Significance
DOT1L is a histone methyltransferase that catalyzes histone H3K79 methylation, a chromatin modification that is correlated with active transcription. Inhibition of DOT1L’s catalytic activity has been studied for cancer therapy; however, the mechanisms underlying its function in development and cancer pathogenesis remain elusive. To understand the catalytic-dependent and catalytic-independent functions of DOT1L, we generated catalytic dead and null DOT1L embryonic stem cells and found that DOT1L, but not its catalytic activity, is required for establishing the proper expression signature of neural progenitor cells, suggesting that DOTlL has biological functions that are independent of its methyltransferase activity. We propose that the loss/degradation of DOT1L could be beneficial for cancer therapeutics.
Keywords: transcription, chromatin, gene expression, epigenetics, cancer
Abstract
Actively transcribed genes in mammals are decorated by H3K79 methylation, which is correlated with transcription levels and is catalyzed by the histone methyltransferase DOT1L. DOT1L is required for mammalian development, and the inhibition of its catalytic activity has been extensively studied for cancer therapy; however, the mechanisms underlying DOT1L’s functions in normal development and cancer pathogenesis remain elusive. To dissect the relationship between H3K79 methylation, cellular differentiation, and transcription regulation, we systematically examined the role of DOT1L and its catalytic activity in embryonic stem cells (ESCs). DOT1L is dispensable for ESC self-renewal but is required for establishing the proper expression signature of neural progenitor cells, while catalytic inactivation of DOT1L has a lesser effect. Furthermore, DOT1L loss, rather than its catalytic inactivation, causes defects in glial cell specification. Although DOT1L loss by itself has no major defect in transcription elongation, transcription elongation defects seen with the super elongation complex inhibitor KL-2 are exacerbated in DOT1L knockout cells, but not in catalytically dead DOT1L cells, revealing a role of DOT1L in promoting productive transcription elongation that is independent of H3K79 methylation. Taken together, our study reveals a catalytic-independent role of DOT1L in modulating cell-fate determination and in transcriptional elongation control.
Epigenetic landscapes of higher eukaryotes are implicated in transcription modulation, cell-fate determination, and diseases such as cancer. Posttranslational modifications of core histone tails have been intensely investigated for the past few decades while the modifications of histone cores are relatively understudied. Lysine 79 methylation of histone H3 (H3K79) was the first core histone modification identified outside the histone tail (1–4), the distribution of which is highly correlated with actively transcribed genes (5–8). DOT1L, the only enzyme catalyzing mono, di-, and trimethylation of H3K79 in metazoans, plays a critical role in embryogenesis and leukemia transformation (9–11), suggesting a potential function of H3K79 methylation in normal development and human disorders.
DOT1L null mouse embryos die around 10.5 d post coitum with developmental arrest and cardiac dilation, while DOT1L null embryonic stem cells (ESCs) cultured in serum-containing media maintain self-renewal capability despite having elongated telomeres and reduced proliferation (9). An independent study showed that DOT1L knockdown by short hairpin RNA led to significant proliferation defects of ESCs only under differentiation conditions (12), supporting the notion that DOT1L facilitates cellular differentiation. Furthermore, DOT1L inhibition enhanced the conversion efficiency of induced pluripotent stem cells from fibroblasts (13), suggesting that DOT1L and H3K79 methylation are major roadblocks of somatic cell reprogramming.
Unlike other known lysine methyltransferases, DOT1L lacks a SET domain and is structurally more similar to arginine methyltransferases (14–16). DOT1L is the core component of the DOT1-containing multisubunit complex named DotCom that includes the MLL translocation partners AF10, AF17, AF9, and ENL (17). AF9 and ENL are also subunits—along with additional MLL translocation partners AFF1, AFF4, and ELL—of the super elongation complex (SEC).
SEC is a positive transcription elongation factor (P-TEFb)-containing complex that is required for rapid transcriptional induction and expression of key leukemia genes (18–20). The majority of P-TEFb in cells is sequestered by HEXIM1 and HEXIM2 in the 7SK small nuclear RNA-containing complex (21, 22). Once released from the 7SK-P-TEFb complex, P-TEFb can associate with SEC or BRD4 to form active complexes, phosphorylate the serine 2 residues of the RNA Polymerase II C-terminal domain (Pol II CTD), and facilitate the release of paused Pol II into gene bodies (20, 23, 24). Multiple lines of evidence have pointed to a critical role for SEC in human diseases including MLL-rearranged leukemia (19, 25), HIV (26, 27), and high-MYC–expressing solid tumors (24, 28). The existence of common subunits between SEC and DotCom, together with the observation that the bodies of active genes are decorated by H3K79 methylation, suggests that DOT1L may play a role in regulating transcription elongation and disorders related to it. Here, we aim to elucidate the functions of DOT1L and H3K79 methylation in cellular differentiation and transcription elongation. Unexpectedly, our results reveal that DOT1L harbors H3K79 methyltransferase-activity-independent functions in modulating neural differentiation and productive transcription elongation.
Results
DOT1L and H3K79 Methylation Are Dispensable for ESC Self-Renewal.
