Significance
The obstacles limiting the practical applications of promising titanium carbide MXene macroscopic sheets are poor mechanical and oxidation-resistant properties. Herein, we demonstrate strong and highly electrically conductive MXene sheets through sequential bridging of hydrogen and ionic bonding, also achieving high shielding efficiency and excellent fatigue and oxidation resistance. The synergistic strengthening and toughening mechanism was thoroughly revealed by experiments and molecular dynamics simulations. The proposed sequential bridging strategy in this article provides an avenue for assembling high-performance MXene materials having potential applications in flexible electronic devices and aerospace in the near future.
Keywords: MXene, interface interactions, mechanical properties, electromagnetic interference shielding
Abstract
Titanium carbide (Ti3C2Tx) MXene has great potential for use in aerospace and flexible electronics due to its excellent electrical conductivity and mechanical properties. However, the assembly of MXene nanosheets into macroscopic high-performance nanocomposites is challenging, limiting MXene’s practical applications. Here we describe our work fabricating strong and highly conductive MXene sheets through sequential bridging of hydrogen and ionic bonding. The ionic bonding agent decreases interplanar spacing and increases MXene nanosheet alignment, while the hydrogen bonding agent increases interplanar spacing and decreases MXene nanosheet alignment. Successive application of hydrogen and ionic bonding agents optimizes toughness, tensile strength, oxidation resistance in a humid environment, and resistance to sonication disintegration and mechanical abuse. The tensile strength of these MXene sheets reaches up to 436 MPa. The electrical conductivity and weight-normalized shielding efficiency are also as high as 2,988 S/cm and 58,929 dB∙cm2/g, respectively. The toughening and strengthening mechanisms are revealed by molecular-dynamics simulations. Our sequential bridging strategy opens an avenue for the assembly of other high-performance MXene nanocomposites.
Titanium carbide (Ti3C2Tx) MXene, an emerging two-dimensional transition-metal carbide, is a promising building block for constructing functional materials used in flexible electronic devices and aerospace (1–9), such as supercapacitors (10–13), lithium-ion batteries (14), and electromagnetic interference (EMI) shielding (15, 16), among many others, due to its excellent mechanical and electrical properties (17, 18). The surface polar functional groups (Tx), such as −F, =O, and −OH, enable MXene nanosheets to be easily bridged by hydrogen (12, 16, 19–23), ionic (24–27), and covalent bonding (28, 29). Numerous efforts have been made to assemble MXene nanosheets, which were discovered in 2011 (30), into macroscopic high-performance MXene-based nanocomposites through various interfacial interactions (3, 4). For example, various polymers or nanomaterials with oxygen-containing polar groups, such as polyvinyl alcohol (PVA) (12), chitosan (31), sodium alginate (SA) (16), poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) (19), cellulose nanofiber (CNF) (10, 20, 22), aramid nanofiber (ANF) (21, 23), montmorillonite (32), and graphene oxide (33), were introduced to MXene interlayers, improving the mechanical performances of MXene sheets by hydrogen bonding. Liu et al. (27) used ionic cross-linking to strengthen MXene sheets. Shen et al. (29) covalently bridged adjacent MXene platelets to improve the adhesive force and stiffness of MXene sheets. However, these strategies usually caused only slight improvement in mechanical properties of MXene-based sheets at the expense of substantial decrease in electrical conductivity, limiting their practical applications. For example, the MXene-PVA sheets have a moderate tensile strength of 91 MPa but an ultralow electrical conductivity of 0.0004 S/cm (12).
Recently a proton acid colloidal processing approach (34) was demonstrated to promote both the electrical and mechanical properties of MXene sheets, but the achieved tensile strength (102 MPa) is much lower than for the previously reported strongest MXene-CNF sheets (341 MPa) (10) due to weak intersheet connectivity. Thus, despite some progress, it is still very challenging to integrate excellent mechanical and electrical properties in MXene-based sheets.
Herein, we report the construction of high-performance MXene-based sheets through a sequential bridging process, in which the MXene nanosheets are first bridged with SA by hydrogen bonding, and then the resulting hybrid MXene-SA building blocks are bridged with calcium ion (Ca2+) by ionic bonding. The resultant sequentially bridged MXene (SBM) sheets have a high in-plane tensile strength of 436 MPa, an excellent toughness of 8.39 MJ/m3, and a good Young’s modulus of 14.0 GPa, which are 6.9, 13.5, and 2.5 times those for pure MXene sheets, respectively. Additionally, the SBM sheets exhibit a high electrical conductivity (2,988 S/cm) and an extraordinary weight-normalized EMI shielding effectiveness (SE) (58,929 dB∙cm2/g). The fatigue life of the SBM sheets is more than 2 × 105 times at a maximum tensile stress level of 245 MPa. The SBM sheets can retain 78.5% of their original electrical conductivity and 87.2% of their original tensile strength after 100 cycles of 360° folding. They also show outstanding long-term electrical stability in a humid environment. Molecular-dynamics (MD) simulations reveal that the simultaneous improvement in tensile strength and toughness is due to efficient load transfer and large sliding of MXene nanosheets.
