Abstract
BACKGROUND AND PURPOSE:
Although multishot EPI (readout-segmented EPI) has been touted as a robust DWI sequence for cholesteatoma evaluation, its efficacy in disease detection compared with a non-EPI (eg, HASTE) technique is unknown. This study sought to compare the accuracy of readout-segmented EPI with that of HASTE DWI in cholesteatoma detection.
MATERIALS AND METHODS:
A retrospective review was completed of consecutive patients who underwent MR imaging for the evaluation of suspected primary or recurrent/residual cholesteatomas. Included patients had MR imaging examinations that included both HASTE and readout-segmented EPI sequences and confirmed cholesteatomas on a subsequent operation. Two neuroradiologist reviewers assessed all images, with discrepancies resolved by consensus. The ratio of signal intensity between the cerebellum and any observed lesion was noted.
RESULTS:
Of 23 included patients, 12 (52.2%) were women (average age, 47.8 [SD, 25.2] years). All patients had surgically confirmed cholesteatomas: Six (26.1%) were primary and 17 (73.9%) were recidivistic. HASTE images correctly identified cholesteatomas in 100.0% of patients. On readout-segmented EPI sequences, 16 (69.6%) were positive, 5 (21.7%) were equivocal, and 2 (8.7%) were falsely negative. Excellent interobserver agreement was noted between reviews on both HASTE (κ = 1.0) and readout-segmented EPI (κ = 0.9) sequences. The average signal intensity ratio was significantly higher on HASTE than in readout-segmented EPI, facilitating enhanced detection (mean difference 0.5; 95% CI, 0.3–0.8; P = .003).
CONCLUSIONS:
HASTE outperforms readout-segmented EPI in the detection of primary cholesteatoma and disease recidivism.
Middle ear cholesteatomas are ectopic, keratinizing squamous epithelium, which may be acquired or, much less commonly, congenital.1,2 Although not considered a true neoplasm, cholesteatomas are locally destructive and have a high propensity for recurrence following surgical removal. As they gradually expand, cholesteatomas erode the osseous structures within and adjacent to the middle ear cavity, including the ossicles, labyrinth, fallopian canal, and middle fossa bone plate.3 Current mainstay therapy includes microsurgical extirpation, with the chief goal of complete disease removal and prevention of intratemporal and intracranial complications.4 The most common microsurgical approach for cholesteatoma is an canal wall up tympanomastoidectomy, in which the posterior bony ear canal is left intact and the tympanic membrane is reconstructed. Depending on the extent of disease at surgery, a planned second-look procedure may be performed approximately 1 year after the initial operation to evaluate residual disease and potentially reconstruct the ossicular chain when indicated. Nevertheless, residual and/or recurrent (ie, recidivism) disease occurs in up to 30% of cases. Imaging is crucial in cholesteatoma management; it aids in the initial diagnosis and may obviate the need for second-look surgery.5,6
During the past decade, DWI has emerged as a powerful diagnostic tool for detection of both primary and residual or recurrent cholesteatomas.7-9 Cholesteatomas demonstrate marked hyperintensity on DWI , likely related to either T2 shinethrough or intralesional restricted diffusion related to keratin debris.10 Across the years, there have been many iterations of DWI optimization for cholesteatoma identification. The EPI trajectory used by conventional DWI makes such sequences prone to substantial susceptibility artifacts, and single-shot EPI sequences were found to be poor at identifying lesions of <4–5 mm.11-13 Consequently, non-EPI DWI techniques began to be favored; such algorithms minimize susceptibility artifacts and geometric distortion related to the skull base and are able to detect lesions as small as 2 mm.14,15 BLADE (Siemens) and other such PROPELLER sequences are subtypes of non-EPI techniques that minimize susceptibility artifacts and geometric distortions by sampling k-space in a rotating fashion.16,17
More recently, HASTE DWI (Siemens) has emerged as a particularly effective sequence, which is relatively insensitive to motion and has been shown in prior studies to detect cholesteatomas with promising accuracy.18,19 Although traditional EPI techniques have been largely abandoned in the setting of cholesteatoma detection, readout-segmented EPI (RESOLVE; Siemens) DWI is a relatively new technique that has been promoted as a possible alternative diffusion sequence. RESOLVE is a multishot (MS) EPI sequence that is able to reduce geometric distortions at the expense of longer imaging time. Recent reports have indicated that RESOLVE may be a useful sequence in cases of suspected cholesteatoma.7,20
Despite its proposed efficacy in cholesteatoma imaging, the diagnostic utility of RESOLVE sequences has yet to be robustly evaluated against non-EPI DWI, the current criterion standard. Thus, this study was conceived with the chief goal of assessing the accuracy of RESOLVE in the detection of cholesteatomas and comparing the ability of RESOLVE with that of HASTE sequences in this context.
