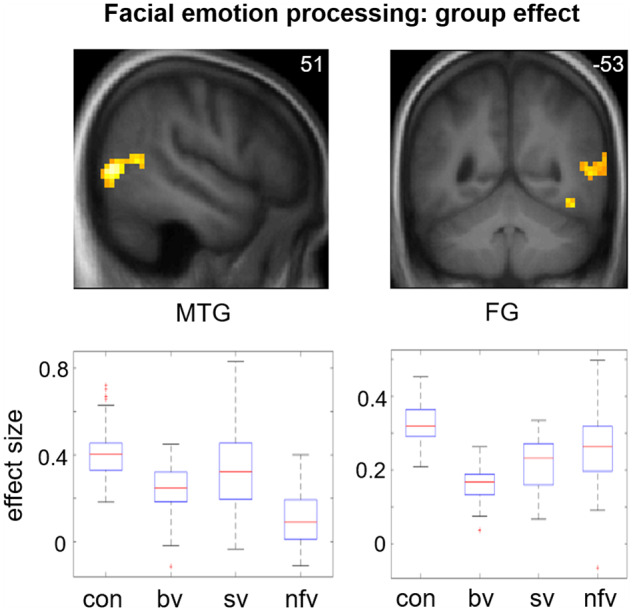
Functional neuroanatomy of facial emotion viewing: effect of participant group. Statistical parametric maps (SPMs) for the F-contrast (main effect of participant group; facial emotion > dynamic mosaic contrast; top row), together with plots of effect sizes (beta-values) demonstrating differential patterns of attenuated BOLD response across groups in the two significant clusters (bottom row; box and whisker plots display median, interquartile range, minimum and maximum values, with outliers appearing as red crosses). SPMs are thresholded at the cluster-defining threshold of P < 0.005 and displayed on sections of the structural group mean T1-weighted template brain image. The plane of each section (in mm in MNI space) is shown in the top right of each image; the coronal section shows the right hemisphere on the right. bv = patient group with bvFTD; con = healthy control group; FG = fusiform gyrus; MTG = middle temporal gyrus; nfv = patient group with nfvPPA; sv = patient group with svPPA.
Activations predicting facial emotion identification performance after covarying for semantic competence were found in syndrome-specific loci (Fig. 3): for the bvFTD group, left anterior insula and caudate; for the svPPA group, right temporal polar cortex; and for the nfvPPA group, right frontal operculum.
Complex syndromic activation profiles correlating with autonomic reactivity were identified (Figs 4 and 5). Within the svPPA group, cardiac deceleration (reflecting parasympathetic activity) was associated with activation of fusiform gyrus bilaterally, left middle temporal and superior frontal gyri; while pupil dilatation was associated with activation of fusiform and angular gyri bilaterally and left temporal pole. Within the nfvPPA group, cardiac deceleration was associated with activation of medial prefrontal cortex bilaterally, right superior temporal sulcus, insula and anterior cingulate and left frontal operculum; while cardiac acceleration (reflecting sympathetic activity) was associated with activation of right temporo-parietal junction and orbitofrontal cortex, left insula and brainstem (central pons in the vicinity of locus coeruleus, parabrachial complex and ventrolateral medulla); and pupil dilatation was associated with activation of right anterior cingulate. No significant associations of autonomic reactivity were identified in the healthy control or bvFTD groups at the prescribed threshold.
Discussion
Here we have shown that canonical FTD syndromes have functional neuroanatomical signatures across three core dimensions of facial emotion processing—perceptual decoding, explicit categorization and autonomic arousal. These signatures map onto the hierarchical network architecture implicated in the processing of socio-emotional signals in the healthy brain (Alcalá-López et al., 2017).