Preparation of the SBM Sheets
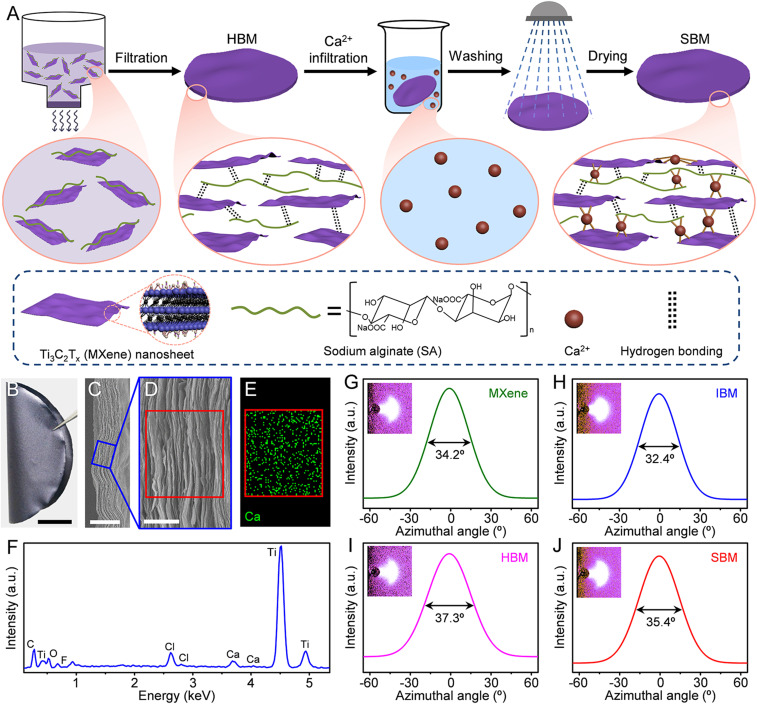
SA, an environmentally friendly biopolymer, is an ideal hydrogen-bonding agent due to its numerous oxygen-containing polar groups (−OH, −COO−, and =O). MXene (Ti3C2Tx) nanosheets, having an average lateral size of 2.3 μm (SI Appendix, Fig. S1) and a thickness of about 1.5 nm (SI Appendix, Fig. S2 A and B), were prepared by selectively etching the Al element from the Ti3AlC2 MAX phase (the precursor for Ti3C2Tx MXene) using lithium fluoride/hydrochloric acid (LiF/HCl) solution (35). Fig. 1A illustrates the fabrication process of the SBM sheets. First, an SA solution was added dropwise into the MXene suspension under continuous stirring, resulting in a homogeneous dispersion of MXene-SA hybrid building blocks (SI Appendix, Fig. S3). Subsequently, the MXene-SA dispersion was vacuum filtered to form a hydrogen bonding-bridged MXene (HBM) sheet. Finally, the HBM sheet was immersed into calcium chloride (CaCl2) solution and then washed using deionized water (DIW) and dried to produce an SBM sheet.
Fig. 1.
Fabrication and structural characterization of the SBM sheets. (A) The MXene-SA hybrid building blocks were first assembled into an HBM sheet by vacuum filtration. The Ca2+ was then infiltrated into the HBM sheet, resulting in an SBM sheet. (B) Photograph of an SBM sheet displaying its flexibility. (C) Low-resolution SEM image of the fracture surface of the SBM sheet. (D) High-resolution SEM image of the area outlined in C. (E) EDS mapping of Ca2+ and (F) EDS spectrum of the area outlined in D. WAXS patterns for an incident Cu-Kα X-ray beam parallel to the sheet plane and corresponding azimuthal scan profiles for the 002 peak for the (G) MXene, (H) IBM, (I) HBM, and (J) SBM sheets. [Scale bars: (B) 1 cm, (C) 5 μm, and (D) 1 μm.]
Five types of HBM sheets, from HBM-I to HBM-V with increasing SA contents, were fabricated to optimize their mechanical properties. The results indicated that the HBM-IV sheets (with SA content of 20 wt %) have optimal mechanical properties. The exact composition has been confirmed by thermogravimetric analysis (TGA, SI Appendix, Fig. S4). The ratio of hydrogen bonding to ionic bonding can be optimized by adjusting the concentration of CaCl2 solution. We constructed four types of SBM sheets, SBM-I to SBM-IV, having increasing Ca2+ content. All of the SBM sheets have a constant SA addition of 20 wt %, which corresponds to that of the optimized HBM sheets. For comparison, the ionic bonding-bridged MXene (IBM) sheets were fabricated by removing the addition of SA. The actual weight fraction of Ca2+ and SA (SI Appendix, Table S1) in these bridged MXene sheets can be obtained by TGA (SI Appendix, Fig. S4) and energy-dispersive X-ray spectroscopy (EDS). In the following sections, unless otherwise noted, optimized results for the SBM sheets are discussed and compared with those for the optimized process using alternative fabrication methods.
Structural Characterization of the SBM Sheets
A resultant SBM sheet (containing 17.8 wt % SA and 1.24 wt % Ca2+, Fig. 1B) shows excellent flexibility, which is similar to previously reported MXene-polymer composites (12) and can accommodate complex folding into a paper crane without any breakage (SI Appendix, Fig. S5). The cross-sectional scanning electron microscope (SEM) images (Fig. 1 C and D) of the SBM sheets show a layered structure, which is analogous to that for MXene sheets and other bridged MXene sheets (SI Appendix, Fig. S6). Additionally, the EDS results (Fig. 1 E and F) of the fracture surface of the SBM sheets demonstrate the uniform infiltration of Ca2+ into the sheets. Furthermore, the orientation degree of MXene nanosheets is characterized by wide-angle X-ray scattering (WAXS) patterns (Fig. 1 G–J and SI Appendix, Fig. S7), which derived from the diffraction of an incident X-ray beam parallel to the surface of sheet. The IBM sheets (containing 0.61 wt % Ca2+, 32.4°) have a narrower full width at half maximum (FWHM) obtained from azimuthal scans of the 002 peak than do the MXene sheets (34.2°), indicating that ionic bonding can effectively improve the alignment of MXene nanosheets. The ionic bonding is formed from the electrostatic attraction between calcium ions and the polar groups on the surface of MXene nanosheets. Thus, the MXene nanosheets might rearrange due to electrostatic attraction in the process of ionic bonding formation, resulting in alignment improvement. By contrast, the HBM sheets (containing 18.4 wt % SA, 37.3°) have a wider FWHM than do the MXene sheets, indicating that the hydrogen bonding decreases the alignment of MXene nanosheets. The FWHM for the SBM sheets (35.4°) is slightly wider than for the MXene sheets, but much narrower than for the HBM sheets.
