Abstract
BACKGROUND AND PURPOSE:
Few data are available regarding the influence of the timing of ischemic stroke management, such as daytime and nighttime hours, on the delay of mechanical thrombectomy, the effectiveness of revascularization, and clinical outcomes. We aimed to investigate whether admission during nighttime hours could impact the clinical outcome (mRS at 90 days) of patients with acute ischemic stroke treated by mechanical thrombectomy.
MATERIALS AND METHODS:
We retrospectively analyzed 169 patients (112 treated during daytime hours and 57 treated during nighttime hours) with acute ischemic stroke in the anterior cerebral circulation. The main outcome was the rate of patients achieving functional independence at 90 days (mRS ≤2), depending on admission time.
RESULTS:
In patients admitted during nighttime hours, the rate of mRS ≤ 2 at 90 days was significantly higher (51% versus 35%, P = .05) compared with those admitted in daytime hours. Patients in daytime and nighttime hours were comparable regarding admission and treatment characteristics. However, patients in nighttime hours tended to have a higher median NIHSS score at admission (P = .08) and to be younger (P = .08), especially among the mothership group (P = .09). The multivariate logistic regression analysis confirmed that patients in nighttime hours had better functional outcomes at 90 days than those in daytime hours (P = .018; 95% CI, 0.064–0.770; OR = 0.221).
CONCLUSIONS:
In a highly organized stroke care network, mechanical thrombectomy is quite effective in the nighttime hours among acute ischemic stroke presentations. Unexpectedly, we found that those patients achieved favorable clinical outcomes more frequently than those treated during daytime hours. Larger series are needed to confirm these results.
Blood flow restoration is the principal therapeutic goal in acute ischemic stroke (AIS). IV rtPA is recommended for all eligible patients within 4.5 hours of of symptoms onset. For patients with AIS with acute large-vessel occlusion, mechanical thrombectomy (MT) is highly beneficial and recommended as a standard of care.1 Functional outcomes are better when the MT is performed early after stroke onset.2
The impact of admission hours on short-term prognosis of patients with AIS is still controversial. Some series investigated whether patients with AIS admitted during off-hours (Monday to Friday between 6 PM and 8 AM and weekends) had different outcomes compared with patients admitted during on-hours. One study reported that patients in off-hours had higher short-term mortality, greater disability at discharge, and worse outcomes at 90 days than patients admitted during working hours.3 Conversely, another study suggested that rates of poor 90-day outcomes (mRS >2) were similar between off- and on-hours admissions.4
Furthermore, in a recent large cohort of Dutch patients, the overall outcome was not influenced by time of admission.5 Results of these studies may be influenced by local stroke center organization and may not be generalized to other centers with different organizations.
The only study focusing on the outcomes after MT performed during on-versus-off hours was a recent analysis of the Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) registry group (https://mrclean-trial.org/), which showed comparable functional outcomes and complication rates among the 2 groups.6
Accordingly, outcomes after MT performed during working hours versus off-hours have not been accurately examined and require further research.
Night presentation and sleep deprivation have been reported as potential risk factors for patients presenting with unplanned critical illness and requiring rapid diagnostics and interventions.7 This can cause worse outcome in these patients, that can be attributed to increased complications, fatigue, and differential staffing.8 Accordingly, our hypothesis was that performance of the workflow and operators could be impacted during the night, reflecting worse outcomes after MT performed during nighttime hours. In addition, our institution (Millau hospital, Mende hospital and Perpignan hospital) receives patients with stroke from a 200-km perimeter; therefore, delay in transportation may negatively influence outcomes. We hypothesized that transport delays could be higher during nighttime hours compared with daytime hours due to less availability of helicopter transport at night. We aimed to investigate whether admission during nighttime hours could impact the clinical outcomes (in-hospital mortality and mRS at 90 days) of patients with AIS treated by MT.
MATERIALS and METHODS
Population
Since 2015, a neuroradiologic data base (Commission Nationale de l’Informatique et des Libertés 1724786; https://www.cnil.fr/en/home) includes, prospectively, all patients admitted to our comprehensive stroke center (CSC). The patients were managed directly in the CSC (mothership patients) or first admitted to 1 of the 4 primary stroke centers with or without IV rtPA before transfer for MT (patients experiencing drip and ship). These primary stroke centers are a distant 50–200 km from our CSC.
