Significance
Food system collapse incurs major societal costs that may extend across generations. The Great Chinese Famine—widely acknowledged as the largest famine in human history—was associated with tremendous short-term adverse health consequences, yet its long-term effect on infectious disease incidence has not been estimated. We conducted a cohort analysis of >1 million pulmonary tuberculosis (PTB) cases in a major center of ongoing transmission that experienced high famine mortality, finding a substantial burden of active PTB potentially attributable to the famine in the birth cohort that experienced prenatal and early-life exposure to famine, as well in their putative offspring. The Great Chinese Famine occurred >60 y ago, yet continues to contribute to the high PTB burden in China.
Keywords: food systems, tuberculosis, Great Chinese Famine, infectious disease, nutrition
Abstract
Global food security is a major driver of population health, and food system collapse may have complex and long-lasting effects on health outcomes. We examined the effect of prenatal exposure to the Great Chinese Famine (1958–1962)—the largest famine in human history—on pulmonary tuberculosis (PTB) across consecutive generations in a major center of ongoing transmission in China. We analyzed >1 million PTB cases diagnosed between 2005 and 2018 in Sichuan Province using age–period–cohort analysis and mixed-effects metaregression to estimate the effect of the famine on PTB risk in the directly affected birth cohort (F1) and their likely offspring (F2). The analysis was repeated on certain sexually transmitted and blood-borne infections (STBBI) to explore potential mechanisms of the intergenerational effects. A substantial burden of active PTB in the exposed F1 cohort and their offspring was attributable to the Great Chinese Famine, with more than 12,000 famine-attributable active PTB cases (>1.23% of all cases reported between 2005 and 2018). An interquartile range increase in famine intensity resulted in a 6.53% (95% confidence interval [CI]: 1.19–12.14%) increase in the ratio of observed to expected incidence rate (incidence rate ratio, IRR) in the absence of famine in F1, and an 8.32% (95% CI: 0.59–16.6%) increase in F2 IRR. Increased risk of STBBI was also observed in F2. Prenatal and early-life exposure to malnutrition may increase the risk of active PTB in the exposed generation and their offspring, with the intergenerational effect potentially due to both within-household transmission and increases in host susceptibility.
The impact of prenatal exposure to famine on the risk of chronic conditions—including hypertension, coronary heart disease, type 2 diabetes, metabolic syndrome, and schizophrenia—in adulthood has been widely studied (1–5). Higher risks of chronic diseases have also been identified among the children of individuals from famine birth cohorts (6–8). While there is an extensive literature on short-term effects of prenatal exposure to famine and malnutrition on infectious diseases (9, 10), research on long-term and intergenerational effects is limited (11–13).
Tuberculosis (TB) is a primarily airborne bacterial infection caused by the pathogen Mycobacterium tuberculosis. After infection, the latent period can range from months to years, with a relatively small proportion (∼5–10%) of infected individuals progressing to active disease (14). Worldwide, TB caused 1.6 million deaths in 2017 and was the leading cause of death from a single infectious agent in 2016 (15, 16). According to China’s web-based National Infectious Disease Reporting System (NIDRS) that collects case-level data on 40 notifiable infectious diseases from almost all healthcare facilities in the country (17), pulmonary tuberculosis (PTB) contributed the fourth highest number of incident cases in 2018 (823,342 out of 7,770,749 cases, 10.6%) and the second highest number of deaths (3,149 out of 23,377 deaths, 13.5%) (18). In the short term, exposure to famine is associated with an increased likelihood of latent PTB progressing to active disease, an increased probability of complications and deaths in active cases, and a reduced immune response following bacille Calmette–Guérin (bacillus Calmette–Guérin) vaccination (10). However, the long-term impacts of famine on the burden of PTB at a population level is poorly understood.
The Great Chinese Famine of 1959–1961 is widely considered the largest famine in human history in terms of total lives lost (19). An estimated 30 million people died from starvation, and an estimated 33 million births were lost or postponed (19). Rural residents were affected more severely because of shortages in grain supplies following their fulfillment of compulsory grain procurement quotas, while urban residents’ food supplies were secured by legal and other protections (20). This urban bias in the food distribution system, combined with provincial differences in the rural and urban population shares, led to substantial spatial heterogeneity in famine severity across China (21, 22).
The province of Sichuan is located in southwest China and has a population of more than 80 million people. Sichuan remains a high–PTB-transmission area within China (23), with an estimated prevalence rate of active PTB of 598 cases per 100,000 persons (95% confidence interval [CI]: 480–717), substantially higher than the national average of 442 (95% CI: 417–469) cases per 100,000 persons, according to a cross-sectional survey conducted in 2010 (24). Furthermore, Sichuan had the highest total fertility loss and excess mortality of all Chinese provinces during the Great Chinese Famine (25). As a leading producer of rice, Sichuan transferred grain to other affected provinces while also aggressively implementing a “communal dining” system in which food stocks and cookware were confiscated from rural residents, but free meals were provided (21). This led to an illusion of abundance, resulting in overconsumption and waste of food, followed by massive food shortages (26). As a consequence, the famine in Sichuan was both more severe and longer in duration than other provinces, lasting from 1958 to 1962 (21, 27). Migration during the period of the Great Chinese Famine was sharply limited, in part because residents were only allowed access to food supplies in the area of their household registration—e.g., through meals at People’s communes and commodity ration certificates for rural and urban residents, respectively (20).