X-ray diffraction (XRD, SI Appendix, Fig. S8A) results show that the 104 peak of Ti3AlC2 MAX (∼39°) is missing in the Ti3C2Tx MXene, indicating successful elimination of the Al element in the process of etching (29, 30). The element Al is also absent in the EDS spectrum of the SBM sheets (Fig. 1F), further verifying the elimination of Al layers. Additionally, the 002 peak was downshifted from 9.50° for Ti3AlC2 MAX to 6.88° for Ti3C2Tx MXene, which is due to the introduction of functional groups and water expanding the interplanar spacing of the MXene sheets (29).
The thickness of MXene-SA hybrid building blocks (2 nm, SI Appendix, Fig. S2) is larger than for MXene nanosheets (1.5 nm), indicating the coating of SA molecules on the MXene surface. Additionally, the interplanar spacing of the HBM sheets (1.41 nm, Fig. 2A and SI Appendix, Fig. S8 and Table S2) is longer than for the MXene sheets (1.29 nm), confirming the insertion of SA molecules into MXene interlayers. By contrast, the interplanar spacing of the IBM sheets (1.20 nm) is shorter than for the MXene sheets. This results from the electrostatic attraction between the negatively charged Ti3C2Tx MXene nanosheets and the inserted interlayer Ca2+, which is consistent with previous reports (36, 37). Similarly, the SBM sheets (1.34 nm) have a shorter interplanar spacing than do the HBM sheets. In addition, further increasing the Ca2+ content causes the slight increase of interplanar spacing, such as that shown in IBM-IV (1.23 nm) and SBM-IV (1.35 nm) sheets. This is likely because the excess Ca2+ cannot effectively improve the electrostatic attraction with MXene nanosheets and in turn acts as the intercalating impurities to expand the interplanar spacing (38, 39).
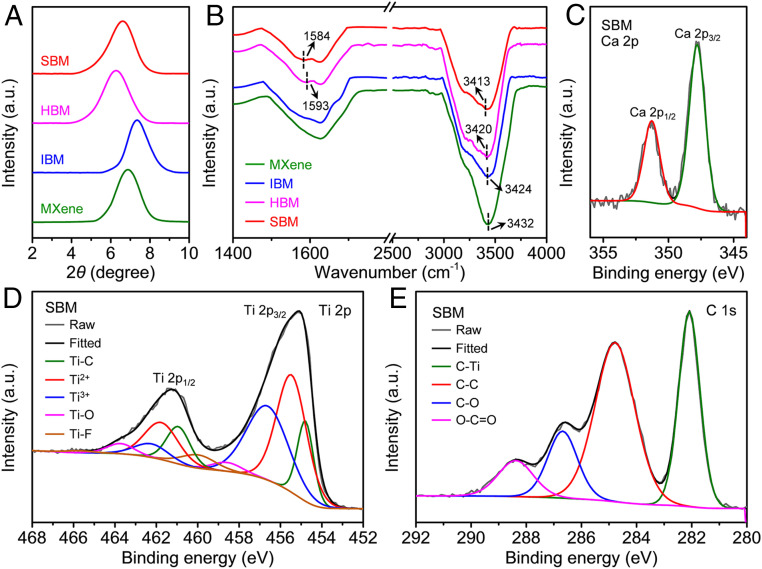
Fig. 2.
XRD and spectra characterization of the SBM sheets. (A) XRD patterns for the MXene, IBM, HBM, and SBM sheets. Compared with the MXene sheets, the IBM sheets have a shorter interlayer spacing, while the HBM sheets have a longer interlayer spacing. (B) FTIR spectra for the MXene, IBM, HBM, and SBM sheets. The –OH peak is redshifted from 3,432 cm−1 for the MXene sheets to 3,424 cm−1 for the IBM sheets and 3,420 cm−1 for the HBM sheets, verifying the ionic bonding and hydrogen bonding, respectively. The redshifted −COO− peak demonstrates the coordination between Ca2+ and −COO− groups. XPS spectra for (C) Ca 2p, (D) Ti 2p, and (E) C 1s of the SBM sheets. The peaks around 347.8 and 351.3 eV indicate the presence of Ca2+. The main Ti 2p3/2 peak can be fitted by five peaks at 454.9, 455.5, 456.8, 458.8, and 460.3 eV, corresponding to Ti−C, Ti2+, Ti3+, Ti−O, and Ti−F, respectively. The C 1s peak can be fitted by four peaks at 282.2, 284.8, 286.6, and 288.4 eV, corresponding to C−Ti, C−C, C−O, and O−C = O, respectively.
Fourier transform infrared (FTIR, Fig. 2B) spectra exhibit that compared with the MXene sheets, the HBM sheets have a new absorption peak (1,593 cm−1) for the antisymmetric stretching vibration mode of −COO−, indicating the presence of SA molecules (40). Additionally, the peak for stretching vibration of –OH is redshifted from 3,432 cm−1 for the MXene sheets to 3,420 cm−1 for the HBM sheets, demonstrating the hydrogen bonding between SA molecules and MXene nanosheets (25). Furthermore, compared with the MXene sheets, the –OH peak of the IBM sheets is weaker and redshifted to 3,424 cm−1, which indicates the possible formation of H–O→Ca2+ coordination (24, 25, 41). This peak change is also observed in the SBM sheets. Moreover, the −COO− peak is redshifted from 1,593 cm−1 for the HBM sheets to 1,584 cm−1 for the SBM sheets, verifying the coordination between Ca2+ and −COO− groups of SA molecules (40). Due to the strong ionic bonding, flocculent precipitate occurs once the Ca2+ is added into the MXene or MXene-SA dispersion (SI Appendix, Fig. S3).