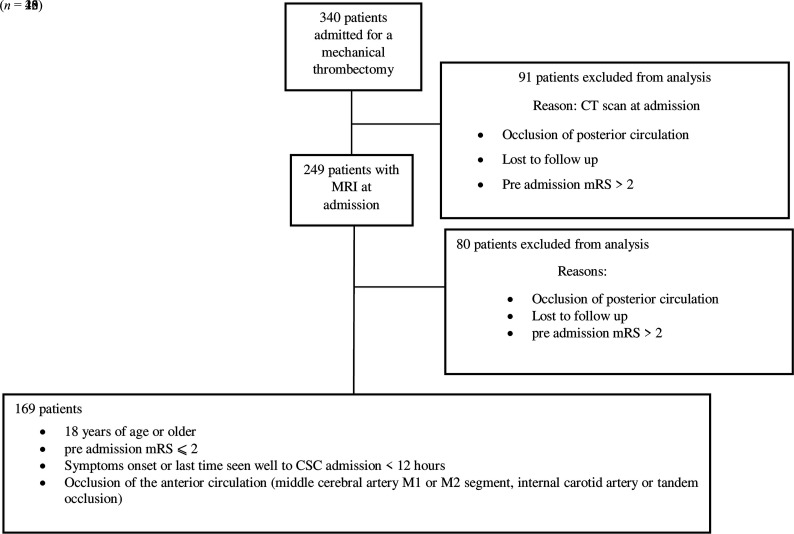
Three hundred forty patients admitted to our stroke unit from January 2017 to December 2018 were studied. In this retrospective cohort study, patients were included if they fulfilled the 4 following inclusion criteria: 1) 18 years of age or older, 2) preadmission mRS of ≤2, 3) symptom onset or last time seen well to CSC admission of <12 hours, and 4) anterior circulation occlusion (middle cerebral artery M1 or M2 segment, internal carotid artery, or tandem occlusion) visible on MR imaging at admission. As shown in Fig 1, our inclusion criteria were observed in 169 patients. We excluded from this study patients with a CT scan at admission (n = 91) to keep 1 imaging technique and compare infarct volume on the basis of only MR imaging.9 Patients presenting with posterior occlusion, a preadmission mRS >2, or lost to follow-up (n = 80) were also excluded from the study.
FIG 1.
Flow chart: exclusion and inclusion criteria.
All patients admitted in the CSC between 6:00 pm and 8:00 am the next morning were grouped as the patients in nighttime hours. All patients admitted to the CSC between 8:00 am and 6:00 pm were grouped as the patients in daytime hours. The whole medical staff was present during the daytime. During the nighttime hours, medical staff was reduced and composed of 1 neuroradiologist resident, 1 senior stroke neurologist, a neurology resident on duty, and 2 technicians.
Scores and Parameters Evaluation
Clinical and Imaging Evaluation.
Stroke severity was assessed by the NIHSS on CSC admission by a stroke neurologist. The following data were collected prospectively with a structured questionnaire: age, sex, cardiovascular risk factors (hypertension, dyslipidemia, diabetes mellitus, and smoking), time of symptom onset, NIHSS at CSC admission and at 24 hours, vital signs before treatment, imaging findings, use of IV rtPA, and clinical outcomes.
All patients underwent multimodal 1.5T (Aera; Siemens) or 3T (Skyra; Siemens) MR imaging before treatment, with a standardized protocol. Infarct volume was estimated in milliliters on DWI using RApid processing of PerfusIon and Diffusion (RAPID; iSchemaView).10
The ASPECTS on DWI was calculated by a neuroradiologist blinded to the results of the MT.
Timing.
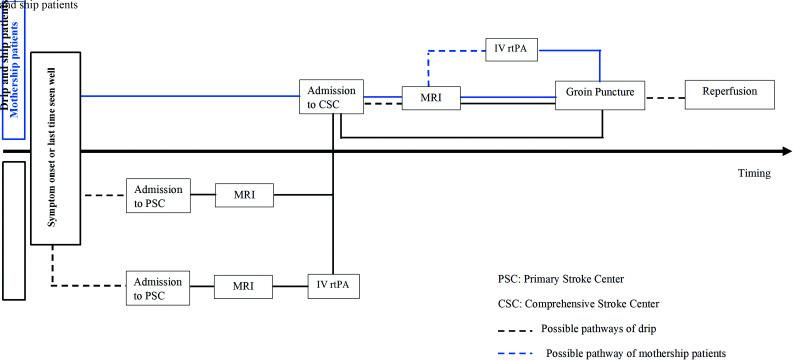
Delays were calculated in minutes. For all the calculated delays, admission was defined by the CSC admission except for admission to imaging. For mothership patients, admission was defined by CSC admission to imaging. Otherwise, for patients experiencing drip and ship, this delay was defined by primary stroke center admission to imaging except if a second imaging was performed in the CSC. In this case, the CSC admission to imaging was used. The other studied delays were the following: symptom to CSC admission, imaging to reperfusion, CSC admission to groin puncture, CSC admission to reperfusion, groin puncture to reperfusion, and symptom to reperfusion. All the crucial points for calculating these delays are shown in Fig 2.