Despite consistent activation of primary visual cortex (Fig. 1), activation of fusiform and occipito-temporal junctional cortices was differentially attenuated across FTD syndromic groups (Fig. 2). In the healthy brain, fusiform gyrus and area MT/V5 participate in a ‘visual-sensory’ processing network (Alcalá-López et al., 2017) that encodes facial expressions and other dynamic signals (Vuilleumier et al., 2001; Kilts et al., 2003; Pelphrey et al., 2007; Haxby and Gobbini, 2011; Foley et al., 2012) while posterior middle temporal gyrus (together with superior temporal sulcus) is a multimodal hub linking encoding of dynamic stimulus features to higher-order associative processes such as behavioural context and theory of mind (Said et al., 2010; Deen et al., 2015; Alcalá-López et al., 2017; Schuwerk et al., 2017; Ballotta et al., 2018). In line with previous evidence in the healthy brain (Fusar-Poli et al., 2009), our data further demonstrate emotion specificity at the level of visual analysis, reflecting the neural resources required to differentiate the valence of facial expressions: positively-valenced (smiling) faces can be distinguished perceptually from other expressions based on elementary configurational feature decoding in early visual areas, whereas differentiation of negatively-valenced facial expressions demands a more fine-grained categorical analysis, engaging higher order visual cortices (fusiform gyrus and MT/V5).
Our findings extend previous work in bvFTD (Virani et al., 2013; De Winter et al., 2016), demonstrating that nfvPPA (but not svPPA) is also associated with reduced engagement of the temporo-occipital hub for dynamic facial expression processing while all major FTD syndromes lead to reduced activity in fusiform face-responsive cortex. Moreover, visual cortical responses were not the key drivers of emotional identification performance. Consistent with previous work (Hutchings et al., 2017), this was impaired across FTD syndromes but predicted by syndrome-specific activation of more anterior cortical regions linked to visual association areas (Alcalá-López et al., 2017) (Fig. 3): anterior insula and caudate in bvFTD, anterior temporal cortex in svPPA and frontal operculum in nfvPPA. These distinct neuroanatomical associations suggest that the mechanism of impaired emotion categorization may differ between syndromes and arise at different levels of the processing hierarchy (Alcalá-López et al., 2017). Emotion identification in the bvFTD and nfvPPA groups was driven by activation of intermediate-integrative network elements: anterior insula plays a key role in integrating body state representations and affective judgements (Jabbi and Keysers, 2008; Craig, 2009), while both caudate and frontal operculum have been implicated in motoric processing of dynamic emotional faces, providing a substrate for ‘mirror’ activity supporting empathic emotion identification (Montgomery et al., 2009; Said et al., 2010; Trinkler et al., 2017). In contrast, emotion identification in the svPPA group was determined by a core element of the higher associative network in right anterior temporal lobe, which instantiates social concepts and person-specific semantics (Zahn et al., 2007; Olson et al., 2013).
The autonomic findings here amplify mounting evidence for central autonomic dysregulation in FTD (Joshi et al., 2014, 2017; Fletcher et al., 2015b; Guo et al., 2016; Marshall et al., 2017, 2018a). The neuroanatomical associations of cardiac responses here conformed broadly to the partitioning of cerebral sympathetic and parasympathetic regulatory mechanisms in previous studies of the healthy brain (Beissner et al., 2013). Cardiac parasympathetic reactivity to facial emotions was impaired in all FTD syndromes, while pupil reactivity was impaired in nfvPPA. Our neuroanatomical findings support distinct mechanisms of altered autonomic reactivity in svPPA and nfvPPA. In the svPPA group, this was mediated by fusiform together with posterior temporo-parietal, temporal polar and prefrontal cortices, previously linked to parasympathetic autonomic responses and pupillary visuomotor tracking (Critchley et al., 2005; Beissner et al., 2013; Hosseini et al., 2017); while in the nfvPPA group, autonomic responses were mediated by cingulo-insular and inferior frontal integrative and higher-order dorsal fronto- and temporo-parietal associative areas conjointly with brainstem sympathetic and parasympathetic pathways (Critchley et al., 2005; Beissner et al., 2013; Alcalá-López et al., 2017). The lack of a group-level functional neuroanatomical correlate of cardiac hyporeactivity for the bvFTD group may reflect the pathological and structural neuroanatomical heterogeneity of this syndrome (Warren et al., 2013a).