MATERIALS AND METHODS
Patient Selection and Electronic Medical Record Review
Institutional review board approval (approval No. 19–008120) was obtained before study commencement. A retrospective review was completed of consecutive patients who underwent high-resolution MR imaging of the temporal bone between September 20, 2011, and March 9, 2020; patients were identified using an institutional electronic medical record search engine. Inclusion criteria encompassed patients who had preoperative MR imaging including dedicated HASTE and RESOLVE sequences for the evaluation of cholesteatoma (both sequences were routinely performed for cholesteatoma work-ups since 2011) and a subsequent operation in which the diagnosis of cholesteatoma was confirmed. Patients were excluded if images were substantially degraded by artifacts (eg, motion) rendering the imaging nondiagnostic.
MR Imaging Protocol
MR imaging was performed on a 1.5T MR imaging scanner (Magnetom Espree; Siemens) using a 32-channel head coil. HASTE (TR/TE = 800/108 ms, flip angle = 180°, section thickness = 2.0 mm, interslice gap = 2.2 mm, matrix = 128 × 90, acquisition time = 4 minutes 17 seconds) and RESOLVE sequences (TR/TE = 3500/76 ms, flip angle = 180°, section thickness = 2.0 mm, interslice gap = 2.6 mm, matrix = 160 × 128, acquisition time = 3 minutes 44 seconds) were performed in all cases per inclusion criteria.
Imaging Analysis
Two board-certified neuroradiologists (J.I.L. and J.C.B.) reviewed all MR imaging examinations. All discrepancies were resolved by consensus. Each sequence was analyzed for the subjective presence or absence of cholesteatoma (presumed on the basis of a focus of hyperintensity) on both HASTE and RESOLVE sequences. Images were noted as being “positive,” “negative,” or “equivocal” for the presence of a lesion. Identified lesions were measured in the longest axial dimensions; measurements were used to calculate the presumed ellipse axial area of all cholesteatomas. Average signal intensities of the presumed cholesteatoma and adjacent cerebellar parenchyma were measured to establish a signal intensity ratio for each diffusion sequence.
Statistical Analysis
Means and SDs for continuous variables were calculated in Excel (Microsoft). A paired t test was used to compare both average signal intensity ratios and measured lesion size on HASTE and RESOLVE. Interobserver agreement was calculated using a Cohen κ statistical test. Statistical analyses were performed with the SAS-based statistical software package (JMP 13.0; SAS Institute). The threshold for significance was set at P < .05.
RESULTS
Patient Characteristics
Twenty-five patients met the inclusion criteria, of whom 2 were excluded (one because the incorrect MR imaging protocol was used and one because DWI did not include the entire temporal bone). Hence, the final cohort comprised 23 patients; 12/23 (52.2%) were women, and the average age was 47.8 [SD, 25.2] years. Six patients (26.1%) were diagnosed with a primary cholesteatoma; the remainder (73.9%) represented disease recidivism diagnosed on follow-up MR imaging after prior cholesteatoma resection. Of the patients who had previously undergone surgical resection, the average time interval between the initial surgery and the second-look MR imaging was 104.6 [SD, 162.9] months (median, 60.5 months). For all patients, the average time between MR imaging and surgical confirmation of cholesteatoma was 1.6 [SD, 2.4] months.
Imaging Analysis
Every patient in the cohort had cholesteatoma confirmed at surgery. All (100.0%) lesions were detected on preoperative HASTE imaging. On RESOLVE sequences, 16 (69.6%) were positive (Fig 1), 5 (21.8%) were equivocal (Fig 2), and 2 (8.7%) were falsely negative (Fig 3). Excellent interobserver agreement was noted between reviews of both HASTE (κ = 1.0) and RESOLVE (κ = 0.9) sequences. The sole disagreement was regarding a RESOLVE sequence in which the 2 reviewers regarded the examination as either equivocal or negative.