FIG 2.
Pathways for management of patients with acute ischemic stroke from symptom onset to mechanical thrombectomy.
IV rtPA and Endovascular Therapy.
IV rtPA was administered according to the current guidelines.11
MT was performed via a femoral artery approach with the patient under general anesthesia or local anesthesia with sedation. Reperfusion was graded using the modified TICI (mTICI) score.12 Successful reperfusion was defined as mTICI2b, 3; and first-pass success was defined as a good reperfusion (TICI 2b or 3) after a 1-pass device was used for MT.
Stroke subtypes were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.13
Measures and Main Outcome.
Follow-up imaging was performed between 16 and 30 hours after MT to assess intracranial hemorrhage. Symptomatic intracranial hemorrhage (sICH) was defined as any hemorrhage occurring within 24 hours associated with an increase of ≥4 points in the NIHSS score or that caused death.11 Early neurologic improvement (ENI) was defined as an improvement of at least 8 points between the NIHSS score at CSC admission and the NIHSS score at 24 hours (compared with baseline) after MT.14 Early neurologic deterioration (END) was defined as a loss of 4 points in the NIHSS score between NIHSS at CSC admission and NIHSS at 24 hours.15 Finally, in-hospital mortality was defined as the rate of patient death during hospitalization.
Functional outcome was assessed by a neurologist using the mRS at 90 days, during the clinical visit, or by a study nurse using a standardized telephone interview. Favorable functional outcome was defined as a mRS ≤ 2.
Statistical Analysis
Continuous variables were reported as median (interquartile range [IQR]). Univariate statistical analysis was performed using the Mann-Whitney U test for continuous variables and the Fisher exact test for categoric variables.
All statistical analyses were performed using MedCalc, Version 18.10 (MedCalc Software). Patients in daytime- and nighttime-hour groups were used in a logistic regression as independent predictors of the follow-up outcome, defined as good (mRS ≤ 2) or bad (mRS > 2) outcome. Variables either known to be potential confounding factors or identified as the most significantly different in the univariate analysis were included in the logistic regression. Regarding our sample size, we chose to limit the number of covariables to 8. A stepwise method was used with an α-to-enter and α-to-exit set at .2 and .001, respectively. Eight variables, age, sex, NIHSS score at CSC admission, IV rtPA, dyslipidemia, atrial fibrillation, hypertension, and CSC admission to groin puncture were thus included in the model.
Finally, specificities of mothership patients and those in the drip and ship group were investigated using a subgroup analysis. For an optimal comparability, a backward method was used to compare patients in daytime and nighttime hours among mothership patients as well as those in the drip and ship group on the basis of the 8 previous variables. Data were adjusted by the NIHSS score at CSC admission, atrial fibrillation, IV rtPA, and CSC admission-to-groin puncture delay for mothership group and by the NIHSS score at CSC admission, dyslipidemia, and hypertension for patients in the drip and ship group.
The statistical threshold was set to P < .05 for all analyses.
RESULTS
Altogether, 169 patients (50% of men; mean age, 75 years; IQR, 63–83 years), of whom 44% (74/169) were transferred from another center, were included. Patient characteristics are reported in the Online Supplemental Data. The median NIHSS score at CSC admission was 17 (IQR = 11–20), and the median ASPECTS was 7 (IQR = 5–8). MT was performed within a median delay of 355 minutes (IQR = 248–544 minutes) from symptom onset. General anesthesia was used in 55% of patients (93/169). Good reperfusion (TICI 2b, 3) was achieved in 74% (125/169). Overall, at 90 days, 40% (68/169) of patients had an mRS ≤ 2.
Daytime-versus-Nighttime Hours
Patient Characteristics.
Of 169 patients, 112 (66%) were treated during daytime hours, and 57 (34%), during nighttime hours. No significant difference was found between the nighttime- and daytime-hour groups regarding demographics and cardiovascular risks factors, though patients in daytime hours were slightly older (77 versus 74 years, P = .08) and tended to have more dyslipidemia (41% versus 28%, P = .09) (Online Supplemental Data).
The daytime- and nighttime-hour groups were comparable in terms of pre-MT (biologic parameters, occlusion site, ASPECTS, and infarct volume) and treatment characteristics (general anesthesia; successful first-pass recanalization; rate of TICI 2b, 3; rate of complications). Patients in nighttime hours tended to have a higher median NIHSS score at CSC admission, 18 (IQR = 14–22), than those in daytime hours, 16 (IQR = 10–20) (P = .08).