Whereas all three FTD syndromes were associated with impaired explicit identification of facial expressions and reduced engagement of face-responsive fusiform cortex, their distinctive syndromic profiles of higher-order evaluative and autonomic dysfunction corroborate previous studies of neural network organization in the healthy brain (Critchley et al., 2005; Beissner et al., 2013; Alcalá-López et al., 2017). In bvFTD, core network dysfunction centred on middle temporal gyrus, anterior insula and dorsal striatum: regions integral to the integration of bodily and relevant environmental signals with output behaviours, including mental state judgments. This is in line with previous evidence for profoundly disturbed emotional mimesis and homeostatic signalling in this syndrome (Marshall et al., 2018a, b) and also with the cardiac parasympathetic deficit here. In svPPA, core network dysfunction centres on areas (notably, anterior temporal cortex) involved in appraisal of salient socio-emotional and other environmental stimuli, and implicated both in emotion categorization and autonomic reactivity (Fletcher et al., 2015b, 2016). In nfvPPA, core frontal opercular dysfunction underpins deficits of both cognitive and autonomic emotional responses, embedded in a distributed cortico-subcortical signature of autonomic dysregulation that extends to brainstem effector circuitry: this aligns with previous evidence for autonomic hyporeactivity in nfvPPA (Fletcher et al., 2015a, b; Marshall et al., 2018a). Moreover, the syndrome of nfvPPA is often a variant of progressive supranuclear palsy (Josephs and Duffy, 2008), with associated midbrain atrophy. This is one possible explanation for the selective loss of pupil reactivity in this syndrome but requires further work to confirm (Fletcher et al., 2016).
This work has several limitations and raises a number of important issues for future clarification. Larger patient cohorts with histopathological and genetic correlation will be required to define the pathophysiological phenotypes delineated here fully (Perry et al., 2017). The interpretation of BOLD signal changes in neurodegenerative disease cohorts is complicated by the presence of grey matter atrophy: it is noteworthy that a number of functional neuroanatomical associations in the present cohort fell outside regional atrophy zones for these syndromes (Supplementary Fig. 1) but on the other hand, certain structures that are integral to emotion processing—notably, amygdala—were conspicuously absent here. This might be attributable (at least in part) to reduced BOLD signal due to severe atrophy but could also reflect the nature of the paradigm. Engagement of amygdala may require stimuli to carry emotional or other behavioural significance for the viewer (Strathearn and Kim, 2013; Kumfor et al., 2018): our facial expression stimuli were relatively banal. This raises the broader issue of paradigm design: an in-scanner task with manipulation of behavioural context would likely modulate activation profiles (Alcalá-López et al., 2017; Kumfor et al., 2018), and indeed, the separable correlates of emotion perception and identification here hint at such a modulatory effect. The absence of a correlated task might also account for the finding of impaired cardiac reactivity in svPPA, in contrast to previous observations (Marshall et al., 2018a).
Conclusions
Our findings in the working brain in FTD suggest a refinement of the influential neural network paradigm of these diseases (Warren et al., 2013b; Perry et al., 2017): rather than a unitary mapping between clinical phenotype and brain network dysfunction, we have demonstrated coactivation of distributed sensory and associative networks across FTD syndromes (Alcalá-López et al., 2017). A further key emerging theme in FTD and related neurodegenerative diseases is the centrality of homeostatic dysfunction to socio-emotional symptoms (Cersosimo and Benarroch, 2013; Marshall et al., 2017; Trinkler et al., 2017; Ahmed et al., 2018; Marshall et al., 2018a, b): integration of functional MRI with simultaneous autonomic recordings here has underscored this, by revealing a rich matrix of central autonomic dysregulatory changes overlapping network profiles of emotional visual and categorization processing in FTD syndromes. An important direction for further work will be to define more precisely the relative contributions of aberrant stimulus decoding and primary failure of central autonomic control to diminished physiological reactivity in different FTD syndromes. The use of naturalistic, dynamic emotional stimuli (as here) is likely to be critical to delineate homeostatic processes that evolve over time; this might also motivate the application of functional neuroimaging techniques such as magnetoencephalography with high temporal resolution (Macey et al., 2015; Hughes et al., 2018).