To investigate the role of DOT1L and its catalytic activity in transcription regulation, we first generated DOT1L knockout (KO) ESCs by deleting exon 5 of the DOT1L gene, which generates a frameshift mutant DOT1L with premature termination (9) (Fig. 1A). DOT1L catalytic inactive (CI) ESCs were derived by mutating the catalytic pocket Gly163 and Ser164 of the endogenous DOT1L as suggested previously (1, 14) (Fig. 1B). The genotypes of mutant cell lines were validated by PCR genotyping and Sanger sequencing of genomic DNA and complementary DNA (cDNA) (SI Appendix, Fig. S1 A and B). The morphology and growth rate of both KO and CI mutant cells are comparable to wild-type (WT) cells (SI Appendix, Fig. S1 C and D). Furthermore, RNA sequencing (RNA-seq) results confirmed the removal of exon 5 in DOT1L KO cell lines (SI Appendix, Fig. S1E). We noted that the deletion of exon 5 leads to a reduction in RNA levels of DOT1L (SI Appendix, Fig. S1E), suggesting that the stability of the exon 5 null transcript is impaired. Western blotting results indicated that DOT1L is undetectable in KO cells, while its levels in CI cells are comparable to that in WT ESCs (Fig. 1C). All three types of H3K79 methylation are abolished in respective DOT1L KO and CI cells (SI Appendix, Fig. S1 F and G and Fig. 1D), indicating the lack of in vivo catalytic activity of the catalytic mutant DOT1L in CI ESCs. Consistent with previous results, H3K79 methylation is enriched at genes with higher RNA Polymerase II (Pol II) levels (SI Appendix, Fig. S1 H and I and Fig. 1D). To examine if the loss of DOT1L or its activity has any impact on the ESC transcriptome, we performed differential gene expression analyses of RNA-seq data from WT, DOT1L KO, and DOT1L CI ESCs. We found that a limited number of genes were altered by DOT1L deletion or catalytic inactivation (Fig. 1E). In addition, RNA Pol II levels at transcription start sites (TSS) and gene bodies are largely unperturbed by DOT1L mutation (SI Appendix, Fig. S1H). These data demonstrate that neither DOT1L nor its catalytic activity plays a major role in the maintenance of the naive pluripotent stem cell transcriptome.
Fig. 1.
DOT1L deletion has limited impact on gene expression in ESCs. (A) Schematic representation of the generation of the DOT1L KO allele. (B) Sequences of WT (Top) and CI DOT1L alleles at exon 5. Mutated bases and amino acid residues are in red. PAM sequences are in orange. (C) Western blotting of total cell lysates of WT, DOT1L KO, and DOT1L CI ESCs. Antibodies used are labeled on the left. (D) Heatmaps showing the occupancy of H3K79me1 (Left), H3K79me2 (Middle), and H3K79me3 (Right) in WT, DOT1L KO, and DOT1L CI ESCs at the 7,362 RNA Pol II-positive genes of ESCs. The profiles are sorted by descending occupancy of Pol II in WT ESCs. cpm, counts per million mapped reads. (E) Correlation analysis of gene expression levels between WT and respective DOT1L KO (Left) and DOT1L CI (Right) ESCs. Significantly down-regulated genes [compared with WT, adjusted P < 0.01, log2 fold change (log2FC) > |1|] are in blue, and up-regulated ones are in red. Unchanged genes are gray dots. The number of up- and down-regulated genes are indicated in the upper left and bottom-right corners, respectively. RPKM, reads per kilobase of transcript per million mapped reads.
DOT1L Is Required for Establishing Transcription Signatures of Neural Progenitor Cells and Neural Differentiation.
DOT1L deletion leads to embryonic lethality in mice (9), suggesting that DOT1L is required for lineage commitment during development. To characterize the functions of DOT1L in cellular differentiation, we first performed embryoid body (EB) differentiation of WT, DOT1L KO, and DOT1L CI ESCs. Surprisingly, we did not observe deficiencies of EBs derived from DOT1L KO and CI ESCs compared with WT cells (SI Appendix, Fig. S2A). RNA-seq analyses of WT and DOT1L mutant EBs further demonstrated that the loss of DOT1L has little impact on gene up-regulation during EB differentiation (SI Appendix, Fig. S2B). These results prompted us to investigate the role of DOT1L in lineage specification. Previous studies indicate that DOT1L is highly expressed in the neural ectoderm and the optic vesicle in embryonic day 9.5 (E9.5) to E12.5 mouse embryos, suggesting a potential role of DOT1L and H3K79 methylation in neural development (9). We thus differentiated DOT1L mutant ESCs toward neural progenitor cells (NPCs) using a monolayer differentiation protocol which bypasses EB generation (Fig. 2A) (29, 30). During the 17-d differentiation course, cells gradually lost the dome-shaped morphology and adopted the spindle-shaped NPC morphology (Fig. 2A). RNA-seq analyses of NPCs and undifferentiated ESCs showed that neural-related genes are significantly up-regulated (Fig. 2 B and C), supporting the success of NPC differentiation. Although DOT1L mutant ESCs were able to differentiate into NPC-like cells (SI Appendix, Fig. S3A), DOT1L deletion led to the significant perturbation of 1,012 genes in NPCs (Fig. 2 D, Left). In contrast, DOT1L CI NPCs have 225 significantly differentially expressed genes in comparison with WT NPCs (Fig. 2 D, Right), suggesting that CI cells have a milder defect in neural differentiation than DOT1L null cells. Moreover, down-regulated genes in DOT1L KO NPCs are enriched for genes important for neuron differentiation and brain development, including Nestin and Sox11 (SI Appendix, Fig. S3 B and E). Furthermore, ∼70% of genes down-regulated in DOT1L null NPCs are elevated during NPC differentiation (SI Appendix, Fig. S3 C and G), further suggesting that DOT1L facilitates neural differentiation and the activation of neural genes in NPCs. The expression levels of NPC marker genes such as Nestin, Sox11, Sox9, and Cd117 are drastically reduced in DOT1L KO cells post differentiation (SI Appendix, Fig. S3 D and E). Interestingly, DOT1L deletion leads to a more substantial down-regulation of these markers than DOT1L catalytic inactivation in NPCs (SI Appendix, Fig. S3D). As expected, the majority of misregulated genes in DOT1L CI NPCs overlaps with that in DOT1L KO NPCs (SI Appendix, Fig. S3 I and J); however, the NPC transcriptome is much less perturbed by DOT1L catalytic inactivation than deletion (Fig. 2D and SI Appendix, Fig. S3H). We detected reduced RNA Pol II levels at the promoters of NPC markers in DOT1L KO NPCs (SI Appendix, Fig. S3F), demonstrating that the reduction in RNA levels of neural genes is due to the impairment in transcription. To further examine whether DOT1L has an impact on glial and neuronal lineage specification, we differentiated ESCs using a previously established protocol (31). After the 27-d culture, we found that DOT1L KO ESCs are defective in generating glial cells as represented by the lack of GFAP-positive cells in comparison with WT and DOT1L CI cells, although the formation of neurons is largely unaffected by DOT1L deletion (Fig. 2E). Collectively, these results indicate that DOT1L is indispensable for the establishment of NPC transcription signatures and glial cell specification, whereas its catalytic activity fine-tunes the transcription outputs in NPC differentiation.