Age–period–cohort (APC) approach can attribute variation in incidence rates across age groups and over time to three temporal processes (28, 29). First, age effects represent the influence of aging on disease risk, which may be explained by age-specific variation in immunity, behavior, or exposure (30–32). Second, period effects represent variation in the outcome associated with the time period in which the data were collected, affecting all age groups equally. These may relate to both underlying risk of the disease and changes in surveillance in a given time period. For example, the scale-up of Directly Observed Therapy Short-course strategy in China in the 1990s reduced the reported prevalence rate of PTB (33), while the shift from paper- to web-based reporting in 2004 increased the reported incidence of PTB in all age groups (34). Third, cohort effects represent differences in disease risk among different birth cohorts, which may be attributable to the common exposures across their life histories. For instance, people born in 1957 in Sichuan, who first experienced the Great Chinese Famine when they were 1 y old, may be at higher risk for various health problems than those born in 1965, after controlling for effects of age and observation period.
Here, we conducted a cohort analysis of surveillance records of more than 1 million PTB cases diagnosed between 2005 and 2018 in Sichuan Province, which experienced especially high mortality during the Great Chinese Famine. APC models and smoothing splines were used to estimate counterfactual PTB incidence for the directly affected birth cohort (F1, see definition in Materials and Methods) and their likely offspring (F2) in the absence of famine, calculating incidence rate ratios (IRRs) and the number of PTB cases potentially attributable to the famine by comparing observed and estimated counterfactual incidence during the 14-y period from 2005 to 2018 for the F1 and F2 birth cohorts. Mixed-effects metaregression analyses were conducted to understand whether the impact was associated with famine intensity at the prefecture level, and the analysis was repeated on certain sexually transmitted and blood-borne infections (STBBI) to explore potential mechanisms of the intergenerational effects. Assuming a low probability of sexual and/or blood contact between the F1 and F2 generations, the existence of intergenerational effects for STBBI would suggest an important role of increased host susceptibility, whereas the absence of these effects would suggest increased contact with high-risk parents among the F2 generation as a dominant mechanism.
Results
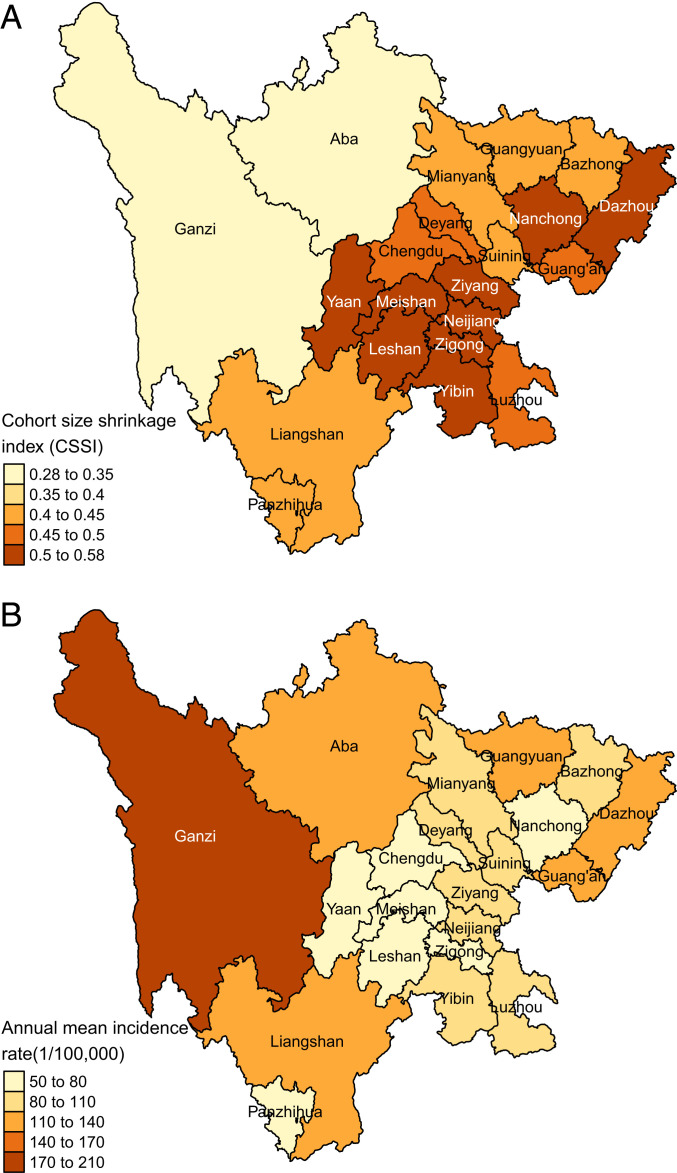
The severity of famine—expressed as cohort size shrinkage index (CSSI; see details in Materials and Methods)—varied widely across regions in the study area (Fig. 1A). Yaan, Meishan, and Leshan prefectures in central Sichuan exhibited the highest famine intensity; these areas had high rural populations and experienced high compulsory grain procurement during the famine period (21, 35). The prefectural CSSIs estimated for only male, only female, and the whole population were highly consistent (SI Appendix, Table S1).
Fig. 1.
