Abstract
BACKGROUND AND PURPOSE:
Both ASPECTS and core volume on CTP are used to estimate infarct volume in acute ischemic stroke. To evaluate the potential role of ASPECTS for acute endovascular treatment decisions, we studied the correlation between ASPECTS and CTP core, depending on the timing and the presence of large-vessel occlusion.
MATERIALS AND METHODS:
We retrospectively reviewed all MCA acute ischemic strokes with standardized reconstructions of CTP maps entered in the Acute STroke Registry and Analysis of Lausanne (ASTRAL) registry. Correlation between ASPECTS and CTP core was determined for early (<6 hours) versus late (6–24 hours) times from stroke onset and in the presence versus absence of large-vessel occlusion. We used correlation coefficients and adjusted multiple linear regression models.
RESULTS:
We included 1046 patients with a median age of 71.4 years (interquartile range, IQR = 59.8–79.4 years), an NIHSS score of 12 (IQR, 6–18), an ASPECTS of 9 (IQR, 7–10), and a CTP core of 13.6 mL (IQR, 0.6–52.8 mL). The overall correlation between ASPECTS and CTP core was moderate (ρ = –0.49, P < .01) but significantly stronger in the late-versus-early window (ρ = –0.56 and ρ = –0.48, respectively; P = .05) and in the presence versus absence of large-vessel occlusion (ρ = –0.40 and ρ = –0.20, respectively; P < .01). In the regression model, the independent association between ASPECTS and CTP core was confirmed and was twice as strong in late-arriving patients with large-vessel occlusion (β = –0.21 per 10 mL; 95% CI, −0.27 to –0.15; P < .01) than in the overall population (β = –0.10; 95% CI, −0.14 to –0.07; P < .01).
CONCLUSIONS:
In a large cohort of patients with acute ischemic stroke, we found a moderate correlation between ASPECTS and CTP core. However, this was stronger in patients with large-vessel occlusion and longer delay from stroke onset. Our results could support the use of ASPECTS as a surrogate marker of CTP core in late-arriving patients with acute ischemic stroke with large-vessel occlusion.
Both the ASPECTS1,2 and automated core volume on CTP3,4 have been used to estimate infarct volume in the acute phase of stroke. However, the level of agreement between the two modalities remains uncertain.
ASPECTS is a useful and easily applicable tool for standardized evaluation of the extent of early ischemic changes in anterior circulation strokes on non-contrast CT scan (NCCT). In the original report describing the ASPECTS1 and in a subsequent observational study involving 1135 patients undergoing intravenous thrombolysis (IVT), the ASPECTS grading was shown to be an independent predictor of functional outcome.5 For mechanical thrombectomy performed within 6 hours after onset, a clear benefit was found for patients with an NCCT ASPECTS of 6–10, while for ASPECTS values of 0–5, the treatment effect was not clear.6
Recently, the efficacy of endovascular treatment (EVT) beyond the 6-hour time window was demonstrated in 2 randomized trials using a tissue-based approach: An advanced neuro-imaging protocol (with CTP or DWI) was used to identify patients with acute ischemic stroke (AIS) with a low infarct core despite late presentation.7,8
The role of ASPECTS in selecting patients likely to benefit from EVT and for predicting clinical outcome has not been clearly established in the late time window.9,10 Its use in the setting of late-presenting AIS could enlarge EVT eligibility in centers without the availability of advanced neuroimaging techniques or in patients who have contraindications to such imaging.
The main purposes of our study were the following: 1) to investigate the correlation between ASPECTS and automated core volume on CTP in a large cohort of patients with AIS with involvement of the middle cerebral artery (MCA territory), 2) to assess the influence of large-vessel occlusion (LVO) and time from stroke onset on this correlation, and 3) to evaluate the association of ASPECTS with clinical outcome at 3 months, with a special focus on late-arriving patients with AIS (ie, 6–24 hours after last proof of good health [LPGH]).
