We read with interest the Letter by Schierwagen et al. (1), which reports that fibrotic liver injury in rodents and in patients is associated with increased whole liver expression of β-arrestin 2 (β-Arr2), appearing to contrast with our recent report (2) demonstrating that injury induced by bile duct ligation (BDL) in mice leads to decreased β-Arr2 expression in liver sinusoidal endothelial cells (LSECs). We demonstrated that reduced β-Arr2 expression in this specific cell type is associated with reduced activation of endothelial nitric oxide synthase and portal hypertension.
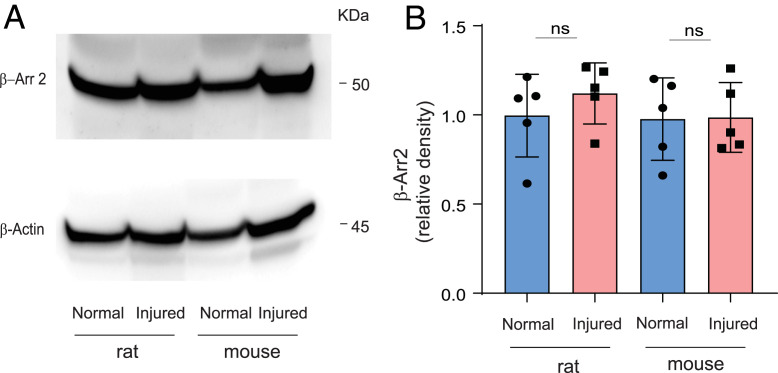
We find that whole liver β-Arr2 appears to be unchanged to slightly elevated (although not statistically so) after BDL in rats and mice (Fig. 1). However, we emphasize that the finding of changes in whole liver β-Arr2 is difficult to translate to the biology of disease, largely because dynamic changes of molecules such as β-Arr2 in specific cells drive phenotypes. Whole liver studies of proteins are always confounded by the balance of parenchymal (hepatocytes) and nonparenchymal cells (stellate, endothelial, and Kupffer cells) in the liver. Given that hepatocytes make up at least 80% of the cells in the liver, changes in protein expression at the whole liver level often reflect expression by hepatocytes—and it is possible that the changes reported by Schierwagen et al. (1) reflect (even small) changes in hepatocyte expression. One of the key strengths of our study (2) is in defining the cellular changes in β-Arr2 in LSECs directly. It should also be noted that we used knockout mice as controls for our immunostaining (2) to stringently validate the specificity of the β-arrestin antibodies used.
Fig. 1.
β-Arr2 expression in normal and BDL-injured liver. (A) Whole liver protein lysates from normal and 14-d BDL-injured rat (Left) and mouse (Right) were subjected to immunoblotting to detect β-Arr2 and β-actin as described (2). (B) Specific β-Arr2 bands were quantitated, normalized to β-actin (n = 5 per group), and plotted with mean ± SD; ns, no significant difference.
A number of recent publications have noted altered expression of β-Arr2 and the related β-Arr1 after various forms of liver injury, and revealed roles for both β-arrestins (3–9). Of particular interest was a transient ischemia/reperfusion study of liver injury, in which β-Arr2 protein increased (β-Arr1 was not assessed), finding that β-Arr2 knockout mice had worse outcomes (7), consistent with vascular dysregulation, similar to our work (2). Notably, cellular localization was not assessed, and/or the specificity of the antisera used was not established in most of these studies.
Although Schierwagen et al. (1) report elevated β-Arr2 in human patient samples with fibrosis, others have reported increased β-Arr1 but unchanged β-Arr2 (6), as well as elevated β-Arr2 (4). Overall, due to the small numbers of patient samples assessed and the inherent variability among patients even within defined fibrotic injury types (viral, alcohol, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, etc.), it seems premature to claim a clear understanding of the role of either β-arrestin protein in human fibrotic liver disease, although β-arrestins certainly are implicated.
Taken together, these studies suggest that β-arrestin expression changes depend on the type and timing of liver injury and are almost certainly different in distinct liver cell types. The general principles and driving stimuli responsible for each of these changes remain unknown, and much more work is needed to encompass the breadth of injury-dependent responses and roles of β-arrestin proteins in the liver (10).
Footnotes
The authors declare no competing interest.
References
- 1.Schierwagen R.et al., β-Arrestin2 is increased in liver fibrosis in humans and rodents. Proc. Natl. Acad. Sci. U.S.A. 117, 27082–27084 (2020).33144522 [Google Scholar]
- 2.Liu S., Luttrell L. M., Premont R. T., Rockey D. C., β-Arrestin2 is a critical component of the GPCR−eNOS signalosome. Proc. Natl. Acad. Sci. U.S.A. 117, 11483–11492 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J. C.et al., Depletion of β-arrestin 2 protects against CCl4-induced liver injury in mice. Biochem. Biophys. Res. Commun. 522, 485–491 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Sun W. Y.et al., β-arrestin2 deficiency protects against hepatic fibrosis in mice and prevents synthesis of extracellular matrix. Cell Death Dis. 11, 389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun W. Y.et al., Depletion of β-arrestin2 in hepatic stellate cells reduces cell proliferation via ERK pathway. J. Cell. Biochem. 114, 1153–1162 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Tan S.et al., β-Arrestin1 enhances liver fibrosis through autophagy-mediated Snail signaling. FASEB J. 33, 2000–2016 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Wen Y.et al., β-arrestin2 inhibits apoptosis and liver inflamation induced by ischemia-reperfusion in mice via AKT and TLR4 pathway. Arch. Med. Res. 50, 413–422 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Xuan J.et al., MiR-29a and miR-652 attenuate liver fibrosis by inhibiting the differentiation of CD4+ T cells. Cell Struct. Funct. 42, 95–103 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Yang Y.et al., Indispensable role of β-arrestin2 in the protection of remifentanil preconditioning against hepatic ischemic reperfusion injury. Sci. Rep. 9, 2087 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe H., Schuppan D., β-arrestin: Dr Jekyll and Mr Hyde in NASH and fibrosis. J. Hepatol. 72, 813–815 (2020). [DOI] [PubMed] [Google Scholar]