FIG 1.
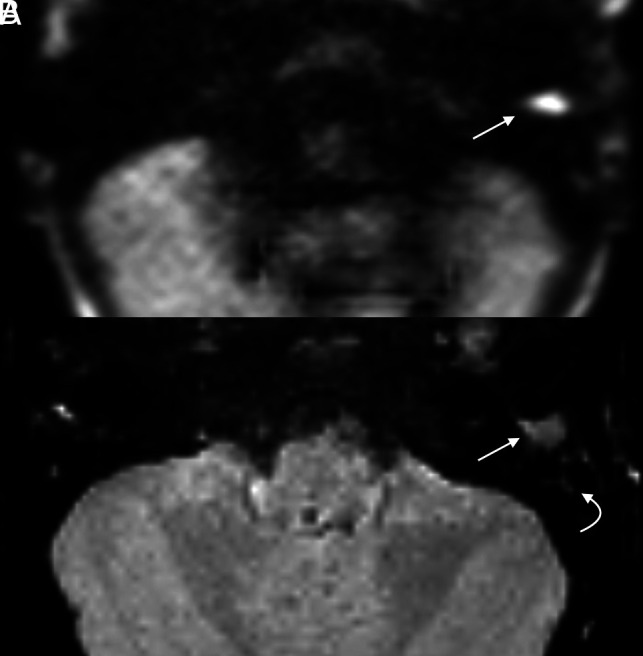
Cholesteatoma with positive findings on both HASTE and RESOLVE in a 4-year-old girl with delayed speech. Both axial HASTE (A) and RESOLVE (B) demonstrate a region of restricted diffusion in the left external auditory canal (arrow). Note greater signal hyperintensity of HASTE compared with the RESOLVE image. Adjacent opacified mastoid air cells demonstrate relatively faint signal (curved arrow).
FIG 2.
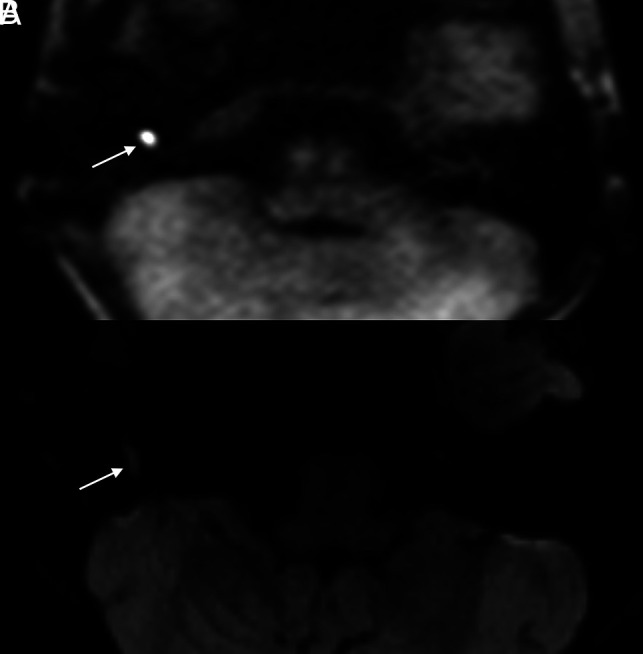
Example of a cholesteatoma seen on HASTE imaging with equivocal findings on RESOLVE. The patient is a 13-year-old boy with a history of bilateral cholesteatomas status post multiple prior surgeries who presented with recurrent hearing loss in his right ear. Axial HASTE (A) image clearly depicts a 0.6-cm focus of restricted diffusion in the right hypotympanum (arrow). Although faint signal is seen in this region on corresponding axial RESOLVE (B) image (arrow), the intralesional signal is insufficiently intense to warrant a certain diagnosis.
FIG 3.