Spatial variation in (A) CSSI, an indicator of famine intensity based on shrinkage in average birth cohort size over the famine period; and (B) annual mean active PTB incidence rate 2005–2018.
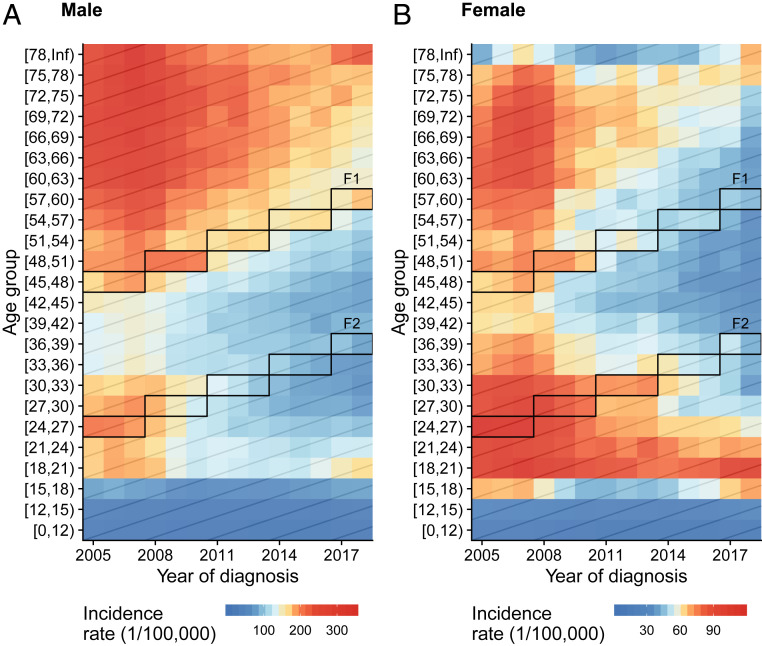
Over the 2005–2018 study period, annual mean PTB incidence rate was higher among men (123.4 per 100,000) than women (54.1 per 100,000). The annual mean incidence rate was higher in less developed, western mountainous regions, and lower in the more affluent eastern plains regions (Fig. 1B). Across years, age-standardized incidence rates decreased for both men (−5.6%, 95% CI: −4.7 to −6.4%) and women (−4.8%, 95% CI: −3.9 to −5.7%) (SI Appendix, Fig. S1A). This downward trend was also observed in most age groups in both sexes (Fig. 2, along each row). When comparing across age groups (Fig. 2, along each column; SI Appendix, Fig. S1B), both men and women exhibited low incidence rates in age groups under 17 y, and the highest incidence rates were observed among both young (ages 18–32 y) and older (ages 60–77 y) adults, although the highest-incidence age group for men (ages 60–77) was older than that for women (ages 18–32, Fig. 2 and SI Appendix, Fig. S1B). Gray diagonal lines in Fig. 2 represent different birth cohorts. Both the famine birth cohort (Fig. 2, Upper boxes; SI Appendix, Fig. S2, red lines) and their putative offspring (Fig. 2, Lower boxes; SI Appendix, Fig. S2, blue lines) had higher incidence rates than their adjacent birth cohorts in both males and females.
Fig. 2.
Active PTB incidence rate by year of diagnosis and age group at diagnosis among (A) men and (B) women. Colors represent the incidence rate, with red representing higher incidence rates and blue representing lower (note the differing scales in A and B). Each row represents the incidence rate across calendar years for that age group; each column represents the incidence rate across age groups for that year; and each diagonal line represents the change in incidence rate for that birth cohort as they age. The boxes outline the famine birth cohort (F1) and their likely offspring (F2). See definitions of F1 and F2 in Materials and Methods.
SI Appendix, Fig. S3 presents the age-specific active PTB incidence rates by year of diagnosis across prefectures. Consistent with the province-level results, age groups 18–32 and 60–77 exhibited the highest incidence rates, except for Aba and Ganzi prefectures, where elevated incidence rates were observed starting at age 15. In general, prefecture-level PTB incidence rates decreased over time, except in Liangshan and Ganzi prefectures. As for the provincial-level results (Fig. 2), incidence rates of the F1 and F2 cohorts were higher than adjacent birth cohorts.
Age, Period, and Cohort Effects.
Age, period, and cohort effect estimates for all of Sichuan Province are shown in Fig. 3. After adjusting for cohort and period effects, the highest risk of active PTB occurred in ages spanning 20–30 and then decreased gradually until age 40 (Fig. 3A). The age effect among women plateaued after age 40, while that of men rose slightly after age 40, peaking again around age 60. The period effect (Fig. 3B) increased in the first 3 y of the reporting period, followed by a gradual decrease in risk for both men and women. Trends toward decreasing risk of active PTB across progressively younger cohorts were generally observed, with apparent interruptions coinciding to the birth years of the F1 and F2 cohorts (Fig. 3C). Age, period, and cohort effects across prefectures for PTB are presented in SI Appendix, Figs. S4–S6.
Fig. 3.
Marginal IRRs for active PTB in each (A) age group, (B) period, and (C) cohort group in relation to the overall geometric mean incidence rate of all groupings given by the intrinsic estimator per model [1]. Estimation was conducted separately for men (blue) and women (red). Colored solid lines and the surrounding shaded areas represent estimated effects and 95% CIs, while dashed lines in C represent counterfactual cohort effects in the absence of famine predicted by smoothing splines. In C, the black vertical lines indicate the midpoint of the F1 and F2 cohorts.