X-ray photoelectron spectroscopy (XPS, Fig. 2C and SI Appendix, Fig. S9) spectra show that compared with the MXene and HBM sheets, the SBM sheets have new peaks for Ca 2p1/2 (351.3 eV) and Ca 2p3/2 (347.8 eV) (42), which are also observed in the IBM sheets. This further confirms the ionic modification. Additionally, the Ti2+ 2p3/2 and Ti3+ 2p3/2 peaks are slightly downshifted from 455.8 and 457.1 eV for the MXene and HBM sheets to 455.5 and 456.8 eV for the IBM and SBM sheets (Fig. 2D and SI Appendix, Fig. S9), respectively. This is because the Ca atoms ionically bonded onto MXene nanosheets have lower electronegativity than do Ti atoms, increasing the electron cloud density of Ti atoms. The HBM and SBM sheets have stronger C−O and O−C = O peaks than the MXene and IBM sheets (Fig. 2E and SI Appendix, Fig. S9), which results from the introduction of SA molecules. Moreover, the O−C = O peak is shifted from 288.9 eV for the HBM sheets to 288.4 eV for the SBM sheets, demonstrating the coordination between Ca2+ and −COO− (41).
Mechanical and Electrical Performances of the SBM Sheets
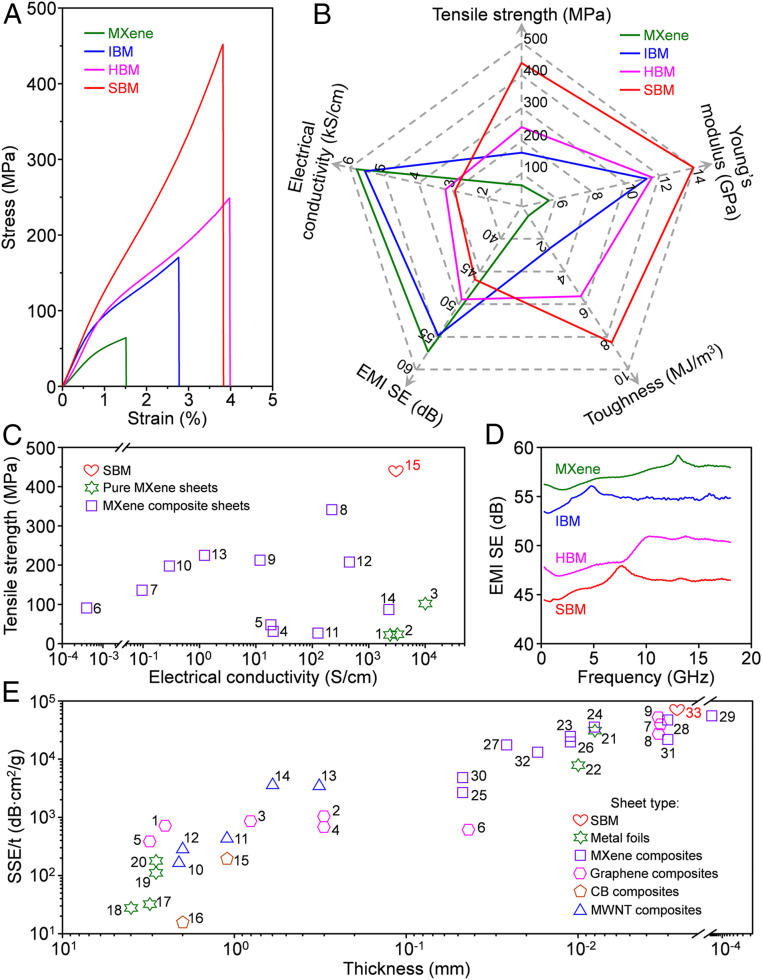
The representative tensile stress–strain curves for the MXene, IBM, HBM, and SBM sheets are exhibited in Fig. 3A and SI Appendix, Fig. S10. The SBM sheets have a tensile strength of 436 ± 15 MPa, a toughness of 8.39 ± 0.35 MJ/m3, and a Young’s modulus of 14.0 ± 0.2 GPa (Fig. 3B and SI Appendix, Table S3), which are 1.8, 1.5, and 1.2 times higher than those for the HBM sheets (241 ± 10 MPa, 5.54 ± 0.08 MJ/m3, and 11.6 ± 1.1 GPa); 2.7, 3.2, and 1.2 times higher than those for the IBM sheets (161 ± 8 MPa, 2.65 ± 0.23 MJ/m3, and 11.3 ± 1.1 GPa); and 6.9, 13.5, and 2.5 times higher than those for the MXene sheets (63 ± 1 MPa, 0.62 ± 0.04 MJ/m3, and 5.6 ± 0.6 GPa), respectively. Additionally, with the Ca2+ content increased from 0.37 to 1.24 wt %, the toughness and tensile strength of the SBM sheets are gradually increased from 6.32 ± 0.41 MJ/m3 and 303 ± 10 MPa to 8.39 ± 0.35 MJ/m3 and 436 ± 15 MPa, respectively, which is a consequence of increased intersheet ionic bridging. By contrast, with the Ca2+ content further increased to 2.38 wt %, the toughness and tensile strength of the SBM sheets go down to 7.03 ± 0.46 MJ/m3 and 367 ± 15 MPa, respectively. This is likely because excess Ca2+ increases the interplanar spacing and the highly cross-linked SA-Ca2+ chelate structure is slightly rigid, resulting in little plastic deformation (38), which is analogous to the mechanical deformation characteristics of the granules in the byssus cuticle of mussel (43).