Procedural Timing and Reperfusion.
All timing variables were comparable among daytime- and nighttime-hour groups, except the delay from CSC admission to groin puncture, which was significantly longer in the nighttime-hour group compared with the daytime-hour group (94 minutes; range, 78–123 minutes) versus (82 minutes; range, 61–105.25 minutes) (P = .009) (Online Supplemental Data).
Outcomes: Comparison between Patients in Nighttime and Daytime Hours.
The in-hospital mortality rate was higher in the daytime-hour group (19/112 = 17%) than in the nighttime-hour group (3/57 = 5%, P = .05; OR =3.6544; 95% CI, 1.007–20.1601). ENI and END were similar between the 2 groups (P = .34). At 90 days, patients in nighttime hours had significantly higher rates of favorable outcome (29/57 = 51%) compared with those in daytime hours (39/112 = 35%) (2-sided Wilcoxon test, P = .05; OR = 1.9308; 95% CI, 0.9619–3.9002) (Online Supplemental Data). After we adjusted for age, sex, NIHSS at CSC admission, dyslipidemia, atrial fibrillation, hypertension, IV rtPA, CSC admission to groin puncture, and delay in a logistic regression model, the nighttime-hour MT appeared to have an even higher significant impact on the 90-day outcome (logistic regression, P = .018; OR = 0.221; 95% CI, 0.064–0.770; Online Supplemental Data).
Subgroup Analysis: Mothership—Daytime-Versus-Nighttime Hours.
In the subgroup of 95 mothership patients (Table 1), among those in nighttime hours, there was a trend toward a lower median age (69 versus 78 years, P = .09) and higher rates of successful first-pass recanalization (45% versus 26%, P = .09), whereas the median NIHSS score (20; IQR = 6–22) versus 15 (IQR = 10–20; P = .03) and CSC admission-to-groin puncture delay (115 versus 86 minutes, P = .001) were higher. In-hospital mortality was lower among patients in nighttime hours (3% versus 26%, P = .01), while 45% of patients in nighttime hours gained independence at 90 days compared with 36% in daytime hours. In logistic regression using the backward method, a model adjusted by atrial fibrillation, IV rtPA, and CSC admission-to-groin puncture delay showed that nighttime-hour MT appeared to have a higher significant impact on the 90-day outcome (P = .018; OR = 0.221; 95% CI, 0.064–0.770; Online Supplemental Data).
Table 1:
Mothership patients—characteristics and comparison between patients in nighttime and daytime hours—univariate analysisa
| All (N = 95) | Nighttime Hours (n = 29) | Daytime Hours (n = 66) | P Value | |
|---|---|---|---|---|
| Age (yr) | 76 (62–84) | 69 (59–83) | 78 (71–84) | .09 |
| Men | 46 (48) | 15 (52) | 31 (47) | .82 |
| Hypertension | 60 (63) | 17 (59) | 43 (65) | .64 |
| Diabetes mellitus | 14 (15) | 5 (17) | 9 (14) | .75 |
| Dyslipidemia | 38 (40) | 10 (34) | 28 (42) | .50 |
| Atrial fibrillation | 29 (31) | 12 (41) | 17 (26) | .20 |
| Smoking | 21 (22) | 9 (31) | 12 (18) | .20 |
| Tandem occlusion | 14 (15) | 4 (14) | 10 (15) | 1.00 |
| NIHSS at admission | 18 (11–21.5) | 20 (6–22) | 15 (10–20) | .03 |
| IV rtPA | 57 (60) | 10 (34) | 28 (42) | .50 |
| ASPECTS | 7 (5–7) | 7 (5–7) | 7 (5–8) | 1.00 |
| Infarct volume (mL) | 18.8 (8.4–41) | 29.8 (12–45.5) | 18.3 (6.3–34) | .20 |
| General anesthesia | 41 (43) | 14 (48) | 27 (41) | .51 |
| First-pass success | 30 (32) | 13 (45) | 17 (26) | .09 |
| mTICI > 2b | 63 (66) | 21 (72) | 42 (64) | .50 |
| Symptom onset to CSC admission (min) | 170 (89.5–404) | 170 (98–406) | 170 (82–392) | .61 |
| CSC admission to groin puncture (min) | 93 (72–118) | 115 (88–132.5) | 86 (66–111) | .001 |
| CSC admission to reperfusion (min) | 168 (129–202) | 176 (144–203) | 168 (128–199) | .55 |
| Groin puncture to reperfusion (min) | 60 (43–90) | 49 (33–81) | 64 (43–95) | .11 |
| ENI | 28 (29) | 10 (33) | 18 (28) | .48 |
| END | 42 (44) | 12 (41) | 30 (46) | .82 |
| sICH | 8 (8) | 1 (3) | 7 (11) | .43 |
| In-hospital mortality | 18 (19) | 1 (3) | 17 (26) | .01 |
| mRS ≤2 at 90 days | 37 (39) | 13 (45) | 24 (36) | .50 |
Categoric variables are expressed as number (%), and continuous variables, as median (IQR).