MATERIALS AND METHODS
Study Design and Patient Selection
We performed a retrospective analysis of all consecutive patients entered in the Acute STroke Registry and Analysis of Lausanne (ASTRAL) from January 2003 to December 2018. The ASTRAL registry includes all patients with AIS admitted to the stroke unit and/or intensive care unit of the Lausanne University Hospital (Centre Hospitalier Universitaire Vaudois) within 24 hours after LPGH. For each patient, >250 prespecified demographic, clinical, and laboratory variables and multimodal neuroimaging items were prospectively collected, as previously reported.11
For the current analysis, we selected patients according to the following criteria: acute CT-based multimodal imaging performed <24 hours after LPGH; stroke involving the MCA based on clinical findings such as new hemispheric deficits (aphasia, hemineglect, eye deviation toward the side of the hemiparesis); the simultaneous absence of neuroimaging findings showing posterior circulation stroke; and availability of good-quality CTP maps (ie, with the arterial input function returning to baseline before the end of the acquisition), reconstructed with a standardized method.12
Demographic data, medical history, and vascular risk factors were reviewed. We collected prestroke modified Rankin scale (mRS) and current medications at the time of the index event. We recorded neurologic symptoms and signs, stroke severity (NIHSS) on admission, and biochemical parameters at baseline. Acute recanalization treatments, including intravenous thrombolysis and/or EVT, were administered in accordance with Swiss and European Stroke Organization guidelines13,14 and were updated with recent positive randomized trial data.7,8 We calculated LPGH to arrival, to first imaging, and to treatment times. Stroke mechanism was classified according to the trial of ORG 10172 in acute stroke treatment classification,15 with dissection, embolic stroke of undetermined source, and multiple causes added as categories.
Clinical outcome was measured at 3 months using the mRS, either at the outpatient stroke clinic or by standardized telephone interview by Rankin-certified medical staff. Favorable outcome was considered as a 3-month mRS of ≤2.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) method was applied, and the STROBE checklist for observational studies is available in the Online Supplemental Data. The local ethics commission of Canton de Vaud approved the scientific use of anonymized data from the ASTRAL registry.
Neuroimaging Protocol
During the study period, patients admitted to our institution with suspected AIS were examined with a multimodal, mostly CT-based neuroimaging protocol as a standard of care. In patients without contraindications for iodinated contrast, this protocol included NCCT, CTA, and CTP.
Cerebral CT was performed on a 16–detector row multidetector CT scanner (LightSpeed; GE Healthcare) up to November 2005 and on a 64–detector row multidetector CT scanner (LightSpeed VCT; GE Healthcare) thereafter. NCCT was acquired in the axial mode using the following parameters: 120-kV(peak) tube voltage; 320-mA tube current; section thickness, 5 mm; 32-cm scan FOV; and 512 × 512 matrix. Raw data were reconstructed in the axial plane using filtered back-projection until 2009 and adaptive statistical iterative reconstruction thereafter. Using NCCT, we searched for intracranial hemorrhage, the hyperdense MCA sign, chronic stroke lesions, the presence of leukoaraiosis, and the presence and extent of early ischemic changes in the MCA territory to calculate the ASPECTS.2
The cervical and cerebral CTAs were acquired in helical scan mode from the aortic arch to the top of the frontal sinuses, according to the following parameters: 120-kVp tube voltage, 1500- to 260-mA tube current, 0.9:1 pitch, 0.625-mm section thickness (1.25 mm before November 2005), and 512 × 512 matrix. Data acquisition was performed after intravenous injection of 50 mL of iodinated contrast material at a flow rate of 5 mL per second, with a delay according to the perfusion data. We defined LVO as an internal carotid artery, M1, or proximal M2 occlusion. We calculated clot burden score (CBS) for each patient as an indicator of clot extension.16 We defined tandem occlusion as arterial occlusion affecting both the extra- and intracranial circulation in the same carotid axis. In patients with LVO, collaterals were graded according to Tan et al.17 We considered collaterals as “good” if >50% of the ischemic territory distal to the occluded artery was filled.
CTP images were acquired for 50 seconds in a cine mode with a delay of 5–7 seconds after beginning the injection of 50 mL of iodinated contrast at a flow rate of 5 mL per second; four 10-mm slices (40-mm coverage) were imaged before November 2005, and 18 groups of sixteen 5-mm slices (80-mm coverage) thereafter. CTP data were transferred to a workstation and analyzed using the Brilliance Workspace Portal (Philips Healthcare). This team performed manual checks and adjustments and correction of artifacts. The locations of early signs of ischemia and infarct core on CTP were not checked for accordance. A deconvolution approach, based on the central volume principle, was used to create parametric maps of MTT. CBV was calculated from the area under the time-enhancement curves, and CBF was derived from the formula CBF = CBV/MTT. Infarct core and ischemic penumbra volumes were calculated by applying appropriate MTT and CBV thresholds, which are MTT >145% of the contralateral side values and CBV >2.0 mL/100 g for the penumbra volume, and MTT >145% of the contralateral side values and CBV <2.0 mL/100 g for the core volume.12
NCCT images were reviewed for ASPECTS, retrospectively, by an experienced vascular neurologist (P.M.), who compared his value with the assessment established by the radiologist in the acute phase and appearing in the official (clinical) radiology report. In cases of disagreement between the 2 ASPECTS values, the case was discussed at the weekly joint neuroradiology meetings to reach a consensus. We had previously assessed interrater variability between the vascular neurologist and senior neuroradiologist using the Cohen κ on 100 consecutive acute CT scans with anterior circulation occlusive stroke for NCCT ASPECTS, CBS, and collateral status (poor versus good). The ASPECTS was scored by the Lausanne team, without considering results of the CTP, the latter being performed by a completely independent team in Stanford. Both ASPECTS and CTP were calculated in the acute phase, without knowledge of the long-term clinical outcome.