Fig. 3.
Mechanical and electrical properties of the SBM sheets. (A) Typical stress–strain curves for the MXene, IBM, HBM, and SBM sheets. (B) A radial plot comparing the tensile strength, Young’s modulus, toughness, electrical conductivity, and EMI SE for the MXene, IBM, HBM, and SBM sheets. (C) Comparison of the tensile strength and electrical conductivity of the SBM sheets (red heart) with those of previously reported MXene sheets: pure MXene sheets (green hexagrams) and bridged MXene composite sheets (purple squares). The references associated with the sample numbers in this plot are in SI Appendix, Table S5. (D) EMI SE as a function of frequency for MXene, IBM, HBM, and SBM sheets having thicknesses of 3.2, 2.9, 2.9, and 2.8 μm, respectively. (E) Comparison of the relationship between weight-normalized shielding effectiveness (SSE/t) and sheet thickness for solid materials: SBM sheets (red heart), metal foils (green hexagrams), graphene composites (pink hexagons), CB composites (brown pentagons), MWNT composites (blue triangles), and previously reported MXene composites (purple squares). The references associated with the sample numbers in this plot are in SI Appendix, Table S7.
The MXene sheets have an electrical conductivity of 5,824 ± 42 S/cm (Fig. 3B and SI Appendix, Table S4), which is consistent with a previous report (11). Despite the interruption of electron transportation by intercalated SA molecules, the HBM sheets still provide a high electrical conductivity of 3,271 ± 45 S/cm. Additionally, due to ionic bridging-induced decrease in interplanar spacing and improvement in MXene nanosheet alignment, the electrical conductivities of the IBM (5,586 ± 42 S/cm) and SBM (2,988 ± 28 S/cm) sheets are close to those of the MXene and HBM sheets, respectively. The tensile strength of the SBM sheets is higher than that of most reported MXene sheets (Fig. 3C and SI Appendix, Table S5). Very recently, Zhang et al. (44) demonstrated the fabrication of strong MXene sheets having a tensile strength of 570 MPa using large MXene flakes. The use of larger MXene flakes of better quality might further improve the properties of the SBM sheets. Moreover, the electrical conductivity of the SBM sheets is superior to that of previously reported MXene composite sheets bridged through various interfacial interactions, such as MXene-CNF (10, 20, 22), MXene-PVA (12), MXene-ANF (21), MXene-PEDOT:PSS (19), and MXene-Al3+ (27), and even comparable to that of some pure MXene sheets (12).
Shahzad et al. (16) firstly demonstrated the excellent EMI shielding performance of Ti3C2Tx MXene and its composites with Na alginate binder, which have potential applications in flexible electronic devices and aerospace. Herein, due to their excellent electrical conductivity, the SBM sheets having a thickness of 2.8 μm also exhibit a high average EMI SE of 46.2 dB for the frequency ranging from 0.3 to 18 GHz (Fig. 3 B and D and SI Appendix, Table S6). The EMI SE of SBM sheets is slightly lower than for similarly thick HBM (49.3 dB and 2.9 μm), IBM (54.8 dB and 2.9 μm), and MXene (57.3 dB and 3.2 μm) sheets. Additionally, the main contribution to shielding in these sheets is from absorption (SI Appendix, Fig. S11), which is consistent with previous reports (16, 20, 21, 45).
For reliable comparisons of weight-sensitive shielding capabilities, the density-normalized shielding effectiveness (SSE) is divided by the thickness of the shield to provide SSE/t. This parameter indicates the effectiveness of a given weight coating on a surface for EMI shielding. Interestingly, the SSE/t value of the SBM sheets (58,929 dB∙cm2/g) is superior to that for most of solid shielding materials, such as metal foils and graphene, carbon black (CB), carbon multiwalled nanotubes (MWNT), and MXene composites (Fig. 3E and SI Appendix, Table S7). Nanometer-thin MXene sheets, which can be transferred onto an arbitrary substrate, have recently demonstrated higher SSE/t than the SBM sheets (15). In addition, although some low-density materials, such as graphene aerogel sheets (46–48), MXene foams (49), and MXene-nanocellulose aerogel (50), can provide a higher SSE/t than the SBM sheets, their mechanical strength is lower than that for the SBM sheets.