Drip and Ship: Daytime-versus-Nighttime Hours.
In the subgroup of 74 patients experiencing drip and ship (Table 2), the rate of favorable outcome at 90 days was significantly higher in those in nighttime-versus-daytime hours (57% versus 33%, P = .05; OR =2.716; 95% CI, 0.9401–8.1342). In a logistic regression using the backward method, a model adjusted by NIHSS at CSC admission, dyslipidemia, and hypertension showed that nighttime-hour MT appeared to have a higher significant impact on the 90-day outcome (P = .024; OR = 0.246; 95% CI, 0.073–0.831; Online Supplemental Data). None of the other parameters differed between the daytime-and nighttime-hour groups.
Table 2:
Patients subject to drip and ship—characteristics and comparison between patients in nighttime and daytime hours—univariate analysisa
| All (N = 74) | Nighttime Hours (n = 28) | Daytime Hours (n = 46) | P Value | |
|---|---|---|---|---|
| Age (yr) | 73 (64.3–82) | 71 (63–82) | 75 (64.5–82) | .60 |
| Men | 39 (53) | 15 (54) | 24 (52) | 1.00 |
| Hypertension | 53 (72) | 17 (61) | 36 (78) | .12 |
| Diabetes mellitus | 14 (19) | 4 (14) | 10 (22) | .55 |
| Dyslipidemia | 24 (32) | 6 (21) | 18 (39) | .13 |
| Atrial fibrillation | 25 (34) | 11 (39) | 14 (30) | .46 |
| Smoking | 23 (31) | 6 (21) | 17 (37) | .20 |
| Tandem occlusion | 16 (22) | 6 (21) | 10 (22) | 1.00 |
| NIHSS at admission | 16 (11–19.8) | 16 (9–19) | 16 (11.5–20) | .90 |
| IV rtPA | 39 (53) | 17 (61) | 22 (48) | .34 |
| ASPECTS | 7 (6–8) | 7 (6–8) | 7 (6–8) | .70 |
| Infarct volume (mL) | 24.9 (10.7–6.8) | 15.4 (9.7–36.7) | 26.2 (11.3–4.1) | .20 |
| General anesthesia | 39 (53) | 13 (46) | 26 (57) | .47 |
| First-pass success | 24 (32) | 8 (29) | 16 (35) | .62 |
| mTICI > 2b | 61 (82) | 23 (82) | 38 (82) | 1.00 |
| Symptom onset to CSC admission (min) | 298 (256–481) | 277 (228–374) | 302 (271–531) | .20 |
| CSC admission to groin puncture (min) | 71 (55–99) | 85 (66–105) | 67 (54–92) | .11 |
| CSC admission to reperfusion (min) | 152 (101–200) | 164 (101–189) | 147 (107–210) | .90 |
| Groin puncture to reperfusion (min) | 70 (47–106) | 68 (47–78) | 75.5 (49–125) | .21 |
| ENI | 28 (38) | 12 (43) | 16 (35) | .62 |
| END | 14 (19) | 4 (14) | 10 (22) | .55 |
| sICH | 8 (11) | 2 (7) | 6 (13) | .70 |
| In-hospital mortality | 5 (7) | 2 (7) | 3 (7) | 1.00 |
| mRS ≤2 at 90 days | 31 (42) | 16 (57) | 15 (33) | .05 |
Categoric variables are expressed as numbers (%), and continuous variables, as median (IQR).
Nighttime-Hour Group: Characteristics of Patients with mRS ≤ 2 and mRS >2.
In the subgroup of 57 patients with nighttime hours, among the patients with an mRS ≤ 2 at 90 days, there was a younger median age (69 versus 79 years, P = .05) with a lower median NIHSS score at CSC admission (16 [IQR, 9–20] versus 19 [IQR = 17–23], P = .01). Also, the rate of first-pass success was significantly higher (52% versus 21%, P = .03). The rate of favorable revascularization mTICI ≥ 2b was significantly higher in patients with mRS ≤2 (97% versus 57%, P < .001, Table 3).