Statistical Analysis
Categoric and binary variables were summarized as frequencies and percentages, while continuous variables were summarized as median and interquartile range.
Statistical correlation between the ASPECTS and core volume on CTP was quantified using the Spearman ρ coefficient. To report the strength and direction of the correlation, we referred to a commonly used interpretation of the Spearman correlation coefficient in medical research.18 We calculated the statistical significance of this association both in the overall study population and in several meaningful subpopulations defined by the following settings: 1) presence and absence of LVO, 2) early- and late-arriving patients, and 3) known and unknown stroke onset. Furthermore, we compared the correlation coefficients between groups defined by variable combinations of the above-mentioned scenarios (eg, late-arriving patients with LVO). Comparisons between the different groups of patients were based on z scores obtained using the Fisher r-to-z transformation of the ρ; this allows determining the statistical significance of the differences by means of tests based on the Student t distributions. We performed a complete case analysis, and no imputations of missing data were performed.
To check for independent factors associated with ASPECTS (used as a dependent variable), we developed a multivariate linear regression model. We included in the model variables likely to influence the ASPECTS based on pathophysiologic considerations (such as age, NIHSS, prestroke treatments, vascular risk factors, clot burden, leukoaraiosis, core, and penumbra volumes on CTP) and variables that were supposed to influence the relationship between ASPECTS and core volumes (such as the above variables and time from LPGH and LVO and onset type). The complete list of variables included in the model, along with the P values obtained using univariate analysis, is shown in the Online Supplemental Data. We used a stepwise backward elimination method based on the Akaike Information Criterion to select relevant covariates for inclusion in the final model. Then, we checked the sensitivity of our findings by fitting the same model (except for LVO) into the subpopulation of late-presenting patients with LVO. Heteroscedasticity and normality of residuals were checked using graphic methods (QQ and residuals versus fitted plots).
To identify independent predictors of good clinical outcome at 3 months (mRS ≤2), we fitted a multivariate logistic regression model with the stepwise backward elimination method. This model included demographic, clinical, and radiologic variables at stroke onset that are known to be related to the functional long-term outcome (Online Supplemental Data).19,20 Then, as before, we performed a separate logistic regression analysis on the subgroup of late-arriving patients with LVO, applying the same model (except for LVO).
Finally, to understand better the capability of NCCT and CTP to predict clinical outcome, we performed receiver operating characteristic (ROC) curve analyses for both imaging modalities in patients showing a concordant or discordant NCCT CTP profile. We defined favorable NCCT as ASPECTS ≥6, and favorable CTP if the core volume was ≤70 mL, as previously suggested.7,21
RESULTS
Study Population and Baseline Characteristics
Of 5049 patients with AIS entered in the ASTRAL registry during the study period, 1046 were included in the current analysis. The flow chart in the Online Supplemental Data describes the reasons for exclusion from the analysis and the main differences between the included and excluded patients.
The median age of the included patients was 71.4 years (interquartile range, IQR = 59.8–79.4 years), and the median NIHSS score was 12 (IQR, 6–18), as described in the Online Supplemental Data. The median time from LPGH to hospital arrival was 2.6 hours (IQR, 1.3–6.9 hours), and the median time from LPGH to imaging was 3.4 hours (IQR, 1.9–8.5 hours). Two hundred ninety-two patients (27.9%) were admitted in the late time window; their median LPGH to hospital arrival time was 10.2 hours (IQR, 7.9–13.4 hours), and their median LPGH to CT time was 11.5 hours (IQR, 8.7–15.3 hours).
We previously assessed interrater agreement measures for the following CT-based neuroimaging variables, finding almost perfect agreement for ASPECTS (κ = 0.82) and collaterals (κ = 0.81) and good agreement for the clot burden score (κ = 0.77).
In the study population, the median ASPECTS was 9 (IQR, 7–10), and median core volume on CTP was 13.6 mL (IQR, 0.6–52.8 mL). On CTA, an LVO was detected in 612 (58.5%) patients and in 151 (51.7%) late-arriving patients (Online Supplemental Data). Additional treatment details, stroke etiology, and clinical outcome measures are available in the Online Supplemental Data.