Fig. 2.
DOT1L is required for neural differentiation. (A) Differentiation strategy for generating NPCs from ESCs. Cell morphologies for each step are shown with phase-contrast images. (Scale bar, 100 µm.) (B) Correlation analysis of gene expression levels between ESCs and NPCs. Genes significantly up-regulated in NPCs are in red, whereas genes with significantly higher levels in ESCs are in blue. (C) Gene ontology analysis of significantly up-regulated genes in NPCs vs. ESCs. The top 300 genes with the highest log2FC (RPKM) values were used as the input for the analysis. (D) Correlation analysis of gene expression levels between WT and respective DOT1L KO (Left) and DOT1L CI (Right) NPCs. Significantly down-regulated genes (compared with WT, adjusted P < 0.01, log2FC > |1|) are in blue, and up-regulated ones are in red. The number of up- and down-regulated genes are red and blue, respectively. (E) Immunostaining analyses of WT, DOT1LKO, and DOT1L CI cells after 27 d of neural differentiation. Antibodies used are labeled on top of the images. Nuclei were stained with Hoechst 33342. (Scale bars, 50 µm.)
The Differential Impact of DOT1L Deletion and SEC Inhibition on RNA Pol II Levels at Promoters.
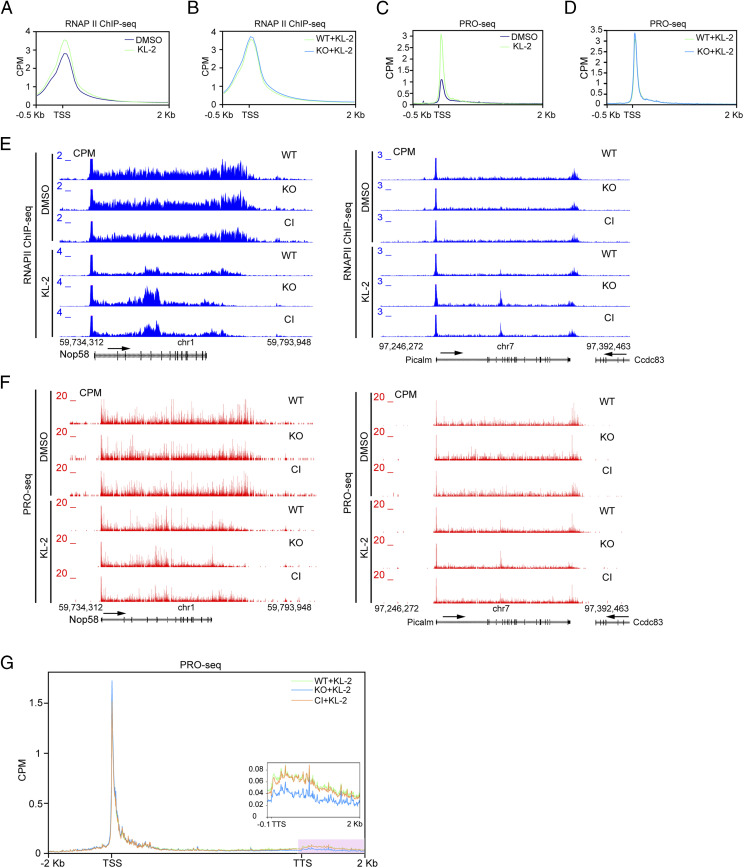
DOT1L has been linked to transcription regulation mainly due to the enrichment of all three H3K79 methylation marks at bodies of actively transcribed genes (Fig. 1D) and the observation that genes with higher levels of H3K79me2 tend to have higher transcription elongation rates (32, 33). Although removal of DOT1L has little effect on genome-wide RNA Pol II distribution or transcript levels in ESCs (SI Appendix, Fig. S1H, and Fig. 1E), we noted that down-regulated genes in DOT1L null NPCs have significantly higher gene length, higher length of the first intron, and higher intron numbers in comparison with up-regulated genes and all protein-coding genes (SI Appendix, Fig. S3 K–M). Together with the observation that the DOT1 complex (DotCom) and SEC share subunits AF9 and ENL (17, 19), these data suggest a possible cooperation of these complexes in promoting productive transcription elongation. To test this hypothesis, we utilized the recently reported SEC inhibitor KL-2 (28) and mutant ESCs generated here to dissect the relationship between SEC and DOT1L in modulating transcription elongation. Consistent with previous findings (28), we found that KL-2 treatment for 6 h leads to a decrease in the global level of the SEC component AFF1 and an elevation of RNA Pol II levels around TSS in ESCs (SI Appendix, Fig. S4A and Fig. 3A), indicating that the inhibition of SEC increases promoter proximal pausing of Pol II in ESCs. Interestingly, despite both ENL and AF9 being shared subunits between SEC and DotCom, the protein levels of ENL but not AF9 were down-regulated by SEC inhibition (SI Appendix, Fig. S4A), indicating the dependency of ENL stability on the integrity of SEC. To precisely map the positions of RNA Pol II, we performed precision nuclear run-on and sequencing (PRO-seq) (34, 35), providing single-nucleotide resolution of Pol II occupancy. Similar to Pol II ChIP sequencing (ChIP-seq) results, we observed the elevation of Pol II occupancy at TSSs after KL-2 treatment by PRO-seq (Fig. 3C). Such effects are more evident at TSSs of highly expressed genes (SI Appendix, Fig. S4 B and C), consistent with SEC being the highly active elongation complex. On the other hand, DOT1L deletion in ESCs has little impact on Pol II occupancy at TSSs of protein-coding genes regardless of SEC activity (Fig. 3 B and D, and SI Appendix, Fig. S4 B and C), suggesting that DOT1L is not a major regulator of Pol II pausing.