Estimated Impact of the Great Chinese Famine on PTB in the Directly Affected Birth Cohort.
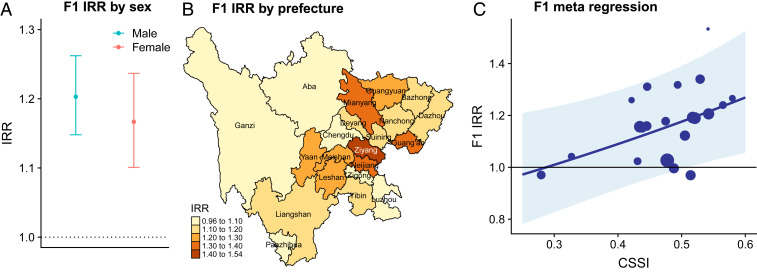
The IRRs of observed active PTB incidence rate over the expected incidence rate in the absence of famine in the male and female F1 cohorts were 1.20 (95% CI: 1.14–1.26) and 1.17 (95% CI: 1.10–1.24), respectively (Fig. 4A). The number of excess active PTB cases in Sichuan potentially attributable to the Great Chinese Famine was 3,654 (95% CI: 2,696–4,525) and 992 (95% CI: 616–1,310) for males and females, respectively; corresponding to 16.9% (95% CI: 12.5–21.0%) and 14.4% (95% CI: 8.9–19.0%) of all male and female observed cases in F1, respectively. Prefecture-level IRRs of observed incidence rate to expected incidence rate in the absence of famine in F1 ranged from 0.97 (95% CI: 0.87–1.10) in Ganzi to 1.53 (95% CI: 1.28–1.81) in Ziyang (Fig. 4B). Elevated F1 IRRs for PTB across prefectures (Fig. 4B) corresponded with higher CSSI (Fig. 1A), with excess estimated famine-attributable incidence concentrated in eastern areas of the province. Metaregression analyses revealed a positive correlation between CSSI and log F1 famine IRR (SI Appendix, Table S2), with an interquartile range increase in CSSI associated with a 6.53% (95% CI: 1.19–12.14%) increase in the F1 famine IRR for active PTB (Fig. 4C). Metaregression analyses stratified by sex revealed similar positive associations between CSSI and log F1 famine IRR (SI Appendix, Table S2).
Fig. 4.
Impact of the Great Chinese Famine on active PTB risk in directly affected birth cohort (F1). (A) Province-level IRR by sex. (B) Spatial heterogeneity in prefecture-level IRR. Prefectures are colored according to their IRRs, with darker colors representing higher IRRs. (C) Scatterplot of CSSI and IRR of F1 across prefectures (dots). The size of the dot is proportional to the inverse variance of the estimated IRR of that prefecture. The line represents the metaregression fit, and the shaded area represents the 95% CI.
Intergenerational Effect of the Great Chinese Famine on Active PTB Risk and Potential Mechanisms.
Increased active PTB risk in F2 was observed at both the province and prefecture level (SI Appendix, Text S1). The mediation analysis suggests that the Great Chinese Famine may have increased PTB risk in the F2 generation via its effects on F1 (SI Appendix, Tables S3 and S4). An interquartile range increase in CSSI was associated with an 8.32% (95% CI: 0.59–16.6%) increase in the F2 famine IRR for active PTB, among which 5.08% (95%CI: 0.07–14.1%) was through F1. Moreover, F2 had significant excess risks for STBBI as well, and the risk was strongly associated with CSSI (SI Appendix, Table S5 and Text S1), which suggests that the intergenerational effect of famine may act through mechanisms other than increased contact rates with infected individuals in the F1 generation, such as changes in disease susceptibility. Additional results for STBBI and intergenerational effect are shown in SI Appendix, Figs. S7–S15 and Text S1.
Discussion
Food security is a major driver of global health, and undernourishment in many parts of the world is often a direct consequence of environmental degradation and biodiversity loss. While unhealthy diets have long been linked to the growing incidence of global noncommunicable diseases, the impact of food system collapse on chronic, infectious disease outcomes has been difficult to establish, leading to an underestimation of the burden of disease attributable to food shortage and famine. We examined the long-term and potential intergenerational effects of prenatal exposure to famine on active PTB risk in later life. Using surveillance data on more than 1 million active PTB cases, results of an APC analysis indicated that the cohort of individuals born during the Great Chinese Famine were at higher risk of PTB in later life (2005–2018) when compared with individuals in adjacent birth cohorts. Our prefecture-level analysis indicated that local famine severity was positively associated with active PTB risk in the famine birth cohort, suggesting a mechanistic link between famine exposure and F1 PTB risk. We found that the Great Chinese Famine was likely to have induced an intergenerational effect on PTB risk, mediated through the increased risk in the famine birth cohort. Our comparison to STBBI suggested that the increased PTB risks for F2 are unlikely to be explained by increased contact rates with infectious F1 individuals alone, but may also indicate increased susceptibility to infectious disease for F2 individuals.