This work has far-reaching clinical as well as pathobiological implications. Functional neuroimaging can reveal disease effects beyond and predating the development of irreversible network degeneration, including presymptomatic changes in genetic cases (Rohrer et al., 2015). More fundamentally, work of this kind promises to deliver a new class of pathophysiological dementia biomarkers: if, as our findings suggest, autonomic measures are surrogates for complex socio-emotional behaviours and neural network dysfunction, this could find powerful applications in early diagnosis, disease tracking and the evaluation of new therapies.
Supplementary Material
Acknowledgements
We are grateful to all participants for their involvement.
Glossary
Abbreviations
- (bv)FTD
(behavioural variant) frontotemporal dementia
- nfv/svPPA
non-fluent variant/semantic variant primary progressive aphasia
Funding
The Dementia Research Centre is supported by Alzheimer’s Research UK, the Brain Research Trust and the Wolfson Foundation. This work was funded by the Alzheimer’s Society, Leonard Wolfson Experimental Neurology Centre, Medical Research Council UK, and the NIHR UCLH Biomedical Research Centre. C.R.M. was supported by a Clinical Research Fellowship from the Leonard Wolfson Experimental Neurology Centre, and now receives funding from Bart’s Charity. H.S. is supported by a Clinical Research Fellowship from the Leonard Wolfson Experimental Neurology Centre. C.J.D.H. and R.L.B. were funded by MRC PhD studentships. K.M.M. is supported by a grant from the Alzheimer’s Society. J.D.R. is an MRC Clinician Scientist.
Competing interests
The authors report no competing interests.
References
- Ahmed RM, Ke YD, Vucic S, Ittner LM, Seeley W, Hodges JRet al. Physiological changes in neurodegeneration: mechanistic insights and clinical utility. Nat Rev Neurol 2018; 14: 259–71. [DOI] [PubMed] [Google Scholar]
- Alcalá-López D, Smallwood J, Jefferies E, Van Overwalle F, Vogeley K, Mars RBet al. Computing the social brain connectome across systems and states. Cereb Cortex 2017: 1–26. [DOI] [PubMed] [Google Scholar]
- Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter Fet al. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci 2014; 6: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballotta D, Lui F, Porro CA, Nichelli PF, Benuzzi F. Modulation of neural circuits underlying temporal production by facial expressions of pain. PLOS ONE 2018; 13: e0193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 2013; 33: 10503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. Br J Psychol 1986; 77: 305–27. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE. Central control of autonomic function and involvement in neurodegenerative disorders. Handb Clin Neurol 2013; 117: 45–57. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Carton AM, Hardy CJ, Golden HL, Clark CN, Fletcher PDet al. Processing emotion from abstract art in frontotemporal lobar degeneration. Neuropsychologia 2015; 81: 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto B, Manes F, Montañés P, Matallana D, Reyes P, Velasquez Met al. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Human Neurosci 2013; 7: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10: 59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage 2005; 24: 751–62. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb Cortex 2015; 25: 4596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter F-L, Van den Stock J, de Gelder B, Peeters R, Jastorff J, Sunaert Set al. Amygdala atrophy affects emotion-related activity in face-responsive regions in frontotemporal degeneration. Cortex 2016; 82: 179–91. [DOI] [PubMed] [Google Scholar]
- Downey LE, Blezat A, Nicholas J, Omar R, Golden HL, Mahoney CJet al. Mentalising music in frontotemporal dementia. Cortex 2013; 49: 1844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL Jr, Le Goualher G, Bruce Pike Get al. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex 2000; 10: 454–63. [DOI] [PubMed] [Google Scholar]
- Ekman P, Sorenson ER, Friesen WV. Pan-cultural elements in facial displays of emotion. Science 1969; 164: 86–8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Hodges SD, Baird JA, Black SE. Empathy in frontotemporal dementia and Alzheimer’s disease. J Clin Exp Neuropsychol 2010; 32: 289–98. [DOI] [PubMed] [Google Scholar]