Correlation between ASPECTS and CTP Core, and Influence of Time and LVO
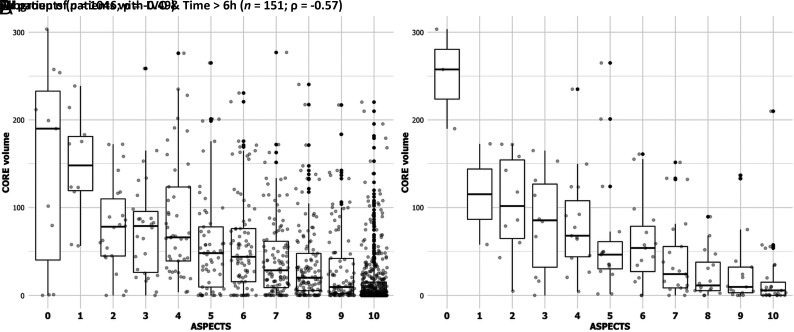
The overall correlation between ASPECTS and CTP core was moderate (ρ = –0.49, P < .01). The distribution of CTP core volumes across ASPECTS grades is depicted in Fig 1A, and it showed a definitive trend of increasing median baseline CTP cores as the ASPECTS decreased. The ASPECTS-CTP core correlation was significantly stronger in the subgroup of patients admitted in the late rather than in the early time window (ρ = –0.56 and ρ = –0.48, respectively; P = .05; Online Supplemental Data). In addition, this correlation was significantly better in the presence versus absence of an LVO (ρ = –0.40 and ρ = –0.20, respectively; P < .01; Online Supplemental Data). We did not find any significant difference in the ASPECTS-CTP core correlation in the subgroup of patients with known-versus-unknown stroke onset (ρ = –0.55 and ρ = –0.44, respectively; P = .12).
FIG 1.
Boxplot of ASPECTS (x-axis) and baseline CTP core volumes (y-axis) in the overall population (n = 1046, A) and in subgroup of late-arriving patients with LVO (n = 151, B). We observe a moderate ASPECTS-CTP core correlation in our study cohort (ρ = –0.49) and a stronger correlation among late-arriving patients with LVO (ρ = –0.57).
Testing the combined covariates LVO and time, we found that the correlation increased up to a moderately strong degree (ρ = –0.57, P < .01) in the subgroup of late-arriving patients with LVO (Fig 1B). On the other hand, it was poor in early patients with and without LVO (ρ = –0.36, P < .01; and ρ = –0.23, P = .01, respectively).
With the linear multiple regression model, we confirmed an independent association between ASPECTS and CTP core (β = –0.10 per 10 mL; 95% CI, −0.14 to –0.07; P < .01). Moreover, a higher ASPECTS was independently associated with older age, shorter delay to arrival time, prestroke statin use, and lower admission glucose levels. Regarding radiologic variables, we found an independent association between a higher ASPECTS and the absence of a hyperdense MCA sign, absence of LVO, higher CBS, and the presence of good collaterals (Table 1). In the subpopulation of late-arriving patients with LVO, the association of ASPECTS and CTP core was twice as strong (β = –0.21 per 10 mL; 95% CI, 0.27 to –0.15; P < .01). Again, older age, shorter delay to arrival time, and higher CBS were independently associated with a higher ASPECTS (Table 1).
Table 1:
Significant results from the multiple regression model with NCCT ASPECTS as dependent variable in the overall population and in late-arriving (>6 hours from LPGH) patients with AIS with LVOa
| Variables Associated with ASPECTS | Study Cohort (n = 1046) | Late AIS with LVO (n = 151) |
|---|---|---|
| Age (yr) | 0.02 (0.01–0.03) | 0.05 (0.02–0.07) |
| LPGH to arrival time (h) | −0.11 (−0.15 to −0.08) | −0.21 (−0.30 to −0.12) |
| Prestroke statin use | 0.67 (0.13–1.21) | NS |
| Acute glucose (g/L) | −0.07 (−0.13 to −0.01) | NS |
| Hyperdense MCA sign | −0.56 (−0.98 to −0.14) | NS |
| LVOb | −0.73 (−1.26 to −0.20) | − |
| CBS | 0.14 (0.06–0.21) | 0.17 (0.03–0.31) |
| Good collaterals | 0.87 (0.51–1.23) | NS |
| Core volume, per 10 mL | −0.10 (−0.14 to −0.07) | −0.21 (−0.27 to −0.15) |
Note:—NS indicates nonsignificant; −, variable was non included in the model.
Results are expressed as β coefficient and relative 95% CI.
Included in the predictive model for the entire study cohort only.