Our finding that prenatal famine exposure is associated with an increase in active PTB risk in adulthood is consistent with results from studies that have observed positive associations between low birth weight (LBW) and TB risk. In cohort and cotwin case-control studies, 500-g increases in birth weight were associated with 11% and 46% decreases in TB risk, respectively (12). Two possible mechanisms could contribute to the observed long-term effect of the Great Chinese Famine on TB risk in the famine-affected cohort. For one, the famine may have generated an enlarged pool of latently infected individuals as a result of increased rates of TB infection during the famine period. Malnutrition is known to accelerate progression of latent TB to active disease and to result in an abrupt increase in the number of active cases in the short term, which, through transmission, can lead to an increased burden of latent TB and subsequent increases in active PTB incidence (10, 36). Alternatively, the famine may have yielded increased and persistent susceptibility across the life-course of affected individuals. Maternal and postnatal malnutrition is known to change lung architecture and compromise immunological development, increasing morbidity or mortality from certain infectious diseases, including TB (12, 37).
Our findings of multigenerational impacts of the Great Chinese Famine on both PTB and STBBI outcomes suggest that in addition to increased household contact between children and their more heavily infected parents, increases in disease susceptibility may have played an important role in the intergenerational effect. Several factors might contribute to increases in susceptibility to disease in F2. For one, F1 was likely a generation born with LBW (38), and because a higher rate of LBW is expected among children born to LBW mothers (39), F2 likely experienced many of the adverse immune effects of LBW (40). What is more, prenatal exposure to famine has been shown to increase type 2 diabetes in the exposed generation and their offspring; F2 may thus have experienced increased susceptibility from the immune dysfunction associated with diabetes (40, 41). Additionally, inherited epigenetic changes have been shown to be affected by prenatal exposure to famine and may have contributed to increased susceptibility in F2 (41). Finally, behavioral factors, including diet, physical activity, and the awareness of risk, may also have contributed to the intergenerational effects of prenatal famine exposure (38, 39). Studies have found that individuals conceived during the famine were more likely to prefer fatty foods and have less physical activity than those conceived in the year before and after the famine, and children raised by them were likely to have the same lifestyle, which may result in immunologic changes that increase risk of TB infection and progression (40, 41).
Our study is limited by the widely acknowledged challenges of identifiability associated with APC analyses (42). However, since only the linear trends of the age, period, and cohort effects are nonidentifiable, our narrow focus on measuring a hypothesized anomaly in cohort effects during the famine period and removal of the long-term trend (including linear trend) suggest that our results were unlikely to be affected. Another limitation was possible misclassifications in the exposure for both the F1 and F2 cohorts. Our definition of the F2 cohort likely included some individuals that were not born to F1 and excluded some individuals that were born to F1. This misclassification of exposure would be expected to bias the estimates of the intergenerational famine effect toward the null. We applied an alternative approach to defining the F2 cohort by estimating the proportion of children born to F1 in each cohort group (SI Appendix, Fig. S16) and found strong agreement between the two methods. Misclassification may also arise from the reliance on passive surveillance data, which may reflect variation in healthcare-seeking behavior, variation in disease reporting, and other factors. Given that the underascertainment and underreporting is unlikely to be related to the exposure status (being born during the famine), this nondifferential misclassification would be expected to bias results toward a null finding. Furthermore, leaving out the selection effects of famine—famine survivors may be healthier than general population—may also bias the results toward a null finding. Although we expect that any potential confounders in the relationship between famine intensity and PTB IRR are controlled for in the meta-analyses because the counterfactual population represents the observed population in all aspects except events that occurred during the famine, it is conceivable that a cooccurring event might have influenced both famine intensity and IRR. Such an event would be expected to have minor impacts when compared to the famine, however. Finally, APC models can be used to infer population-level associations, but cannot establish causality at the individual level. Future epidemiologic studies that directly measure immune function, susceptibility to infection, and contact are needed to confirm the findings in this study and expose potential mechanisms linking famine to PTB and other infectious diseases.
In conclusion, we found that the famine birth cohort and their putative offspring experienced increased risks of active PTB during the observation period and that the respective magnitude of these increases was associated with famine severity at the prefecture level and PTB risk increases in the parent cohort, respectively. Mechanisms driving the observed increase in active PTB in the famine birth cohorts remain uncertain, but may involve a deeper reservoir of latent TB infections that manifest in disease as individuals age and their immune function weakens. Mechanisms underlying intergenerational effects may involve increased contact with the high-risk famine birth cohort, increased susceptibility to infection (e.g., due to malnutrition), as well as other contextual factors. Future studies are needed to investigate this hypothesis, including those employing designs that are capable of differentiating the impacts of prenatal and early life famine exposure; cross-sectional surveys to distinguish between the impact of famine on TB infection and progression; studies examining whether famine exposure impacts TB treatment initiation and success; research in settings outside of China on the long-term impact of famine on the risk of TB; and studies that investigate biological mechanisms underlying intergenerational famine effects on PTB risk.
This work underscores how societal shocks can reverberate in ways that impact national and global development for decades or more. More than one out of four countries on World Health Organization’s list of high–TB-burden countries (15) has experienced famine in the past century—including Democratic Republic of Congo, India, Cambodia, Ethiopia, and others (43)—with interactions that have yet to be explored. It is possible that contemporary food system and other shocks, such as those in employment, food, and nutrition during the COVID-19 pandemic (44), may yield analogous adverse health consequences extending across generations. Understanding the impact of famine on TB risk—and the underlying mechanisms driving this relationship—will be crucial to guarding against increases in TB and other infections in the aftermath of future humanitarian, climate, and public health crises and to accomplishing the Sustainable Development Goal of eliminating TB by 2035.