Table 3:
Characteristics and comparison between good outcome (mRS 0–2) and bad outcome (mRS 3–6) among patients in nighttime hours—predictive factors of favorable outcomea
| All (N = 57) | mRS (0–2) (n = 29) | mRS (3–6) (n = 28) | P Value | |
|---|---|---|---|---|
| Age (yr) | 70 (60–83) | 69 (58–73) | 79 (62–85.5) | .05 |
| Men | 30 (53) | 17 (59) | 13 (46) | .43 |
| Hypertension | 34 (60) | 16 (55) | 18 (64) | .60 |
| Diabetes mellitus | 9 (16) | 4 (14) | 5 (18) | .73 |
| Dyslipidemia | 16 (28) | 8 (28) | 8 (29) | 1.00 |
| Atrial fibrillation | 23 (40) | 10 (34) | 13 (46) | .42 |
| Smoking | 20 (35) | 11 (38) | 9 (32) | .78 |
| Tandem occlusion | 10 (18) | 4 (14) | 6 (21) | .50 |
| NIHSS at admission | 18 (14–22) | 16 (9–20) | 19 (17–23) | .01 |
| IV rtPA | 27 (47) | 15 (52) | 12 (43) | .60 |
| ASPECTS | 7 (5–8) | 7 (5–7) | 7 (5.75–8) | .43 |
| Infarct volume | 23 (11–45) | 23 (11–47) | 25 (10.5–37.3) | .73 |
| General anesthesia | 29 (51) | 13 (45) | 16 (57) | .43 |
| First-pass success | 21(37) | 15 (52) | 6 (21) | .03 |
| mTICI >2 | 44 (77) | 28 (97) | 16 (57) | .0004 |
| CSC admission to groin puncture (min) | 94 (78–123) | 87 (77–110) | 108 (84−135.5) | .11 |
| Symptom to groin puncture (min) | 361 (272–487) | 360 (272–485) | 261 (273–494) | .82 |
| Groin puncture to reperfusion (min) | 56.5 (39–76) | 51 (38–69) | 64 (42–80) | .43 |
| CSC admission to reperfusion (min) | 168 (128–195) | 147 (123–188) | 178 (153–207) | .09 |
| ENI | 22 (39) | 13 (45) | 9 (32) | .42 |
| END | 12 (25) | 3 (10) | 9 (32) | .06 |
| sICH | 3 | 0 (0) | 3 (11) | .11 |
| In-hospital mortality | 3 | 0 (0) | 3 (11) | .11 |
Categoric variables are expressed as numbers (%), and continuous variables, as median (IQR).
There was a trend toward a lower median CSC admission-to-reperfusion delay among patient with mRS ≤ 2 (147 minutes [IQR = 123–188 minutes] versus 178 minutes [IQR = 153–207 minutes], P = .09).
DISCUSSION
Our study investigated outcomes after MT between patients with AIS admitted during nighttime hours compared with those admitted during daytime hours. These results are important because 30% of patients with strokes are admitted during nighttime hours, and both performance of the operators and efficiency of the workflow can be comparable with daytime hours. On the basis of our results, it seems that the nighttime-hour period is not an obstacle to the best treatment-management of patients with AIS and should not be regarded as a dangerous time for patients treated with MT in a highly organized stroke care network. Most interesting, patients treated during nighttime hours had a better outcome at 90 days than those treated during daytime hours. The topic is still controversial; a recent analysis from the MR CLEAN Registry of the workflow intervals of MT for patients presenting during off-hours (including weekends) and on-hours (8 AM–6 PM during weeks) showed no significant difference in functional outcome among these 2 groups, as well as similar reperfusion and complication rates.6 On the contrary, a systematic review and meta-analysis of 21 studies performed by Sorita et al,3 in 2014, in the pre-MT era showed that patients with AIS in off-hours had both higher short-term mortality and greater disability at discharge. Putative explanations included a less experienced staff, less available diagnostic procedures, variations in the processes of care, and a decreased likelihood of delivering IV rtPA or intra-arterial thrombolysis.
In our population, increased CSC admission-to-groin puncture delay during nighttime hours has been observed, possibly relying on the time required for the neuroradiologist on call to get to the hospital. However, this increased delay does not have a pejorative impact on patient outcome at 90 days. Besides CSC admission-to-groin puncture delay, patients in nighttime and daytime hours experienced similar stroke management, with similar symptom onset-to-groin puncture and groin puncture-to-reperfusion delays. The organization of stroke management appears equally as efficient whether during daytime or nighttime hours.