Fig. 3.
DOT1L deletion further impairs transcription elongation upon SEC inhibition. (A) Metagene analysis of Pol II occupancy at TSS of 22,219 protein-coding genes measured by ChIP-seq in ESCs treated with dimethylsulfoxide (DMSO) or KL-2 for 6 h. (B) Metagene analysis of Pol II occupancy at TSS of 22,219 protein-coding genes measured by ChIP-seq in WT and DOT1L KO ESCs treated with KL-2 for 6 h. (C) Metagene analysis of Pol II occupancy at TSS of 22,219 protein-coding genes measured by PRO-seq in ESCs treated with DMSO or KL-2 for 6 h. (D) Metagene analysis of Pol II occupancy at TSS of 22,219 protein-coding genes measured by PRO-seq in WT and DOT1L KO ESCs treated with KL-2 for 6 h. (E) Representative UCSC Genome Browser view of Pol II ChIP-seq in WT, DOT1L KO, and DOT1L CI cells treated with DMSO or KL-2 for the indicated genes. (F) Representative UCSC Genome Browser view of PRO-seq in WT, DOT1L KO, and DOT1L CI cells treated with DMSO or KL-2 for the indicated genes. (G) Metagene analysis of Pol II occupancy at TSS, gene bodies, and TTS of 22,219 protein-coding genes measured by PRO-seq in WT and DOT1L KO ESCs treated with KL-2 for 6 h. The length of each gene (from TSS to TTS) was normalized to 6 kb for plotting. Signals near TTS are highlighted by a pink box. (Inset) Zoomed-in view of the pink area.
SEC and DOT1L Coregulate the Poly(A)-Associated Transcription Elongation Checkpoint.
Although DOT1L deletion or catalytic inactivation has negligible effects on the distribution pattern of Pol II (SI Appendix, Fig. S1H), transcription elongation is more severely impaired by SEC inhibition in DOT1L KO cells compared with WT or DOT1L CI ESCs as evidenced by RNA Pol II ChIP-seq and PRO-seq (Fig. 3 E–G). In DOT1L null cells with SEC inhibited, Pol II is prematurely terminated at Nop58 and Picalm genes with accumulated Pol II signals at gene bodies (Fig. 3 E and F), suggesting that productive transcription elongation is hampered. One of the major transcription elongation checkpoints is located near the Poly(A) sites indicated by Pol II accumulation at transcription termination sites (TTS) (36), which ensures proper 3′ end processing. Interestingly, DOT1L deletion but not catalytic inhibition led to a reduction of Pol II levels near TTS in the presence of SEC inhibition (Fig. 3G and SI Appendix, Fig. S4E), suggesting that this elongation checkpoint is safeguarded by SEC and DOT1L in a partially redundant manner. It is noteworthy that the expression levels of SEC components are comparable in WT and DOT1L mutants and that the chromatin occupancy of AF10, one of the unique components of DotCom, is unperturbed after KL-2 treatment (SI Appendix, Fig. S4 D and F–G), suggesting that alternative mechanisms, rather than compromised SEC levels in DOT1L null cells or the loss of DotCom recruitment when SEC is inhibited, underlie the effect of DOT1L in regulating productive transcription elongation.
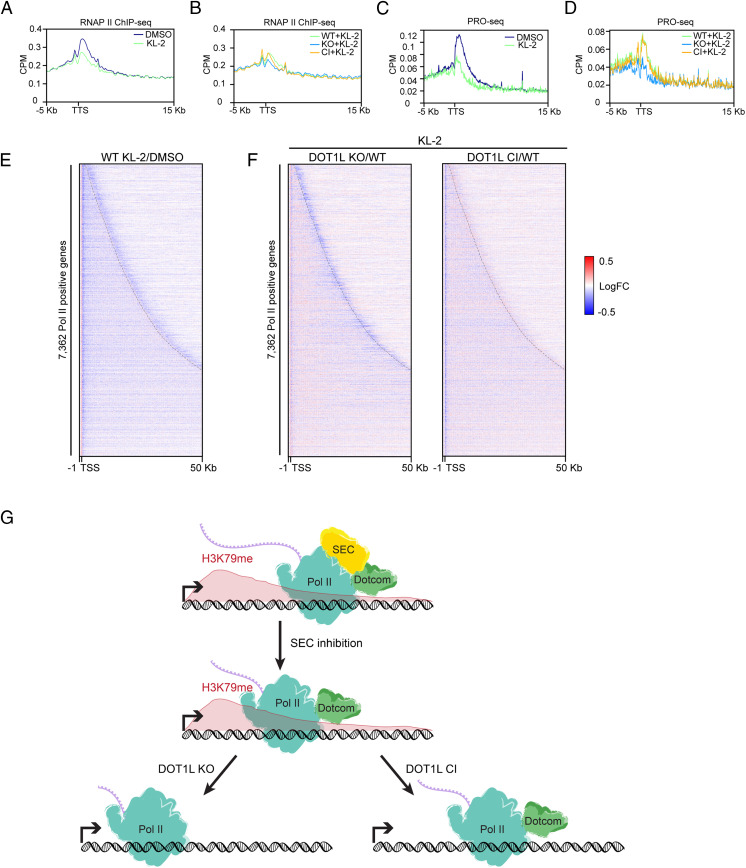
To investigate how transcription elongation is impaired by DOT1L and SEC loss-of-function, we analyzed our data focusing on the 3′ end of genes. Indeed, KL-2 treatment caused defects of Pol II accumulation at the TTS of protein-coding genes in ESCs (Fig. 4 A and C), indicating the reduction of Pol II processivity under SEC inhibition. DOT1L deletion, rather than catalytic inactivation, further inhibited transcription elongation at TTS upon SEC inhibition (Fig. 4 B and D). Such effects are stronger at highly expressed genes (SI Appendix, Fig. S5), indicating that the productive elongation at active genes is a critical step for their transcription regulation and is governed by both SEC and DOT1L largely independently of H3K79 methyltransferase activity. The impact of DOT1L on transcription elongation is further supported by PRO-seq analyses. SEC inhibition leads to the premature transcription termination at the 3′ end of genes (Fig. 4E). DOT1L KO led to a further reduction of Pol II at TTS upon KL-2 treatment while DOT1L catalytic inactivation had limited impact on 3′ Pol II levels (Fig. 4F). Taken together, these results argue that DOT1L, rather than its catalytic activity, participates in the regulation of transcription regulation through cooperating with SEC on safeguarding the Poly(A)-associated elongation checkpoint.