- Fletcher PD, Nicholas JM, Downey LE, Golden HL, Clark CN, Pires Cet al. A physiological signature of sound meaning in dementia. Cortex 2016; 77: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PD, Nicholas JM, Shakespeare TJ, Downey LE, Golden HL, Agustus JLet al. Dementias show differential physiological responses to salient sounds. Front Behav Neurosci 2015a; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PD, Nicholas JM, Shakespeare TJ, Downey LE, Golden HL, Agustus JLet al. Physiological phenotyping of dementias using emotional sounds. Alzheimers Dement 2015b; 1: 170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, Rippon G, Thai NJ, Longe O, Senior C. Dynamic facial expressions evoke distinct activation in the face perception network: a connectivity analysis study. J Cogn Neurosci 2012; 24: 507–20. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze Set al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatr Neurosci 2009; 34: 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SFet al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Brain Networks involved in viewing angry hands or faces. Cereb Cortex 2005; 16: 1087–96. [DOI] [PubMed] [Google Scholar]
- Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AYet al. Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc Natl Acad Sci 2016; 113: E2430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI. Distributed neural systems for face perception. In: Calder A, Rhodes G, Johnson M, Haxby J, editors. Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. p. 93–110. [Google Scholar]
- Hazelton JL, Irish M, Hodges JR, Piguet O, Kumfor F. Cognitive and affective empathy disruption in non-fluent primary progressive aphasia syndromes. Brain Impair 2016; 18: 117–29. [Google Scholar]
- Hosseini SMH, Bruno JL, Baker JM, Gundran A, Harbott LK, Gerdes JCet al. Neural, physiological, and behavioral correlates of visuomotor cognitive load. Sci Rep 2017; 7: 8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol 2013; 26: 174–84. [DOI] [PubMed] [Google Scholar]
- Hughes LE, Rittman T, Robbins TW, Rowe JB. Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia. Brain 2018; 141: 2486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings R, Palermo R, Piguet O, Kumfor F. Disrupted face processing in frontotemporal dementia: a review of the clinical and neuroanatomical evidence. Neuropsychol Rev 2017; 27: 18–30. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion 2008; 8: 775–80. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR. Apraxia of speech and non-fluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol 2008; 21: 688–92. [DOI] [PubMed] [Google Scholar]
- Joshi A, Jimenez E, Mendez MF. Pavlov’s orienting response in frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2017; 29: 351–6. [DOI] [PubMed] [Google Scholar]
- Joshi A, Mendez MF, Kaiser N, Jimenez E, Mather M, Shapira JS. Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci 2014; 26: 227–32. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 1997; 17: 4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. NeuroImage 2003; 18: 156–68. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Hodges JR. Theory of mind in frontotemporal dementia. Soc Neurosci 2006; 1: 235–44. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Ibanez A, Hutchings R, Hazelton JL, Hodges JR, Piguet O. Beyond the face: how context modulates emotion processing in frontotemporal dementia subtypes. Brain 2018; 141: 1172–85. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 2009; 4: 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Ogren JA, Kumar R, Harper RM. Functional imaging of autonomic regulation: methods and key findings. Front Neurosci 2015; 9: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Allen M, Russell LL, Clark CN, Bond RLet al. Cardiac responses to viewing facial emotion differentiate frontotemporal dementias. Ann Clin Transl Neurol 2018a; 5: 687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Russell LL, Clark CN, Bond RL, Dick KMet al. Motor signatures of emotional reactivity in frontotemporal dementia. Sci Rep 2018b; 8: 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Russell LL, Clark CN, Dick KM, Brotherhood EVet al. Impaired interoceptive accuracy in semantic variant primary progressive aphasia. Front Neurol 2017; 8: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJD, Volkmer A, Russell LL, Bond RL, Fletcher PDet al. Primary progressive aphasia: a clinical approach. J Neurol 2018c; 265: 1474–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery KJ, Seeherman KR, Haxby JV. The well-tempered social brain. Psychol Sci 2009; 20: 1211–3. [DOI] [PubMed] [Google Scholar]