Toughening and Strengthening Mechanisms of the SBM Sheets
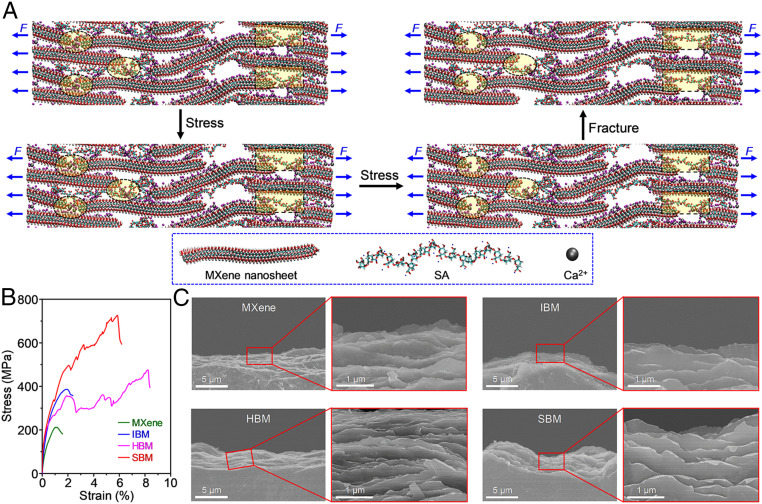
MD simulations were applied to uncover the fracture mechanism for the SBM sheets. Fig. 4A shows the model snapshots for the SBM sheets during simulative tensile stretching. Upon initial loading, the adjacent MXene nanosheets begin to slide each other. With continuous stretching, the coiled SA molecular chains (yellow-shaded rectangular region) are gradually stretched to supply enough slippage space for MXene nanosheets, during which the hydrogen bonding between SA molecules and MXene nanosheets is continuously broken and reformed and numerous energy is dissipated. Meanwhile, some ionic bonding (yellow-shaded elliptical region) begins to fracture. With the loading further increased, the SA molecular chains are sufficiently extended and then ionic bonding and hydrogen bonding are completely broken, leading to efficient load transfer and thereby high strength (51). By contrast, in the simulated fracture process, the HBM sheets show only the breakage of hydrogen bonding and stretching of coiled SA molecular chains, while the IBM sheets show only the breakage of ionic bonding, as shown in SI Appendix, Fig. S12. Additionally, the simulative fracture process of MXene sheets shows that upon stretching, the MXene nanosheets slide mutually only until fracture. Moreover, the simulative tensile mechanical properties (Fig. 4B) vary for different sheet types in the same order as do those measured in our experiments. The SEM images (Fig. 4C and SI Appendix, Fig. S13) of fracture surfaces exhibit curled morphology for the IBM, HBM, and SBM sheets, but not for the MXene sheets, which demonstrates the enhanced intersheet bonding in the bridged sheets.
Fig. 4.
Fracture mechanism of the SBM sheets. (A) Model snapshots for the SBM sheets during simulative tensile stretching showing not only the breakage of hydrogen bonding and stretching of coiled SA molecular chains (yellow-shaded rectangular region) but also the breakage of ionic bonding (yellow-shaded elliptical region). (B) Simulated stress–strain curves for the MXene, IBM, HBM, and SBM sheets. (C) Inclined-view SEM images of the fracture surface of the MXene, IBM, HBM, and SBM sheets, indicating curling of the platelet edges for the IBM, HBM, and SBM sheets but not for the MXene sheets.
Fatigue Resistance of the SBM Sheets to Repeated Severe Mechanical Deformations
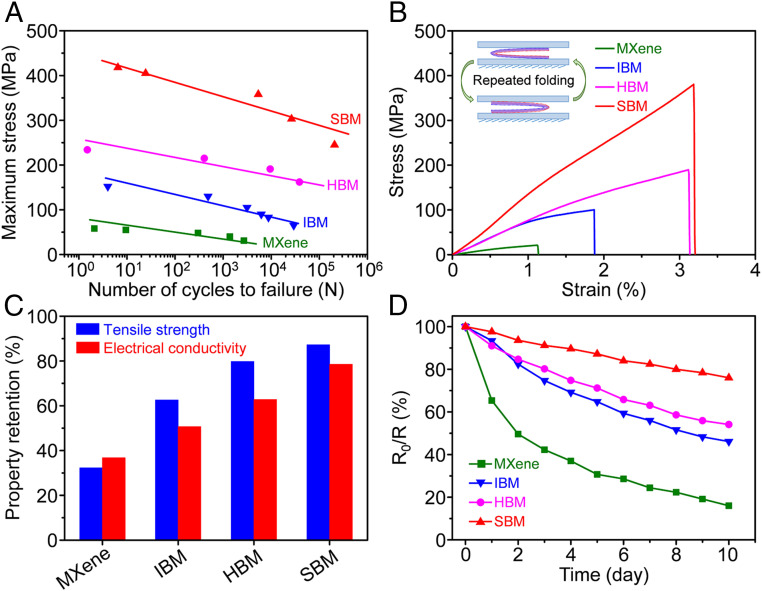
In addition to supplying excellent static electrical and mechanical performances, sequential intersheet bridging increases the fatigue resistance of the SBM sheets to both cyclic 360° folding and cyclic stretching. The relationship between cycle life and maximum applied stress is exhibited in Fig. 5A. The fatigue resistance to stretching is highest for the SBM sheets, whose cycle life is 207,742 cycles for a stress ranging from 215 to 245 MPa. Additionally, the fatigue resistance of the HBM sheets is higher than for either the IBM or MXene sheets. This is likely due to the ability of hydrogen bonding to break and reform in the process of cyclic stretching, thereby absorbing much more energy than ionic bonding. Given that toughness changes for the different sheet types in the identical sequence as does fatigue resistance, the same reason is likely suitable to sheet toughness.
Fig. 5.
Resistance of the SBM sheets to the mechanical damage from repetitive tensile stretching and folding and their long-term electrical stability in a humid environment. (A) Dependence of the number of cycles to failure on the maximum stress level for the MXene, IBM, HBM, and SBM sheets. (B) Typical stress–strain curves of the MXene, IBM, HBM, and SBM sheets after repeated folding for 100 cycles. (Inset) Schematic illustration of the repeated folding process. (C) Retention percentages for the strength and electrical conductivity of the MXene, IBM, HBM, and SBM sheets after repetitive folding for 100 cycles. (D) Change of electrical conductance as a function of time for the MXene, IBM, HBM, and SBM sheets stored in a 100% RH environment for 10 d. In the first day (d = 0), the sheets were in a dry state.
The SBM sheets also provide higher stability in electrical and mechanical performances in the process of cyclic 360° folding than do the HBM, IBM, and MXene sheets. Fig. 5B and SI Appendix, Fig. S14 show the stress–strain curves of these sheets, which were 360° folded for 100 cycles. The retention for electrical conductivity and tensile strength of repeatedly folded SBM sheets are 78.5 and 87.2%, respectively, which are higher than those of repeatedly folded HBM (62.8 and 79.7%), IBM (50.7 and 62.7%), and MXene (36.8 and 31.7%) sheets (Fig. 5C). The detailed electrical and mechanical properties for these repeatedly folded sheets are listed in SI Appendix, Table S8.