Fig. 4.
The poly(A)-associated transcription elongation checkpoint is regulated by SEC and DOT1L. (A) Metagene analysis of Pol II occupancy around TTS of 22,219 protein-coding genes measured by ChIP-seq in ESCs treated with DMSO or KL-2 for 6 h. (B) Metagene analysis of Pol II occupancy around TTS of 22,219 protein-coding genes measured by ChIP-seq in WT, DOT1L KO, and DOT1L CI ESCs treated with KL-2 for 6 h. (C) Metagene analysis of Pol II occupancy around TTS of 22,219 protein-coding genes measured by PRO-seq in ESCs treated with DMSO or KL-2 for 6 h. (D) Metagene analysis of Pol II occupancy around TTS of 22,219 protein-coding genes measured by PRO-seq in WT, DOT1L KO, and DOT1L CI ESCs treated with KL-2 for 6 h. (E) Heatmap depicting the log2 fold change in normalized PRO-seq signal (KL2/DMSO) within 10-bp bins from −1 κb to +50 kb relative to the TSS for WT ESCs. TTS are marked by the dashed line. (F) Heatmaps depicting the log2 fold change in normalized PRO-seq signal within 10-bp bins from −1 κb to +50 kb relative to the TSS for DOT1L KO/WT (Left) or DOT1L CI/WT (Right). (G) A model of catalytic-dependent and -independent function of DOT1L activity. DOT1L removal but not catalytic inactivation further lowers the elongation rate reduced by SEC inhibition.
Discussion
In this study, we report H3K79 methylation independent roles of DOT1L in regulating neural differentiation and transcription elongation. Furthermore, DOT1L’s contribution to transcription elongation appears to be redundant with SEC. Unlike previous reports (9, 12), we did not observe growth differences between DOT1L null and WT ESCs. We also did not observe defects of EB formation in DOT1L KO cells. It is noteworthy that all ESCs described in our study are maintained in the naive pluripotent state with serum-free 2i/leukemia inhibitory factor (LIF) media (37), which is drastically different from serum/LIF conditions used to culture ESCs in previous reports. Naive pluripotency is characterized by homogeneously expressed pluripotency genes such as Nanog and Klf4, DNA hypomethylation, and reduced bivalency (38–40). In addition, DOT1L loss did not trigger major transcriptional changes in ESCs or differentiated EBs, in line with the finding that the developmental retardation of DOT1L null embryos occurs during midgestation (9). EB differentiation generates a population of heterogeneous cells; thus it is possible that the bulk-level RNA-seq technique could not detect transcriptional perturbation in a small group of cells. Single-cell RNA sequencing would be a powerful tool to dissect the roles of DOT1L in regulating expression of lineage-specific genes during EB differentiation in the future. Consistent with DOT1L’s enrichment in neuroectoderm during mouse development, many neural genes fail to properly express in DOT1L null cells during NPC differentiation, suggesting that DOT1L governs the differentiation trajectory of neuroectoderm. Indeed, our neuronal and glial differentiation demonstrates impaired glial cell specification in DOT1L KO cells. Interestingly, the catalytic activity of DOT1L is dispensable for both neuronal and glial specification.
Our results that several NPC marker genes are mildly deregulated in DOT1L CI cells suggest that H3K79 methylation could participate in fine-tuning the transcription program during neural differentiation. Although we did not observe an instructive role of H3K79 methylation in EB formation, NPC differentiation, or neuronal and glial specification, it is possible that H3K79 methylation functions in vivo in a tissue-specific manner, which is worth investigating in the future. Recent reports have demonstrated catalytic-independent functions of yeast DOT1 in promoting histone exchange, nucleosome remodeling, and H2B ubiquitination (H2Bub) (41, 42). Independent structural analyses of DOT1L and H2B ubiquitinated nucleosomes suggest that the interaction of H2Bub and DOT1L could stabilize the binding of DOT1L to nucleosomes (43–47). Intriguingly, such binding of DOT1L to ubiquitinated nucleosomes could in turn destabilize them by unwrapping nucleosomal DNA (44), which could contribute to the catalytic-independent function of DOT1 in promoting transcription elongation.
DOT1L null ESCs do not exhibit apparent transcription elongation defects; however, loss of DOT1L enhances the processivity defects caused by SEC inhibition. This provides experimental evidence that DOT1L has a positive but catalytic-independent role in transcription elongation. It is possible that proper expression of key genes of neural differentiation relies on an elevated transcription elongation rate, which may be impaired by DOT1L depletion alone, which could lead to diminished expression of neural genes and an impairment of glial cell specification. It is worth noting that ENL levels in ESCs are diminished by KL-2 treatment. ENL reductions could contribute to the enhanced elongation defect of combined SEC inhibition and DOT1L loss as it is a common subunit of both complexes. However, we cannot rule out that DOT1L has additional, unknown noncatalytic functions that contribute to the observed defects.
Although the transcriptional perturbation by DOT1L deletion in ESCs is subtle, a more severe misregulation of transcription might be obtained upon acute degradation of DOT1L, as the DOT1L null cells may have adapted to the absence of DOT1L. The 6-h treatment with KL-2 is less likely to have indirect effects, but again, an acute degradation of 1 or 2 h could further minimize indirect effects of these complexes in promoting transcription elongation. Furthermore, elucidating the mechanisms underlying the role of DotCom in transcription elongation with in vitro assays would be important to understand human diseases associated with defects in transcription elongation machineries in the future.