To check whether ASPECTS could reliably identify the CTP core volume thresholds, which have been used in recent clinical trials of the late time window, we performed a ROC analysis using a CTP core of 70 mL (as used in the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke [DEFUSE-3] trial)22 investigating whether a higher ASPECTS was associated with a favorable CTP profile. We found an area under the curve of 0.76 in the overall population and an under the curve of 0.79 in the subpopulation of late-arriving stroke with LVO (Online Supplemental Data). In this latter group, a cutoff of ASPECTS of ≥7 (based on the Youden index) identified patients with a CTP core of <70 mL, with a sensitivity of 65.7% and a specificity of 76.7%.
Association of ASPECTS with Clinical Outcome
The overall percentage of good clinical outcome at 3 months was 51.9%. Fitting a logistic multiple regression model, ASPECTS emerged as an independent predictor of good outcome in both the overall population (OR = 1.10; 95% CI, 1.00–1.20; P = .05) and late-arriving patients with AIS (OR = 1.23; 95% CI, 1.02–1.51; P = .03; Table 2). Moreover, we found younger age, lower NIHSS score on admission, and lower frequency of a decreased level of consciousness as independently associated with a favorable outcome in our study cohort. Among radiologic variables, a smaller clot (ie, higher CBS) and the absence of tandem occlusion also predicted favorable outcome.
Table 2:
Independent predictors of good clinical outcome at 3 months (mRS ≤2) in the overall population and in late-arriving (>6 hours from LPGH) patients with AIS with LVOa
| Variables Associated with Good Outcome | Study Cohort (n = 1046) | Late AIS with LVO (n = 151) |
|---|---|---|
| Age (yr) | 0.96 (0.94–0.97) | NS |
| NIHSS on admission | 0.87 (0.84–0.91) | 0.86 (0.80–0.93) |
| Decreased LOC on admission | 0.45 (0.24–0.83) | NS |
| LPGH to arrival time (h) | 0.95 (0.91–0.99) | NS |
| NCCT ASPECTS | 1.10 (1.00–1.20) | 1.23 (1.02–1.51) |
| CBS | 1.17 (1.08–1.28) | NS |
| Tandem occlusion | 0.54 (0.32–0.92) | 0.23 (0.06–0.76) |
| AIC = 694.76 | AIC = 146.02 |
Note:—LOC indicates level of consciousness; AIC, Akaike Information Criteria.
Results are adjusted for prestroke mRS and expressed as odds ratio and relative 95% CI.
For a sensitivity analysis, we compared the predictive capabilities of the models for good clinical outcome at 3 months in which ASPECTS is replaced with the CTP core (Online Supplemental Data). These models showed very similar performances as indicated by the similar coefficients and the Akaike Information Criteria, in both the overall cohort and in the subpopulation of late-arriving patients with LVO; the Vuong tests for the difference in the Akaike Information Criteria were not significant (P = .39 for the overall cohort; P = .14 for the late-arriving patients with LVO).
Looking at the relationship between imaging concordance and clinical outcome, we identified the following subgroups of patients: patients with favorable NCCT/CTP (n = 756/1046, 72%), patients with poor NCCT/CTP (n = 79, 8%), and patients with discordant NCCT/CTP (n = 211, 20%). The percentages of good outcome across the subgroups were the following: 61% in patients with both images being favorable, 14% in patients with both unfavorable images, and 32% in patients with discordant images. The areas under the curve (AUC) for predicting good outcome for each imaging technique in the 3 subgroups of patients are reported in the Online Supplemental Data. We observed a similar but poor prognostic performance of NCCT and CTP in patients with favorable profiles on both modalities. The performance was higher in patients showing both unfavorable NCCT and CTP, without any statistical difference between the ASPECTS and CTP. In patients with discordant NCCT and CTP profiles, the performance of both modalities was again modest, without a higher accuracy for CTP core compared with ASPECTS.
DISCUSSION
In a large cohort of consecutive patients with AIS involving the MCA territory, we showed a moderate correlation between the ASPECTS and core volume on CTP in the acute phase of stroke. This correlation was significantly better in the presence of an LVO (ICA, M1, or proximal M2 occlusion) and was time-dependent, being stronger in the subgroup of patients potentially eligible for late endovascular treatment (ie, LVO-positive and arriving after 6 hours of last proof of good health). In the latter, we confirmed an independent role of ASPECTS in determining good clinical outcome at 3 months, which was similar to the CTP core.
Compared with previous studies reporting a weak23-to-moderate24 ASPECTS-CTP core correlation, correlations in a larger study cohort were tested, applying multiple adjustments. We demonstrated that the ASPECTS-CTP core association was stronger in patients with LVO than without it. This finding was very robust, given that the association was present even after correction for several clinical and radiologic variables. We suppose that the presence of a proximal intracranial occlusion leads to higher ischemic core volume and, therefore, to a higher likelihood of detecting early ischemic changes on NCCT and, as a consequence, to a higher accuracy of ASPECTS on estimating core volume.25 This was especially evident in patients assessed 6 hours after symptom onset, which probably reflects the progressive development of cytotoxic edema, the histologic equivalent of early ischemic changes on NCCT.