Long-Term Electrical Stability of the SBM Sheets in a Humid Environment
Compared with the MXene sheets, the conductance of IBM and HBM sheets decreases by smaller percentages during storage in a 100% relative humidity (RH) environment for 10 d (Fig. 5D). This is because the ionic bridging decreases the interplanar spacing of the IBM sheets, impeding the intercalation of H2O/O2, while the SA molecules are coated on the surface of MXene nanosheets within the HBM sheets, hindering the contact of H2O/O2 and MXene nanosheets. Moreover, the SBM sheets have the highest resistance to the oxidation-induced conductance degradation. More specifically, the SBM sheets, after being stored in a 100% RH environment for 10 d, provide a conductance retention percentage of 76.0%, which is higher than the percentages for the HBM (54.1%), IBM (46.1%), and MXene (16%) sheets. Additionally, the structural stability for different sheet types with respect to the mechanical abuse by sonication (100 W, 40 kHz) in water increases monotonically with increasing sheet strength, namely that SBM > HBM > IBM > MXene. As shown in SI Appendix, Fig. S15, the MXene, IBM, and HBM sheets begin to disintegrate after ultrasonication for 0.5, 5, and 30 min, respectively, while the SBM sheets are still intact after ultrasonication for 30 min.
Conclusion
In summary, we demonstrate that the sequential application of hydrogen and ionic bonding agents can optimize toughness, tensile strength, oxidation resistance in a humid environment, and resistance to sonication disintegration and mechanical abuse. The tensile strength of these MXene sheets reaches up to 436 MPa. For all of the differently bonded MXene sheets, these aforementioned properties increase as follows: SBM > HBM > IBM > MXene. Although both hydrogen and ionic bonding agents degrade electrical properties, the SBM sheets retain excellent electrical conductivity and EMI shielding capability. Additionally, since ionic bridging can decrease interplanar spacing and improve MXene nanosheet alignment, the ionic bonding agent is much more beneficial to maintain the high electrical performances of MXene sheets than the hydrogen bonding agent. Moreover, MD simulations reveal the effectiveness of hydrogen and ionic bonding agents in synergistically transferring local stress and providing large slippage space for MXene nanosheets, inspiring to integrate both high strength and toughness to traditional composites (51). Our sequential bridging process can potentially be used to assemble other MXene nanosheets into high-performance MXene composites with potential applications in flexible electronic devices and aerospace.
Materials and Methods
LiF (≥99.99%) and SA (200 ± 20 mPa∙s) were received from Shanghai Macklin Biochemical Co., Ltd. HCl (36∼38%) was purchased from Sinopharm Chemical Reagents Co., Ltd. Calcium chloride anhydrous (CaCl2, 99%) was provided by Adamas-beta. These reagents were applied as received without additional purification. DIW (resistivity >18 MΩ∙cm) was obtained from a Milli-Q Biocel system. Ti3C2Tx nanosheets were exfoliated from Ti3AlC2 based on the minimally intensive layer delamination method (35). Some simulation experiments in this work are carried out on the High Performance Computing Platform at Beihang University. More details on the materials and methods can be found in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the Excellent Young Scientist Foundation of National Natural Science Foundation of China (Grant 51522301), the National Natural Science Foundation of China (Grants 22075009, 51961130388, 21875010, 51103004, and 52003011), Newton Advanced Fellowship (Grant NAF\R1\191235), Beijing Natural Science Foundation (Grant JQ19006), the 111 Project (Grant B14009), the National Postdoctoral Program for Innovative Talents (Grant BX20200038), the China Postdoctoral Science Foundation (Grant 2019M660387), the Postdoctoral Research Program on Innovative Practice in Jiangmen, Excellent Sino-Foreign Young Scientist Exchange Program of China Association for Science and Technology, and the National Training Program on Innovation and Entrepreneurship of China for Undergraduates (Grant 201910006167).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009432117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Naguib M., Mochalin V. N., Barsoum M. W., Gogotsi Y., 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 26, 992–1005 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Anasori B., Lukatskaya M. R., Gogotsi Y.,2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017). [Google Scholar]
- 3.Wu Z., Shang T., Deng Y., Tao Y., Yang Q.-H., The assembly of MXenes from 2D to 3D. Adv. Sci. 7, 1903077 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao L., et al., MXene/polymer membranes: Synthesis, properties, and emerging applications. Chem. Mater. 32, 1703–1747 (2020). [Google Scholar]
- 5.Luo J., Gao J., Wang A., Huang J., Bulk nanostructured materials based on two-dimensional building blocks: A roadmap. ACS Nano 9, 9432–9436 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Brar V. W., Koltonow A. R., Huang J., New discoveries and opportunities from two-dimensional materials. ACS Photonics 4, 407–411 (2017). [Google Scholar]
- 7.Zhang X., et al., Ultrathin nanosheets of MAX phases with enhanced thermal and mechanical properties in polymeric compositions: Ti3Si(0.75)Al(0.25)C2. Angew. Chem. Int. Ed. Engl. 52, 4361–4365 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Li S., et al., Ultrathin MXene nanosheets with rich fluorine termination groups realizing efficient electrocatalytic hydrogen evolution. Nano Energy 47, 512–518 (2018). [Google Scholar]
- 9.Verger L., et al., Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 23, 149–163 (2019). [Google Scholar]
- 10.Tian W., et al., Multifunctional nanocomposites with high strength and capacitance using 2D MXene and 1D nanocellulose. Adv. Mater. 31, e1902977 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Zhang C. J., et al., Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 29, 1702678 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Ling Z., et al., Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. U.S.A. 111, 16676–16681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukatskaya M. R., et al., Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Zhang C., et al., Layered orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx hierarchical composites for high performance Li-ion batteries. Adv. Funct. Mater. 26, 4143–4151 (2016). [Google Scholar]