In summary, our study defines catalytic-independent roles of H3K79 methyltransferase DOT1L in cellular differentiation and transcription elongation. Given that DOT1L has been implicated in leukemogenesis, our study suggests that degrading DOT1L rather than solely inhibiting its catalytic activity could be instrumental in cancer therapy. More importantly, the concept that combining SEC inhibition and targeted DOT1L degradation further slows down RNA Polymerase II processivity (Fig. 4G) may facilitate the development of therapy to treat transcription-addicted human developmental diseases and cancer (48).
Materials and Methods
Antibodies.
The following antibodies were used in this study: anti-DOT1L [generated in house (17), 1: 500 for Western blotting]; anti-H3K79me1 (generated in house, 1:1,000 for Western blotting, 1:100 for ChIP-seq); anti-H3K79me2 (Abcam 3594, 1 µg/mL for Western blotting); anti-H3K79me2 (generated in house, 1:100 for ChIP-seq); anti-H3K79me3 (Abcam 2621, 1 µg/mL for Western blotting, 1:100 for ChIP-seq); anti-GFAP (Abcam 7260, 1:1,000 for immunostaining); anti-βIII tubulin/TUJ1 (Millipore MAB1637, 1:50 for immunostaining); anti-AFF1 (Bethyl A302-344A, 1:1,000 for Western blotting); anti-AF9 (generated in house, 1:4,000 for Western blotting); anti-ENL (Cell Signaling Technology 14893, 1:1,000 for Western blotting); anti-RBBP5 (Bethyl A300-109A, 1:5,000 for Western blotting); anti-AF10 (Santa Cruz 53156, 1:100 for ChIP-seq); and anti-RNA Pol II (Cell Signaling Technology 14958, 1:100 for ChIP-seq).
ESC Culture, CRISPR/Cas9-Guided Gene Editing, and Differentiation.
The v6.5 ESCs (49) were grown in N2B27 media supplemented with two inhibitors and LIF as described previously (40). Briefly, cells were grown on plates coated with 0.1% gelatin and passaged when they reach 60 to 75% confluency. All KL-2 treatments were performed at 10 µM of KL-2 for 6 h. Plasmids containing desired guide RNAs (gRNAs) were generated, and transfections were performed as previously described (50). Briefly, 20 µg of plasmids were transfected into ESCs using Nucleofector (Lonza). Single-cell clones were picked 10 d after transfection, and PCR was used for genotyping the clones. For DOT1L KO ESCs, a plasmid carrying 500-bp homology arms flanking the inserted sequences including two loxP sites were cotransfected with the gRNA plasmid. Cre recombinase was then transiently expressed in the loxP sites containing cells to remove the desired region. For generating DOT1L CI ESCs, asymmetric single-stranded oligonucleotides were designed as described (51) and cotransfected as donors together with the gRNA plasmid. The sequence of sgRNA used for generating the KO allele and the CI allele was 5′-CACTGCCCAGGTCGACAAAC-3′.
EB differentiation was performed using the hanging-drop method (31) with adaptation as previously described (52). Briefly, hanging drops were loaded on the lids of 15-cm plates and cultured for 6 d. NPC differentiation protocol was adapted from ref. 30. Briefly, cells were seeded on a gelatinized dish in 2i/LIF media for a day. The two inhibitors and LIF were then withdrawn from the media, and cells were kept in plain N2B27 media for 7 d. Cells were then dissociated and seeded in ultra-low attachment plates (Sigma) in N2B27 media containing 10 ng/mL epidermal growth factor (EGF) (Peprotech) and 10 ng/mL basic fibroblast growth factor (FGF2) (Peprotech) for 3 d to form neurospheres. Floating aggregates (neurospheres) were then seeded on gelatinized plates in N2B27 containing 10 ng/mL EGF and 10 ng/mL FGF2 for 2 to 4 d. Attached cells were dissociated and seeded again on gelatinized plates in N2B27 containing 10 ng/mL EGF and 10 ng/mL FGF2. Cells were passaged one more time and harvested for different assays. Neural differentiation was performed as previously published (31). Briefly, EBs were generated from ESCs using the hanging-drop method. EBs were then treated with 1 µM all-trans retinoic acid for 2 d and reseeded on plates coated with poly-L-ornithine (5 µg/mL) and laminin (5 µg/mL). EBs were cultured with NeuroCult NSC proliferation medium (StemCell Technologies) supplemented with 10 ng/mL FGF2 (Peprotech) for 21 d. Media was changed every 3 d.
Immunofluorescence.
Immunostaining was performed as previously described (31). Briefly, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% TritonX-100 in phosphate-buffered saline (PBS), and blocked with 10% fetal bovine serum (FBS) in PBS. Cells were then stained with primary antibody diluted with 10% FBS in PBS, washed with 0.2% TritonX-100 in PBS, stained with fluorescent secondary antibody and Hoechst dye diluted with 10% FBS in PBS in the dark, and washed again with 0.2% TritonX-100 in PBS. Coverslips were mounted to glass slides using Fluoromount-G (Thermo Fisher) and sealed with nail polish. Images were collected using the Nikon confocal microscope A1 located at the imaging center of Northwestern University.
RNA-seq, ChIP-seq, and PRO-seq.
RNA- and ChIP-seq experiments and library generation were performed following previously published protocols (50). For RNA-seq, cells were lysed with TRIzol reagent (Thermo Fisher), and RNA was purified according to the manufacturer’s instructions. RNA was further treated with RNase free DNase I (Sigma), and the treated RNA was purified with an RNeasy mini kit (Qiagen). RNA-seq libraries were prepared using a TruSeq-stranded total RNA sample preparation kit (Illumina). For ChIP-seq, ESCs were fixed with 1% formaldehyde for 10 min. Chromatin was sheared using an E220 focused ultrasonicator (Covaris). Sheared chromatin was mixed with antibody and protein A/G beads (Santa Cruz Biotechnology) and incubated overnight at 4 °C. Immunoprecipitated DNA was purified and submitted for library preparation. ChIP-seq libraries were generated with a KAPA HTP library preparation kit (KAPA Biosystems) following the manufacturer’s instruction.