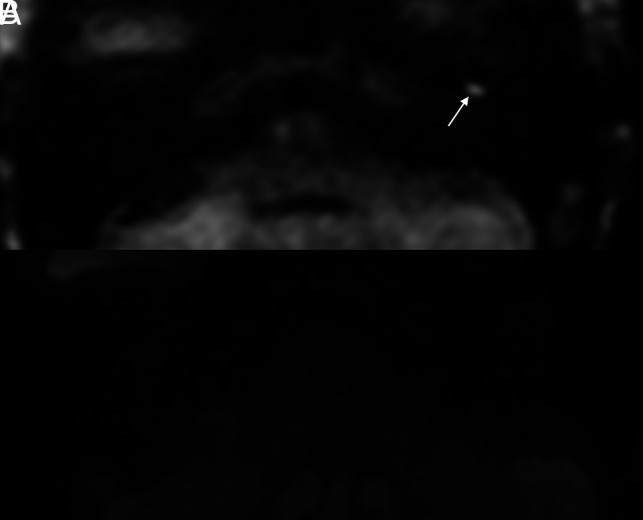
Cholesteatoma visible on HASTE but not on RESOLVE in a 69-year-old woman who had undergone a left tympanomastoidectomy approximately 15 months prior for resection of a cholesteatoma. MR imaging was performed to assess residual or recurrent disease. Axial (A) HASTE image demonstrates a well-demarcated 0.6-cm focus of restricted diffusion in the left anterior mesotympanum (arrow). No abnormal signal was seen on corresponding RESOLVE image (B).
The average axial area of cholesteatoma on HASTE images was 3.7 [SD, 4.2] cm2 measured in the maximum diameter (range = 0.5–15.6 cm2); the average size on RESOLVE images was 2.9 [SD, 3.8] cm2 (range, 0.4–15.6 cm2). The measured size on HASTE images was significantly larger than that measured on RESOLVE images (mean difference, 1.0 cm2; 95% CI, 0.6–1.5 cm2; P < .001). Both of the lesions not identified on RESOLVE images were among the smallest cholesteatomas in the cohort, each measuring 0.6 cm2. Two other similarly sized lesions (0.5 and 0.6 cm2) were each regarded as “equivocal” on RESOLVE images. Lesions <1.0 cm2 measured on HASTE were more likely to be equivocal or falsely negative on RESOLVE than those of ≥1.0 cm2 (P = .02).
The average ratio between cholesteatoma and cerebellar signal intensities was 1.8 [SD, 0.8] on HASTE and 1.3 [SD, 0.7] on RESOLVE. The average signal intensity ratio was significantly higher on HASTE than on RESOLVE (mean difference, 0.5; 95% CI, 0.3–0.8; P = .003).
DISCUSSION
The results of this study indicate that HASTE outperforms RESOLVE in the detection of primary and recidivistic cholesteatoma. HASTE imaging correctly identified lesions in all cases, while RESOLVE results were equivocal in more than one-fifth of cases and falsely negative in 2 patients. These findings have immediate implications in terms of how MR cholesteatoma imaging is approached. Specifically, the results indicated that RESOLVE images are more likely to be equivocal or falsely negative in cases of surgically confirmed cholesteatomas, particularly in the case of small lesions.
The most likely contributor to the superior performance of HASTE imaging is the greater relative intensity of lesions compared with the adjacent cerebellar parenchyma. Cholesteatomas were both subjectively positive and objectively brighter on the HASTE sequence. This observed appearance is similar to that described by Dhepnorrarat et al,21 in which cholesteatomas were distinctly apparent, even in the setting of postoperative changes with adjacent scar tissue. Cholesteatomas on RESOLVE images, conversely, frequently had signal more similar to that of the adjacent parenchyma, thereby accounting for the equivocal results garnered from RESOLVE images. Size, too, appeared to be an important factor: Smaller lesions were more likely to be missed or called equivocal on RESOLVE sequences, and the axial dimensions of lesions were greater when measured on HASTE compared with RESOLVE. Because many studies have demonstrated a size threshold of about 2 mm for adequate detection, this would favor HASTE over RESOLVE to improve cholesteatoma detection.
The results of this study are in agreement with prior analyses that have found non-EPI (eg, HASTE) sequences to be both sensitive and specific for cholesteatoma detection.10,22 A systematic review by Jindal et al23 found the sensitivity, specificity, and positive predictive value of non-EPI DWI to be 91%, 96%, and 97%, respectively. Among postoperative patients, Bakaj et al24 found that non-EPI DWI had a 100% sensitivity after a canal wall down mastoidectomy. Nevertheless, false-positives have been reported, related to abscess, wax/debris, and encephaloceles.25 False-negatives, too, occur, particularly in small (<3–5 mm) lesions.26–28 Horn et al,27 for example, concluded that non-EPI DWI does not eliminate the need for second-look surgery due to the rate of false-negative examinations. Because the cohort of the current study was made up exclusively of patients with known cholesteatomas, the results should be specifically interpreted as being related to the superiority of HASTE over RESOLVE; any comments regarding the false-negative rate or negative predictive value of either sequence are beyond the scope of this study.