We also found that multiple other clinical and radiologic variables, in addition to time and presence of an LVO, influenced the ASPECTS. Patients with prestroke statin use presented with higher ASPECTS, which is in line with previous studies reporting that statin pretreatment enhances collateral perfusion and reduces final infarct volume.26,27 We also demonstrated that admission hyperglycemia was associated with poorer ASPECTS; this finding is consistent with previous human and animal studies showing that hyperglycemia is associated with early infarct expansion in AIS.28,29 Regarding radiologic variables, we found higher ASPECTS in patients without the hyperdense MCA sign (which is a marker of proximal MCA occlusion) and with higher CBS (which means smaller clots). Taken together, these results suggest a favorable NCCT profile in patients with a distal or small area of vascular occlusion. Moreover, we identified an independent association between higher ASPECTS and good collaterals, further supporting the role of collateral circulation in the early prevention of tissue loss.25
Our results confirm that in a mixed population of patients with AIS, some treated with intravenous thrombolysis and/or EVT, baseline ASPECTS is a major determinant of good clinical outcome at 3 months, after adjusting for known confounders (including age, prestroke disability, stroke severity, and admission glycemia). This finding has already been shown in the early time window for patients without revascularization treatment,30 treated with intravenous thrombolysis5 and with early EVT.6,31,32 In our subgroup of patients admitted late and who had LVO (a minority of whom underwent EVT), we confirmed that a higher ASPECTS also remained independently associated with good clinical outcome, and we showed that the prognostic value of ASPECTS was similar to that of core volume on CTP.
Nevertheless, relying on imaging alone could lead to erroneous outcome prediction. Our results showed that a good and concordant NCCT/CTP imaging profile on admission was not a sufficient condition for sure translation to a positive clinical outcome, and the performance of both imaging modalities did not seem to contribute to prediction. We can probably explain this outcome by the other numerous variables that may have an impact on the outcome (as emerged from our multivariate model of prediction of good clinical outcome at 3 months). In addition, the small number of patients with LVO who underwent EVT in our cohort might have influenced this finding. We also showed that 20% of patients presented with a discordant NCCT/CTP profile, of whom 30% achieved a good outcome. These patients include patients with good ASPECTS despite a large infarct volume, in whom it was demonstrated that a quick and successful revascularization of the hypoperfused region was still associated with a high probability of good outcome. In fact, up to 20% of such patients might present with a final infarct volume lower than that of the admission volume, due to an overestimation of the latter by CTP in the early hours after stroke onset.33,34 In the opposite situation of low ASPECTS associated with acceptable core volumes on CTP, a revision of early ischemic NCCT changes should be considered (eg, to exclude old infarctions or NCCT artifacts), especially for patients imaged in the extended time window and with good collateral circulation.
The clinical implications of our findings are that ASPECTS appears a quite reliable surrogate marker for the ischemic core in patients with LVO in the later time window. Such a finding supports the possible role of ASPECTS as a selection tool for late mechanical thrombectomy. We previously demonstrated that the strict application of trial criteria (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo [DAWN] and DEFUSE-3) translated into a low proportion of patients eligible for late EVT in the real-word scenario and that this treatment could be offered to a larger population of patients if more liberal criteria were adopted.22 In this setting, the use of ASPECTS could help with the decision to proceed to thrombectomy in cases of absent, failed, or contraindicated advanced imaging or in situations of CT and CTP discordant profiles.21 The success of late revascularization therapies according to trial criteria could be hopefully replicated by simpler selection criteria. This strategy is currently being evaluated in ongoing randomized clinical trials (Endovascular Treatment of Acute Stroke for Late arrivals, MR CLEAN-LATE;35 Tenecteplase in Wake-up Ischaemia Trial [TWIST]36).
Several limitations of our study need to be acknowledged. First, its single-center retrospective design and the exclusion of patients due to the absence of reconstructed CTP volumes could lead to a selection bias; furthermore, an external validation of the study results is lacking. Second, we did not assess the spatial agreement between the ASPECTS and CTP core; therefore, we could not assess whether unequal weighting of brain regions in the ASPECTS rating could hamper its correlation with core volumes. Third, the thresholds model used for core and penumbra volume reconstructions was different from those adopted in recent EVT trials;37 however, it is a well-established model, based on a systematic evaluation of all PCT parameters and is the most suitable for the software used in the analysis.12 Finally, given the small number of patients treated with late EVT in our cohort, we could not analyze the impact of ASPECTS on the response to revascularization treatments.