PRO-seq was performed as previously described (28, 35). In brief, 0.5 million Drosophila S2 cell nuclei were spiked into 10 million ESC nuclei. Nuclear run-on assays were then performed, and RNA was fragmented. Biotinylated RNA was purified by streptavidin beads M-280 (Thermo Fisher), 5’ cap removed, the 5' hydroxyl was repaired, and ligated with adaptors. After reverse transcription, cDNA was amplified using Phusion Hot Start II DNA polymerase (ThermoFisher). DNA Libraries were size selected by PippinHT (Sage).
RNA-seq libraries were prepared using a TruSeq-stranded total RNA sample preparation kit (Illumina), and ChIP-seq libraries were prepared with a KAPA HTP library preparation kit (KAPA Biosystems) following the manufacturer’s instructions. ChIP-seq and RNA-seq libraries were sequenced on the NextSEq. 500 sequencer (Illumina) using single-end sequencing for 50 cycles. The Ceto modular pipeline (53) was used for generating bam files and genome browser tracks. Briefly, after BCL2FASTQ conversion, reads were trimmed with Trimomatic, version 0.33 (54), aligned with Bowtie version 1.1.2 [(55), ChIP-seq] or Tophat version 2.1.0 [(56), RNA-seq] to the mm9 genome. Raw reads were normalized to total read counts per million (cpm) and visualized in the UCSC Genome Browser as bigWig-formatted coverage tracks.
PRO-seq libraries were sequenced on the NextSEq. 500 sequencer using single-end sequencing for 75 cycles, and after running BCL2FASTQ, reads were processed as described below. Adapters were removed from raw reads with cutadapt version 1.14 (57). Reads were trimmed from the 3′ end to 36 bp to remove low-quality bases using Trimmomatic version 0.33 (54) requiring a minimal read length of 16 bp. Reads were then mapped to the mouse genome (mm9) and fruit fly genome (dm3) using Bowtie version 1.1.2 (55). Only uniquely mapped reads with up to two mismatches in the entire read were used for further analysis. The total number of mouse reads was normalized by the number of Drosophila reads for each experiment. Normalized reads were then converted to single-nucleotide 3′ bigWig strand-specific tracks by taking 5′ positions of the read using BEDtools genomecov version 2.17 (58) with the options -strand -bg -5. Strands were then swapped to give the correct orientation with the 5′ end, now becoming the 3′ end of the read (59).
Data Analysis.
Using Ceto (53), RNA-seq gene counts were computed by HTSeq. (60) and used as input for edgeR version 3.12.1 (61). Genes with cpm less than 1 were filtered out in each comparison. A Benjamini–Hochburg-adjusted P value threshold of 0.01 and a log2 cutoff of 1 were used to identify genes significantly differentially expressed in one experimental condition relative to another. RNA-seq correlation plots were generated using cutsomized R scripts. Gene ontology analyses were performed using Metascape (62).
The 7,362 genes used for ChIP-seq analysis were selected as follows. Genes filtered from an mm9 TxDb were required to be Ensembl protein-coding genes that were also RefSeq validated. The best transcript with the highest coverage in the TSS region from 0 to +100 bp was selected, after which the genes were required to be at least 1 kb in length, have a minimal distance of 1 kb to the nearest gene, and have a maximum cpm in the TSS region of at least 1. Genes were then organized in a descending order based on their maximum signals at the TSS. All ChIP-seq heat maps were generated using ngsplot version 2.47 (63), and metagene plots were generated using deepTools version 3.1.1 (64), where signal is grouped into 10-bp bins in figures depicting signal relative to the TSS or TTS. AF10 peaks were called using model-based analysis of ChIP-seq (MACS) v1.4.2 with default parameters (65) with sonicated input DNA as the control.
PRO-seq metagene plots and heat maps were generated using deepTools version 3.1.1, where signal is grouped into 10-bp bins in figures depicting signal relative to the TSS and in 50-bp bins in figures depicting signal relative to the TTS.
For RNA-seq box plot analyses in SI Appendix, Figs. S3 and S4, Student’s t test and Wilcoxon signed-rank test were performed to calculate the statistical significance. Sample size and P values are reported in the figures and figure legends.
Supplementary Material
Acknowledgments
We thank the members of the A.S. laboratory for helpful discussions; Fei X. Chen for insightful suggestions; and Nicole Ethen for illustrations in Fig. 4G. K.C. was supported by the NIH Pathway to Independence Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant K99HD094906). E.R.S. is supported by the Research Specialist Award from the National Cancer Institute (NCI) (Grant R50CA211428). This study was supported in part by Grants R01CA214035 and R35CA197569 from the NCI (to A.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001075117/-/DCSupplemental.
Data Availability.
All sequencing data generated by this study have been deposited in the Gene Expression Omnibus under accession number GSE134083 (66).