MS EPI sequences such as RESOLVE, conversely, have not been extensively investigated in cholesteatoma imaging. A study by Yamashita et al20 found the sensitivity (76.7%) and accuracy (87.9%) of MS EPI superior to that of single-shot EPI; the specificity of both was 100%. Henninger and Kremser7 reported, in 2017, that MS EPI sequences were subjectively reliable for cholesteatoma imaging but did not provide supportive quantitative data. The only prior study that directly compared MS EPI with non-EPI DWI was by Dudau et al.11 Although the authors found good (κ = 0.75) agreement between the techniques, non-EPI was better able to predict the presence of cholesteatoma in cases of disagreement. Nevertheless, the authors concluded that MS EPI could be used in conjunction with non-EPI for cholesteatoma detection. The results of the current study, in contrast, indicate that RESOLVE sequences are prone to false-negative or equivocal results in the setting of surgically confirmed cholesteatomas.
Nevertheless, MR cholesteatoma imaging remains a dynamic field. Recently, an optimized BLADE DWI sequence using 2D turbo gradient- and spin-echo imaging was shown to significantly reduce distortion, blurring, and magnetic-sensitive artifacts.29 A subsequent study demonstrated that turbo gradient- and spin-echo BLADE had fewer ghosting artifacts and higher image quality than REOSLVE and was superior to RESOLVE in identifying particularly small cholesteatomas.30 To date, however, no studies have sought to compare turbo gradient- and spin-echo imaging with HASTE sequences. Such a comparison could be the focus of future research studies and is a poignant example of the still-evolving landscape of MR imaging used for cholesteatoma detection.
Finally, the effect of MR imaging field strength on cholesteatoma imaging deserves mention. Although the superior signal-to-noise ratio of a 3T scanner could theoretically provide better detection of smaller lesions, the lower field strength of a 1.5T scanner is less susceptible to magnetic field inhomogeneities that often arise near the temporal bone. A recent study by Lips et al6 found that non-EPI DWI had superior sensitivity and specificity for cholesteatoma detection if imaged on a 1.5T scanner rather than 3T. These results stood in contrast to a prior report by Lincot et al31 in which the authors found near-equal diagnostic capabilities on 3T and 1.5T scanners. Like most prior studies, the current study was performed using a 1.5T scanner. However, this issue may be of further interest to future investigations.
This study has limitations shared by all retrospective analyses. In addition, the number of included patients is relatively small (n = 23). Also, because both imaging sequences were completed as part of the same examination, the neuroradiologists reviewing the cases were not blinded to the results of other corresponding images. Both reviewers were also aware that the cohort consisted of only patients with surgically confirmed cholesteatomas, thereby potentially introducing bias to the results. Similarly, because all patients in the cohort had surgery-proved primary or recurrent/residual cholesteatomas and the decision to operate was influenced by the results of the second-look MR imaging, it is possible that a selection bias influenced the results. Furthermore, the lack of patients without cholesteatoma prevents calculation of test specificity and negative predictive value. Finally, both DWI sequences used in this study were vendor-specific, which may reduce the applicability of the results of this study to the capabilities of all non-EPI and MS EPI DWI techniques.
CONCLUSIONS
HASTE is superior to RESOLVE in the detection of both primary and residual/recurrent cholesteatomas. The discrepancy between sequences may be related to the greater relative intralesional signal intensity and size on HASTE images. Because cholesteatomas appear smaller and less hyperintense on RESOLVE images, such sequences are more likely to provide equivocal and sometimes falsely negative results.