Materials and Methods
Disease Data.
Sichuan Province is the fourth most populous province in China. Because of its large area and heterogeneous landscape, the spatial distribution of population and socioeconomic development in the province is highly uneven. There are a total of 21 prefectures in Sichuan, with the proportion of rural residents within each prefecture ranging from 43.4% to 87.8% in 2010 (45).
We included all clinically diagnosed and laboratory-confirmed active PTB cases reported to NIDRS with home addresses located in Sichuan and diagnosed between 2005 and 2018, constructing a dataset of case counts by sex, age at diagnosis, year of diagnosis, and residential prefecture. The data and case definition have been described in detail elsewhere (23). Our dataset included 1,006,702 active PTB cases, including 704,837 (70.0%) among men and 301,865 (30.0%) among women.
To examine possible mechanisms underlying the intergenerational effect, we compared the TB data to clinically diagnosed and laboratory-confirmed STBBI (see details in SI Appendix, Text S2) data reported to NIDRS. All personal identifiers were removed prior to analyses, and the study was approved by the University of California, Berkeley, Committee for Protection of Human Subjects.
Population Data.
Population sizes for 2005–2018 by age and sex at province and prefecture levels were projected based on the 2000 census data using the cohort-component method (SI Appendix, Text S3) (35, 46). We validated the projections using 2010 census data (47). Province-level projections were accurate for all age groups (SI Appendix, Fig. ST3.6). Prefecture-level projections for F1 were accurate for all prefectures, while projections for F2 were less accurate for Chengdu, Guangyuan, Guang’an, and Ziyang (SI Appendix, Fig. ST3.7). These four prefectures were excluded from analyses estimating intergenerational effects, and sensitivity analyses were conducted examining the effect of these exclusions.
Definition of F1 and F2.
We defined F1 as the generation of individuals born during 1958–1962, which corresponds to the famine period in Sichuan. In keeping with the famine duration and consistent with the span of F1, we defined F2 to also span 5 y. To define the birth year range for F2, we examined the population age structure across prefectures in 2000 to identify age ranges where lower than expected population was apparent. Two such ranges are evident, corresponding to people born during the famine and their possible offspring. Within the younger range, we defined the 5-y age window with the smallest population size as F2, allowing it to vary by prefecture since typical age at reproduction may not have been the same in all areas (SI Appendix, Table S6). At the province level, the F2 cohort was defined as those born between 1979 and 1983.
Famine Intensity Measure.
To account for the potential impact of variable famine intensity on TB outcomes, we measured prefecture-level famine intensity as the degree of the shrinkage in birth cohort size during the famine using the CSSI (2, 48):
where Nfamine is the average number of births per year during the famine years (1958–1962) and Nnonfamine is the average number of births per year in the 5 y before (1953–1957) and after the famine (1963–1967). The CSSI ranges from 0 to 1, with a larger CSSI indicating a greater reduction in the average cohort size and therefore a higher famine intensity in the prefecture. We used 2000 census data to estimate CSSIs for the male, female, and total populations (35).
Statistical Analysis.
Estimating age, period, and cohort effects.
To isolate the cohort effect from age- and period-specific effects, we fit APC models: 1) for all of Sichuan Province and 2) for each of its 21 prefectures. We used these models to decompose log-incidence rates as (42):
| [1] |
where Yijk, Nijk, and λijk denote the number of cases, population size, and incidence rate in the ith age, jth period, and kth birth cohort, respectively. The parameter μ represents the overall mean; αi represents the ith age effect; πj represents the jth period effect; and γk represents the kth cohort effect. To ensure the intercept, μ, is identifiable, the parameters of APC models must be constrained (42, 49). Here we imposed the constraint that Σαi = Σπj = Σγk = 0. Furthermore, due to the fact that the index of one effect is entirely determined by the indices of the other two effects—i.e., linear dependence among the indices of the three effects (i − j + k = A, where A is the total number of age groups)—the linear trends of these effects will also be nonidentifiable unless an extra constraint on the parameters is specified (29, 50). We dealt with this by using the intrinsic estimator, in which a principal components transformation is applied to the design matrix to decorrelate model inputs (42, 51). This approach has been widely used in epidemiologic studies, and its theoretical justification and statistical properties are well-studied (51, 52). Finally, to account for potential overdispersion, we modeled the outcome as quasi-Poisson. More details are shown in SI Appendix, Text S4.
Impact of the Great Chinese Famine on PTB risk.
To estimate excess PTB risk in F1 potentially attributable to the Great Chinese Famine, we first constructed counterfactual incidence rates by fitting a smoothing spline to all cohort effects excluding the older age ranges with apparent lower than expected population (F1 birth cohort). We then used the resulting model to impute a potential F1 cohort effect in the absence of famine. Counterfactual cohort effects for F2 were estimated in the same fashion. We next predicted the incidence rate for the F1 birth cohort as if the famine had not occurred by Eq. 1 using the predicted cohort effect and estimated age and period effects from the APC model. We calculated the IRR as the observed active PTB incidence rate divided by the counterfactual incidence rate in the same cohort in the absence of famine. We then estimated the total number of excess cases attributable to the Great Chinese Famine using the difference between the number of observed and expected cases in the absence of famine. We also constructed posterior intervals for the F1 and F2 famine IRRs and the number of excess cases via Monte Carlo simulation, drawing from the parametric distributions of the fitted models (SI Appendix, Text S4). The analysis was conducted first for all of Sichuan Province, then for each of its prefectures.