References
- 1.Feng Q.et al., Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr. Biol. 12, 1052–1058 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Lacoste N., Utley R. T., Hunter J. M., Poirier G. G., Côte J., Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J. Biol. Chem. 277, 30421–30424 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Ng H. H.et al., Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 16, 1518–1527 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Leeuwen F., Gafken P. R., Gottschling D. E., Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109, 745–756 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Schübeler D.et al., The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 18, 1263–1271 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vakoc C. R., Sachdeva M. M., Wang H., Blobel G. A., Profile of histone lysine methylation across transcribed mammalian chromatin. Mol. Cell. Biol. 26, 9185–9195 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steger D. J.et al., DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol. Cell. Biol. 28, 2825–2839 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z.et al., Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 40, 897–903 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones B.et al., The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet. 4, e1000190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada Y.et al., hDOT1L links histone methylation to leukemogenesis. Cell 121, 167–178 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Bernt K. M.et al., MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell 20, 66–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry E. R.et al., ES cell cycle progression and differentiation require the action of the histone methyltransferase Dot1L. Stem Cells 27, 1538–1547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onder T. T.et al., Chromatin-modifying enzymes as modulators of reprogramming. Nature 483, 598–602 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min J., Feng Q., Li Z., Zhang Y., Xu R. M., Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell 112, 711–723 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Dlakić M., Chromatin silencing protein and pachytene checkpoint regulator Dot1p has a methyltransferase fold. Trends Biochem. Sci. 26, 405–407 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Sawada K.et al., Structure of the conserved core of the yeast Dot1p, a nucleosomal histone H3 lysine 79 methyltransferase. J. Biol. Chem. 279, 43296–43306 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan M.et al., Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom). Genes Dev. 24, 574–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Z., Lin C., Shilatifard A., The super elongation complex (SEC) family in transcriptional control. Nat. Rev. Mol. Cell Biol. 13, 543–547 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Lin C.et al., AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol. Cell 37, 429–437 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C.et al., Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC). Genes Dev. 25, 1486–1498 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen V. T., Kiss T., Michels A. A., Bensaude O., 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322–325 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Yang Z., Zhu Q., Luo K., Zhou Q., The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317–322 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Jang M. K.et al., The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell 19, 523–534 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Luo Z.et al., The super elongation complex family of RNA polymerase II elongation factors: Gene target specificity and transcriptional output. Mol. Cell. Biol. 32, 2608–2617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama A., Lin M., Naresh A., Kitabayashi I., Cleary M. L., A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell 17, 198–212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He N.et al., HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol. Cell 38, 428–438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobhian B.et al., HIV-1 Tat assembles a multifunctional transcription elongation complex and stably associates with the 7SK snRNP. Mol. Cell 38, 439–451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang K.et al., Targeting processive transcription elongation via SEC disruption for MYC-induced cancer therapy. Cell 175, 766–779.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A., Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Nora E. P.et al., Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell 169, 930–944.e22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.et al., Histone h1 depletion impairs embryonic stem cell differentiation. PLoS Genet. 8, e1002691 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonkers I., Kwak H., Lis J. T., Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife 3, e02407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veloso A.et al., Rate of elongation by RNA polymerase II is associated with specific gene features and epigenetic modifications. Genome Res. 24, 896–905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak H., Fuda N. J., Core L. J., Lis J. T., Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science 339, 950–953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mas G.et al., Promoter bivalency favors an open chromatin architecture in embryonic stem cells. Nat. Genet. 50, 1452–1462 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Laitem C.et al., CDK9 inhibitors define elongation checkpoints at both ends of RNA polymerase II-transcribed genes. Nat. Struct. Mol. Biol. 22, 396–403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying Q. L.et al., The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marks H.et al., The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590–604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Los Angeles A.et al., Hallmarks of pluripotency. Nature 525, 469–478 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Cao K.et al., An Mll4/COMPASS-Lsd1 epigenetic axis governs enhancer function and pluripotency transition in embryonic stem cells. Sci. Adv. 4, eaap8747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.et al., Dot1 regulates nucleosome dynamics by its inherent histone chaperone activity in yeast. Nat. Commun. 9, 240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Welsem T.et al., Dot1 promotes H2B ubiquitination by a methyltransferase-independent mechanism. Nucleic Acids Res. 46, 11251–11261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson C. J.et al., Structural basis for recognition of ubiquitylated nucleosome by Dot1L methyltransferase. Cell Rep. 26, 1681–1690.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang S.et al., Structural basis of recognition and destabilization of the histone H2B ubiquitinated nucleosome by the DOT1L histone H3 Lys79 methyltransferase. Genes Dev. 33, 620–625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valencia-Sanchez M. I.et al., Structural basis of Dot1L stimulation by histone H2B lysine 120 ubiquitination. Mol. Cell 74, 1010–1019.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worden E. J., Hoffmann N. A., Hicks C. W., Wolberger C., Mechanism of cross-talk between H2B ubiquitination and H3 methylation by Dot1L. Cell 176, 1490–1501.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao T.et al., Structural basis of the crosstalk between histone H2B monoubiquitination and H3 lysine 79 methylation on nucleosome. Cell Res. 29, 330–333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen F. X., Smith E. R., Shilatifard A., Born to run: Control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 19, 464–478 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Rideout W. M. IIIet al., Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat. Genet. 24, 109–110 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Cao K.et al., SET1A/COMPASS and shadow enhancers in the regulation of homeotic gene expression. Genes Dev. 31, 787–801 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson C. D., Ray G. J., DeWitt M. A., Curie G. L., Corn J. E., Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34, 339–344 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Sze C. C.et al., Histone H3K4 methylation-dependent and -independent functions of Set1A/COMPASS in embryonic stem cell self-renewal and differentiation. Genes Dev. 31, 1732–1737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodfin A. R., Ozark P. A., Shilatifard A., Bartom E. T., Ceto: An extensible, modular pipeline ecosystem for Next Generation Sequence Analysis. GitHub. https://github.com/ebartom/NGSbartom. Accessed 15 October 2020.
- 54.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langmead B., Trapnell C., Pop M., Salzberg S. L., Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D.et al., TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin M., Cutadapt removes adapter sequences from high-throughput sequencing read. EMBnet. J. 17, 10–12 (2011). [Google Scholar]
- 58.Quinlan A. R., Hall I. M., BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahat D. B.et al., Base-pair-resolution genome-wide mapping of active RNA polymerases using precision nuclear run-on (PRO-seq). Nat. Protoc. 11, 1455–1476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anders S., Pyl P. T., Huber W., HTSeq: A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson M. D., McCarthy D. J., Smyth G. K., edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y.et al., Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen L., Shao N., Liu X., Nestler E., ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics 15, 284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramírez F.et al., deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y.et al., Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao K., Ozark P. A., Shilatifard A., DOT1L-controlled cell-fate determination and transcription elongation are independent of H3K79 methylation Gene Expression Omnibus. http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE134083. Deposited 15 June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data generated by this study have been deposited in the Gene Expression Omnibus under accession number GSE134083 (66).