ABBREVIATIONS:
- MS
multishot
- RESOLVE
readout-segmented EPI
References
- 1.Gilberto N, Custódio S, Colaço T, et al. Middle ear congenital cholesteatoma: systematic review, meta-analysis and insights on its pathogenesis. Eur Arch Otorhinolaryngol Head Neck Dis 2020;277:987–98 10.1007/s00405-020-05792-4 [DOI] [PubMed] [Google Scholar]
- 2.Nevoux J, Lenoir M, Roger G, et al. Childhood cholesteatoma. Eur Ann Otorhinolaryngol Head Neck Dis 2010;127:143–50 10.1016/j.anorl.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 3.Olszewska E, Wagner M, Bernal-Sprekelsen M, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngo l 2004;261:6–24 10.1007/s00405-003-0623-x [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Teh BM, Hurtado G, et al. Can endoscopic ear surgery replace microscopic surgery in the treatment of acquired cholesteatoma? A contemporary review. Int J Pediatr Otorhinolaryngol 2020;131:109872 10.1016/j.ijporl.2020.109872 [DOI] [PubMed] [Google Scholar]
- 5.Choi DL, Gupta MK, Rebello R, et al. Cost-comparison analysis of diffusion weighted magnetic resonance imaging (DWMRI) versus second look surgery for the detection of residual and recurrent cholesteatoma. J Otolaryngol Head Neck Surg 2019;48:58 10.1186/s40463-019-0384-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lips LM, Nelemans PJ, Theunissen FM, et al. The diagnostic accuracy of 1.5 T versus 3 T non-echo-planar diffusion-weighted imaging in the detection of residual or recurrent cholesteatoma in the middle ear and mastoid. J Neuroradiol 2020;47:433–40 10.1016/j.neurad.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 7.Henninger B, Kremser C. Diffusion weighted imaging for the detection and evaluation of cholesteatoma. World J Radiol 2017;9:217–22 10.4329/wjr.v9.i5.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ide S, Ganaha A, Tono T, et al. Value of DW-MRI in the preoperative evaluation of congenital cholesteatoma. Int J Pediatr Otorhinolaryngol 2019;124:34–38 10.1016/j.ijporl.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 9.Kavanagh RG, Liddy S, Carroll AG, et al. Rapid diffusion-weighted MRI for the investigation of recurrent temporal bone cholesteatoma. Neuroradiol J 2020;33:210–15 10.1177/1971400920920784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Egmond SL, Stegeman I, Grolman W, et al. A systematic review of non-echo planar diffusion-weighted magnetic resonance imaging for detection of primary and postoperative cholesteatoma. Otolaryngol Head Neck Surg 2016;154:233–40 10.1177/0194599815613073 [DOI] [PubMed] [Google Scholar]
- 11.Dudau C, Draper A, Gkagkanasiou M, et al. Cholesteatoma: multishot echo-planar vs non echo-planar diffusion-weighted MRI for the prediction of middle ear and mastoid cholesteatoma. BJR Open 2019;1:20180015 10.1259/bjro.20180015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vercruysse JP, De Foer B, Pouillon M, et al. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol 2006;16:1461–67 10.1007/s00330-006-0160-2 [DOI] [PubMed] [Google Scholar]
- 13.Lingam RK, Connor SE, Casselman JW, et al. MRI in otology: applications in cholesteatoma and Ménière’s disease. Clin Radiol 2018;73:35–44 10.1016/j.crad.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Aarts MCJRovers MM, van der Veen EL, et al. The diagnostic value of diffusion-weighted magnetic resonance imaging in detecting a residual cholesteatoma. Otolaryngol Head Neck Surg 2010;143:12–16 10.1016/j.otohns.2010.03.023 [DOI] [PubMed] [Google Scholar]
- 15.Cavaliere M, Di Lullo AM, Cantone E, et al. Cholesteatoma vs granulation tissue: a differential diagnosis by DWI-MRI apparent diffusion coefficient. Eur Arch Otorhinolaryngol 2018;275:2237–43 10.1007/s00405-018-5082-5 [DOI] [PubMed] [Google Scholar]
- 16.McJunkin J, Chole R. Clinical utility of MRI for cholesteatoma recurrence. Curr Surg Rep 2014;2:63 10.1007/s40137-014-0063-0 [DOI] [Google Scholar]