- 15.Yun T., et al., Electromagnetic shielding of monolayer MXene assemblies. Adv. Mater. 32, e1906769 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Shahzad F., et al., Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 353, 1137–1140 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Miranda A., Halim J., Barsoum M., Lorke A., Electronic properties of freestanding Ti3C2Tx MXene monolayers. Appl. Phys. Lett. 108, 033102 (2016). [Google Scholar]
- 18.Lipatov A., et al., Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 4, eaat0491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R., et al., Ultrathin biomimetic polymeric Ti3C2Tx MXene composite films for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 10, 44787–44795 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Cao W.-T., et al., Binary strengthening and toughening of MXene/cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 12, 4583–4593 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Xie F., et al., Ultrathin MXene/aramid nanofiber composite paper with excellent mechanical properties for efficient electromagnetic interference shielding. Nanoscale 11, 23382–23391 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Zhan Z., Song Q., Zhou Z., Lu C., Ultrastrong and conductive MXene/cellulose nanofiber films enhanced by hierarchical nano-architecture and interfacial interaction for flexible electromagnetic interference shielding. J. Mater. Chem. C Mater. Opt. Electron. Devices 7, 9820–9829 (2019). [Google Scholar]
- 23.Zhang Z., et al., Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 10, 2920 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng Y., et al., Fast gelation of Ti3C2Tx MXene initiated by metal ions. Adv. Mater. 31, 1902432 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Shi X., et al., Bioinspired ultrasensitive and stretchable MXene-based strain sensor via nacre-mimetic microscale “brick-and-mortar” architecture. ACS Nano 13, 649–659 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Ding L., et al., Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 3, 296–302 (2020). [Google Scholar]
- 27.Liu Z., et al., Electrically conductive aluminum ion-reinforced MXene films for efficient electromagnetic interference shielding. J. Mater. Chem. C Mater. Opt. Electron. Devices 8, 1673–1678 (2020). [Google Scholar]
- 28.Shang T., et al., 3D macroscopic architectures from self-assembled MXene hydrogels. Adv. Funct. Mater. 29, 1903960 (2019). [Google Scholar]
- 29.Shen J., et al., 2D MXene nanofilms with tunable gas transport channels. Adv. Funct. Mater. 28, 1801511 (2018). [Google Scholar]
- 30.Naguib M., et al., Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv. Mater. 23, 4248–4253 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Hu C., et al., Characteristics of Ti3C2X-chitosan films with enhanced mechanical properties. Front. Energy Res. 4, 41 (2017). [Google Scholar]
- 32.Lipton J., et al., Mechanically strong and electrically conductive multilayer MXene nanocomposites. Nanoscale 11, 20295–20300 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Liu J., et al., Ultrastrong and highly conductive MXene-based films for high-performance electromagnetic interference shielding. Adv. Electron. Mater. 6, 1901094 (2020). [Google Scholar]
- 34.Chen H., et al., Pristine titanium carbide MXene films with environmentally stable conductivity and superior mechanical strength. Adv. Funct. Mater. 30, 1906996 (2020). [Google Scholar]
- 35.Alhabeb M., et al., Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 29, 7633–7644 (2017). [Google Scholar]
- 36.Ren C. E., et al., Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes. J. Phys. Chem. Lett. 6, 4026–4031 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Come J., et al., Controlling the actuation properties of MXene paper electrodes upon cation intercalation. Nano Energy 17, 27–35 (2015). [Google Scholar]
- 38.Wan S., Xu F., Jiang L., Cheng Q., Superior fatigue resistant bioinspired graphene-based nanocomposite via synergistic interfacial interactions. Adv. Funct. Mater. 27, 1605636 (2017). [Google Scholar]
- 39.Wan S., Fang S., Jiang L., Cheng Q., Baughman R. H., Strong, conductive, foldable graphene sheets by sequential ionic and π bridging. Adv. Mater. 30, e1802733 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Lawrie G., et al., Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 8, 2533–2541 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Park S., et al., Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2, 572–578 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Hanawa T., Ota M., Characterization of surface film formed on titanium in electrolyte using XPS. Appl. Surf. Sci. 55, 269–276 (1992). [Google Scholar]
- 43.Harrington M. J., Masic A., Holten-Andersen N., Waite J. H., Fratzl P., Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 328, 216–220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., et al., Scalable manufacturing of free-standing, strong Ti3C2Tx MXene films with outstanding conductivity. Adv. Mater. 32, 2001093 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Wei Q., et al., Superhigh electromagnetic interference shielding of ultrathin aligned pristine graphene nanosheets film. Adv. Mater. 32, e1907411 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Xi J., et al., Graphene aerogel films with expansion enhancement effect of high-performance electromagnetic interference shielding. Carbon 135, 44–51 (2018). [Google Scholar]
- 47.Zhou T., et al., Second time-scale synthesis of high-quality graphite films by quenching for effective electromagnetic interference shielding. ACS Nano 14, 3121–3128 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Xu C., Ma C., Ren W., Cheng H.-M., Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 25, 1296–1300 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Liu J., et al., Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 29, 1702367 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Zeng Z., et al., Nanocellulose-MXene biomimetic aerogels with orientation-tunable electromagnetic interference shielding performance. Adv. Sci. 7, 2000979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan S., et al., Ultrastrong graphene films via long-chain π-bridging. Matter 1, 389–401 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.