Not considering stroke management, a plausible explanation for the better neurologic outcome after nighttime MT would be the intrinsic variability of our patients between the samples in terms of stroke characteristics and clinical variables. The former does not differ between patients in nighttime and daytime hours regarding neither the ASPECTS, the infarct volume, nor the technical characteristic–related complications. However, the latter shows some slight age (P = .08) differences. Patients in nighttime hours are slightly younger than those in daytime hours (3 years), especially among the mothership group (9 years). Age is an important factor influencing the probability of achieving a good outcome among patients with AIS. In a recent study, Jayaraman et al16 quantified the interaction between age and outcomes after MT. The authors found a deleterious influence of age: With each 1-year advance in age, the increase in the mRS change worsened among recanalized patients (TICI 2b, 3) and approached the value of mRS change in the TICI 0-2a group.16 Age could then explain, at least partially, the better outcome among the patients in nighttime hours.
However, adjusting statistical analysis by age is not sufficient to remove the observed outcome differences between groups. This issue suggests a more complex, multifactorial explanation. For more insight, we conducted a subgroup analysis, comparing characteristics of patients in nighttime hours between good (mRS ≤ 2) and poor (mRS > 2) outcome groups. First-pass success, favorable recanalization (mTICI ≥ 2), and a low NIHSS score at CSC admission appear to be predictors of good outcome. The NIHSS score at admission is known to be strongly associated with outcome.17 Most interesting, except for age, patients do not clearly differ in terms of history characteristics, supporting our hypothesis that age is an important variable.
Impact of Drip and Ship versus Mothership Patients
The drip and ship scenario implies additional delays before performing MT, which might reduce the chance of success.2 We, thus, investigated mothership patients and those experiencing drip and ship, independently. Mourand et al18 reported no significant difference in 90-day outcomes when comparing mothership patients with those in the drip and ship group independent of admission time.
In our series, the proportion of patients having functional independence at 90 days was higher among patients in nighttime compared with daytime hours, both with the mothership and the drip and ship strategy. However, the latter was associated with a significantly higher rate of mRS ≤ 2 among patients in nighttime hours. Although it is difficult to explain why patients in nighttime hours treated in a drip and ship strategy had a good outcome more frequently compared with the mothership strategy, we can underline some differences between the 2 groups: The NIHSS score was lower (20 versus 16) among patients in nighttime hours treated in the drip and ship group; the rate of IV rtPA was lower among mothership patients (34% versus 61%); and the rate of ENI was higher in the drip and ship group (43% versus 33%). All these differences may, in part, explain why patients in nighttime compared with daytime hours gained independence more frequently at 90 days after the drip and ship strategy compared with the mothership strategy. However, bias related to the small sample size of the subgroups should be evaluated. In conclusion, we can demonstrate with this analysis that although potential confounders related to selection bias should be considered, workflow is quite efficient in the nighttime, leading to good treatment results among patients with AIS.
Limitations
Our study has some limitations. Although prospectively collected, our results were retrospectively analyzed. It is a single-center cohort study, a representation of the 2017–2018 clinical practices of our CSC. As in similar works, generalization to other centers can be difficult and highly depends on the management of each local patient.
Our work was focused on patients who underwent MT; thus, we have no access to information on patients not retained for it. We cannot exclude the hypothesis that during nighttime hours, patients are more carefully selected for MT due to reduced medical team availability. Such observations could explain a part of the nighttime-hour/daytime-hour differences but should be obvious when comparing characteristics of patients in daytime and nighttime hours, which is not clearly the case, except for age.
CONCLUSIONS
In a highly organized stroke care network, MT is effective both in nighttime- and daytime-hour AIS presentations. Nighttime-hour management does not lead to deleterious effects on outcome at 90 days, and treatment times are similar between daytime hours and nighttime hours. Unexpectedly, we found that patients treated during nighttime hours achieved a favorable clinical outcome more frequently at 90 days and had less in-hospital mortality than those treated during daytime hours. There was a difference in age between the groups. It might partially explain this observation and introduce a potential sample bias. Our results provide an important insight for later studies aiming to improve clinical practices in AIS care organization.
ABBREVIATIONS:
- AIS
acute ischemic stroke
- CSC
comprehensive stroke center
- END
early neurologic deterioration
- ENI
early neurologic improvement
- IQR
interquartile range
- MT
mechanical thrombectomy
- mTICI
modified TICI
- sICH
symptomatic intracranial hemorrhage
Footnotes
Emmanuelle Le Bars and Vincent Costalat contributed equally to this work.