A future potential development of this study includes the comparison between visual ASPECTS and scoring with automated software applications able to detect and quantify early ischemic changes.38
CONCLUSIONS
In our series of 1046 patients with MCA stroke, ASPECTS showed a moderate correlation with CTP-based infarct core, which is stronger in late-arriving patients with large-vessel occlusion. This could support the use of ASPECTS as a surrogate marker for CTP core for selection of late endovascular treatment and for estimation of prognosis. Further studies on the effect of an ASPECTS-based selection for late revascularization therapies are strongly welcomed.
Acknowledgments
We thank Melanie Price Hirt for English language correction and editing.
ABBREVIATIONS:
- AIS
acute ischemic stroke
- ASTRAL
Acute STroke Registry and Analysis of Lausanne
- CBS
clot burden score
- CTP
Computed tomographic perfusion
- EVT
endovascular treatment
- LPGH
last proof of good health
- LVO
large-vessel occlusion
- MCA
middle cerebral artery
- NCCT
non-contrast CT scan
Footnotes
The study was funded by the Swiss National Science Foundation (grant No. 320030_182654).
Preliminary results of the study previously presented as an abstract at: European Stroke Organization Conference, May 22–24, 2019; Milan, Italy (E-poster AS10-019).
Disclosures: Stefania Nannoni—RELATED: Grant: Swiss National Science Foundation, Comments: grant 320030_182654*; Support for Travel to Meetings for the Study or Other Purposes: Bristol Myers Squibb, Bayer AG, Comments: Congress travel grants,* Gaia Sirimarco—UNRELATED: Board Membership: Daichii-Sankio, Bayer AG, Comments: Advisory Board*; Grants/Grants Pending: Swiss Heart Foundation*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Bayer AG, Shire, Comments: support for Congress.* Max Wintermark—UNRELATED: Consultancy: Nous, Icometrix. Vincent Dunet—UNRELATED: Grants/Grants Pending: Swiss National Science Foundation.* Patrik Michel—RELATED: Grant: Swiss National Science Foundation, Swiss Heart Foundation, Employee Retirement Income Security Act of 1974 program (Bristol Myers Squibb/Pfizer)*; Consulting Fee or Honorarium: Medtronic.* *Money paid to the institution.
References
- 1.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 10.1016/s0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 2.Puetz V, Dzialowski I, Hill MD, et al. The Alberta Stroke Program Early CT Score in clinical practice: what have we learned? Int J Stroke 2009;4:354–64 10.1111/j.1747-4949.2009.00337.x [DOI] [PubMed] [Google Scholar]
- 3.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–32 10.1002/ana.10136 [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Goyal M, Jahan R, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol 2016;79:76–89 10.1002/ana.24543 [DOI] [PubMed] [Google Scholar]
- 5.Hill MD, Buchan AM. Canadian Alteplase for Stroke Effectiveness Study (CASES) Investigators. Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 2005;172:1307–12 10.1503/cmaj.1041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 7.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 9.Konstas AA, Minaeian A, Ross IB. Mechanical thrombectomy in wake-up strokes: a case series using Alberta Stroke Program Early CT Score (ASPECTS) for patient selection. J Stroke Cerebrovasc Dis 2017;26:1609–14 10.1016/j.jstrokecerebrovasdis.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 10.Nagel S, Herweh C, Pfaff JA, et al. Simplified selection criteria for patients with longer or unknown time to treatment predict good outcome after mechanical thrombectomy. J Neurointerv Surg 2019;11:559–62 10.1136/neurintsurg-2018-014347 [DOI] [PubMed] [Google Scholar]
- 11.Michel P, Odier C, Rutgers M, et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke 2010;41:2491–98 10.1161/STROKEAHA.110.596189 [DOI] [PubMed] [Google Scholar]
- 12.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006;37:979–85 10.1161/01.STR.0000209238.61459.39 [DOI] [PubMed] [Google Scholar]
- 13.Michel P, Engelter S, Arnold M, et al. Thrombolyse de l'attaque cérébrale ischémique: recommandations actualisées. Swiss Medical Forum 2009;9:225–28 10.4414/fms.2009.07010 [DOI] [Google Scholar]
- 14.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO): European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic Stroke Endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019;4:6–12 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke J 1993. January;24(1):35–41 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 16.Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke 2008;3:230–36 10.1111/j.1747-4949.2008.00221.x [DOI] [PubMed] [Google Scholar]