- Oliver LD, Mitchell DG, Dziobek I, MacKinley J, Coleman K, Rankin KPet al. Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia 2015; 67: 14–26. [DOI] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci 2013; 8: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar R, Henley SM, Bartlett JW, Hailstone JC, Gordon E, Sauter DAet al. The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration. NeuroImage 2011a; 56: 1814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar R, Rohrer JD, Hailstone JC, Warren JD. Structural neuroanatomy of face processing in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2011b; 82: 1341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus PC, Castegnetti G, Bach DR. Modeling event-related heart period responses. Psychophysiology 2016; 53: 837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, LaBar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci 2007; 2: 140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke Aet al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 2017; 140: 3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus Jet al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EBet al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci 2006; 103: 8900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. NeuroImage 2009; 44: 99–111. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot Let al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the genetic frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol 2015; 14: 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD. Receptive prosody in nonfluent primary progressive aphasias. Cortex 2012; 48: 308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci 2010; 293: 35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff Net al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 2002; 125: 2286–95. [DOI] [PubMed] [Google Scholar]
- Said CP, Moore CD, Engell AD, Todorov A, Haxby JV. Distributed representations of dynamic facial expressions in the superior temporal sulcus. J Vis 2010; 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S. Spatiotemporal neural network dynamics for the processing of dynamic facial expressions. Sci Rep 2015; 5: 12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuwerk T, Schurz M, Muller F, Rupprecht R, Sommer M. The rTPJ’s overarching cognitive function in networks for attention and theory of mind. Soc Cogn Affect Neurosci 2017; 12: 157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 2001; 70: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Front Neurosci 2013; 7: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suess F, Rabovsky M, Abdel Rahman R. Perceiving emotions in neutral faces: expression processing is biased by affective person knowledge. Soc Cogn Affect Neurosci 2014; 10: 531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkler I, Devignevielle S, Achaibou A, Ligneul RV, Brugières P, Cleret de Langavant Let al. Embodied emotion impairment in Huntington’s Disease. Cortex 2017; 92: 44–56. [DOI] [PubMed] [Google Scholar]
- Virani K, Jesso S, Kertesz A, Mitchell D, Finger E. Functional neural correlates of emotional expression processing deficits in behavioural variant frontotemporal dementia. J Psychiatry Neurosci 2013; 38: 174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 2001; 30: 829–41. [DOI] [PubMed] [Google Scholar]
- Wallbott HG, Ricci-Bitti P. Decoders’ processing of emotional facial expression: a top-down or bottom-up mechanism? Eur J Soc Psychol 1993; 23: 427–43. [Google Scholar]
- Wallhoff F. Facial expressions and emotion database. München: Technische Universität München; 2006–2015. [Google Scholar]
- Warren JD, Rohrer JD, Rossor MN. Clinical review. Frontotemporal dementia. BMJ 2013a; 347: f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Rohrer JD, Schott JM, Fox NC, Hardy J, Rossor MN. Molecular nexopathies: a new paradigm of neurodegenerative disease. Trends Neurosci 2013b; 36: 561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sciences 2007; 104: 6430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available as they include information that could compromise the privacy of the research participants.