Disclosures: Alain Bonafe—UNRELATED: Consultancy: Stryker, Medtronic, MicroVention, phenox.* Anne Ducros—UNRELATED: Board Membership: Novartis, Teva Pharmaceutical Industries, Eli Lilly, Comments: Advisory Boards in the field of migraine treatments; Consultancy: Wefight, Comments: consultancy for the development of a chatbot on improving self-management of patients with migraine; Payment for Lectures Including Service on Speakers Bureaus: Novartis; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Novartis, Teva Pharmaceutical Industries. Vincent Costalat—UNRELATED: Consultancy: Stryker, Medtronic, Balt, Cerenovus, MicroVention; Grants/Grants Pending: Stryker, Medtronic, Balt, Cerenovus, MicroVention*; Payment for Development of Educational Presentations: Stryker, Medtronic, Balt, Cerenovus, MicroVention. *Money paid to the institution.
References
- 1.Powers WJ, Derdeyn CP, Biller J, et al. ; American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020–35 10.1161/STR.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 2.Ota T, Nishiyama Y, Koizumi S, et al. Impact of onset-to-groin puncture time within three hours on functional outcomes in mechanical thrombectomy for acute large-vessel occlusion. Interv Neuroradiol 2018;24:162–67 10.1177/1591019917747247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute ischemic stroke: a systematic review and meta-analysis. Eur J Intern Med 2014;25:394–400 10.1016/j.ejim.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Streifler JY, Benderly M, Molshatzki N, et al. Off-hours admission for acute stroke is not associated with worse outcome: a nationwide Israeli stroke project: outcome of stroke off-hours admissions. Eur J Neurol 2012;19:643–47 10.1111/j.1468-1331.2011.03603.x [DOI] [PubMed] [Google Scholar]
- 5.Tuinman MP, van Golde EG, Portier RP, et al. Comparison of outcome in stroke patients admitted during working hours vs. off-hours; a single-center cohort study. J Neurol 2019;266:782–89 10.1007/s00415-018-9079-1 [DOI] [PubMed] [Google Scholar]
- 6.Hinsenveld WH, de Ridder IR, van Oostenbrugge RJ, et al. ; MR CLEAN Registry Investigators. Workflow intervals of endovascular acute stroke therapy during on-versus off-hours. Stroke 2019;50:2842–50 10.1161/STROKEAHA.119.025381 [DOI] [PubMed] [Google Scholar]
- 7.Carr BG, Reilly PM, Schwab CW, et al. Weekend and night outcomes in a statewide trauma system. Arch Surg 2011;146:810–17 10.1001/archsurg.2011.60 [DOI] [PubMed] [Google Scholar]
- 8.Shulkin DJ. Like night and day: shedding light on off-hours care. N Engl J Med 2008;358:2091–93 10.1056/NEJMp0707144 [DOI] [PubMed] [Google Scholar]
- 9.Sotoudeh H, Shafaat O, Sotoudeh E. Misleading CT perfusion in subacute ischemic stroke. Emerg Radiol 2019;26:581–86 10.1007/s10140-019-01719-7 [DOI] [PubMed] [Google Scholar]
- 10.Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke 2011;42:1608–14 10.1161/STROKEAHA.110.609008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 12.Fugate JE, Klunder AM, Kallmes DF. What is meant by “TICI”? AJNR Am J Neuroradiol 2013;34:1792–97 10.3174/ajnr.A3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke; definitions for use in a multicenter clinical trial; TOAST—Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 14.Ong CT, Sung SF, Wu CS, et al. Early neurological improvement after intravenous tissue plasminogen activator infusion in patients with ischemic stroke aged 80 years or older. J Chin Medi Assoc 2014;77:179–83 10.1016/j.jcma.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Seners P, Turc G, Oppenheim C, et al. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 2015;86:87–94 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- 16.Jayaraman MV, Kishkovich T, Baird GL, et al. Association between age and outcomes following thrombectomy for anterior circulation emergent large vessel occlusion is determined by degree of recanalisation. J Neurointerv Surg 2019;11:114–18 10.1136/neurintsurg-2018-013964 [DOI] [PubMed] [Google Scholar]
- 17.Wouters A, Nysten C, Thijs V, et al. Prediction of outcome in patients with acute ischemic stroke based on initial severity and improvement in the first 24 h. Front Neurol 2018;9:308 10.3389/fneur.2018.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourand I, Malissart P, Dargazanli C, et al. A regional network organization for thrombectomy for acute ischemic stroke in the anterior circulation; timing, safety, and effectiveness. J Stroke Cerebrovasc Dis 2019;28:259–66 10.1016/j.jstrokecerebrovasdis.2018.09.051 [DOI] [PubMed] [Google Scholar]