- 17.Tan JC, Dillon WP, Liu S, et al. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007;61:533–43 10.1002/ana.21130 [DOI] [PubMed] [Google Scholar]
- 18.Fowler J, Cohen L, Jarvis P. Practical Statistics for Field Biology 2nd ed. Wiley; 2013:132–33 [Google Scholar]
- 19.Quinn TJ, Singh S, Lees KR, et al. ; VISTA Collaborators. Validating and comparing stroke prognosis scales. Neurology 2017;89:997–1002 10.1212/WNL.0000000000004332 [DOI] [PubMed] [Google Scholar]
- 20.Ntaios G, Faouzi M, Ferrari J, et al. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. Neurology 2012;78:1916–22 10.1212/WNL.0b013e318259e221 [DOI] [PubMed] [Google Scholar]
- 21.Sarraj A, Hassan AE, Grotta J, et al. Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT): a prospective, multicenter cohort study of imaging selection. Ann Neurol 2020;87:419–33 10.1002/ana.25669 [DOI] [PubMed] [Google Scholar]
- 22.Nannoni S, Strambo D, Sirimarco G, et al. Eligibility for late endovascular treatment using DAWN, DEFUSE-3, and more liberal selection criteria in a stroke center. J Neurointerv Surg 2020;12:842–47 10.1136/neurintsurg-2019-015382 [DOI] [PubMed] [Google Scholar]
- 23.Demeestere J, Scheldeman L, Cornelissen SA, et al. Alberta Stroke Program Early CT Score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic stroke. Stroke 2018;49:2361–67 10.1161/STROKEAHA.118.021961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olive-Gadea M, Martins N, Boned S, et al. Baseline ASPECTS and e-ASPECTS correlation with infarct volume and functional outcome in patients undergoing mechanical thrombectomy. J Neuroimaging 2019;29:198–202 10.1111/jon.12564 [DOI] [PubMed] [Google Scholar]
- 25.Nannoni S, Cereda CW, Sirimarco G, et al. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 2019;61:971–78 10.1007/s00234-019-02224-x [DOI] [PubMed] [Google Scholar]
- 26.Shook SJ, Gupta R, Vora NA, et al. Statin use is independently associated with smaller infarct volume in nonlacunar MCA territory stroke. J Neuroimaging 2006;16:341–46 10.1111/j.1552-6569.2006.00061.x [DOI] [PubMed] [Google Scholar]
- 27.Malhotra K, Safouris A, Goyal N, et al. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: a meta-analysis. Atherosclerosis 2019;282:75–79 10.1016/j.atherosclerosis.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 28.MacDougall NJ, Muir KW. Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and meta-analysis. J Cereb Blood Flow Metab 2011;31:807–18 10.1038/jcbfm.2010.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoyama T, Shibazaki K, Kimura K, et al. Admission hyperglycemia causes infarct volume expansion in patients with ICA or MCA occlusion: association of collateral grade on conventional angiography. Eur J Neurol 2013;20:109–16 10.1111/j.1468-1331.2012.03801.x [DOI] [PubMed] [Google Scholar]
- 30.Demchuk AM, Hill MD, Barber PA, et al. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA stroke study. Stroke 2005;36:2110–15 10.1161/01.STR.0000181116.15426.58 [DOI] [PubMed] [Google Scholar]
- 31.Pfaff J, Herweh C, Schieber S, et al. e-ASPECTS correlates with and is predictive of outcome after mechanical thrombectomy. AJNR Am J Neuroradiol 2017;38:1594–99 10.3174/ajnr.A5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haussen DC, Dehkharghani S, Rangaraju S, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke 2016;47:2318–22 10.1161/STROKEAHA.116.014117 [DOI] [PubMed] [Google Scholar]
- 33.Martins N, Aires A, Mendez B, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol 2018;7:513–21 10.1159/000490117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotem SH, Mor S, Chen B, et al. Infarct core reliability by CT perfusion is a time-dependent phenomenon. J Neuroimaging 2020;30:240–45 10.1111/jon.12692 [DOI] [PubMed] [Google Scholar]
- 35.van Oostenbrugge R, van Zwam W. MR CLEAN LATE Trial Protocol. 2018. https://mrclean-late.nl/trial-protocol.html. Accessed September 11, 2019
- 36.Tenecteplase in Wake-up Ischaemic Stroke Trial (TWIST) study protocol. https://clinicaltrials.gov/ProvidedDocs/60/NCT03181360/Prot_SAP_000.pdf. Accessed September 11, 2019
- 37.Lansberg MG, Lee J, Christensen S, et al. RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study 3. Stroke 2011;42:1608–14 10.1161/STROKEAHA.110.609008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoelter P, Muehlen I, Goelitz P, et al. Automated ASPECTS scoring in acute ischemic stroke: comparison of three software tools. Neuroradiology 2020;62:1231–38 10.1007/s00234-020-02439-3 [DOI] [PubMed] [Google Scholar]