- 17.Kim TH, Baek MY, Park JE, et al. Comparison of DWI methods in the pediatric brain: PROPELLER turbo spin-echo imaging versus readout-segmented echo-planar imaging versus single-shot echo-planar imaging. AJR Am J Roentgenol 2018;210:1352–58 10.2214/AJR.17.18796 [DOI] [PubMed] [Google Scholar]
- 18.Más-Estellés F, Mateos-Fernández M, Carrascosa-Bisquert B, et al. Contemporary non–echo-planar diffusion-weighted imaging of middle ear cholesteatomas. Radiographics 2012;32:1197–13 10.1148/rg.324115109 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz KM, Lane JI, Bolster BD, et al. The utility of diffusion-weighted imaging for cholesteatoma evaluation. AJNR Am J Neuroradiol 2011;32:430–36 10.3174/ajnr.A2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita K, Yoshiura T, Hiwatashi A, et al. Detection of middle ear cholesteatoma by diffusion-weighted MR imaging: multishot echo-planar imaging compared with single-shot echo-planar imaging. AJNR Am J Neuroradiol 2011;32:1915–18 10.3174/ajnr.A2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol 2009;30:54–58 10.1097/MAO.0b013e31818edf4a [DOI] [PubMed] [Google Scholar]
- 22.Romano A, Covelli E, Confaloni V, et al. Role of non-echo-planar diffusion-weighted images in the identification of recurrent cholesteatoma of the temporal bone. Radiol Med 2020;125:75–79 10.1007/s11547-019-01085-x [DOI] [PubMed] [Google Scholar]
- 23.Jindal M, Riskalla A, Jiang D, et al. A systematic review of diffusion-weighted magnetic resonance imaging in the assessment of postoperative cholesteatoma. Otol Neurotol 2011;32:1243–49 10.1097/MAO.0b013e31822e938d [DOI] [PubMed] [Google Scholar]
- 24.Bakaj T, Zbrozkova LB, Salzman R, et al. Recidivous cholesteatoma: DWI MR after canal wall up and canal wall down mastoidectomy. Bratisl Lek Listy 2016;117:515–20 10.4149/bll_2016_100 [DOI] [PubMed] [Google Scholar]
- 25.Muhonen EG, Mahboubi H, Moshtaghi O, et al. False-positive cholesteatomas on non-echoplanar diffusion-weighted magnetic resonance imaging. Otol Neurotol 2020;41:e588–92 10.1097/MAO.0000000000002606 [DOI] [PubMed] [Google Scholar]
- 26.Geoffray A, Guesmi M, Nebbia JF, et al. MRI for the diagnosis of recurrent middle ear cholesteatoma in children: can we optimize the technique? Preliminary study. Pediatr Radiol 2013;43:464–73 10.1007/s00247-012-2502-3 [DOI] [PubMed] [Google Scholar]
- 27.Horn RJ, Gratama JW, van der Zaag-Loonen HJ, et al. Negative predictive value of non-echo-planar diffusion weighted MR imaging for the detection of residual cholesteatoma done at 9 months after primary surgery is not high enough to omit second look surgery. Otol Neurotol 2019;40:911–19 10.1097/MAO.0000000000002270 [DOI] [PubMed] [Google Scholar]
- 28.Velthuis S, van Everdingen KJ, Quak JJ, et al. The value of non echo planar, diffusion-weighted magnetic resonance imaging for the detection of residual or recurrent middle-ear cholesteatoma. J Laryngol Otol 2014;128:599–603 10.1017/S0022215114001418 [DOI] [PubMed] [Google Scholar]
- 29.Hu HH, McAllister AS, Jin N, et al. Comparison of 2D BLADE turbo gradient- and spin-echo and 2D spin-echo echo-planar diffusion-weighted brain MRI at 3T: preliminary experience in children. Acad Radiol 2019;26:1597–1604 10.1016/j.acra.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Sheng Y, Hong R, Sha Y, et al. Performance of TGSE BLADE DWI compared with RESOLVE DWI in the diagnosis of cholesteatoma. BMC Med Imaging 2020;20:40 10.1186/s12880-020-00438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lincot J, Veillon F, Riehm S, et al. Middle ear cholesteatoma: compared diagnostic performances of two incremental MRI protocols including non-echo planar diffusion-weighted imaging acquired on 3T and 1.5T scanners. J Neuroradiol 2015;42:193–201 10.1016/j.neurad.2014.02.003 [DOI] [PubMed] [Google Scholar]