Association of famine intensity with excess PTB risk.
We conducted a mixed-effects metaregression to evaluate associations between the famine severity and PTB risk in F1 and F2 at the prefecture level. The metaregression model allowed us to account for measured and unmeasured sources of heterogeneity in the impact of the famine on PTB within each prefecture (53). For F1, we fitted linear models to examine the effects of famine intensity represented by CSSI on the log of the ratio of the observed vs. expected nonfamine incidence rates in the F1 cohort. For F2, along with the metaregression model, mediation analysis was also conducted to examine the intergenerational effect of the famine mediated through its impacts on the F1 cohort. More details are shown in SI Appendix, Text S4.
Investigating mechanisms underlying intergenerational risks.
To understand whether intergenerational effects were limited to diseases in which increased household contact with infectious individuals (e.g., increased contact of F2 with infectious individuals in F1) was likely to explain increased risks for the F2 cohort, we repeated the APC analysis and counterfactual effect estimation for STBBI. Assuming a low probability of sexual and/or blood contact between the F1 and F2 generations, the existence of intergenerational effects for STBBI would suggest an important role of increased host susceptibility, whereas the absence of these effects would suggest increased contact with high-risk parents among the F2 generation as a dominant mechanism.
Supplementary Material
Acknowledgments
This work was supported in part by NSF Grant 2032210; NIH Grants R01AI125842, R01AI148336, and R01TW010286; and by the University of California Multicampus Research Programs and Initiatives Award #17-446315.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008336117/-/DCSupplemental.
Data Availability.
All data and code necessary to reproduce the findings of this study are available at the Github repository https://github.com/qu-cheng/TB_famine.
References
- 1.Roseboom T. J.et al., Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart 84, 595–598 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Li Z., Wang M., Martorell R., Early life exposure to the 1959-1961 Chinese famine has long-term health consequences. J. Nutr. 140, 1874–1878 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Lumey L. H., Khalangot M. D., Vaiserman A. M., Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: A retrospective cohort study. Lancet Diabetes Endocrinol. 3, 787–794 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Li C., Lumey L. H., Exposure to the Chinese famine of 1959-61 in early life and long-term health conditions: A systematic review and meta-analysis. Int. J. Epidemiol. 46, 1157–1170 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Bhutta Z. A., Early nutrition and adult outcomes: Pieces of the puzzle. Lancet 382, 486–487 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Veenendaal M. V.et al., Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 120, 548–553 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Li J., et al., Multigenerational effects of parental prenatal exposure to famine on adult offspring cognitive function. Sci. Rep. 5, 13792 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J.et al., Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: A population-based cohort study of families in Suihua, China. Am. J. Clin. Nutr. 105, 221–227 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Black R. E.et al.; Maternal and Child Nutrition Study Group , Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Cegielski J. P., McMurray D. N., The relationship between malnutrition and tuberculosis: Evidence from studies in humans and experimental animals. Int. J. Tuberc. Lung Dis. 8, 286–298 (2004). [PubMed] [Google Scholar]
- 11.McDade T. W., Beck M. A., Kuzawa C., Adair L. S., Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am. J. Clin. Nutr. 74, 543–548 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Villamor E., Iliadou A., Cnattingius S., Evidence for an effect of fetal growth on the risk of tuberculosis. J. Infect. Dis. 201, 409–413 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Moore S. E.et al., Season of birth predicts mortality in rural Gambia. Nature 388, 434 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Behr M. A., Edelstein P. H., Ramakrishnan L., Revisiting the timetable of tuberculosis. BMJ 362, k2738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization , Global Tuberculosis Report 2018, (World Health Organization, 2018). [Google Scholar]
- 16.World Health Organization , The Top 10 Causes of Death, (World Health Organization, 2018). [Google Scholar]
- 17.Liang S.et al., Surveillance systems for neglected tropical diseases: Global lessons from China’s evolving schistosomiasis reporting systems, 1949-2014. Emerg. Themes Epidemiol. 11, 19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Commission of the People’s Republic of China , Overview of national notifiable infectious diseases in 2018. http://www.nhc.gov.cn/jkj/s3578/201904/050427ff32704a5db64f4ae1f6d57c6c.shtml. Accessed 22 June 2019.
- 19.Ashton B., Hill K., Piazza A., Zeitz R., “Famine in China, 1958–61” in The Population of Modern China, Poston D. L. Jr., Yaukey D., Eds. (Springer, 1992), pp. 225–271. [Google Scholar]
- 20.Lin J. Y., Yang D. T., Food availability, entitlements and the Chinese famine of 1959–61. Econ. J. (Lond.) 110, 136–158 (2000). [Google Scholar]
- 21.Yang D. L., Su F., The politics of famine and reform in rural China. China Econ. Rev. 9, 141–155 (1998). [Google Scholar]
- 22.Xu H., Li L., Zhang Z., Liu J., Is natural experiment a cure? Re-examining the long-term health effects of China’s 1959-1961 famine. Soc. Sci. Med. 148, 110–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T.et al., Evidence for heterogeneity in China’s progress against pulmonary tuberculosis: Uneven reductions in a major center of ongoing transmission, 2005-2017. BMC Infect. Dis. 19, 615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.et al., Tuberculosis prevalence in China, 1990-2010; A longitudinal analysis of national survey data. Lancet 383, 2057–2064 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Peng X., Demographic consequences of the great leap forward in China’s provinces. Popul. Dev. Rev. 13, 639–670 (1987). [Google Scholar]
- 26.Chang G. H., Wen G. J., Communal dining and the Chinese famine of 1958–1961. Econ. Dev. Cult. Change 46, 1–34 (1997). [Google Scholar]
- 27.Cao S., Mortality in Sichuan during 1958-1962. Chin. J. Popul. Sci. 2004, 57–67 (2004). [Google Scholar]
- 28.Yang Y., Land K. C., Age-Period-Cohort Analysis: New Models, Methods, and Empirical Applications, (Chapman and Hall/CRC, 2016). [Google Scholar]
- 29.Fosse E., Winship C., Analyzing age-period-cohort data: A review and critique. Annu. Rev. Sociol. 45, 467–492 (2019). [Google Scholar]
- 30.Vasto S., Malavolta M., Pawelec G., Age and immunity. Immun. Ageing 3, 2 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettifor A. E., van der Straten A., Dunbar M. S., Shiboski S. C., Padian N. S., Early age of first sex: A risk factor for HIV infection among women in Zimbabwe. AIDS 18, 1435–1442 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Niccoli T., Partridge L., Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Liu J., Chin D. P., Progress in tuberculosis control and the evolving public-health system in China. Lancet 369, 691–696 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X. Y., Zhang N. M., Diao X., Mao X., Li Y. P., Epidemiological analysis of pulmonary tuberculosis in Sichuan Province, China, 2000-2006. Int. J. Infect. Dis. 12, 534–541 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Sichuan Population Census Office , Tabulation on the 2000 Population Census of Sichuan Province, (China Statistics Press, Beijing, 2002). [Google Scholar]
- 36.Kimbrough W., Saliba V., Dahab M., Haskew C., Checchi F., The burden of tuberculosis in crisis-affected populations: A systematic review. Lancet Infect. Dis. 12, 950–965 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Harding R., Maritz G., Maternal and fetal origins of lung disease in adulthood. Semin. Fetal. Neonatal. Med. 17, 67–72 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Stein A. D., Lumey L. H., The relationship between maternal and offspring birth weights after maternal prenatal famine exposure: The Dutch famine birth cohort study. Hum. Biol. 72, 641–654 (2000). [PubMed] [Google Scholar]
- 39.Coutinho R., David R. J., Collins J. W. Jr., Relation of parental birth weights to infant birth weight among African Americans and whites in Illinois: A transgenerational study. Am. J. Epidemiol. 146, 804–809 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Raqib R.et al., Low birth weight is associated with altered immune function in rural Bangladeshi children: A birth cohort study. Am. J. Clin. Nutr. 85, 845–852 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Heijmans B. T.et al., Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. U.S.A. 105, 17046–17049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu W., A Practical Guide to Age-Period-Cohort Analysis: The Identification Problem and Beyond, (Chapman and Hall/CRC, 2018). [Google Scholar]
- 43.Hasell J., Famines. https://ourworldindata.org/famines. Accessed 27 July 2020.
- 44.Food and Agriculture Organization of the United Nations , World Food Programme, FAO-WFP early warning analysis of acute food insecurity hotspots. 10.4060/cb0258en. Accessed 6 October 2020. [DOI]
- 45.Sichuan Statistical Bureau , Sichuan Statistical Yearbook 2006-2018, (China Statistics Press, Beijing, 2018). [Google Scholar]
- 46.Whelpton P. K., An empirical method of calculating future population. J. Am. Stat. Assoc. 31, 457–473 (1936). [Google Scholar]
- 47.Sichuan Population Census Office , Tabulation on the 2010 Population Census of Sichuan Province, (China Statistics Press, Beijing, 2012). [Google Scholar]
- 48.Xu H., Zhang Z., Li L., Liu J., Early life exposure to China’s 1959-61 famine and midlife cognition. Int. J. Epidemiol. 47, 109–120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu Y.-K., Krämer N., Lee W. C., Addressing the identification problem in age-period-cohort analysis: A tutorial on the use of partial least squares and principal components analysis. Epidemiology 23, 583–593 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Smith T. R., Wakefield J., A review and comparison of age–period–cohort models for cancer incidence. Stat. Sci. 31, 591–610 (2016). [Google Scholar]
- 51.Fu W., Constrained estimators and consistency of a regression model on a lexis diagram. J. Am. Stat. Assoc. 111, 180–199 (2016). [Google Scholar]
- 52.Keyes K. M.et al., Joint effects of age, period, and cohort on conduct problems among American adolescents from 1991 through 2015. Am. J. Epidemiol. 187, 548–557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viechtbauer W., Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code necessary to reproduce the findings of this study are available at the Github repository https://github.com/qu-cheng/TB_famine.